Abstract
Mercaptoacetate (MA) is an orexigenic agent reported to block fatty acid (FA) oxidation. Recently, however, we reported evidence from isolated nodose ganglion neurons that MA antagonizes the G protein-coupled long- and medium-chain FA receptor GPR40. GPR40 mediates FA-induced secretion of the satietogenic incretin peptide glucagon-like peptide 1 (GLP-1), by enteroendocrine L cells, as well as FA-induced enhancement of glucose-stimulated insulin secretion. Our results in cultured nodose neurons suggest that MA would also block GPR40 in enteroendocrine cells controlling GLP-1 secretion. If so, this would suggest an alternative mechanism by which MA increases food intake. We tested the hypothesis that MA blocks FA-induced GLP-1 secretion in vitro using cultured STC-1 cells (a murine enteroendocrine cell line) and in vivo in adult male rats. In vitro, MA blocked the increase in both cytosolic Ca2+ and GLP-1 release stimulated by FAs and also reduced (but less effectively) the response of STC-1 cells to grifolic acid, a partial agonist of the GPR120 FA receptor. In vivo, MA reduced GLP-1 secretion following olive oil gavage while also increasing glucose and decreasing insulin levels. The carnitine palmatoyltransferase 1 antagonist etomoxir did not alter these responses. Results indicate that MA's actions, including its orexigenic effect, are mediated by GPR40 (and possibly GPR120) receptor antagonism and not by blockade of fat oxidation, as previously believed. Analysis of MA's interaction with GPR40 may facilitate understanding of the multiple functions of this receptor and the manner in which FAs participate in the control of hunger and satiety.
Keywords: glucagon-like peptide-1, G protein-coupled receptor 40, G protein-coupled receptor 120, fatty acids, mercaptoacetate
mercaptoacetate (MA) is an orexigenic agent (51) that has been used extensively for studies of feeding behavior (46, 47, 51). Because MA inhibits mitochondrial acyl-CoA dehydrogenase, thereby reducing fatty acid (FA) oxidation (3, 4), it has been widely assumed that this mechanism mediates MA's orexigenic action (35, 36, 37, 40, 53). Recently, however, we reported evidence for a novel mechanism through which MA potentially could increase feeding and that provides additional information regarding MA's potential sites of action (10), which are currently unknown. Using cultured nodose ganglion neurons, we found that MA antagonizes G protein-coupled receptor 40 (GPR40), a membrane receptor for long- and medium-chain FAs (7), also known as free fatty acid receptor 1. Linoleic acid (LA) and caprylic acid (long- and medium-chain FAs, respectively) and GW-9508 [a synthetic agonist of GPR40 (6)] increased cytosolic Ca2+ levels ([Ca2+]i) in nodose neurons, whereas MA reversibly blocked those effects without altering responses to agents that increase [Ca2+]i by other mechanisms (10). We also confirmed the expression of GPR40 by nodose neurons using RT-PCR.
Currently, neither the function of GPR40 on vagal afferent neurons nor the consequences of MA's direct actions on these nodose FA receptors are known. Given the dependence of MA-induced feeding on vagal afferent neurons (5, 48, 49), it is possible that this vagal site of action may account for MA's orexigenic effects. However, GPR40 is expressed in a variety of tissues with prominent roles in energy homeostasis and feeding, including taste bud cells (12), cholecystokinin (CCK)-secreting I cells (41), glucagon-like peptide 1 (GLP-1)-secreting L cells (11), and pancreatic α- and β-cells (62) (for review, see Ref. 21). Therefore, it is possible that MA-induced stimulation of feeding is mediated by its effects on taste cells and/or on secretion of satietogenic gastrointestinal or pancreatic hormones, or by widespread action at multiple GPR40 receptor sites.
Here we used in vitro and in vivo approaches to examine effects of MA on FA-induced secretion of the satietogenic peptide GLP-1, a response mediated by GPR40 (11, 58, 61). GLP-1 is an incretin hormone that significantly influences systemic metabolism by enhancing insulin secretion and reducing blood glucose (1, 2, 16, 44), inhibiting gastric emptying (20), and promoting satiety (1, 50, 59, 65). Our results reveal that MA, but not the FA oxidation inhibitor etomoxir, blocks GPR40 and reduces GLP-1 secretion in vivo and in vitro. These results indicate that MA's orexigenic effect arises, at least in part, from blockade of FA membrane receptors rather than from blockade of FA oxidation. Results not only clarify MA's mechanism of action but also expand our appreciation of the avenues through which FAs control food intake and metabolism. Moreover, because of its role in secretion of gastrointestinal incretin hormones and insulin, GPR40 has become a prominent target for development of therapeutic drugs for treatment of obesity and diabetes and disorders of appetite (14, 24). Understanding MA's effects on GPR40′s receptor function would be of potential importance for development of such drugs.
RESEARCH DESIGN AND METHODS
Animals.
Adult male Sprague-Dawley rats were purchased from Simonsen Laboratories (Gilroy, CA) and housed individually in an animal care facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were maintained on a 12:12-h light-dark cycle, under controlled temperature conditions (22°C), with ad libitum access to pelleted rodent food (Rodent diet 5001; LabDiet, St. Louis, MO) and tap water unless otherwise noted. All experimental procedures were approved by the Washington State University Institutional Animal Care and Use Committee, which conforms to National Institutes of Health guidelines.
Cell culture.
STC-1 cells, derived from murine enteroendocrine cells (a gift from Dr. Helen Raybould, University of California Davis), were cultured in DMEM (SH3002201; ThermoFisher Scientific, Waltham, MA) supplemented with 100 IU/ml penicillin, 0.1 mg/ml streptomycin, 0.25 μg/ml amphotericin B, and 10% fetal calf serum (64). Every 3–4 days (at ∼90% confluence) cells were treated with 0.25% trypsin/EDTA and subcultured in culture flasks.
Ca2+ imaging and data analysis.
Ca2+ imaging experiments were performed at room temperature (22°C) using a Nikon Eclipse inverted microscope and a ×40 objective. [Ca2+]i was measured ratiometrically using fura 2-AM as previously reported (29, 66). STC-1 cells were cultured on coverslips (∼105 cells/coverslip) for 1 day in culture medium. On the day of the experiment, coverslips were rinsed and incubated in physiological buffer (standard bath) composed of (in mM) 40 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 6 glucose, and 10 HEPES (pH adjusted to 7.4 with Tris base) with 2 μM fura 2-AM for 40 min followed by a 20-min wash. Next, the coverslips were mounted in a closed chamber (260 μl). LA (0.1–10 μM; Sigma-Aldrich, St. Louis, MO), GW-9508 (GW, a GPR40 agonist, 1–5 μM; Tocris, Minneapolis, MN), grifolic acid (GA, a GPR120 agonist, 0.1–1 μM; Tocris), and MA (β-mercaptoacetic acid, 0.2 mM; Sigma-Aldrich) were diluted in standard buffer from stock solutions and applied via inflow lines that were switched through a common manifold upstream of the perfusion chamber. After switching, new solutions were delivered to the cultured cells after a delay of ∼15 s. Stock solutions of GW (50 μM) and GA (10 μM) were made in DMSO, LA stock solution (5 mM) was made in water, and MA solution was made in standard buffer just before each application. The working concentrations of LA, GW, GA, and MA were modified from the published data from our lab and others (6, 10, 15) and were confirmed in STC-1 cells by preliminary experiments. At the end of each run a high-potassium (Hi-K) solution (90 mM NaCl and 55 mM KCl, otherwise the same as the above physiological buffer) was applied for 1 min to depolarize the cells and to determine whether cells were viable. Cells were included in the analysis if they responded to the Hi-K challenge with a rapid and reversible Ca2+ response of at least three times over baseline in amplitude. [Ca2+]i was monitored and analyzed by MetaFluor Software (Universal Imaging, West Chester, PA). Image pairs (340 and 380 nm excitation, 510 emissions) were collected every 6 s. The ratios of the fluorescence intensity were then converted to Ca2+ concentrations using a calibration curve generated from a bath containing 10 μM fura 2, 130 mM KCl, 10 mM MOPS buffered to pH 7.4 with KOH, 10 mM EGTA, and concentrations of CaCl2 between 0 and 10 mM. Free Ca2+ concentrations were calculated using the computer program EQCAL (Biosoft, Ferguson, MO). Ca2+ responses were calculated and presented as baseline levels (averaged during the 1-min period before each treatment), and as peaks (1-min average at the point of maximal change from the baseline level during the 10-min period after treatment). Data were collected from at least three coverslips in experiments performed on different days for each treatment. Cell viability was tested after treatments with 0.1, 0.2, and 0.5 mM MA for 30 min; 5 μM LA, 1 μM GA, and 5 μM GW for 10 min; and 100 μM LA for 30 min, and live or dead cells were counted after staining in 0.4% trypan blue. The viability ratio after these treatments was 98–103% of that resulting from the control treatment (saline or 10% DMSO), and there were no significant differences.
Measurements of GLP-1 release in STC-1 cells.
STC-1 cells (106 cells/ml) were cultured in a 24-well plate (1 ml each well) for 2 days to reach ∼70% confluence. The method for collecting cell culture medium was modified from a previous report (19). On the test day, cells were rinsed three times with Hanks' balanced salt solution, and then new Hanks' balanced salt solution (1 ml) containing LA with or without MA was added, and cells were incubated for 30 min at 37°C. After incubation, conditioned medium was collected, centrifuged, and stored at −80°C until GLP-1 determination. Cells were stored at −20°C for isolation of proteins. Concentration of GLP-1 was determined by enzyme immunoassay with a GLP-1 EIA Kit (RAB0201; Sigma-Aldrich). To test effects of FA and MA on GLP-1 secretion, we used optimal doses of LA (100 μM) and MA (0.1 mM) determined from pilot studies. MA was applied 2 min before LA treatment. Total protein in each well was measured using a BCA protein assays kit (ThermoFisher Scientific), and GLP-1 levels were normalized with the total protein in each well.
Catheter implantation and blood sampling for in vivo measurement of GLP-1, glucose, and insulin secretion.
Adult male rats were anesthetized, and a catheter made of Silastic tubing (inside diameter, 0.64 mm; outside diameter, 1.19 mm; Dow Corning, Midland, MI) was implanted in the atrium through the right jugular vein. When not in use, catheters were locked with heparin saline (10 U/ml heparin in 0.9% saline) and temporarily occluded with a polyvinylpyrrolidone solution (40,000 molecular wt; Sigma-Aldrich), 0.55 g/ml polyvinylpyrrolidone, 50 U/ml heparin (Elkins-Sinn, Cherry Hill, NJ), and 2 mg/ml gentamicin (Boehringer Ingelheim Vetmedica, St. Joseph, MO) in 0.9% saline. After recovery and before testing, rats were habituated during five daily sessions to 30 × 10 cm opaque Plexiglas chambers used for blood sampling experiments.
The effects of intragastric gavage of olive oil (10 ml/kg) on blood levels of GLP-1, insulin, and glucose were then tested in rats adapted to a high-fat diet (D12451, 45.0% calories from fat; Research Diets, New Brunswick, NJ). A high-fat diet was used to be consistent with the diet most commonly used for MA-induced feeding experiments. On test days, 4-h-fasted rats were placed in the chambers without food 2 h before the first blood sample. For MA experiments, MA (68 mg·kg−1·2 ml−1 in saline) or saline was injected intraperitoneally 15 min before olive oil gavage. This route of MA administration is nearly always used for feeding studies and, based on recent data, is appropriate for measuring interactions with enteroendocrine GPR40 (9). Olive oil, a rich source of long-chain FAs, was given by gavage to enable consistency across rats with respect to amount consumed and timing of consumption. Blood samples were collected remotely 55 min, 35 min before, and 15, 30, and 60 min after olive oil gavage. For etomoxir experiments, etomoxir (10 mg·kg−1·3 ml−1 in 0.5% methyl cellulose; Sigma-Aldrich) or control 0.5% methyl cellulose was gavaged 30 min before olive oil gavage, and blood samples were collected remotely 70 min, 50 min before, and 15, 30, and 60 min after olive oil gavage. At each time point, blood samples were immediately mixed with potassium-EDTA (13.6 mg/ml) and 1% vol/vol dipeptidyl peptidase IV inhibitor (EMD Millipore, Billerica, MA) and centrifuged. After centrifugation, plasma was separated into aliquots and stored at −80°C. Plasma glucose was analyzed using the glucose oxidase method. The concentration of insulin and active GLP-1 was measured using a rat/mouse insulin ELISA kit (EZRMI-13K; EMD Millipore) and a high-sensitivity GLP-1 active ELISA kit (EZGLPHS-35K; EMD Millipore), respectively, following protocols provided by the company.
Statistical analysis.
All results are presented as means ± SE. For statistical analysis of data, we used one-way ANOVA or two-way repeated-measures ANOVA, as appropriate. After significance was determined by ANOVA, multiple comparisons between individual groups were tested using a post hoc Fisher's least-significant difference test. P < 0.05 was considered to be statistically significant.
RESULTS
MA blocked LA-induced increase in [Ca2+]i in STC-1 cells.
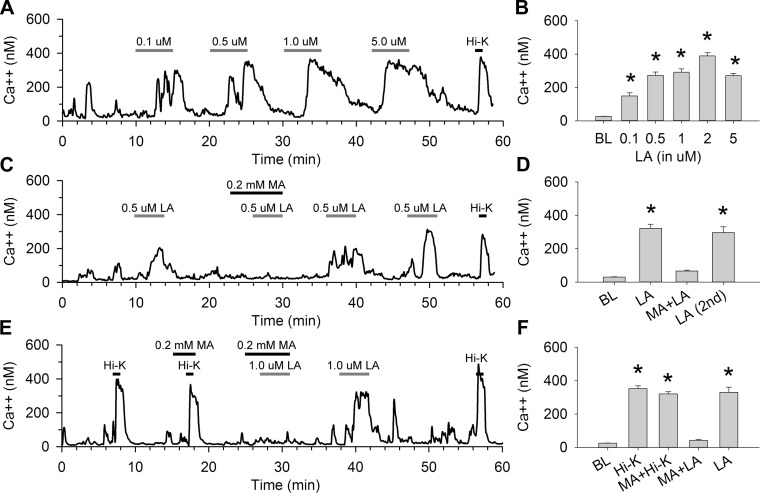
To further investigate the mechanism of MA on FA-induced insulin and GLP-1 secretion, we studied [Ca2+]i in STC-1 cells. STC-1 cells are enteroendocrine cells that have been shown to release GLP-1 in response to FA action at GPR40 and/or GPR120. These receptors are Gq linked (17) and increase [Ca2+]i when activated. Accordingly, we found that application of LA dose dependently increased [Ca2+]i in STC-1 cells. Figure 1A shows a Ca2+ trace from an individual STC-1 cell perfused with different concentrations of LA, and Fig. 1B shows the average peak cellular response to these concentrations compared with baseline. Figure 1, C and D, shows the antagonistic effect of MA on these responses in an individual cell and the average peak response in the population of cells tested. Ca2+ levels were increased significantly in response to 0.1, 0.5, 1, 2, and 5 μM LA compared with baseline responses (n = 8–24 cells/treatment; P < 0.001 vs. baseline). Preperfusion of MA (0.2 mM) in the bath completely blocked 0.5 μM LA-induced [Ca2+]i (P > 0.2, MA + LA vs. baseline and P < 0.001, MA + LA vs. LA; n = 17 cells for each treatment; Fig. 1, C and D). This blockade by MA was specific, since 0.2 mM MA completely blocked [Ca2+]i induced by 1.0 μM LA (P < 0.001, LA vs. baseline and P > 0.4, MA + LA vs. baseline) but did not block Hi-K-induced [Ca2+]i (P > 0.1, MA + Hi-K vs. Hi-K) in the same cells (n = 22 cells/treatment) (Fig. 1, E and F). In addition, MA at 0.2 or 1.0 mM had no effects on the basal [Ca2+]i, even during longer exposure (10 min, data not shown).
Fig. 1.
Effects of linoleic acid (LA) on cytosolic Ca2+ levels ([Ca2+]i) in STC-1 cells. A and B: LA increased [Ca2+]i in STC-1 cells. A: trace showing [Ca2+]i in one individual STC-1 cell perfused with different doses of LA (0.1–5 μM) and a high-potassium (Hi-K) solution (55 mM), indicated by bars above traces. B: averages of peak responses vs. baseline (BL). n = 8–24 cells/treatment. *P < 0.001 vs. BL. C and D: antagonistic effects of mercaptoacetate (MA) on LA-induced [Ca2+]i in STC-1 cells. C: trace of Ca2+ levels of one STC-1 cell perfused with LA (0.5 μM) with or without a pretreatment with MA (0.2 mM) applied 2 min before LA (indicated by bars above traces). D: averages of peak responses vs. BL. n = 17 cells/treatment. *P < 0.001 vs. BL. E and F: effects of MA on Hi-K-induced [Ca2+]i in STC-1 cells. E: trace showing one STC-1 cell perfused with Hi-K (55 mM) and LA (1 μM) with or without MA (0.2 mM). F: averages of peak responses vs. BL. n = 22 cells/treatment. *P < 0.001 vs. BL.
MA blocked effects of GPR40 and GPR120 synthetic agonists on [Ca2+]i in STC-1 cells.
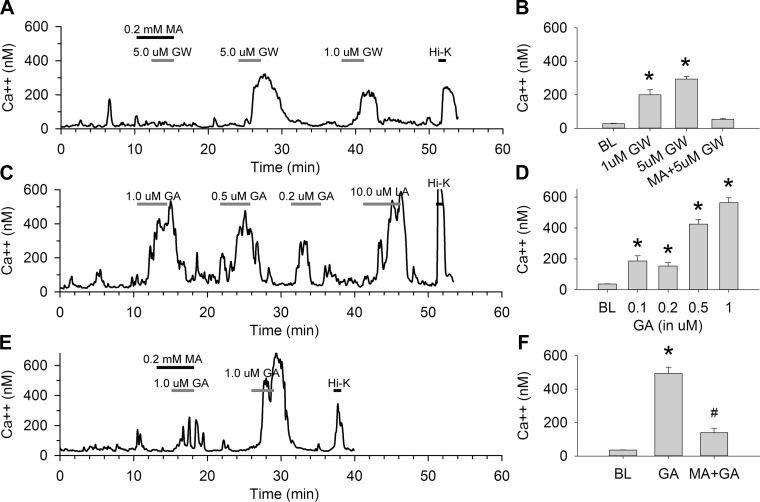
Figure 2 shows effects of the GPR40 agonist GW, and GPR120 partial agonist GA, on [Ca2+]i in STC-1 cells with and without MA. The GPR40 agonist GW increased [Ca2+]i at both 1 and 5 μM in individual STC-1 cells (Fig. 2A). MA (0.2 mM) blocked the response to the 5 μM concentration. The average peak response of the population of cells at each GW concentration and the blocking effect of MA at 5 μM are shown in Fig. 2B (n = 29 cells/treatment, P < 0.001 vs. baseline). Figure 2, C and D, shows similar effects using different doses of the GPR120 agonist GA (0.1, 0.2, 0.5, and 1 μM) on [Ca2+]i in an individual STC-1 cell and the averaged peak response of the population of STC-1 cells at each concentration (n = 14–57 cells/treatment, P < 0.001 vs. baseline). Figure 2, E and F, shows that MA (0.2 mM) significantly reduced [Ca2+]i to 1.0 μM GA (P < 0.001, MA + GA vs. GA; n = 22 cells/treatment), although MA + GA yielded higher [Ca2+]i than observed under baseline conditions (P < 0.01, MA + GA vs. baseline).
Fig. 2.
Effects of G protein-coupled receptor (GPR) 40 agonist GW-9508 (GW) and GPR120 agonist grifolic acid (GA) on [Ca2+]i in STC-1 cells. A and B: MA blocked increases in [Ca2+]i induced by the GPR40 agonist GW in STC-1 cells. A: trace showing responses of one STC-1 cell perfused with different concentrations of GW (1–5 μM) with or without MA (0.2 mM). B: averages of peak responses vs. BL. n = 29 cells/treatment. *P < 0.001 vs. BL. C and D: GA increased [Ca2+]i in STC-1 cells. C: trace showing responses of one STC-1 cell perfused with different concentrations of GA (0.1–1 μM) and LA (10 μM). D: averages of peak responses vs. BL. n = 14–57 cells/treatment. *P < 0.001 vs. BL. E and F: MA blocked increases in [Ca2+]i induced by GA in STC-1 cells. E: antagonistic effects of MA on [Ca2+]i in one STC-1 cell perfused with GA (1 μM), LA (5 μM) with or without pretreatment of MA (0.2 mM). F: averages of peak responses vs. BL. n = 22 cells/treatment. #P < 0.01 and *P < 0.001 vs. BL.
MA blocks LA-induced GLP-1 secretion from STC-1 cells.
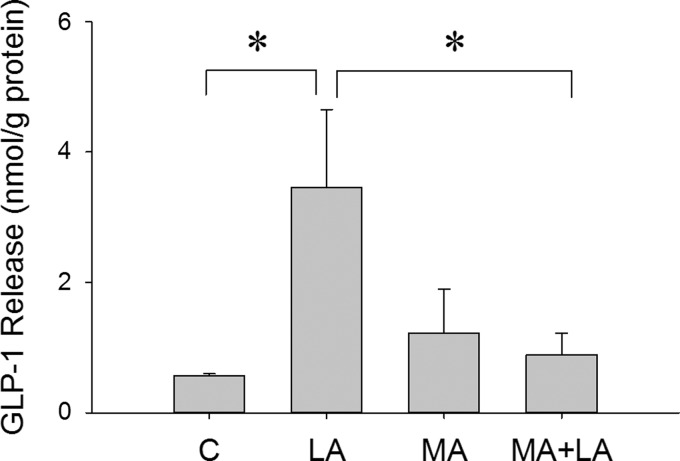
Secretion of the incretin hormone GLP-1 is stimulated by FA activation of GPR40 and/or GPR120. Therefore, we tested effects of LA on GLP-1 release in STC-1 cell culture medium with or without MA (0.1 mM). As shown in Fig. 3, LA (100 μM) significantly increased GLP-1 release in culture medium (P < 0.05, LA vs. control; n = 5 wells/treatment), whereas MA blocked the LA-induced GLP-1 release in STC-1 cells (P > 0.5, MA + LA vs. control; n = 5 wells/treatment). The same results were observed in a repeated experiment.
Fig. 3.
LA-induced glucagon-like peptide 1 (GLP-1) secretion from STC-1 cells. GLP-1 release from cultured STC-1 cells 30 min after treatment with LA (100 μM) with or without MA (0.1 mM). Data were normalized based on total protein in each well. *P < 0.05 vs. control (C) or MA + LA. n = 5 wells/treatment.
MA reduces GLP-1, insulin, and glucose concentration induced in vivo in rats following olive oil gavage.
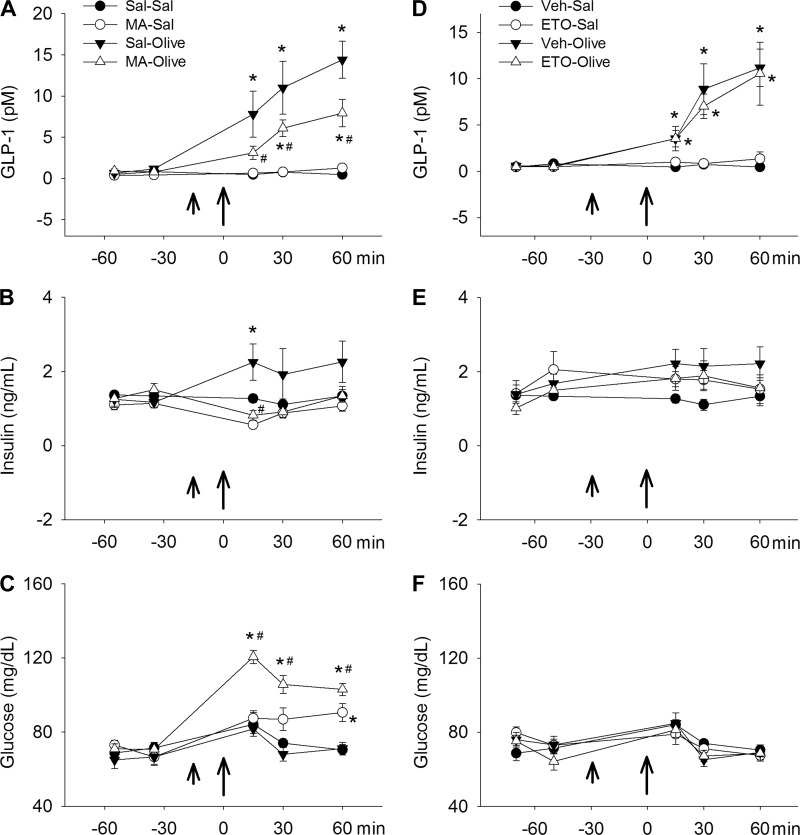
We next conducted experiments to determine whether effects of MA on FA-induced secretion of GLP-1 in vitro are also present in vivo. Because GPR40 mediates FA-induced GLP-1 secretion, as well as FA-induced potentiation of glucose-stimulated insulin secretion (thereby decreasing plasma glucose levels), glucose and insulin levels were also measured in this experiment. Results are shown in Fig. 4. In 6-h-fasted control rats, gavage feeding of olive oil significantly increased plasma GLP-1 concentrations, and preinjection of MA (68 mg/kg) significantly reduced that effect (n = 7–9 rats/group; P < 0.05, saline-olive oil vs. saline-saline or MA-olive oil vs. saline-olive oil; Fig. 4A). Insulin levels were increased significantly by olive oil gavage, and MA reduced insulin secretion to saline baseline levels (Fig. 4B). MA-injected rats also had increased blood glucose concentrations following olive oil gavage compared with rats injected with saline before olive oil gavage (Fig. 4C). In addition, MA also produced a delayed increase in glucose in animals that received saline instead of olive oil. Possibly, the latter increase was the result of MA-induced blockade of constitutive FAs on GLP-1 secretion. These effects of MA on insulin and glucose levels are consistent with the effects of MA on FA-induced GLP-1 secretion we observed in vitro in the STC-1 cell line and with the effects that would be predicted by blockade of GLP-1 secretion in vivo.
Fig. 4.
Effects of MA on olive oil-induced GLP-1 and insulin release. A–C: MA reduced GLP-1 (A) and insulin (B) secretion and increased plasma glucose levels (C) in 6-h-fasted rats after olive oil gavage. MA (68 mg/kg ip) or saline vehicle (Sal) (short arrows) was injected 15 min before an olive oil gavage (10 ml/kg body wt; long arrows). D–F: etomoxir (ETO; 10 mg/kg in 0.5% methylcellulose) or vehicle 0.5% methyl cellulose (Veh), gavaged 30 min to 10 ml/kg olive oil gavage, did not reduce olive oil-induced GLP-1 release (D) and had no effect on insulin (E) or blood glucose (F) levels. n = 7–9 rats/group. *P < 0.05 vs. Sal-Sal (or Veh-Sal) and #P < 0.05, Sal-olive vs. MA-olive.
The FA oxidation inhibitor etomoxir did not alter olive oil-induced GLP-1, insulin, and glucose concentrations in vivo in rats.
Because differentiating effects of MA that may be the result of blockade of FA oxidation from effects mediated via GPR40 are important aspects of this study, we also conducted in vivo experiments using etomoxir in place of MA. Etomoxir reduces FA oxidation by reducing transport of long-chain FAs to mitochondrial oxidation sites, specifically by inhibiting carnitine palmatoyltranserase 1 (34). This mechanism differs from that described for MA (blockade of acyl-CoA dehydrogenase) (3, 4). As shown in Fig. 4, D, E, and F, etomoxir did not alter levels of GLP-1, insulin, or glucose induced by olive oil gavage.
DISCUSSION
Mercaptoacetate inhibits FA oxidation (3, 4), the mechanism of action assumed for nearly three decades to mediate MA-induced stimulation of food intake (35, 36, 51). However, our data indicate that MA is an antagonist of GPR40, a cell membrane receptor for long- and medium-chain FAs. Based on the known function of this receptor, it is likely that GPR40 antagonism may partially or totally mediate MA's orexigenic properties. Consistent with its inhibition of GPR40 activation, we report in vitro and in vivo evidence that MA blocks FA-induced release of GLP-1, a satietogenic peptide with potent incretin effects. We found that MA blocked increases in [Ca2+]i levels triggered by natural (LA) and synthetic (GW) GPR40 agonists in STC-1 cells, a murine enteroendocrine tumor cell line that expresses GPR40 (19). In contrast, MA did not reduce the [Ca2+]i response evoked by high potassium, indicating that MA's blockade of FA-induced increases in [Ca2+]i was not the result of nonspecific impairment of STC-1 cell viability. These data extend our previously reported results from cultured nodose ganglion neurons (10) to STC-1 cells, which have been an important model system for characterizing GPR40 function. Consistent with the effects of MA on [Ca2+]i in STC-1 cells, we also found that MA potently suppressed LA-evoked secretion of GLP-1 from these cells. Taken together, our in vitro results suggest that MA is a direct antagonist of GPR40 and that the interaction of MA with these FA receptors has functional significance for controls that influence metabolism and food intake.
Our current results also reveal for the first time that, as predicted by our in vitro results, MA reduces GLP-1 secretion following gavage of olive oil in vivo. After olive oil gavage, MA-treated rats also exhibited reduced plasma insulin and elevated blood glucose concentrations compared with saline-treated controls gavaged with olive oil. GLP-1 is a potent incretin known to enhance glucose-mediated insulin secretion (2). Therefore, MA's lowering of plasma insulin and elevation of blood glucose are consistent with MA's reduction of FA-induced GLP-1 secretion. Key evidence that these effects are produced by MA-induced blockade of GPR40 receptors includes the following: FA-induced GLP-1 secretion is mediated by this receptor (11, 61, 62); these same incretin effects are produced by administration of GPR40 synthetic agonists (for review, see Ref. 44); and MA blocks increases in [Ca2+]i induced in cultured cells by both LA and the GPR40 synthetic agonist GW and blocks FA-induced GLP-1 secretion in cultured STC-1 cells, as reported here, indicating that this effect of MA is directly mediated. In addition, GLP-1 secretion does not appear to be mediated by MA-induced blockade of FA oxidation, since etomoxir, a drug that antagonizes carnitine palmitolytransferase 1 (thus reducing FA oxidation) (34), did not alter plasma GLP-1, insulin, or glucose concentrations under basal conditions or in response to olive oil gavage.
Although reduced GLP-1 and insulin secretion and increased glucose levels are physiological effects that are consistent with blockade of GLP-1 secretion, glucose-induced insulin secretion is also enhanced by FA binding to pancreatic β-cell GPR40 (26, 27). Therefore, the reduction of insulin level by MA in vivo could be mediated both directly and indirectly: directly by blockade of β-cell GPR40 and indirectly by reducing GLP-1 secretion. It is interesting in this respect that, in 1995, we reported results similar to those reported here in a study examining MA's effects on metabolic parameters in exercising and 48-h-fasted rats (60). Compared with exercised and fasted rats injected with saline, rats injected with MA exhibited a sustained increase in blood glucose levels in combination with an almost complete suppression of insulin secretion both during exercise and after fasting. This result, which could not be explained at that time, is consistent with MA's antagonism of GPR40-mediated GLP-1 secretion that we observed here and indicates that MA exerts anti-incretin effects across various metabolic conditions.
Given the distribution of GPR40, it is likely that MA may act at a variety of sites, in addition to enteroendocrine L cells, to influence feeding and metabolic parameters. The fact that MA blocks FA- and GW-evoked increases in [Ca2+]i in cultured rat nodose neurons (10) suggests that direct reduction of activity in some vagal afferents is a potential mechanism contributing to these effects of MA. In addition, secretion of the satietogenic peptide CCK by enteroendocrine I cells is mediated by GPR40 (41), as demonstrated in isolated transgenically labeled native I cells and in vivo in GPR40−/− mice after olive oil gavage. Therefore, it is likely that MA-induced blockade of both FA-induced CCK secretion and GLP-1 secretion contributes to MA-induced feeding, since both peptides are satietogenic (1, 55) and since GPR40 mediates effects of FA on secretion of both (11, 41). In addition, the satiety effects of both CCK and GLP-1 are, at least in part, mediated by the vagus nerve (18, 50, 56), as is the orexigenic effect of MA (5, 48, 49).
Mercaptoacetate elicits food intake under a variety of circumstances, including chronic leptin treatment, in nondeprived animals, or during maintenance on either high-fat or standard chow diets (22, 35, 36, 38, 54). In this context, the data indicating that MA antagonizes FA activation of GPR40 suggest that these receptors may contribute to maintenance of a basal level of GLP-1 secretion that in turn contributes to control of blood glucose and suppression of food intake between meals (45), as well as in response to a meal. If so, GPR40 might provide a means of targeting the intermeal interval for treatment of eating disorders and obesity.
In addition to its secretion by intestinal L cells, GLP-1 also is released by a population of neurons in the hindbrain (39). It is possible that hindbrain GLP-1 neurons express FA receptors that would provide a substrate for a central action of MA. However, because attempts to stimulate feeding by central administration of MA have been ineffective and because MA-induced feeding is abolished by peripheral vagotomy (5, 46, 47), it is unlikely that MA acts centrally to mediate responses thus far attributed to MA. Furthermore, we would not expect that MA would directly antagonize GLP-1 receptors in the brain, since our data indicate that MA reduces GLP-1 secretion rather than blocks GLP-1 receptors. However, MA-induced reduction of circulating GLP-1 would potentially reduce availability of GLP-1 at central binding sites.
In addition to blockade of GPR40, we found that MA also antagonized (to a lesser extent) the increase in [Ca2+]i in STC-1 cells stimulated by activation of the GPR120 partial agonist GA (15). Although at present we cannot rule out the possibility that GA also has some effects at GPR40 that complicate interpretation of this finding, published results suggest overlapping roles for GPR40 and GPR120 in multiple functions. For example, both receptors have been implicated in GLP-1 secretion (11, 27, 58, 61). However, Xiong et al. (61) have reported recently that corn oil does not stimulate GLP-1 secretion in GPR40−/− mice but does stimulate secretion in GPR120−/− mice and in GPR40+/+ mice. Although these results (59) do not rule out the participation of GPR120 in some aspect of GLP-1 secretion, they clearly indicate that GPR40, but not GPR120, is required for FA stimulation of GLP-1 secretion. Nevertheless, it will be important in future studies to consider how GPR120 interacts with GPR40 and whether it contributes to the feeding and metabolic effects of MA, especially in light of reports that both Gpr120 deletion in mice and genetic mutation of Gpr120 in humans produce obesity (23, 25) and that diet-induced obesity is associated with decreased responsiveness of both intestinal GPR40 and GPR120 to the orexigenic hormone ghrelin (28, 42). MA-induced blockade of this GPR120 effect would be expected to increase feeding under appropriate testing conditions and thus would be consistent with MA's orexigenic actions.
Recently, published results have shown that the FA receptor GPR119, like GPR40 and GPR120, is also expressed by enteroendocrine L cells (43) and that GPR119 agonists increase FA-induced secretion of GLP-1 from primary murine colonic L cells in vitro and in vivo in wild-type mice but not GPR119−/− mice. Therefore, the interactions of FA receptor types and the possible modulation of their binding properties by other nutrients or hormones is an important area for future study. In mediating some responses, GPR40 and GPR120 and possibly other FA receptors (e.g., GPR119) may function cooperatively in response to FA stimulation. Recent studies in GPR40 and GPR120 double-knockout mice indicate that these two FA receptors together play a critical role in the postoral stimulation of appetite by fat. Stimulation of appetite was observed in the double-knockout mice, whereas deletion of the genes individually did not have this effect (52). Mechanisms such as synergism and allosteric modulation of FA receptors (for example, by partial agonists) have been reported and may contribute to these interactive effects (58, 61). The GPR40 receptor itself is linked to both Gq and Gs signaling cascades (17). Thus, GPR40-mediated responses could be modulated by other Gq or Gs activators or inhibitors acting simultaneously on the same cell via other receptor types. It will be important to determine how factors such as these alter GPR40 signaling, the response of the cell to MA, and the control of meal onset and duration.
Overall, our in vivo and in vitro results strongly suggest that the orexigenic effect of MA is not the result of direct blockade of mitochondrial FA oxidation, as previously thought. Rather, they identify membrane receptors responsive to long- and medium-chain FAs as primary sites of action for MA. These receptors are widespread in the gastrointestinal tract and pancreas and constitute critical substrates for control of food intake and metabolism. Although the mechanism of action responsible for MA-induced feeding has been misunderstood for nearly three decades, MA nevertheless has been an important tool for studying feeding behavior and has yielded significant data differentiating neural substrates and pathways responsive to FA and responsible for alteration of appetite (8, 30–33, 47). Findings reported here will provide a clearer focus for investigating physiological mechanisms through which FAs shape appetite and feeding behavior.
Perspectives and Significance
The specific and critical metabolic functions associated with GPR40 and GPR120 have made them prominent targets for development of therapeutic drugs to address obesity, diabetes, and other metabolic disorders (13, 14, 24, 57, 63). Nevertheless, many questions regarding the signaling cascades, receptor-binding properties, and interactions of the FA receptors with each other and with the metabolic environment remain unanswered. A more detailed understanding of how MA blocks FA receptors may enhance understanding of the properties of these receptors and in doing so could facilitate the development of targeted drugs for control of metabolic dysfunction.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-040498, DK-081546, and DK-097437 to S. Ritter.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.-J.L., S.M.S., and S.R. conception and design of research; A.-J.L., Q.W., and T.T.D. performed experiments; A.-J.L. analyzed data; A.-J.L., S.M.S., and S.R. interpreted results of experiments; A.-J.L. prepared figures; A.-J.L. and S.R. drafted manuscript; A.-J.L., S.M.S., and S.R. edited and revised manuscript; A.-J.L., Q.W., T.T.D., S.M.S., and S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Helen Raybould for providing STC-1 cells.
REFERENCES
- 1.Balkan B. Effects of glucagon-like peptide-1 (GLP-1) on glucose homeostasis and food intake. Appetite 35: 269–270, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol 279: R1449–R1454, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bauche F, Sabourault D, Giudicelli Y, Nordmann J, Nordmann R. 2-Mercaptoacetate administration depresses the beta-oxidation pathway through an inhibition of long-chain acyl-CoA dehydrogenase activity. Biochem J 196: 803–809, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauche F, Sabourault D, Giudicelli Y, Nordmann J, Nordmann R. Inhibition in vitro of acyl-CoA dehydrogenases by 2-mercaptoacetate in rat liver mitochondria. Biochem J 215: 457–464, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt K, Arnold M, Geary N, Langhans W, Leonhardt M. Vagal afferents mediate the feeding response to mercaptoacetate but not to the beta (3) adrenergic receptor agonist CL 316,243. Neurosci Lett 411: 104–107, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 148: 619–628, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Calingasan NY, Ritter S. Presence of galanin in rat vagal sensory neurons: evidence from immunohistochemistry and in situ hybridization. J Auton Nerv Syst 40: 229–238, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Christensen LW, Kuhre RE, Janus C, Svendsen B, Holst JJ. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol Rep 3: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darling RA, Zhao H, Kinch D, Li AJ, Simasko SM, Ritter S. Mercaptoacetate and fatty acids exert direct and antagonistic effects on nodose neurons via GPR40 fatty acid receptors. Am J Physiol Regul Integr Comp Physiol 307: R35–R43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 53: 82–92, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Halder S, Kumar S, Sharma R. The therapeutic potential of GPR120: a patent review. Expert Opin Ther Pat 23: 1581–1590, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Hara T, Hirasawa A, Ichimura A, Kimura I, Tsujimoto G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J Pharm Sci 100: 3594–3601, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Hara T, Hirasawa A, Sun Q, Sadakane K, Itsubo C, Iga T, Adachi T, Koshimizu TA, Hashimoto T, Asakawa Y, Tsujimoto G. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch Pharmacol 380: 247–255, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Hara T, Kashihara D, Ichimura A, Kimura I, Tsujimoto G, Hirasawa A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim Biophys Acta 1841: 1292–1300, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Hauge M, Vestmar MA, Husted AS, Ekberg JP, Wright MJ, Di Salvo J, Weinglass AB, Engelstoft MS, Madsen AN, Luckmann M, Miller MW, Trujillo ME, Frimurer TM, Holst B, Howard AD, Schwartz TW. GPR40 (FFAR1): combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metab 4: 3–14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes MR, Kanoski SE, De Jonghe BC, Leichner TM, Alhadeff AL, Fortin SM, Arnold M, Langhans W, Grill HJ. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol 301: R1479–R1485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol 587: 4749–4759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Dai MH, Tao YX. Physiology and therapeutics of the free fatty acid receptor GPR40. Prog Mol Biol Transl Sci 121: 67–94, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Hudson BD, Emanuel AJ, Wiater MF, Ritter S. The lipoprivic control of feeding is governed by fat metabolism, not by leptin or adipose depletion. Endocrinology 151: 2087–2096, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichimura A, Hara T, Hirasawa A. Regulation of Energy Homeostasis via GPR120. Front Endocrinol (Lausanne) 5: 111, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichimura A, Hasegawa S, Kasubuchi M, Kimura I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol 5: 236, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Korner A, Kiess W, Pigeyre M, Caiazzo R, Van Hul W, Van Gaal L, Horber F, Balkau B, Levy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G, Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483: 350–354, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Itoh Y, Hinuma S. GPR40, a free fatty acid receptor on pancreatic beta cells, regulates insulin secretion. Hepatol Res 33: 171–173, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Janssen S, Laermans J, Iwakura H, Tack J, Depoortere I. Sensing of fatty acids for octanoylation of ghrelin involves a gustatory G-protein. PLoS One 7: e40168, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinch DC, Peters JH, Simasko SM. Comparative pharmacology of cholecystokinin induced activation of cultured vagal afferent neurons from rats and mice. PLoS One 7: e34755, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koegler FH, Ritter S. Aqueduct occlusion does not impair feeding induced by either third or fourth ventricle galanin injection. Obes Res 5: 262–267, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Koegler FH, Ritter S. Feeding induced by pharmacological blockade of fatty acid metabolism is selectively attenuated by hindbrain injections of the galanin receptor antagonist, M40. Obes Res 4: 329–336, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Koegler FH, Ritter S. Galanin injection into the nucleus of the solitary tract stimulates feeding in rats with lesions of the paraventricular nucleus of the hypothalamus. Physiol Behav 63: 521–527, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Koegler FH, York DA, Bray GA. The effects on feeding of galanin and M40 when injected into the nucleus of the solitary tract, the lateral parabrachial nucleus, and the third ventricle. Physiol Behav 67: 259–267, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Kruszynska YT, Sherratt HS. Glucose kinetics during acute and chronic treatment of rats with 2[6(4-chloro-phenoxy)hexyl]oxirane-2-carboxylate, etomoxir. Biochem Pharmacol 36: 3917–3921, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Langhans W. Fatty acid oxidation in the energostatic control of eating–a new idea. Appetite 51: 446–451, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Langhans W, Leitner C, Arnold M. Dietary fat sensing via fatty acid oxidation in enterocytes: possible role in the control of eating. Am J Physiol Regul Integr Comp Physiol 300: R554–R565, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Langhans W, Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic fatty acid oxidation. J Auton Nerv Syst 18: 13–18, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Langhans W, Scharrer E. Role of fatty acid oxidation in control of meal pattern. Behav Neural Biol 47: 7–16, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Leonhardt M, Langhans W. Fatty acid oxidation and control of food intake. Physiol Behav 83: 645–651, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 140: 903–912, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X, Zhao X, Feng J, Liou AP, Anthony S, Pechhold S, Sun Y, Lu H, Wank S. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am J Physiol Gastrointest Liver Physiol 303: G367–G376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moss CE, Glass LL, Diakogiannaki E, Pais R, Lenaghan C, Smith DM, Wedin M, Bohlooly YM, Gribble FM, Reimann F. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides In press. [DOI] [PMC free article] [PubMed]
- 44.Nadkarni P, Chepurny OG, Holz GG. Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci 121: 23–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritter RC. Increased food intake and CCK receptor antagonists: beyond abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol 286: R991–R993, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Ritter S, Calingasan N. Neural substrates for metabolic control of feeding. In: Appetite and Body Weight Regulation: Sugar, Fat, and Macronutrient Substitutes, edited by Fernstrom JD and Miller GD. Boca Raton, FL: CRC, 1994. [Google Scholar]
- 47.Ritter S, Ritter JB, Cromer L. 2-Deoxy-d-glucose and mercaptoacetate induce different patterns of macronutrient ingestion. Physiol Behav 66: 709–715, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Ritter S, Taylor JS. Capsaicin abolishes lipoprivic but not glucoprivic feeding in rats. Am J Physiol Regul Integr Comp Physiol 256: R1232–R1239, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Ritter S, Taylor JS. Vagal sensory neurons are required for lipoprivic but not glucoprivic feeding in rats. Am J Physiol Regul Integr Comp Physiol 258: R1395–R1401, 1990. [DOI] [PubMed] [Google Scholar]
- 50.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scharrer E, Langhans W. Control of food intake by fatty acid oxidation. Am J Physiol Regul Integr Comp Physiol 250: R1003–R1006, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 305: R1490–R1497, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singer LK, Ritter S. Differential effects of infused nutrients on 2-deoxy-d-glucose- and 2-mercaptoacetate-induced feeding. Physiol Behav 56: 193–196, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Singer-Koegler LK, Magluyan P, Ritter S. The effects of low-, medium-, and high-fat diets on 2-deoxy-d-glucose- and mercaptoacetate-induced feeding. Physiol Behav 60: 321–323, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Smith GP, Gibbs J. Cholecystokinin: a putative satiety signal. Pharmacol Biochem Behav 3: 135–138, 1975. [PubMed] [Google Scholar]
- 56.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213: 1036–1037, 1981. [DOI] [PubMed] [Google Scholar]
- 57.Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol Sci 32: 543–550, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka H, Yoshida S, Minoura H, Negoro K, Shimaya A, Shimokawa T, Shibasaki M. Novel GPR40 agonist AS2575959 exhibits glucose metabolism improvement and synergistic effect with sitagliptin on insulin and incretin secretion. Life Sci 94: 115–121, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol Regul Integr Comp Physiol 271: R848–R856, 1996. [DOI] [PubMed] [Google Scholar]
- 60.van Dijk G, Scheurink A, Ritter S, Steffens A. Glucose homeostasis and sympathoadrenal activity in mercaptoacetate-treated rats. Physiol Behav 57: 759–764, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Xiong Y, Swaminath G, Cao Q, Yang L, Guo Q, Salomonis H, Lu J, Houze JB, Dransfield PJ, Wang Y, Liu JJ, Wong S, Schwandner R, Steger F, Baribault H, Liu L, Coberly S, Miao L, Zhang J, Lin DC, Schwarz M. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol Cell Endocrinol 369: 119–129, 2013. [DOI] [PubMed] [Google Scholar]
- 62.Yashiro H, Tsujihata Y, Takeuchi K, Hazama M, Johnson PR, Rorsman P. The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J Pharmacol Exp Ther 340: 483–489, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Yonezawa T, Kurata R, Yoshida K, Murayama MA, Cui X, Hasegawa A. Free fatty acids-sensing G protein-coupled receptors in drug targeting and therapeutics. Curr Med Chem 20: 3855–3871, 2013. [DOI] [PubMed] [Google Scholar]
- 64.Young SH, Rey O, Sternini C, Rozengurt E. Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2. Am J Physiol Cell Physiol 298: C1401–C1413, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Ritter RC. Circulating GLP-1 and CCK-8 reduce food intake by capsaicin-insensitive, nonvagal mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R264–R273, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao H, Simasko SM. Role of transient receptor potential channels in cholecystokinin-induced activation of cultured vagal afferent neurons. Endocrinology 151: 5237–5246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]