Abstract
To clarify the lower urinary tract function in mice, we compared bladder and urethral activity between rats and mice with or without spinal cord injury (SCI). Female Sprague-Dawley rats and C57BL/6N mice were divided into five groups: 1) spinal intact (SI) rats, 2) SI mice, 3) pudendal nerve transection (PNT) SI mice, 4) spinal cord injury (SCI) rats, and 5) SCI mice. Continuous cystometry (CMG) and external urethral sphincter (EUS)-electromyogram (EMG) analyses were conducted under an awake, restrained condition. During voiding bladder contractions, SI animals exhibited EUS bursting with alternating active and silent periods, which, in rats but not mice, coincided with small-amplitude intravesical pressure oscillations in CMG recordings. In SI mice with bursting-like EUS activity, the duration of active periods was significantly shorter by 46% (32 ± 5 ms) compared with SI rats (59 ± 9 ms). In PNT-SI mice, there were no significant differences in any of cystometric parameters compared with SI mice. In SCI rats, fluid elimination from the urethra and the EUS bursting occurred during small-amplitude intravesical pressure oscillations. However, SCI mice did not exhibit clear EUS bursting activity or intravesical pressure oscillations but rather exhibited intermittent voiding with slow large-amplitude reductions in intravesical pressure, which occurred during periods of reduced EUS activity. These results indicate that EUS pumping activity is essential for generating efficient voiding in rats with or without spinal cord injury. However, EUS bursting activity is not required for efficient voiding in SI mice and does not reemerge in SCI mice in which inefficient voiding occurs during periods of reduced tonic EUS activity.
Keywords: urinary bladder, external urethral sphincter, mouse, pudendal nerve transection, electromyogram
the storage and release of urine are dependent on the coordinated activity of the urinary bladder and the external urethral sphincter (EUS). This coordination is mediated by neural mechanisms in the brain and spinal cord that are stimulated by afferent input from the bladder. However, spinal cord injury (SCI) rostral to the lumbosacral level alters the coordination between bladder and EUS activity in many species (e.g., human, cat, and rat), leading to detrusor-sphincter dyssynergia (3, 5), which results in inefficient voiding and bladder wall tissue remodeling, such as hypertrophy and fibrosis (6). Although these abnormalities can lead to renal failure and also decrease the quality of life for patients (3, 5, 6), currently available treatment modalities are often unsatisfactory. Therefore, the investigation of bladder and EUS function in animal models is needed to clarify the mechanisms of neurogenic lower urinary tract dysfunction and develop new treatments.
EUS electromyogram (EMG) recordings, which are widely used for evaluating the urethral function, exhibit tonic activity before the onset of voiding and bursting activity during voiding in normal rats. This EUS bursting during voiding is characterized by clusters of high-frequency spikes (active periods: APs) separated by low tonic activity (silent periods: SPs), and produces rhythmic contractions and relaxations of EUS that represent a pumping action of EUS, which is seen as intravesical pressure oscillations during cystometrograms (CMGs) (Fig. 1) (3, 4, 11–13). The EUS bursting activity and pressure oscillations in CMG recordings are abolished by neuromuscular blockers or pudendal nerve transection in rats, and voiding efficiency is markedly decreased, suggesting that the EUS pumping activity plays an important role in efficient bladder emptying (13, 17, 20). However, this EUS-pumping activity during voiding does not occur in humans (1, 16, 20).
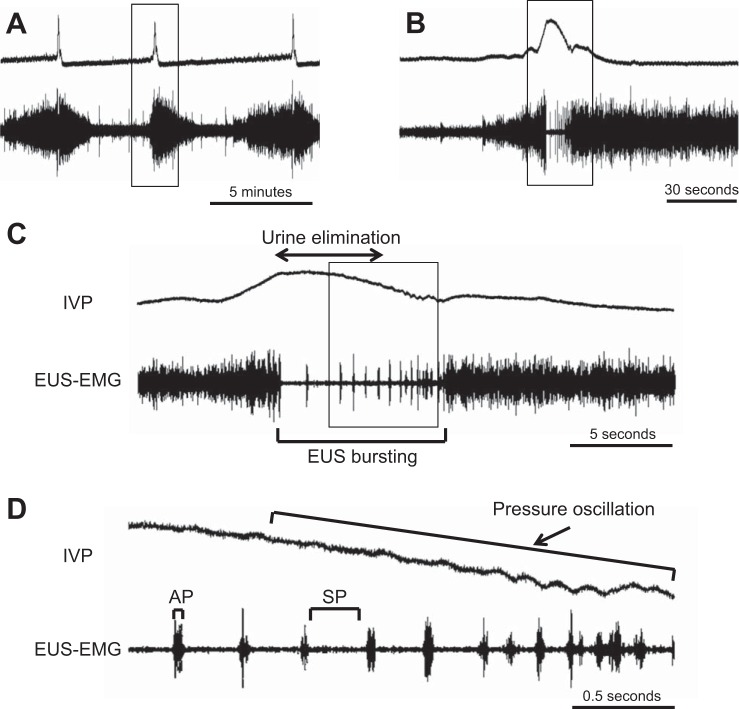
Fig. 1.
Representative recordings of simultaneous measurement of intravesical pressure (IVP) and external urethral sphincter (EUS)-electromyogram (EMG) activity in a spinal intact unanesthetized rat. Traces B, C, and D depict sections of trace A on expanded time scales. Traces B, C, and D are the expanded portion of traces A, B, and C, respectively, indicated by rectangular boxes. The EUS-EMG exhibits tonic activity before the onset of voiding and bursting activity during voiding (A, B, and C). The EUS bursting is characterized by clusters of high-frequency spikes (active periods: APs) separated by low tonic activity (silent periods: SPs) (C and D). The bursting produces rhythmic contractions and relaxations of the EUS and is thought to generate a urethral pumping action during voiding, which is seen as pressure oscillations on the continuous cystometry (CMG) tracing (D).
Animal modeling is a key component of basic research on various human diseases, including lower urinary tract dysfunction. Mouse models have become highly useful because of the feasibility for genetic modification. However, because of their small body size and difficulties in functional analysis of the urethra, coordinating activity of bladder and urethra under normal and pathological conditions has not been well characterized in mice compared with numerous studies in rats (3, 4, 11–13). For the accurate examination of urethral function, differences between mice and other animals should be established. Therefore, to clarify urethral function in mice, we compared bladder and urethral activity in rats and mice with or without SCI using continuous CMG and EUS-EMG recordings.
MATERIALS AND METHODS
Animal model.
A total of 28 female C57BL/6N mice (9 wk in age) weighing 18–20 g and eight female Sprague-Dawley rats (8 wk age) weighing 198–220 g were used according to the experimental protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Animals were divided into five groups: 1) spinal intact (SI) rats (n = 4), 2) SI mice (n = 12), 3) pudendal nerve transection (PNT) SI mice (n = 6), 4) SCI rats (n = 4), and 5) SCI mice (n = 10) groups.
The Th8/9 spinal cord of mice and rats was completely transected under isoflurane anesthesia, according to the methods described in our previous studies in rats (3, 20) and mice (7, 15), and 4 wk after surgery, continuous CMGs and EUS-EMG recordings were conducted under an awake condition. The animals were treated with ampicillin (100 mg/kg sc) for 5 days post-SCI, and this treatment was continued twice a week afterward to prevent urinary tract infection. The bladder of SCI animals was emptied twice a day after spinal cord transection.
In PNT-SI group, the ischiorectal fossa was exposed bilaterally via a dorsal longitudinal incision, and the pudendal nerves were transected bilaterally with microscissors, according to the methods previously described (9, 14). Six hours after PNT, continuous CMG and EUS-EMG analyses were conducted under an awake condition.
Continuous cystometry.
The animals were anesthetized with isoflurane, and the bladder was exposed via a lower abdominal incision. A polyethylene catheter (PE-50; Clay-Adams, Parsippany, NJ) was inserted through the bladder dome, and a purse suture was placed around the catheter. Thereafter, mice and rats were placed in restraining cages (mouse cage; Economy holder 15 to 30 g, Torrington, CT; and rat cage: W 80 mm × L 300 mm × H 150 mm; Yamanaka Chemical Industries, Tokyo, Japan). After the recovery from anesthesia, animals were acclimated to the cages for at least 2 h, and then bladder activity under an awake condition was monitored via the bladder catheter, which was connected via a three-way stopcock to a pressure transducer and a pump for infusion of physiological saline at a rate of 0.01 and 0.1 ml/min for mice and rats, respectively. After rhythmic bladder contractions became stable for at least 60 min, intercontraction interval (ICI), which was the average of intervals between two consecutive peaks of voiding pressures, maximum voiding bladder contraction pressure (MCP), intravesical baseline pressure (IVBP), which was bladder pressure just after voiding, and the number of nonvoiding contractions (NVCs) were measured during the following 30 min. NVCs were defined as small-amplitude bladder contractions without voiding, which were greater than 8 cmH2O (8). The average number of NVCs per minute between voiding contractions was also determined. After the measurement of these parameters, voided volume was estimated from infusion volume between two consecutive voiding contractions. Residual saline in the bladder after voiding was withdrawn through the bladder catheter by gravity to determine residual volumes, and then the bladder was completely emptied by manual compression of the abdominal wall. Bladder capacity was calculated as the sum of voided and residual volumes. Voiding efficiency was also calculated as the ratio of voided volume divided by bladder capacity. Bladder compliance was calculated according to the following formula: compliance = bladder capacity/(pressure at volume threshold for inducing a voiding contraction − initial pressure at the start of saline infusion) (18). The evaluation of bladder function by continuous CMGs was performed without EUS-EMG recordings because, in our preliminary experiments, EMG wire insertion to the EUS induced changes in CMG parameters during the storage phase, such as a reduction in ICI and an increase in NVCs, possibly due to the stimulation of pudendal nerve afferents.
EUS-EMG.
After continuous CMGs, animals were anesthetized with isoflurane again, and epoxy-coated stainless-steel wire EMG electrodes (50 μm diameter; M. T. Giken, Tokyo, Japan) were placed percutaneously into the EUS. This was performed using a 30-gauge needle with a tip portion of EMG electrode hooked at the needle tip. The needle was inserted into the sphincter and then withdrawn to leave the EMG wires embedded in the muscle. After every EUS-EMG experiment, the location of EMG wire ends were visually examined by dissecting each animal, and if they were located more than 1.0 mm from the urethra, the data were excluded from analysis. The EUS-EMG activity was passed through a discriminator, and the output was recorded with an amplifier and data-acquisition software (sampling rate 400 Hz; Chart, AD Instruments, Colorado Springs, CO) on a computer system equipped with an analog-to-digital converter (PowerLab, AD Instruments). The animals were placed in restraining cages as mentioned above, and simultaneous measurements of intravesical pressure and EUS-EMG activity under an awake condition were performed during continuous CMGs after the recovery from anesthesia. After rhythmic bladder contractions became stable for at least 60 min, the EUS-EMG activity, including APs and SPs during voiding bladder contractions, was recorded during the micturition cycle. In mice, contraction duration (CD) with voiding and total duration of reduced EUS activity (R-EA) during voiding, which was calculated by the sum of SP durations in SI mice with EMG bursting or reduced EMG durations in those without EMG bursting and the sum of durations of reduced tonic EUS-EMG activity during a voiding contraction in SCI mice, were evaluated. Also, the ratio of R-EA to CD was compared between SI and SCI mice.
Statistics.
Results are reported as the means ± SE. Comparisons between the groups were done by Mann-Whitney U-test. P < 0.05 was considered to be statistically significant.
RESULTS
Manual bladder expressions.
The bladder of SCI rats was emptied twice a day until the recovery of reflex voiding, which occurred about 2 wk postsurgery. However, SCI mice had large residual urine volumes, and manual bladder expressions with perineal stimulation (1–2 times a day) were needed for sufficient urine elimination during 4 wk postsurgery.
Continuous cystometry.
In SCI rats, cystometric analyses showed significant increases in ICI, voided volume, residual volume, bladder capacity, bladder compliance, and the number of NVCs and a decrease in voiding efficiency compared with SI rats (Table 1). In PNT-SI mice, there were no significant differences in any cystometric parameters, including voiding efficiency compared with the parameters in SI mice (Table 1). In SCI mice, continuous CMGs showed significant increases in MCP, IVBP, residual volume, bladder capacity, bladder compliance, and the number of NVCs and decreases in ICI, voided volume, and voiding efficiency compared with these parameters in SI mice.
Table 1.
Parameters of bladder activity in transvesical cystometrograms
| SI Rats (n = 4) | SCI Rats (n = 4) | SI Mice (n = 12) | SCI Mice (n = 10) | PNT Mice (n = 6) | |
|---|---|---|---|---|---|
| ICI, min | 2.5 ± 0.9 | 8.3 ± 3.1## | 5.5 ± 0.7 | 3.1 ± 1.4*** | 5.5 ± 2.3 |
| MCP, cmH2O | 42.5 ± 13.6 | 41.3 ± 9.7 | 32.0 ± 5.0 | 58.9 ± 6.4*** | 40.3 ± 7.2 |
| IVBP, cmH2O | 9.8 ± 2.4 | 9.9 ± 3.3 | 8.1 ± 1.3 | 12.6 ± 2.2** | 8.2 ± 1.5 |
| NVCs, number/min | 0.00 ± 0.00 | 1.01 ± 0.32## | 0.13 ± 0.08 | 1.31 ± 0.77** | 0.13 ± 0.08 |
| Voided volume, ml | 0.31 ± 0.08 | 0.74 ± 0.31## | 0.05 ± 0.02 | 0.03 ± 0.01** | 0.05 ± 0.02 |
| Residual volume, ml | 0.02 ± 0.02 | 0.18 ± 0.03## | 0.01 ± 0.01 | 0.17 ± 0.09*** | 0.01 ± 0.01 |
| Bladder compliance, ml/cmH2O | 0.12 ± 0.07 | 0.21 ± 0.06# | 0.02 ± 0.01 | 0.03 ± 0.01** | 0.02 ± 0.01 |
| Bladder capacity, ml | 0.32 ± 0.07 | 0.92 ± 0.28## | 0.07 ± 0.02 | 0.20 ± 0.09*** | 0.07 ± 0.02 |
| Voiding efficiency, % | 95 ± 5 | 78 ± 9## | 87 ± 12 | 18 ± 9*** | 82 ± 11 |
Values are expressed as means ± SE. SI, spinally intact, SCI, spinal cord injury; PNT, pudendal nerve transection. Significantly different in SI rats vs. SCI rats, using Mann-Whitney U-test,
P < 0.05,
P < 0.01. Significantly different in SI mice vs. SCI mice or PNT mice. Mann-Whitney U-test,
P < 0.01,
P < 0.001.
Intravesical pressure and EUS-EMG activity.
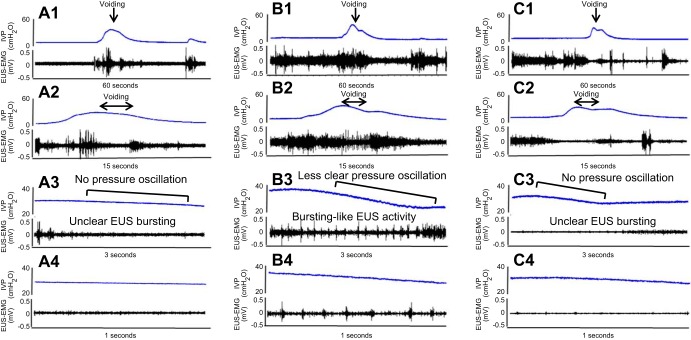
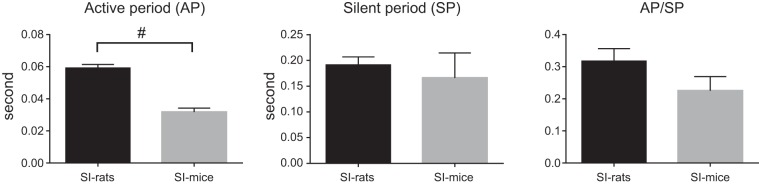
During voiding bladder contractions, SI rats exhibited EUS bursting consisting of alternating APs and SPs, which coincided with rapid intravesical pressure oscillations in the CMG tracing (Fig. 1, A–D). During voiding bladder contractions in SI mice, two patterns of EUS-EMG were noted: unclear EUS bursting (n = 8) compared with that in SI rats (Fig. 2, A1–A4) and bursting-like EUS activity characterized by alternating tonic and reduced EMG activity periods (n = 4) (Fig. 2, B1–B4). A comparison of EUS bursting in SI rats and SI mice with bursting-like EUS activity revealed that the duration of APs was significantly shorter by 46% (32 ± 5 ms; n = 4) in SI mice compared with SI rats (59 ± 9 ms; n = 4) (Fig. 3). Also, the intravesical pressure oscillations were not obvious in SI mice (Fig. 2, A3–A4 and B3–B4) compared with those in SI rats (Fig. 1). In PNT-SI mice (n = 6), EUS-EMG activity did occur before and after voiding, but this activity was reduced during voiding (Fig. 2, C1–C4).
Fig. 2.
Simultaneous measurements of IVP and EUS-EMG in spinal intact (SI) mouse without pressure oscillation (PO) (A1–A4), a SI mouse with PO (B1–B4), and a SI mouse with pudendal nerve transection (PNT) (C1–C4). Traces A2–A4, B2–B4, and C2–C4 are expanded from the traces A1, B1, and C1, respectively, with different time scales. Eight of twelve SI mice exhibited reduced EUS activity without bursting and no obvious pressure oscillation during voiding (A1–A4). The remaining four SI mice exhibited bursting-like EUS activity during voiding bladder contractions, similar to EUS busting in SI rats (B1–B4), consisting of alternating tonic (APs), and reduced EMG activity (SPs), which coincided with pressure oscillations on CMG tracing, but pressure oscillations of CMG tracing was less obvious compared with SI rats. In the PNT-SI-mouse (C1–C4), EUS was silent during voiding.
Fig. 3.
Comparison of duration of APs and SPs of EUS bursting during voiding bladder contractions in SI rats and SI mice. The total duration of APs or SPs were calculated by the sum of each AP or SP duration during a voiding bladder contraction, respectively. The duration of APs was significantly shorter (32 ± 5 ms, 46% shorter) in SI mice compared with SI rats (59 ± 9 ms). #P < 0.05, significant difference between SI rats and SI mice, using Mann-Whitney U-test.
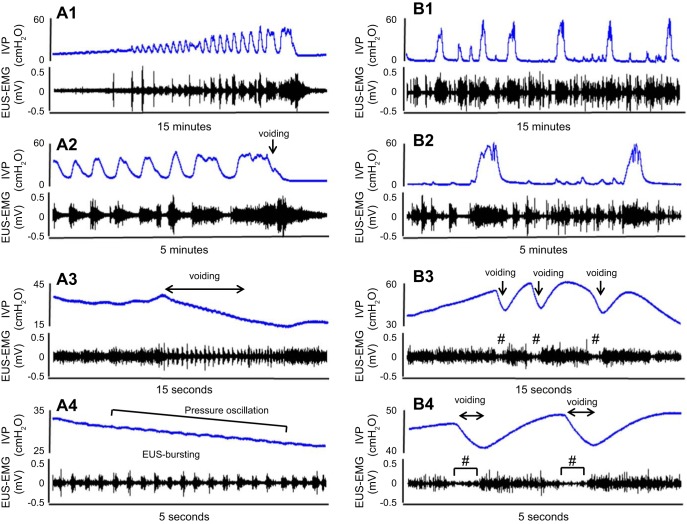
During voiding in SCI rats, fluid elimination from the urethra coincided with rapid intravesical pressure oscillations and the EUS bursting (Fig. 4 A1–A4). However, SCI mice exhibited intermittent voiding with large-amplitude, long-duration reductions in intravesical pressure, which coincided with periods of reduced tonic EUS-EMG activity (Fig. 4B4). Rapid intravesical pressure oscillation and EUS bursting activity were not seen in SCI mice (Fig. 4, B1–B4).
Fig. 4.
Simultaneous measurements of IVP and EUS-EMG in a spinal cord-injured (SCI) rat (A1–A4) and a SCI mouse (B1–B4). Traces A2–A4 and B2–B4 are expanded from the traces A1 and B1, respectively, with different time scales. The SCI rat (A1–A4) exhibited EUS bursting with alternating APs and SPs during voiding bladder contractions, which coincided with rapid pressure oscillations in the CMG tracing. The SCI mouse (B1–B4) had intermittent voiding coinciding with more prolonged reductions in intravesical pressure in the CMG recording, which occurred during periods of low-tonic, synergic EUS-EMG activity (B3 and B4). Clear EUS bursting activity or intravesical pressure oscillations were not seen in the SCI mouse. # symbols in B3 and B4 indicate the reduced EUS activity (R-EA) during voiding. Please note that the time scales of SCI mouse traces in Fig. 4, B1–B4 are different from those used for SI mice in Fig. 2, A–C.
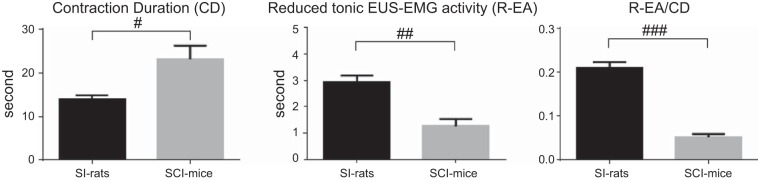
CD was significantly longer (23.1 ± 7.5 s; 65% longer) in SCI mice compared with CD in SI mice (14.0 ± 2.4 s), and R-EA was significantly shorter (1.3 ± 0.7 s; 55% shorter) in SCI mice compared with R-EA of SI mice (2.9 ± 0.8 s). The ratio of R-EA to CD was significantly smaller (0.05 ± 0.02; 76% smaller) in SCI mice compared with the ratio in SI mice (0.21 ± 0.04) (Fig. 5).
Fig. 5.
Comparison of contraction duration (CD) and total duration of reduced EUS activity (R-EA) during voiding bladder contractions in SI and SCI mice. The CD was significantly longer (23.1 ± 7.5 s; 65% longer) in SCI mice compared with SI mice (14.0 ± 2.4 s). The R-EA, which was calculated by the sum of SP durations in SI mice with EMG bursting or reduced EMG durations in those without EMG bursting and the sum of durations of reduced tonic EUS EMG activity during a voiding contraction in SCI mice, was significantly shorter (1.3 ± 0.7 s: 55% shorter) in SCI mice compared with SI mice (2.9 ± 0.8 s). The ratio of R-EA to CD was significantly smaller (0.05 ± 0.02; 76% smaller) in SCI mice compared with SI mice (0.21 ± 0.04). #P < 0.05, ##P < 0.01, ###P < 0.001, significant difference between SI rats and SI mice, using Mann-Whitney U-test.
DISCUSSION
The current study revealed that EUS-EMG activity during voiding can be markedly different in female rats and mice. During voiding bladder contractions, SI rats exhibited EUS bursting with alternating APs and SPs, which coincided with rapid intravesical pressure oscillations in the CMG tracing. In the majority of SI mice (8 of 12), such EUS bursting activity was not detected. When bursting-like EUS activity did occur in SI mice; the duration of APs was shorter compared with that in SI rats. Intravesical pressure oscillations, which occurred during voiding in SI rats, did not occur or were less obvious in SI mice. The pressure oscillations are thought to reflect EUS pumping activity, which contributes to efficient voiding (3, 4, 11, 12, 13, 17, 21). Previous studies demonstrated that bilateral PNT decreases voiding efficiency in SI rats (13, 17, 21), whereas PNT in SI mice, which eliminated EUS activity during voiding, did not affect voiding efficiency in this study, indicating that EUS pumping activity is not essential for efficient voiding in SI mice.
In this study, we observed EUS-EMG activity before and after voiding in PNT-SI mice (Fig. 2C); therefore, it is possible that the EMG electrodes picked up EMG activity not only from the EUS, but also from other pelvic floor muscles, such as the levator ani muscle, whose activity during the storage phase still remained after transection of pudendal nerves that innervate the EUS. Nevertheless, the absence of tonic or bursting EMG activity during voiding in PNT-SI mice (Fig. 2C) indicates that EUS activity during voiding was eliminated after pudendal nerve transection, without affecting voiding efficiency. Thus, it is assumed that a contribution of the EUS pumping activity to expel urine in SI mice is likely to be less than that in SI rats.
Although voiding efficiency was reduced in SCI rats, fluid elimination from the urethra still coincided with rapid intravesical pressure oscillations and EUS bursting. However, in SCI mice, intermittent voiding occurred during reduced tonic EUS (synergic) activity and more prolonged reductions in intravesical pressure fluctuations without obvious EUS bursting activity or rapid pressure oscillations. These findings suggest that EUS pumping activity and bladder pressure oscillation are necessary for voiding in SCI rats; however, longer periods of reduced EUS (synergic) activity rather than high-frequency EUS pumping activity are more important for voiding in SCI mice.
Continuous CMG recordings in the present study showed that the ICI was increased in SCI rats compared with SI rats, but was decreased in SCI mice compared with SI mice. This difference is likely due to the difference in voiding efficiency between SCI rats (78%) and SCI mice (18%). Because SCI rats had larger bladder capacity than SI rats with relatively high voiding efficiency (78%), SCI rats still can void larger volumes with increased ICI than SI rats despite a reduction in voiding efficiency after SCI (Table 1). However, the large residual volume in SCI mice results in more frequent voiding (i.e., a shorter ICI) with smaller voided volume despite the larger bladder capacity after SCI. This discrepancy in voiding efficiency is also reflected in the postoperative condition after SCI in rats and mice in this study. None of the SCI rats required manual expression to drain urine from the bladder beyond 2 wk posttransection. However, after this recovery period, SCI mice continued to exhibit urinary retention with large residual urine volumes, and manual bladder expression along with perineal stimulation 1–2 times per day were needed to obtain efficient urine elimination for 4 wk after surgery. In addition, the bladder and urethral activity in SCI mice was evaluated only at 4 wk after SCI surgery in this study. It is possible that the time course of functional recovery of the EUS after SCI may be different between mice and rats. Therefore, the time-dependent changes in urethral reflex activity in SCI mice after 4 wk should be examined in future studies. Furthermore, in the present study, bladder compliance was increased after SCI at 4 wk in both rats and mice (Table 1). These results are in line with a previous study that showed the similar change in bladder compliance 4 wk after SCI in rats (19).
Because CMG recordings in both rats and mice indicate that reflex bladder contractions recover completely after SCI surgery (Table 1), it is reasonable to conclude that the difference in voiding efficiency in these two species after SCI is due to the difference in urethral activity. On the basis of the findings of this study, we presume that, in rats, reflex EUS pumping activity enables them to achieve efficient voiding even after SCI, whereas mice require more prolonged periods of synergic urethral relaxation during voiding bladder contractions for efficient voiding. After SCI in mice, this relaxation period (shown in Fig. 4, B3–B4, and Fig. 5), which occurs between periods of high-tonic EUS activity during voiding bladder contractions is not long enough to achieve efficient voiding. In SCI mice, voiding efficiency was reduced approximately five-fold, and voided volume was 40% less compared with SI mice. The total duration of R-EA during a voiding bladder contraction, which presumably represents the time of urethral opening, was 60% shorter in SCI mice than in SI mice (Fig. 5). If the urethra is only open during periods of reduced tonic EUS activity, then the decreased voiding efficiency in SCI mice might be due to a shorter period of urethral sphincter relaxation. Previous studies showed that the neurons located in the L3–L4 spinal segment are involved in the generation of EUS bursting activity during voiding (2) and that EUS bursting activity recovers 4–6 wk after SCI in rats (3). It is possible that this type of coordinating reflex activity that controls EUS bursting activity in the mouse spinal cord might be weak. In addition, the possibility that different strains or sex of mice or rats could exhibit different CMG and EUS activity patterns after SCI cannot be excluded. Therefore, further studies are needed to clarify these points.
In conclusion, the present experiments revealed that certain lower urinary tract functions are different in female rats and mice. During micturition, SI rats commonly exhibit EUS-EMG bursting, which is essential for generating high-efficiency voiding. However, only a minority of SI mice exhibit bursting-like EUS activity, and it has little impact on voiding efficiency. Relatively high voiding efficiency is maintained in chronic SCI rats due to the recovery of EUS bursting activity. On the other hand, voiding is less efficient in SCI mice and occurs during periods of reduced tonic EUS-EMG activity but without bursting. These results demonstrate subtle differences between rats and mice regarding the voluntary control of bladder and sphincter function in SI animals, as well as more prominent differences in the spinal reflex mechanisms that underlie the recovery of voiding function after SCI. These differences should be taken into account when performing basic research on neurogenic lower urinary tract dysfunction in mice and rats or when analyzing the effects of drugs on urethral function.
GRANTS
This work was supported by a grant from the National Institutes of Health (Grant P01DK-093424).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.K., N.Y., L.A.B., A.J.K., W.C.d.G., K.S., and M.Y. conception and design of research; K.K., T.M., N.W., and T.S. performed experiments; K.K. analyzed data; K.K., N.Y., N.W., T.S., W.C.d.G., and M.Y. interpreted results of experiments; K.K. and N.Y. prepared figures; K.K. and N.Y. drafted manuscript; K.K., N.Y., N.W., T.S., L.A.B., A.J.K., W.C.d.G., K.S., and M.Y. approved final version of manuscript; N.Y., T.S., L.A.B., A.J.K., W.C.d.G., K.S., and M.Y. edited and revised manuscript.
REFERENCES
- 1.Asselt EV, Groen J, Mastrigt RV. A comparative study of voiding in rat and guinea pig: simultaneous measurement of flow rate and pressure. Am J Physiol Regul Integr Comp Physiol 269: R98–R103, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol 292: F1044–F1053, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187: 445–454, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chen SC, Fan WJ, Lai CH, Jason Chen JJ, Peng CW. Effect of a 5-HT(1A) receptor agonist (8-OH-DPAT) on the external urethral sphincter activity in the rat. J Formos Med Assoc 111: 67–76, 2012. [DOI] [PubMed] [Google Scholar]
- 5.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res 152: 59–84, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol 160: 1518–1527, 1998. [PubMed] [Google Scholar]
- 7.Ikeda Y, Zabbarova IV, Birder LA, de Groat WC, McCarthy CJ, Hanna-Mitchell AT, Kanai AJ. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol 62: 1157–1164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadekawa K, Sugaya K, Nishijima S, Ashitomi K, Miyazato M, Ueda T, Yamamoto H. Effect of naftopidil, an alpha1D/A-adrenoceptor antagonist, on the urinary bladder in rats with spinal cord injury. Life Sci 92: 1024–8, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder to urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol 287: F435–F441, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol Regul Integr Comp Physiol 264: R1157–R1163, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Lin YH, Yamada Y, Daneshgari F. External urethral sphincter activity in diabetic rats. Neurourol Urodyn 27: 429–434, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am J Physiol Regul Integr Comp Physiol 251: R250–R257, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Manzo J, Vazquez MI, Cruz MR, Hernandez ME, Carrillo P, Pacheco P. Fertility ratio in male rats: effects after denervation of two pelvic floor muscles. Physiol Behav 68: 611–618, 2000. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J Urol 181: 1459–1466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa T, Seki S, Masuda H, Igawa Y, Nishizawa O, Kuno S, Chancellor MB, de Groat WC, Yoshimura N. Dopaminergic mechanisms controlling urethral function in rats. Neurourol Urodyn 25: 480–489, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Peng CW, Chen JJ, Chen CL, Grill WM. Role of pudendal afferent in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol 294: R660–R672, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol 302: R577–R586, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toosi KK, Nagatomi J, Chancellor MB, Sacks MS. The effects of long-term spinal cord injury on mechanical properties of the rat urinary bladder. Ann Biomed Eng 36: 1470–1480, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Vera PL, Nadelhaft I. Effects of the atypical neuroleptic clozapine on micturition parameters in anesthetized rats. Neurourol Urodyn 20: 623–639, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Yoshiyama M, de Groat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology 55: 956–960, 2000. [DOI] [PubMed] [Google Scholar]