Abstract
Adipose tissue (AT) inflammation is a hallmark characteristic of obesity and an important determinant of insulin resistance and cardiovascular disease; therefore, a better understanding of factors regulating AT inflammation is critical. It is well established that reduced vascular endothelial nitric oxide (NO) bioavailability promotes arterial inflammation; however, the role of NO in modulating inflammation in AT remains disputed. In the present study, 10-wk-old C57BL6 wild-type and endothelial nitric oxide synthase (eNOS) knockout male mice were randomized to either a control diet (10% kcal from fat) or a Western diet (44.9% kcal from fat, 17% sucrose, and 1% cholesterol) for 18 wk (n = 7 or 8/group). In wild-type mice, Western diet-induced obesity led to increased visceral white AT expression of inflammatory genes (e.g., MCP1, TNF-α, and CCL5 mRNAs) and markers of macrophage infiltration (e.g., CD68, ITGAM, EMR1, CD11C mRNAs, and Mac-2 protein), as well as reduced markers of mitochondrial content (e.g., OXPHOS complex I and IV protein). Unexpectedly, these effects of Western diet on visceral white AT were not accompanied by decreases in eNOS phosphorylation at Ser-1177 or increases in eNOS phosphorylation at Thr-495. Also counter to expectations, eNOS knockout mice, independent of the diet, were leaner and did not exhibit greater white or brown AT inflammation compared with wild-type mice. Collectively, these findings do not support the hypothesis that reduced NO production from eNOS contributes to obesity-related AT inflammation.
Keywords: obesity, Western diet, adipose tissue, endothelial nitric oxide synthase
with obesity, expansion of visceral white adipose tissue (AT) is accompanied by infiltration of immune cells and increased expression of inflammatory cytokines (7). A better understanding of the factors regulating AT inflammation is important, given the cumulative evidence implicating AT inflammation as a determinant of obesity-related insulin resistance and cardiovascular disease (3, 14). Although it is well known that reduced vascular endothelial nitric oxide (NO) bioavailability, a hallmark characteristic of obesity and Type 2 diabetes, contributes to arterial inflammation (4, 6, 9, 10, 15), conflicting evidence exists regarding the role of NO in modulating inflammation in AT. One study revealed that male endothelial nitric oxide synthase (eNOS) knockout mice exhibit increased expression of inflammatory genes and markers of immune cell infiltration in visceral white (epididymal) AT (5); however, that finding does not appear to be reinforced by data from others (12, 13). In a microarray analysis of visceral white AT from female eNOS knockout vs. wild-type mice, inflammatory genes were not reported among the genes with significantly altered expression (13); however, since data on inflammatory genes were not reported, conclusions cannot be drawn regarding whether or not those eNOS knockout mice had greater or equivalent AT inflammation compared with controls. We recently showed in lean and obese male rats that chronic administration of Nitro-l-arginine methyl ester (l-NAME), a NOS inhibitor, did not increase markers of inflammation in AT (12) but did in the liver (17); nevertheless, these data should be interpreted with the understanding that l-NAME inhibits production of NO from all three NOS enzymes [eNOS, inducible NOS (iNOS), and neuronal NOS (nNOS)].
On the basis of these contrasting findings, we set out the present study to more comprehensively interrogate the role of NO in modulating AT inflammation. We assessed several markers of inflammation in visceral white AT of eNOS knockout and wild-type male mice fed a control vs. Western diet. Given that brown AT is more vascularized than white AT and that eNOS is predominantly expressed in endothelial cells, the impact of eNOS deficiency on inflammatory markers was also examined in the interscapular brown AT depot. Indeed, if obesity-induced AT inflammation is partially mediated by reduced NO signaling, we would expect the AT of lean eNOS-deficient mice to phenotypically resemble that of obese mice. That is, we would hypothesize that the proinflammatory effect of eNOS deletion would normalize differences in AT inflammation between lean and obese mice. Further, we hypothesized that eNOS ablation would have greater inflammatory effects in brown AT compared with white AT.
METHODS
Experimental design.
Male C57BL6 wild-type and eNOS knockout mice (B6.129P2-Nos3tm1Unc/J) on a C57BL6 background were purchased from Jackson Laboratory (Bar Harbor, ME) at 10 wk of age. Mice were randomized to either normal chow (i.e., control diet) or Western diet (n = 7 or 8/group) ad libitum for 18 wk. Control diet (3.85 kcal/g of food) contained 10% kcal fat, 70% kcal carbohydrate, and 20% kcal protein, with 3.5% kcal sucrose (D12110704, custom formulated; Research Diets, New Brunswick, NJ). Western diet (4.68 kcal/g of food) contained 44.9% kcal fat, 35.1% kcal carbohydrate, and 20% kcal protein, with 1% cholesterol and 17% kcal sucrose (D09071604, custom formulated; Research Diets). All mice were pair-housed under standard temperature conditions (∼22°C) and humidity with a light cycle from 0700 to 1900 and a dark cycle from 1900 to 0700. At 28 wk of age, mice were euthanized following a 5-h fast, and tissues were harvested for analysis. All procedures were approved in advance by the University of Missouri Institutional Animal Care and Use Committee.
Body composition.
The percent body fat was measured by a nuclear magnetic resonance imaging whole-body composition analyzer (EchoMRI 4in1/1100; Echo Medical Systems, Houston, TX). This noninvasive measure was performed on conscious mice.
Fasting blood parameters.
Plasma glucose, cholesterol, triglycerides, alanine aminotransferase, aspartate aminotransferase, and nonesterified fatty acids (NEFA) assays were performed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) on an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) using assays according to manufacturer's guidelines. Plasma insulin concentrations were determined using a commercially available, mouse-specific ELISA (Alpco Diagnostics, Salem, NH). Plasma nitrate + nitrite (NOx) levels were measured using the nitrate/nitrite fluorometric assay kit (no. 780051; Cayman Chemical, Ann Arbor, MI), according to manufacturer's instructions.
Histological assessments.
Formalin-fixed epididymal white AT samples were processed through paraffin embedment, sectioned at 5 μm, stained with Mac-2 antibody (CL8942AP, Cedarlane), a macrophage marker. Sections were evaluated via an Olympus BX34 photomicroscope (Olympus, Melville, NY), and images were taken at ×10 magnification via an Olympus SC30 optical microscope accessory CMOS color camera. Objective quantification of macrophage infiltration was done by determining the positive Mac-2-stained area per ×10 field of view using ImageJ software (NIH public domain; National Institutes of Health, Bethesda, MD). The average of six ×10 fields of view was used per animal. Adipocyte size was calculated based on 100 adipocytes/animal obtained from the same six ×10 fields, as performed previously (19). Briefly, cross-sectional areas of the adipocytes were obtained from perimeter tracings using ImageJ software. All procedures were performed by an investigator who was blinded to the groups.
RNA extraction and quantitative real-time RT-PCR.
Epididymal white and interscapular brown AT, as well as aorta samples, were homogenized in TRIzol solution using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA). Total RNA was isolated, according to the Qiagen's RNeasy lipid tissue protocol, and was assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration. The MU DNA Core confirmed our RNA extraction produced optimal RNA integrity (all RQN's > 8.0). First-strand cDNA was synthesized from total RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed, as previously described using the ABI StepOne Plus sequence detection system (Applied Biosystems). Primer sequences were designed using the NCBI Primer Design tool and have been previously published (19). All primers were purchased from Integrated DNA Technologies (Coralville, IA). A 20-μl reaction mixture containing 10 μl iTaq UniverSYBR Green SMX (Bio-Rad, Hercules, CA), and the appropriate concentrations of gene-specific primers plus 4 μl of cDNA template were loaded in a single well of a 96-well plate. All PCR reactions were performed in duplicate under thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 45 s. A dissociation melt curve analysis was performed to verify the specificity of the PCR products. GAPDH was used as a house-keeping control gene. GAPDH cycle threshold (CT) was not different among the groups of animals. mRNA expression values are presented as 2ΔCT, whereby ΔCT = GAPDH CT − gene of interest CT. mRNA levels were normalized to the wild-type control diet group.
Western blot analysis.
Triton X-100 tissue lysates were used to produce Western blot-ready Laemmli samples. Protein samples (10 μg/lane) were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with primary antibodies. eNOS (no. 610296, 1:500) and phospho-specific eNOS at Ser-1177 (no. 61239, 1:250) and Thr-495 (no. 612706, 1:500) antibodies were purchased from BD Biosciences (San Jose, CA). Akt (no. 4691, 1:500), phospho-specific Akt at Ser-473 (no. 4060, 1:250), Mac-2 (no. 127335, 1:1000), iNOS (no. 13120, 1:500), and GAPDH (no. 5174, 1:1,000) antibodies were purchased from Cell Signaling (Beverly, MA). OXPHOS (no. ab110413, 1:2,000) cocktail antibody was purchased from Abcam (Cambridge, MA). Nitrotyrosine (no. 5411; 1:500) antibody was purchased from Millipore (Billerica, MA). Intensity of individual protein bands was quantified using FluoroChem HD2 (AlphaView, version 3.4.0.0) and were expressed as a ratio to control band GAPDH.
Statistical analysis.
A 2 × 2 (genotype × diet) ANOVA was used to evaluate the effects of eNOS deficiency and Western diet for all dependent variables. When a significant genotype by diet interaction existed, least significant difference post hoc test was used for pair-wise comparisons. All data are presented as means ± SE. For all statistical tests, the α level was set at 0.05. All statistical analyses were performed with SPSS (version 23.0).
RESULTS
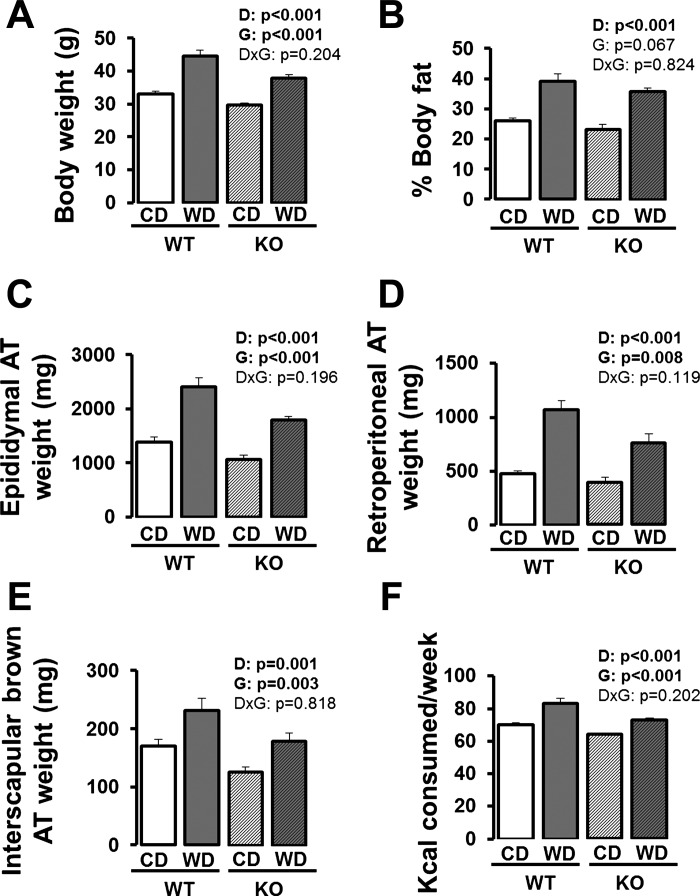
As shown in Fig. 1, independent of genotype, mice fed a Western diet had larger body weights, greater adiposity, and consumed more kilocalories per day compared with control diet-fed mice (P < 0.05). eNOS knockout mice weighed less, had reduced adiposity, and consumed fewer kilocalories per week compared with wild-type mice (P < 0.05). Table 1 summarizes the fasting blood characteristics. Independent of genotype, compared with control-fed mice, Western diet-fed mice had greater plasma total cholesterol, low-density lipoprotein, high-density lipoprotein, alanine aminotransferase, insulin, and HOMA-IR, and reduced plasma nitrite + nitrate (P < 0.05). Despite being leaner, eNOS knockout mice had greater plasma NEFAs compared with wild-type mice (P < 0.05).
Fig. 1.
Body weight (A), percent body fat (B), epididymal adipose tissue (AT) weight (C), retroperitoneal AT weight (D), interscapular brown AT weight (E), and kilocalories consumed per week (F) in wild-type and eNOS knockout mice fed a control diet vs. Western diet. Values for reported body weight, percent fat, and fat pad weights are from the time of death. Values for kilocalories consumed/week are calculated as an average over the 18-wk feeding period. Data are expressed as means ± SE. D denotes main effect of diet, G denotes main effect of genotype, and D×G denotes diet by genotype interaction. Significant P values (<0.05) are highlighted in bold; n = 7 to 8/group.
Table 1.
Fasting blood characteristics
| Wild-Type |
eNOS Knockout |
||||
|---|---|---|---|---|---|
| Variable | Control Diet | Western Diet | Control Diet | Western Diet | Two-Way ANOVA |
| Total cholesterol, mg/dl | 145.4 ± 6.9 | 215.6 ± 25.7 | 134.6 ± 4.9 | 247.1 ± 25.6 | D: P < 0.001 |
| G: P = 0.590 | |||||
| D×G: P = 0.276 | |||||
| LDL cholesterol, mg/dl | 7.8 ± 0.6 | 16.9 ± 2.4 | 6.0 ± 0.4 | 20.4 ± 5.3 | D: P = 0.001 |
| G: P = 0.776 | |||||
| D×G: P = 0.400 | |||||
| HDL cholesterol, mg/dl | 64.3 ± 2.0 | 96.0 ± 10.5 | 65.3 ± 3.9 | 105.9 ± 4.2 | D: P < 0.001 |
| G: P = 0.354 | |||||
| D×G: 0.452 | |||||
| Triglycerides, mg/dl | 51.1 ± 4.8 | 42.0 ± 3.3 | 59.9 ± 4.1 | 52.1 ± 9.7 | D: P = 0.194 |
| G: P = 0.148 | |||||
| D×G: P = 0.913 | |||||
| NEFA, mmol/l | 0.73 ± 0.05 | 0.69 ± 0.07 | 0.80 ± 0.05 | 0.92 ± 0.10 | D: P = 0.585 |
| G: P = 0.042 | |||||
| D×G: P = 0.285 | |||||
| ALT, U/l | 38.3 ± 4.8 | 210.6 ± 50.3 | 42.3 ± 5.3 | 250.8 ± 72.3 | D: P < 0.001 |
| G: P = 0.631 | |||||
| D×G: P = 0.694 | |||||
| AST, U/l | 226.9 ± 19.6 | 288.7 ± 52.9 | 273.0 ± 50.0 | 321.9 ± 38.4 | D: P = 0.189 |
| G: P = 0.342 | |||||
| D×G: P = 0.876 | |||||
| Insulin, ng/ml | 0.8 ± 0.1 | 2.4 ± 0.5 | 1.2 ± 0.2 | 3.2 ± 1.2 | D: P = 0.017 |
| G: P = 0.415 | |||||
| D×G: P = 0.786 | |||||
| Glucose, mg/dl | 290.5 ± 25.5 | 309.0 ± 30.4 | 316.1 ± 14.6 | 315.5 ± 46.4 | D: P = 0.786 |
| G: P = 0.625 | |||||
| D×G: P = 0.770 | |||||
| HOMA-IR | 17.5 ± 2.5 | 57.3 ± 12.4 | 27.9 ± 5.8 | 74.9 ± 30.6 | D: P = 0.021 |
| G: P = 0.436 | |||||
| D×G: P = 0.840 | |||||
| Nitrate + Nitrite, pmol/l | 20.6 ± 3.1 | 11.6 ± 1.4 | 16.4 ± 2.6 | 11.2 ± 2.4 | D: P = 0.011 |
| G: P = 0.382 | |||||
| D×G: P = 0.455 | |||||
Data are expressed as means ± SE.
D, main effect of diet; G, main effect of genotype; D×G, diet by genotype interaction. LDL, low-density lipoprotein; HDL, high-density lipoprotein; NEFA, nonesterified fatty acids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA-IR, homeostatic model assessment insulin resistance index. Significant P values (<0.05) are highlighted in bold; n = 7 or 8/group.
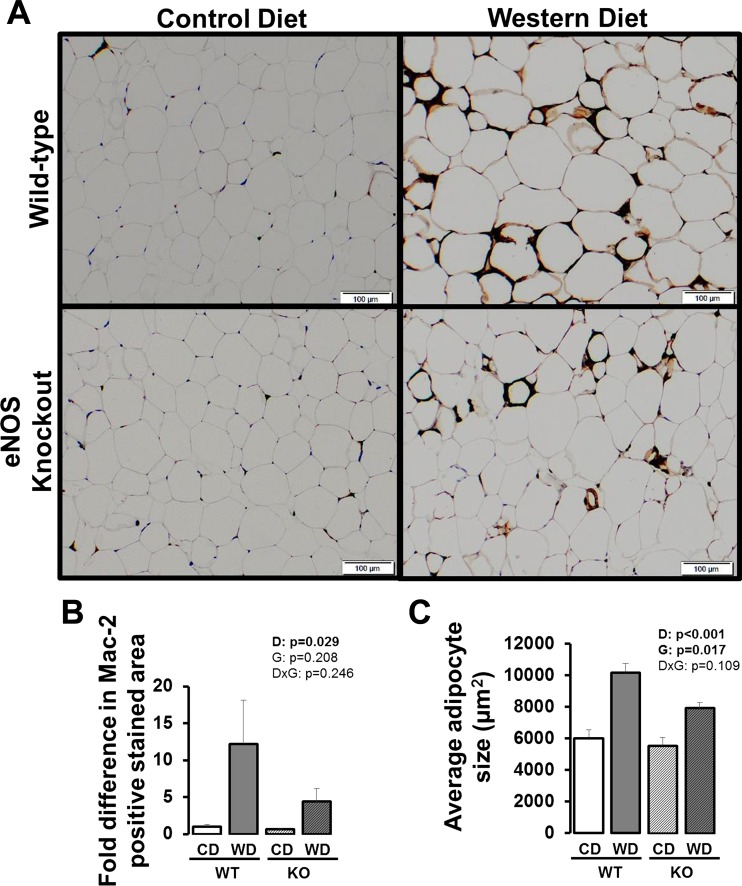
Figure 2A illustrates representative histological images of epididymal white AT stained with Mac-2, a macrophage marker, and quantification is presented in Fig. 2B. Western diet-fed mice had greater Mac-2 staining in white AT than mice fed a control diet (P < 0.05), independent of genotype. Similarly, adipocyte size was greater in Western diet-fed mice compared with control diet-fed mice (P < 0.05) and smaller in eNOS knockout mice compared with wild-type mice (Fig 2C, P < 0.05).
Fig. 2.
Representative histology images (×10) of visceral white (i.e., epididymal) AT stained for Mac-2 (A), fold differences in Mac-2-positive stained area (B), and average adipocyte size (C) in wild-type and eNOS knockout mice fed a control diet vs. Western diet. Data are expressed as means ± SE. D denotes main effect of diet, G denotes main effect of genotype, D×G denotes diet by genotype interaction. Significant P values (<0.05) are highlighted in bold; n = 5/group.
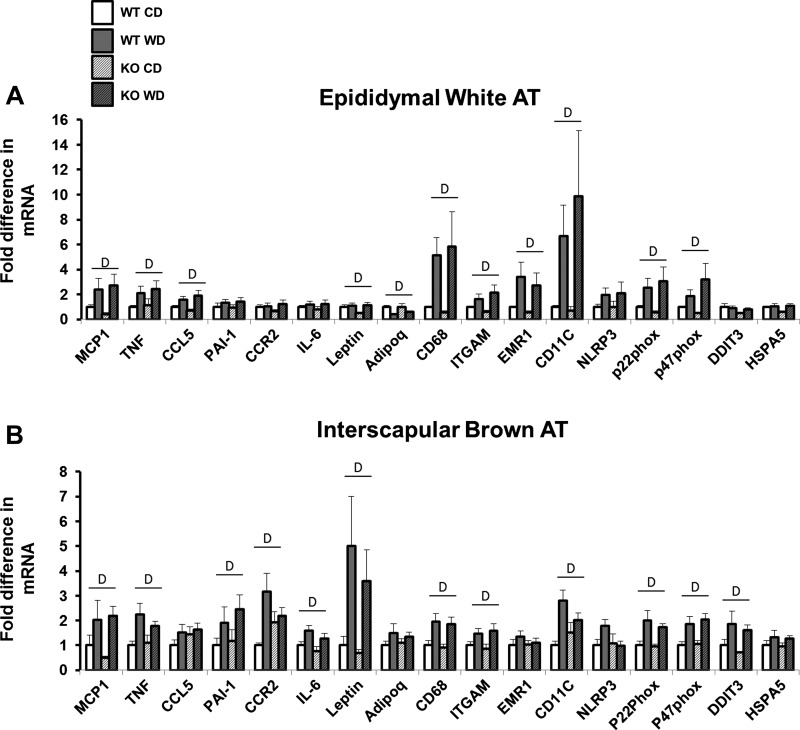
Gene expression data in epididymal white AT and interscapular brown AT are summarized in Fig. 3, A and B, respectively. In white AT, mRNA levels related to inflammation [e.g., MCP-1, TNF-α, and CCL5], immune cell infiltration [e.g., CD68, ITGAM, EMR1, and CD11c], and oxidative stress [e.g., p22phox and p47phox] were all elevated with Western diet (P < 0.05) and not affected by eNOS ablation (P > 0.05). Adipokine leptin mRNA was increased, and adiponectin mRNA was decreased with the Western diet (P < 0.05) but not affected by loss of eNOS (P > 0.05). In brown AT, mRNA levels related to inflammation [MCP1, TNF-α, CCR2, and IL-6], immune cell infiltration [e.g., CD68, ITGAM, and CD11c], oxidative stress [e.g., p22phox and p47phox], endoplasmic reticulum stress [e.g., DDIT3], anti-clotting protein PAI-1, and the adipokine leptin were all elevated with Western diet (P < 0.05) and, again, not affected by eNOS abrogation (P > 0.05).
Fig. 3.
Visceral white (i.e., epididymal) (A) and brown (i.e., interscapular) (B) AT gene expression in wild-type and eNOS knockout mice fed a control diet vs. Western diet. Data are expressed as means ± SE. D denotes main effect of diet (P < 0.05). No significant main effects of genotype or interactions were found (all P > 0.05); n = 7 to 8/group.
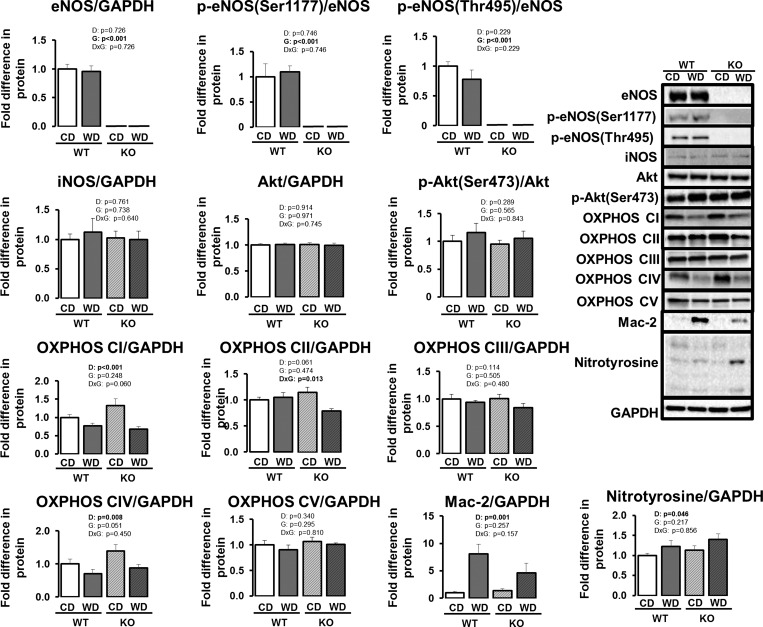
eNOS protein content in epididymal white AT was undetectable in eNOS knockout mice (Fig. 4, P < 0.05). No differences in total eNOS, p-eNOS at Ser-1177, or p-eNOS at Thr-495 were observed with Western diet in wild-type mice (P > 0.05). iNOS protein content was also unaltered with Western diet or eNOS ablation (P > 0.05). In addition, there were no effects of diet or genotype on Akt and p-Akt at Ser-473 (P > 0.05). Similar to findings obtained by immunohistochemistry (Fig. 2) and gene expression (Fig. 3), Mac-2 protein content was greater in Western diet-fed mice (Fig. 4, P < 0.05), independent of genotype. Furthermore, consistent with findings in gene expression (Fig. 3), we found a Western diet-induced increase in nitrotyrosine, a marker of oxidative stress (P < 0.05). Western diet reduced mitochondrial oxidative phosphorylation complex I and IV protein content in both genotypes (P ≤ 0.05) and complex II protein content in eNOS knockout (P < 0.05). No effects of diet or genotype were observed in complexes III or V (P > 0.05).
Fig. 4.
Visceral white (i.e., epididymal) AT protein content in wild-type and eNOS knockout mice fed a control diet vs. Western diet. Data are expressed as means ± SE. D denotes main effect of diet, G denotes main effect of genotype, D×G denotes diet by genotype interaction. Significant P values (<0.05) are highlighted in bold; n = 7 to 8/group. For the nitrotyrosine blot, an average of all bands (between 75 kDa and 25 kDa) was calculated.
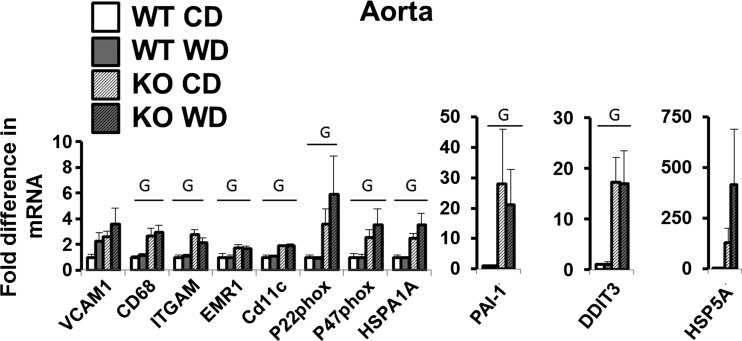
In stark contrast with AT data (Fig. 3), aortic gene expression markers of immune cell infiltration [CD68, ITGAM, EMR1, and CD11c], oxidative stress [p22phox and p47phox], endoplasmic reticulum stress [DDIT3, HSPA1A, HSP5A (P = 0.072)], and anti-clotting protein PAI-1 were significantly upregulated in eNOS knockout compared with wild-type mice (Fig. 5, P < 0.05, unless stated otherwise). Also, in contrast to the results observed in AT, no diet effects were found in the aorta (P > 0.05).
Fig. 5.
Aorta gene expression in wild-type and eNOS knockout mice fed a control diet vs. Western diet. Data are expressed as means ± SE. G denotes main effect of genotype (P < 0.05). No significant main effects of diet or interactions were found (all P > 0.05); n = 4 to 8/group.
DISCUSSION
We found that increased white AT inflammation with Western diet-induced obesity was not accompanied by changes in eNOS phosphorylation at Ser-1177 and Thr-495, the two phosphorylation sites that mainly regulate eNOS activity. Furthermore, ablation of eNOS did not increase AT inflammation in either white or brown AT. Collectively, these findings do not support the hypothesis that reduced NO production from eNOS contributes to obesity-related AT inflammation.
Our findings are in discordance with results from Handa et al. (5), a study also using the eNOS knockout mouse model. In that previous study, the authors reported that eNOS deficiency, but surprisingly not 4 wk of high-fat diet feeding, promoted inflammation in epididymal AT (5). Our results indicate that markers of inflammation in white AT were upregulated by 18 wk of Western diet-induced obesity, but not by eNOS deficiency. We do not have a clear explanation for the discrepancies in results between these two studies. One possibility could be the use of different strains of eNOS knockout mice; however, the strain used in that previous publication (5) was not reported. Another explanation could be related to the use of different diets. Indeed, in Handa et al. (5), obesity was induced with a diet containing 60% kcal from fat, 20% kcal from carbohydrate, and 20% kcal from protein (5.24 kcal/g of food); whereas in our study, the Western diet contained 44.9% kcal from fat, 35.1% kcal from carbohydrate, and 20% kcal protein, with 1% cholesterol (4.68 kcal/g of food). The control diets were comparable between studies in macronutrient composition (10% kcal from fat, 70% kcal from carbohydrate, and 20% kcal from protein; 3.85 kcal/g of food), but levels of sucrose were markedly different [34.5% kcal in Handa et al. (5) vs. 3.5% kcal in our study]. Another important difference between study designs to highlight is that our mice were given the respective diets for 18 wk, whereas in Handa et al.'s study, the diet intervention was 4 wk. To determine whether the lack of effect of eNOS deficiency was limited to white AT, we examined the same genes in interscapular brown AT, a fat depot that is more vascularized. Similar to white AT, we found no evidence of increased inflammation in brown AT from eNOS knockout mice. Interestingly, however, the lack of inflammation could be specific to AT, as we have preliminary data suggesting that there are robust differences in inflammatory markers in the liver of eNOS knockout mice compared with wild-type mice (R. S. Rector, unpublished observations). As will be discussed later, inflammation was also detected in vascular tissue of eNOS knockout mice.
The present findings are congruent with findings from a genome-wide microarray analysis of visceral white AT from eNOS knockout vs. wild-type female mice (13). In that study, no significant differences between eNOS knockout and wild-type mice were reported in regard to inflammatory genes. In support of the notion that reduced production of NO does not contribute to AT inflammation, we recently found that expression of inflammatory genes and markers of immune cell infiltration in white and brown AT were, by and large, unchanged with chronic administration of l-NAME in drinking water in both lean and obese rats (12). However, because l-NAME inhibits all NOS isoforms, we were unable to exclusively test the role of eNOS as a source of NO.
In the present study, the lack of effect of eNOS deficiency on white and brown AT inflammation was in marked contrast with findings in the aorta where loss of eNOS produced increased expression of inflammatory genes and markers of immune cell infiltration. Our data expand on previous work demonstrating the atheroprotective role of vascular NO. Indeed, previous research demonstrates that inhibition of NOS with l-NAME upregulates inflammatory and prooxidant genes, increases endothelial cell adhesion, and promotes atherosclerotic lesions (1, 4, 6, 12). Further, there is evidence that eNOS-deficient mice display aberrant vascular remodeling in response to external carotid artery ligation (15), and mice with eNOS/apoE double knockout reveal accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease (8, 9).
A novel finding of the present study was the observation in the aorta that eNOS deficiency was distinctly associated with increased expression of endoplasmic reticulum (ER) stress markers, including DDIT3, HSP5A, and HSPA1A mRNA (genes encoding for CHOP, GRP78, and HSP72, respectively). ER stress has been implicated in the etiology and progression of atherosclerosis (2, 11, 16, 18), and these data suggest that reduced eNOS-derived NO production promotes ER stress; however, more research is needed to elucidate the intracellular signaling mechanisms underlying the link between reduced NO and ER stress.
This study's findings should be considered in light of its limitations. First, our study did not establish whether the reported changes in adipose tissue mRNA and protein levels are attributable to alterations in the phenotype of adipocytes and/or resident immune cells within AT. It should be noted that expression of some inflammatory genes is markedly different when assessed in the stromal vascular fractions vs. whole tissue (20). As such, future research using FACS analysis is needed to examine the differential inflammatory effects of obesity and eNOS ablation on adipose tissue immune cells (i.e., macrophages, T-cells) vs. adipocytes. Second, we have attempted to measure nitrate + nitrite levels in AT homogenates as a surrogate measure of NO; however, the levels were below the detection limits of the assay. Thus, it remains unknown the extent to which AT NO bioavailability is affected by Western diet and eNOS ablation. Our finding that markers of oxidative stress were increased with Western diet suggests that NO bioavailabilty was likely reduced due to NO scavenging by superoxide radicals. Third, despite eNOS-deficient mice being smaller, leaner, and revealing a decrease in adipocyte size, they displayed an equivalent degree of AT inflammation. One interpretation of this finding is that, relative to the degree of adiposity, the eNOS knockout mice are more inflamed (i.e., more inflammation per unit of adipose tissue). To examine the effect of eNOS ablation independent of adiposity differences, we performed a secondary analysis that involved the comparison of the four leanest mice from the wild-type Western diet group (weight = 41.0 ± 2.0 g; 37.9 ± 4.4% fat) vs. the four heaviest mice from the eNOS knockout Western diet group (weight = 40.2 ± 1.7 g; 38.5 ± 1.2% fat). We found no significant differences in any of the markers of inflammation between these two subgroups (P values ranged from 0.233 to 0.934), thus suggesting that the lack of genotype effect on AT inflammation may be unrelated to differences in body weight or adiposity. However, to more precisely evaluate the influence of adiposity, future studies should experimentally match body weight and adiposity between wild-type and eNOS knockout mice using a pair-feeding paradigm. These effects of eNOS deficiency on body weight and composition may be due to central effects, resulting in reduced food and caloric intake (Fig. 1F). In our previous chronic l-NAME treatment study in rats, it was also noted that NOS inhibition diminished food intake and adiposity (12).
In conclusion, we found that increased white AT inflammation with Western diet-induced obesity was not accompanied by changes in eNOS phosphorylation at Ser-1177 and Thr-495, two sites of phosphorylation that largely control eNOS activation. In addition, we found no evidence of increased AT inflammation in mice with genetic deletion of eNOS. Therefore, obesity and insulin resistance-associated AT inflammation appear to be unrelated to eNOS-derived NO production.
GRANTS
This work was supported by a University of Missouri Life Sciences Fellowship (to R. D. Sheldon), Veterans Health Administration Grant CDA2 1BX001299 (to R. S. Rector), and the National Institutes of Health Grants K01 HL-125503 and R21 DK-105368 (to J. Padilla). This work was completed with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.J.J., R.D.S., M.L.G., M.L.W., T.M.Z., and R.S.R. performed experiments; T.J.J., R.D.S., M.L.G., M.L.W., R.S.R., and J.P. analyzed data; T.J.J., R.D.S., R.S.R., V.J.V.-P., and J.P. interpreted results of experiments; T.J.J., R.D.S., M.L.G., T.M.Z., R.S.R., V.J.V.-P., and J.P. edited and revised manuscript; T.J.J., R.D.S., M.L.G., M.L.W., T.M.Z., R.S.R., V.J.V.-P., and J.P. approved final version of manuscript; R.D.S., R.S.R., V.J.V.-P., and J.P. conception and design of research; J.P. prepared figures; J.P. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Grace Meers, Rebecca Scroggins, Nick Fleming, Youngmin Park, and T'Keaya Gaines for excellent technical assistance.
REFERENCES
- 1.Cayatte A, Palacino J, Horten K, Cohen R. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb 14: 753–759, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Role of endoplasmic reticulum stress in atherosclerosis and diabetic macrovascular complications. Biomed Res Int 2014: 610140, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndrome 4: 113–119, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gomez-Guzman M, Jimenez R, Sanchez M, Romero M, O'Valle F, Lopez-Sepulveda R, Quintela A, Galindo P, Zarzuelo M, Bailon E, Delpon E, Perez-Vizcaino F, Duarte J. Chronic (-)-epicatechin improves vascular oxidative and inflammatory status but not hypertension in chronic nitirc oxide-deficient rats. Br J Nutr 106: 1337–1348, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Handa P, Tateya S, Rizzo N, Cheng A, Morgan-Stevenson V, Han C, Clowes A, Daum G, O'Brien K, Schwartz M, Chait A, Kim F. Reduced vascular nitric oxide-cGMP signaling contributes to adipose tissue inflammation during high-fat feeding. Arterioscler Thromb Vasc Biol 31: 2827–2835, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossain M, Qadri S, Liu L. Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J Inflamm 9: 28, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobi D, Stanya KJ, Lee CH. Adipose tissue signaling by nuclear receptors in metabolic complications of obesity. Adipocyte 1: 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhlencordt P, Gyurko R, Han F, Scherrer-Crosbie M, Aretz T, Hajjar R, Picard M, Huang P. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104: 448–454, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Kuhlencordt P, Padmapriya P, Rutzel S, Schodel J, Hu K, Schafer A, Huang P, Ertl G, Bauersachs J. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE KO mice. Atherosclerosis 202: 48–57, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laroux F, Pavlick K, Hines I, Kawachi S, Harada H, Bharwani S, Hoffman J, Grisham M. Role of nitric oxide in inflammation. Acta Physiol Scand 173: 113–118, 2001. [DOI] [PubMed] [Google Scholar]
- 11.McAlpine CS, Werstuck GH. The development and progression of atherosclerosis: evidence supporting a role for endoplasmic reticulum (ER) stress signaling. Cardiovasc Hematolog Disord Drug Targets 13: 158–164, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Padilla J, Jenkins NT, Thorne PK, Lansford KA, Fleming NJ, Bayless DS, Sheldon RD, Rector RS, Laughlin MH. Differential regulation of adipose tissue and vascular inflammatory gene expression by chronic systemic inhibition of NOS in lean and obese rats. Physiol Rep 2: e00225, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razny U, Kiec-Wilk B, Polus A, Wator L, Dyduch G, Partyka L, Bodzioch M, Tomaszewska R, Wybranska I. The adipose tissue gene expression in mice with different nitric oxide availability. J Physiol Pharmacol 61: 607–618, 2010. [PubMed] [Google Scholar]
- 14.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin NA 95: 875–892, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Rudic R, Shesely E, Maeda N, Smithies O, Segal S, Sessa W. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101: 731–736, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol 31: 2792–2797, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheldon RD, Padilla J, Jenkins NT, Laughlin MH, Rector RS. Chronic NOS inhibition accelerates NAFLD progression in an obese rat model. Am J Physiol Gastrointest Liver Physiol 308: G540–G549, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res 107: 839–850, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. Am J Physiol Regul Integr Comp Physiol 309: R594–R602, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]