Abstract
Hv1 is a voltage-gated proton channel highly expressed in phagocytic cells, where it participates in the NADPH oxidase-dependent respiratory burst. We have recently identified Hv1 as a novel renal channel, expressed in the renal medullary thick ascending limb that appears to importantly contribute to the pathogenesis of renal hypertensive injury in the Dahl salt-sensitive rat model. The purpose of this review is to describe the experimental approaches that we have undertaken to identify the source of excess reactive oxygen species production in the renal outer medulla of Dahl salt-sensitive rats and the resulting evidence that the voltage-gated proton channel Hv1 mediates augmented superoxide production and contributes to renal medullary oxidative stress and renal injury. In addition, we will attempt to point out areas of current controversy, as well as propose areas in which further experimental studies are likely to move the field forward. The content of the following review was presented as part of the Water and Electrolyte Homeostasis Section New Investigator Award talk at Experimental Biology 2014.
Keywords: chronic kidney disease, bicarbonate, hydrogen ion, hypertension, kidney, nephrosclerosis, thick ascending limb
the dahl salt-sensitive rat has been used for more than 50 years as a model organism to dissect the underlying causes of salt-sensitive hypertension and hypertensive renal injury (74). Two main approaches have been used: 1) a top-down approach, in which phenotypic traits associated with the development of hypertension and renal injury, have been investigated in greater and greater detail in an attempt to reveal the underlying cellular and genetic causes of disease and 2) more recently, modern genetic and proteomic approaches that have been used in a “bottom-up” effort to identify genes and proteins associated with disease traits in these animals (12). This review describes our own use of this first approach and the resulting evidence that voltage-gated proton channels contribute to disease progression in the Dahl salt-sensitive rat model. The content of the following review was presented as part of the Water and Electrolyte Homeostasis Section New Investigator Award talk at Experimental Biology 2014.
Dahl Salt-Sensitive Rat as a Model of Salt-Sensitive Hypertension and Hypertensive Renal Injury
The Dahl salt-sensitive rat model was first developed in the early 1960s by Lewis K. Dahl by selectively breeding together either salt-susceptible (those that exhibited a rise in arterial pressure in response to high-salt feeding) or salt-resistant out-bred Sprague-Dawley rats (74). In modern Western populations, in which salt intake is high, human salt-sensitive hypertension is normally defined as a reduction in blood pressure from a relatively stable hypertensive level following a decrease in dietary Na+ intake (68). In contrast, the Dahl salt-sensitive rat develops a rapid and progressive malignant hypertension upon high-salt feeding, which continues to rise as long as the diet is high in salt. Despite these differences in the pathogenesis of the hypertensive phenotype, the Dahl salt-sensitive rat model shares many of the traits associated with human hypertension and may be of particular relevance to phenotypes observed commonly in the African-American population in which salt sensitivity is prevalent (50, 64). Following the development of hypertension, the Dahl salt-sensitive rat also develops marked and progressive renal disease, which is blood pressure-dependent (48, 54). The Dahl salt-sensitive rat model is relatively unique in this regard, with most hypertensive animal models developing only modest renal injury following the development of hypertension. Much of the renal injury that occurs in hypertensive Dahl salt-sensitive rats resembles renal injury observed in human chronic kidney disease patients, in which hypertension is the primary diagnosis, including renal ischemia and a pitted appearance of the kidney surface, focal and segmental glomerular sclerosis, medial-intimal vascular thickening, and the formation of proteinacious casts (54). Therefore, in addition to an experimental model of salt-sensitive hypertension, the Dahl salt-sensitive rat model also provides a potential window into the pathophysiological mechanisms linking hypertension to the development of renal barotrauma and tissue injury.
Traditionally salt-resistant (Dahl R) rats have been the preferred control strain for the Dahl salt-sensitive rat (74). As mentioned, in an effort to understand the complex genetics underlying salt-sensitive hypertension in the Dahl salt-sensitive model, in recent years, numerous consomic, and later congenic, strains have been developed by introgressing select chromosomes or regions of chromosomes, respectively, from the salt-resistant Brown-Norway rat strain into the genetic background of Dahl salt-sensitive rats (17). Phenotyping of these strains has narrowed the search for genes or regions of genes (quantitative trait loci) responsible for the various pathogenic traits observed in the Dahl salt-sensitive rat model. A second benefit of the development of these strains, however, has been the use of these genetically more similar strains as control animals for the study of Dahl salt-sensitive rats. The inbred consomic SS.13BN rat, which was derived by introgressing chromosome 13 of the Brown-Norway rat into the MCW-bred salt-sensitive (SS) genetic background, exhibits a marked reduction of salt-induced hypertension, proteinuria, and glomerular disease when fed a high-salt diet compared with the parental SS strain (12, 15). As these consomic strains exhibit less genetic differences to the parental Dahl salt-sensitive rat model, phenotypic differences observed when comparing these strains are more likely to be related to the pathogenesis of the selected trait (12, 15). For these reasons, we have chosen to use the SS.13BN rat as a salt-resistant control strain in studies in which the Dahl salt-sensitive rat model is used to understand the underlying physiological mechanisms mediating salt-sensitive hypertension and renal injury.
Medullary Ischemia and the Development of Sustained Hypertension
Arthur Guyton was the first to propose the role of the kidneys (specifically, the pressure natriuresis mechanism) as the dominant controller of long-term arterial pressure (33). This hypothesis is supported by a wealth of experimental data, including 1) evidence that after renal transplant, blood pressure follows the kidney (including the Dahl salt-sensitive rat model) (10, 20, 21, 52); 2) evidence that, of the identified single nucleotide polymorphisms that result in significant alterations in blood pressure in humans, all act to affect salt and water reabsorption by the kidney(40); 3) a multitude of experimental studies, indicating that maneuvers that alter the renal pressure-natriuresis relationship by shifting the set point to higher pressures, result in the development of sustained hypertension (11, 16). Given this evidence, our own studies investigating the pathogenesis of hypertension in the Dahl salt-sensitive rat have focused on renal function.
One maneuver that has been demonstrated to shift the renal pressure-natriuresis relationship to higher pressures and, thereby, promote sustained hypertension, is the induction of selective renal medullary ischemia (13). Medullary ischemia brought about by a variety of stimuli, including medullary infusion of a vasopressin V1 receptor agonist (18), the nitric oxide inhibitor l-NAME (56), or a rarefaction of the medullary circulation following ischemia-reperfusion injury (5, 61), have all been demonstrated to be associated with the development of hypertension. In addition, and with particular relevance to our own work, artificially elevating superoxide (O2·−) levels selectively in the renal medulla by infusing the superoxide-dismutase inhibitor diethyldithiocarbonate, has also been shown to reduce medullary perfusion and promote the development of sustained hypertension in rats (47). O2·− is an oxygen-radical produced from a variety of endogenous sources (3). As O2·− can scavenge NO, which is a potent vasodilator of the medullary circulation, elevated medullary O2·− levels may promote renal medullary ischemia by reducing the bioavailability of NO (14). O2·− may also have additional biological actions that promote the development of hypertension independent of its actions of renal hemodynamics. Much evidence indicates that within the renal medulla, O2·− acts as a signaling molecule, stimulating a number of transporters involved in renal Na reabsorption either directly or by reducing NO bioavailbilty (67). As such, elevated O2·− levels are thought to be prohypertensive in that they are likely to promote both renal Na reabsorption and reduced medullary perfusion (14, 57).
The enzyme NADPH oxidase is an endogenous source of O2·−, of which a number of isoforms are expressed in the kidney (3). To determine whether augmented levels of endogenous O2·− production produced by NADPH oxidase in the renal medulla contributed to the development of salt-sensitive hypertension in the Dahl SS rat model, Taylor et al. (69) infused the NADPH oxidase inhibitor apocynin directly into the renal medulla of Dahl salt-sensitive and salt-resistant SS.13BN rats. In this study, Taylor et al. found that indices of O2·− production were elevated in the renal medulla of Dahl SS rats compared with SS.13BN rats and that infusion of apocynin lowered these selectively in SS rats to levels similar to that observed in salt-resistant SS.13BN rats. Importantly, infusion of apocynin significantly limited the development of hypertension in Dahl SS rats fed a high-salt diet, suggesting that augmented O2·− production by NADPH oxidase contributed to the development of hypertension in this strain (69). The concept that NADPH oxidase contributes to the development of hypertension and renal injury in the Dahl SS rat model is further supported by more recent studies in Dahl SS rats, in which a key subunit of the NADPH oxidase complex, p67phox, has been genetically targeted, resulting in a null mutation of this gene (31). Unlike wild-type Dahl SS rats, p67phox−/− mutant rats do not exhibit a reduction in medullary blood flow (29), are greatly protected from the development of salt-induced hypertension and renal injury (29, 31), and do not demonstrate a reduction in glomerular filtration rate following 2 wk of high-salt feeding (29). It should be pointed out that in these null mutant animals, global loss of gene function occurs. Therefore, the phenotype of these animals may not be specifically related to loss of p67phox in the kidney. Together, these studies indicated an important role of NADPH oxidase in contributing to the pathogenic hypertensive and renal injury phenotype observed in Dahl SS rats, and they led us to hypothesize that “elevated levels of O2·− production in the renal outer medulla are causal in the development of hypertension in Dahl SS rats”.
Searching for the Source of Augmented O2·− in the Kidney of Dahl SS Rats
The results of the Taylor et al. study (69), indicating a role of NADPH oxidase-derived O2·− in the development of hypertension in Dahl salt-sensitive rats prompted a number of questions. First, if unchallenged by a high-salt diet, blood pressure in Dahl SS rats remains relatively stable. If augmented, NADPH oxidase activity promotes the rapid development of hypertension in this strain, how then does a high-salt diet stimulate NADPH oxidase activity? Secondly, what cell type is responsible for elevated NADPH oxidase activity in Dahl salt-sensitive rats? We hypothesized that the answer to these questions was that the elevation of NADPH oxidase activity was likely of tubular origin and resulted from increased Na+ delivery and tubular work following initiation of high-salt feeding. The renal medulla contains a number of tubular segments, including the straight portion of the proximal tubule, the thin descending and ascending limbs of the loop of Henle, the thick ascending limb of the loop of Henle, and the medullary collecting ducts. As O2·− production, measured as ethidium fluorescence in microdissection nephron segments, had been shown to be greatest in the thick ascending limb (44), we decided to investigate whether this nephron segment may be the source of augmented O2·− production in the renal medulla of Dahl SS rats.
To determine whether NADPH oxidase-dependent O2·− production was elevated in medullary thick ascending limb (mTAL) tubular segments from Dahl SS, we utilized live-cell imaging of microdissected nephron segments loaded with the free radical indicator dihydroethidium (DHE) and compared the relative rates of O2·− production between mTAL from SS and SS.13BN rats (59). Given our hypothesis that “elevated O2·− production resulted from increased Na+ delivery and tubular work following initiation of high-salt feeding,” the most physiologically relevant stimuli would have been to perfuse isolated nephron segments at various flow rates to mimic alterations in Na+ intake and luminal NaCl delivery. Because of the relative technical difficulty of tubular perfusion studies, however, we instead decided to promote tubular O2·− production by increasing extracellular NaCl concentration. This stimulus, while less physiologically relevant, had previously been demonstrated to result in robust increases in O2·− production in mTAL (53), allowing us to rapidly test our hypothesis. Importantly, we found that the rate of O2·− production in SS mTAL was greater than that of SS.13BN in response to increments in extracellular NaCl concentration (59). Furthermore, this difference was enhanced in mTAL taken from rats 3 days after the advent of high-salt feeding (59). Consistent with the results of Taylor et al.'s (69) in vivo study, indicating NADPH oxidase as the source of elevated outer-medullary reactive oxygen species in Dahl SS rats, we found that the NADPH oxidase inhibitor apocynin selectively reduced O2·− production in mTAL from SS rats and normalized strain differences (59). Together, these data led us to hypothesize that “mTAL are the source of augmented NADPH oxidase-dependent O2·− production in the renal outer medulla of Dahl SS rats and may contribute to the development of hypertension in these animals when fed a high-NaCl diet.”
As we had hypothesized that a high-Na+ diet may promote NADPH oxidase activity by stimulating tubular reabsorptive work, next, we attempted to determine whether tubular Na+ reabsorption was linked to activation of NADPH oxidase activity in SS mTAL. The primary transcellular pathways for Na+ reabsorption in mTAL are the Na/K/2 Cl− cotransporter (NKCC2) and apical Na/H exchangers (NHE3), while the electrochemical gradient driving Na+ reabsorption is provided by primary active transport by Na/K-ATPase on the basolateral membrane. Pharmacological inhibition of each of these transport pathways limited O2·− production in response to increments in bath NaCl concentration in SS mTAL; however, only dimethyl-amiloride, which we used to inhibit Na/H exchange normalized the response between SS and SS.13BN rats (59). These data suggested that activation of Na/H exchangers may stimulate NADPH oxidase activity in SS mTAL. The hypothesis that “Na/H exchange activation was critical in promoting NADPH oxidase activity in SS mTAL” was consistent with a report by Li et al. (45), indicating that H+ efflux promoted NADPH oxidase activity in mTAL and that this response was dependent on the presence of extracellular Na+. This hypothesis was also consistent with our own finding that increments in extracellular Na+ concentration promoted O2·− production by an osmotic effect (59), as Na/H exchangers are known to be activated in responses to osmotic cell shrinkage (65).
H+ Efflux Stimulates Superoxide but not Through NHE
A number of Na/H exchanger isoforms are expressed in mTAL (59). As we hypothesized that “the increase in outer-medullary oxidative stress observed following high-salt feeding in Dahl SS rats was secondary to increased tubular reabsorptive work,” we speculated that the activity of apical NHE-3 would be linked to stimulation of the oxidase, as this Na/H exchange isoform is importantly involved in luminal Na transport in this segment (22). In our study, we used dimethyl-amiloride, and later N-methyl-amiloride, to identify Na/H exchange as a potential trigger of NADPH oxidase activity in the mTAL (59). Both of these amiloride analogs are relatively promiscuous inhibitors of a number of Na transporters, including Na/H exchanger isoforms with greater potency against NHE-1 than NHE-3 (70). As such, to clarify the Na/H exchanger isoform or isoforms involved in mediating NADPH oxidase-dependent O2·− production in mTAL, we performed a series of experiments using more selective and potent NHE-1 and NHE3 inhibitors. Surprisingly, we found that neither selective NHE-1 inhibition using cariporide or KR32568, nor dual inhibition of NHE-1 and NHE-3 isoforms using cariporide in combination with the potent NHE-3 inhibitor S3226, reduced O2·− production in response to increments in bath NaCl concentration or normalized the rate of O2·− production in SS and SS.13BN rats (58). In fact, O2·− production under these conditions was elevated relative to vehicle controls. Furthermore, we did not detect any difference in protein expression of NHE-1 or NHE-3 isoforms in outer-medullary homogenates of Dahl SS and SS.13BN rats (58). These results cast doubt over our earlier conclusion that NHE activation in mTAL was linked to O2·− production in mTAL, as dual inhibition with cariporide and S3226 should have been even more effective in inhibiting Na/H exchange in mTAL than the less specific analogs dimethyl-amiloride and N-methyl-amiloride that we had used to identify Na/H exchangers as potential mediators of O2·− production.
To address the concerns stemming from our disparate results using pharmacological inhibitors of Na/H exchange, we chose to use a more direct stimulus to activate Na/H exchangers within mTAL and measure the resulting rate of O2·− production. To do this, we used NH4Cl to acid-load the cells. This technique involves the addition of NH4Cl to the bath media, which results in an initial alkalization of the mTAL due to more rapid NH3 influx. After a period of equilibration in which cellular pH returns toward normal, the bath NH4Cl is removed, NH3 exits the cell rapidly down in concentration gradient, and as a result, the cell is acidified. If this technique is performed in media lacking HCO3−, NHE activity dominates the recovery of cell pH (41). We measured intracellular pH to confirm pH recovery and ethidium/DHE ratio in a separate group using the same conditions to determine changes in the rate of O2·− production following acidification. Importantly, despite conformation of significant Na-dependent pH recovery (presumed to be Na/H exchanger activation), we did not observe a significant increase in the rate of cellular O2·− production in either SS or SS.13BN mTAL following cellular acidification in Na-replete media (58). This result strongly refuted our earlier conclusion that NHE activity was linked directly to NADPH oxidase-dependent O2·− production in mTAL, as NHE activation itself did not appear to stimulate NADPH oxidase activity.
Characterizing O2·−-Producing H+ Transport Pathways in mTAL
Although we have not observed evidence of O2·− production in response to cellular acidification using NH4Cl in Na+-replete media, interestingly, in control experiments that we performed in 0 Na+ media to inhibit all Na/H exchange, we did see a significant increase in O2·− production during the period of pH recovery following acidification (58). These data suggested that H+ efflux through other secondary pathways, which may not be highly activated by this acidification stimuli when Na/H exchanger activity is present, may, indeed, promote O2·− production. These data, however, were in direct contrast to the findings of Li et al. (45), who had performed a similar study using NH4Cl to acidify mTAL and found significant O2·− production in Na+-replete media during the period of pH recovery, which was inhibited in 0 Na+ media. It remains unclear how our own data and that of Li et al. (45) could come to such disparate conclusions using similar methods. One possibility that could account for our disparate results may be the measurement of O2·− and pH. Li et al. (69) determined O2·− production (Eth/DHE) and pH (BCECF) simultaneously in the same tissue. As one of the excitation filters reported to be used for BCECF (440 ± 10 nM) overlaps with the emission filter reported for DHE (430 ± 15), it is unclear how Li et al. separated these wavelengths (the dichroic mirror was not reported), introducing the possibility of significant measurement artifact in their results. Despite the disparate results, we are confident in our conclusion that “Na/H exchange does not promote NADPH oxidase activity in mTAL.” Our findings are supported by both pharmacological data using the most potent and selective NHE antagonists, as well as cell physiological data using Na+-free media to inhibit NHE activity.
As we suspected a H+ channel or transporter other than NHE was linked to O2·− production in mTAL, we attempted to characterize this pathway further. It had been reported that Ba2+-sensitive channels could transport NH4+ in mTAL and, therefore, may act as a pathway for proton efflux (2, 38, 42). Therefore, we decided to test whether these Ba2+-sensitive pathways may be involved in O2·− production in response to cellular acidification. In 0 Na+ media with Ba2+, we found that O2·− production in response to cellular acidification was enhanced in SS mTAL (58). Importantly, under these conditions, both O2·− production and the rate of cellular pH recovery were significantly greater in mTAL from SS rats compared with SS.13BN rats (58). Furthermore, this O2·− production was sensitive to inhibition by the NADPH oxidase inhibitor apocynin (∼50% reduction) or N-methyl amiloride (58). We concluded that activation of an as yet unidentified H+ transport pathway (or pathways) was capable of promoting O2·− production by NADPH oxidase in mTAL and that the activity of this pathway was enhanced in mTAL of SS rats compared with SS.13BN (58). As we have demonstrated, amiloride-sensitive O2·− production was enhanced in SS mTAL in response to a number of stimuli, including osmotic stress and ANG II (58, 59), we suspected that this pathway was responsible for the elevated outer-medullary ROS levels observed in the outer medulla of Dahl SS rats and contributed to the development of hypertension in this strain.
Voltage-Gated Proton Channels in the Kidney of Dahl SS Rats
While we had grossly characterized this O2·−-producing H+ transport pathway in mTAL, its molecular identity remained a mystery. Some clues from our own data, however, made us focus on the voltage-gated proton channel (Hv1) as a potential candidate. Voltage-gated proton channels (encoded Hvcn1 in humans) are ion channels that mediate acid extrusion from cells (8). Although the existence of proton channels has been recognized for some time (71), the genes encoding Hv1 were not identified until 2006 (25, 62, 66). Despite this, much is known regarding the biophysical properties and function of proton channels in mammalian species. As their name suggests, voltage-gated proton channels are voltage-sensitive, opening in response to membrane depolarization (25). The opening threshold for voltage-gated proton channels is 20 mV positive, far from the resting potential of most cells at around 60 mV negative (25). An important property of voltage-gated proton channels is their pH dependence. The voltage threshold for opening of proton channels is shifted ∼40 mV to more negative potentials for every one unit outward pH gradient (9, 25). The result of this is that, within physiological ranges, voltage-gated proton channels only open in cells in which there is a large outward pH gradient and almost exclusively mediate the outward movement of H+ (25). The most well-recognized function of proton channels is to prevent H+-mediated depolarization and feedback inhibition of NADPH oxidase during the respiratory burst in phagocytic cells (8, 63). Hv1 has been identified in numerous cell types in addition to phagocytic cells, including lung epithelial cells, where it contributes to H+ efflux and possibly gas exchange (24, 55); in spermatozoa, where it plays a role in motility (46); in microglial cells in the brain, where it has been demonstrated to be involved in the pathogenesis of ischemic stroke (73); and in B cells, where it contributes to B-cell antigen receptor signaling and cell activation (7). Prior to our own studies, there was no published evidence of Hv1 expression or activity in the mammalian kidney, although voltage-dependent H+ currents had previously been detected in the frog proximal tubule (Rana pippens) (32).
The currently accepted view of proton channels is that their activation in phagocytic cells promotes NADPH oxidase activity, not by actively stimulating the oxidase, but rather by preventing feedback inhibition of the oxidase by cellular depolarization and acidification (25). The enormous quantities of O2·− produced during the respiratory burst rapidly depolarize and acidify phagocytic cells (25). Hv1 opening during these conditions limits this via the efflux of H+, preventing feedback inhibition of NADPH oxidase, the activity of which is inhibited by cellular depolarization and low pH (28, 51). Stimuli that activate the oxidase can also promote Hv1 opening via phosphorylation of Hv1, which results in “enhanced gating” shifting the opening threshold 30–40 mV more negative (4, 25, 27).
Our own data in mTAL indicated to us that activation of some H+ channel or transporter promoted O2·− production, likely via activation of NADPH oxidase. As opposed to studies in which Hv1 was investigated in phagocytes, we stimulated O2·− production by acidification of the cell, rather than acidification being secondary to stimulation of the NADPH oxidase (58). As such, our stimuli would be expected to potentially open Hv1, but inhibit the NADPH oxidase itself (51). Of course, cellular acidification may have secondary effects that activate the oxidase, such as cell depolarization or an increase in intracellular Ca2+, which could, in turn, promote NADPH-oxidase activity. The specific mechanisms by which cellular acidification promotes O2·− production in the mTAL remain unknown, although it should be pointed out that the currently accepted view of the role of Hv1 in the respiratory burst is that it simply facilitates NADPH oxidase activity by limiting the negative feedback effects of cellular depolarization and acidification, and in no way is H+ flux through Hv1 thought to actively promote NADPH oxidase activity (25, 28).
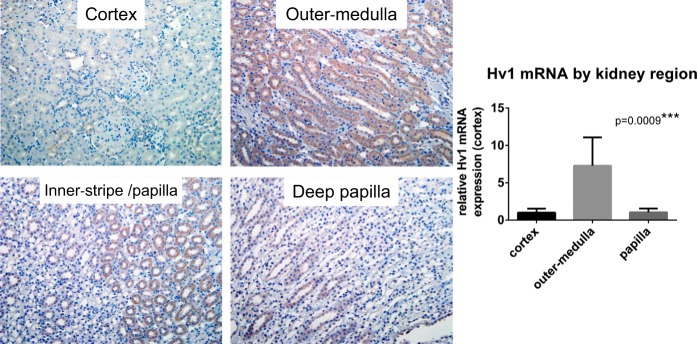
If we entertain the possibility that in our studies, O2·− production in the mTAL reflected an opening of an unidentified channel or transporter, many similarities became apparent between the properties of Hv1 and our unidentified H+ transport pathway. First, increasing the outward pH gradient by cellular acidification promoted O2·− production (58). Hv1 opening is enhanced by an outward pH gradient (25). Second, by using NH4+ loading, over the course of our experiment, we promoted both an outward and inward pH gradient (58). During the alkalization phase immediately after adding NH4+ to the bath, O2·− production was inhibited relative to baseline. This was more evident when valinomycin/high extracellular K+ was present in the media to promote greater depolarization (Fig. 1). This is consistent with Hv1 opening being prevented by an inward pH gradient (25). Finally, O2·− production in mTAL was enhanced in 0 Na+ media, in which NHE activity would be inhibited or in the presence of more specific NHE-1 inhibitors (KR32568, cariporide) (58). Hv1 activity has been demonstrated to be inversely related to NHE activity in cells that express both Hv1 and NHE (26). Given these similarities between our unidentified H+ pathway and Hv1, we investigated the expression of Hv1 in the kidney of rats. Hv1 mRNA could be detected in the outer medulla of rats by PCR, and immunohistochemical analysis indicated Hv1 staining was localized to mTAL (Fig. 2). Taken together, these data led us to hypothesize that “Hv1 was the as yet unidentified H+ transport pathway responsible for augmented O2·− production following cellular acidification in mTAL from Dahl SS rats.”
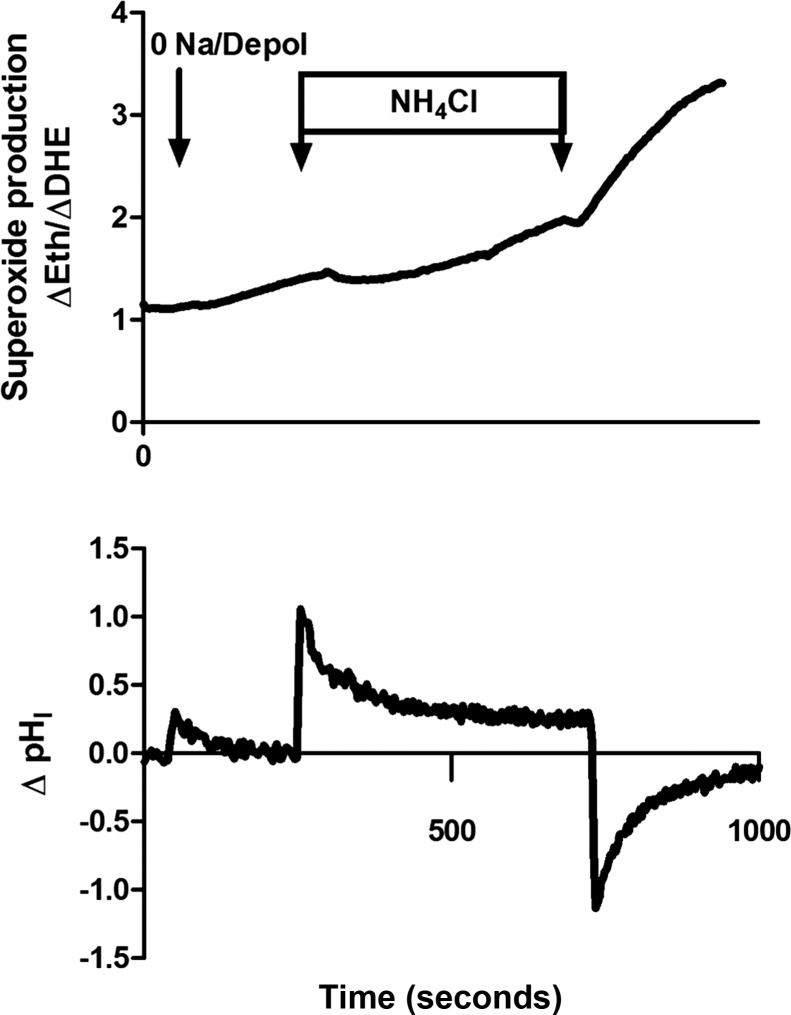
Fig. 1.
Superoxide production following cellular depolarization and internal pH changes in medullary thick ascending limb from wild-type and Hv1−/− mutant Dahl salt-sensitive rats. Superoxide production was stimulated in isolated mTAL from wild-type Dahl salt-sensitive rats (wild-type SS) and Dahl SS rats, in which Hv1 had been mutated, resulting in loss of Hv1 protein function (SS Hv1−/− mutant), as previously reported (39). Shown are typical examples of the superoxide response measured by ethidium/dihydroethidium fluorescence ratio [Eth/DHE (arbitrary units)] along with changes in intracellular pH (ΔpHI) to cellular depolarization and 0 Na+ media and subsequent addition and removal of 20 mM NH4Cl to the bath. x-axis, time in seconds. As observed in the top panel, in medullary thick ascending limb from wild-type SS, switching media from Hank's balanced salt solution to 0 Na+ media (isotonic with choline chloride) containing the ionophore valinomycin and 25 mM KCl to depolarize the cells results in an increase in the rate of superoxide production (1). The rate of superoxide production was reduced following the addition of 20 mM NH4Cl to the bath media, which alkalinized the cell (2). The rate of superoxide production recovers, as pHI returns toward baseline following NH4Cl addition. Rapid removal of NH4Cl from the bath, acidifies the cells, and increases the rate of superoxide production (3). In medullary thick ascending limb from SS Hv1−/− mutant animals, 0 Na+ media and depolarization had a small effect to promote superoxide production; however, changes in intracellular pH in response to NH4Cl did not alter superoxide production rate as in mTAL from wild-type animals, and the overall superoxide production rate was much lower than that observed in wild-type SS (middle panel). Each panel is a typical example (n = 1) obtained from measurements in separate mTAL. pHI responses are shown for wild-type only.
Fig. 2.
Hv1 expression in the rat kidney. Immunohistochemical analysis of paraffin-embedded rat kidney using an antibody raised against human Hv1 (Abcam cat. no. ab117520) indicates significant positive staining (brown) localized to medullary thick ascending limb tubular segments (mTAL) within the renal outer-medullary region. mRNA expression of Hv1 was also greatest in the renal outer medulla compared with renal cortex and papilla. PCR performed and Hv1 primers are as previously reported (39). P is the result of ANOVA.
While inhibitors of Hv1 have been reported (36), to the authors' knowledge, to date, there are no commercially available pharmacological inhibitors of Hv1, which would allow us to easily test our hypothesis. Zn2+ is known to inhibit proton currents (25); however, Zn2+ may also have nonspecific effects on mTAL and cannot be used to inhibit channel opening in vivo. Further, we have found that primary cultures of mTAL rapidly lose the typical H+ currents, and O2·− responses observed in response to cellular acidification in freshly isolated tubules (O'Connor PM, unpublished data), limiting our ability to test our hypothesis ex vivo using genetic approaches, such as siRNA. Given these limitations, with the help of our collaborators, we decided to produce a Hv1null mutant rat on the Dahl SS genetic background using Zn-finger nuclease gene targeting. Zinc finger nucleases targeting a sequence within exon 4 of rat Hv1 were used to produce an 8-bp deletion within the target sequence in Dahl SS rats, and a colony of Hv1−/+ mutant rats was established (39). We confirmed the 8-bp deletion in Hv1 both by DNA sequencing and PCR (39). Complete loss of Hv1 channel activity was confirmed by voltage-clamp studies of peritoneal macrophages from Hv1−/− and wild-type animals (39). We also confirmed expression of Hv1 within mTAL using Hv1−/− rats as negative control tissue, both by traditional immunohistochemistry and with Immunogold staining and transmission electron microscopy analysis. Qualitative analysis of these sections appeared to show both cytosolic and membrane staining with positive staining localized to the apical membrane of the mTAL (39). As has been the case with many of the mutant rat strains produced using these techniques, we had difficulty confirming loss of protein expression of Hv1 by Western blot analysis (despite testing with a number of commercially available antibodies, in addition to those that had demonstrated loss of protein expression by immunohistochemistry). We suspect that our inability to detect Hv1 by Western blot analysis in outer-medullary tissue or isolated mTAL is due to the relatively low expression of this protein coupled with reduced sensitivity of the antibodies used for rat Hv1. In support of this explanation, we were able to detect a band at 32 kDa (the predicted molecular weight of Hv1), which was present in wild-type, but not Hv1−/−, rats in the membrane fraction of splenic tissue, where Hv1 expression is high (39). Most importantly, in isolated mTAL, under the same experimental conditions used to characterize O2·− in response to cellular pHi recovery (0 Na+ media, Ba2+), we observed both reduced cellular pHi recovery following acidification using NH4Cl and complete loss of pH-dependent O2·− production (39) (note: limited stimulation of O2·− production was still observed to depolarizing stimuli). From these data, we have concluded that Hv1 is present in the mTAL of Dahl salt-sensitive rats, that it is the previously unidentified H+ pathway that we had characterized and that it is critical to the production of O2·− production following cellular acidification (39).
Blood Pressure and Renal Injury in Dahl SS Rats Lacking Hv1
The overall goal of our studies was to identify the mechanisms promoting augmented O2·− production in the renal outer medulla of Dahl SS rats, which has been strongly implicated in the development of hypertension. Because of the short half-life of reactive oxygen species (ROS), including O2·−, direct measurement of ROS in living animals is difficult. To gain insight into the relative levels of ROS production in Hv1−/− and wild-type Dahl SS rats, we used 4-HNE staining, a marker of oxidized lipids as an index of in vivo ROS production in the renal outer medulla. We have previously found that 4-HNE staining in the renal outer medulla is significantly reduced in apocynin-treated rats, confirming both the contribution of NADPH oxidase to ROS production in this region and the utility of this measurement to measure changes in tissue ROS levels (30). Using this marker, we found ROS production in the outer medulla to be significantly lower in high-salt diet-fed Hv1−/− Dahl SS rats compared with wild-type animals (39). In addition to comparing vehicle-treated Hv1−/− and wild-type Dahl SS rats fed high a high-salt diet, we also compared these strains when treated with the Na/H exchanger inhibitor KR32568. The rationale for this treatment group was that specific inhibition of Na/H exchange activity would stimulate the activity of Hv1 and the production of O2·− by mTAL [in a similar manner to that seen in our isolated tissue preparations(58)] as the activity of these H+ pathways has been demonstrated to be interrelated, allowing us to study a group with high Hv1 activity(26). Consistent with this hypothesis, the difference in 4HNE-levels between wild-type and Hv1−/− rats was more pronounced in animals treated with the Na/H exchange inhibitor (39). Surprisingly, given the previous studies implicating elevated ROS levels in the outer-medullary region with the development of hypertension (47, 69), blood pressure as measured by 24-h telemetry was only modestly different between Hv1−/− and wild-type Dahl SS rats (less than 8 mmHg) across 2 wk of high-salt feeding, despite observed differences in 4-HNE staining (39). It should be noted that the largest difference in blood pressure was observed between groups treated with the Na/H exchange inhibitor, where differences in outer-medullary ROS indices were also most pronounced, which is consistent with the hypothesis that elevated ROS in this region promote hypertension (39). Given the small difference in blood pressure between the groups, however, our data do indicate that the presence of Hv1 is not critical for the development of significant hypertension in this strain. Furthermore, in subsequent studies in which KR32568 was not administered, we have not observed a significant difference in blood pressure between Hv1−/− and wild-type Dahl SS rats when fed either a low- or high-salt diet by telemetry (O'Connor PM, unpublished observation). Therefore, while we are confident we have identified Hv1 as a novel source of augmented ROS production in the outer medulla of SS rats, it would appear that this pathway of ROS production may not be critical to the development of hypertension.
Although we did not observe the expected blood pressure phenotype in Hv1−/− Dahl SS rats, we did find that renal injury was markedly reduced in SS rats lacking Hv1(39), particularly in the outer-medullary region, where a significant reduction in tubular casts is observed. We have since confirmed that this phenotype is present, even when comparing groups of animals, where there is no difference in blood pressure. Therefore, it would appear Hv1 may contribute to the susceptibility of these animals to pressure-induced renal injury. Like other models in which gene mutation was achieved by Zn-finger nuclease gene targeting, in our colony, the Hv1 mutation is global, resulting in loss of Hv1 not only in the kidney but systemically, including from immune cells. As immune cell infiltration has been implicated in both the development of hypertension and renal injury in the Dahl SS rat model (23, 49), loss of Hv1 from cells other than mTAL, such as immune cells, may be responsible for the phenotype of reduced injury in these animals.
Hv1 and O2·− Production in the Kidney of Salt-Resistant and Salt-Sensitive Rats
Given our previous findings that in addition to enhanced O2·− production, pH recovery was greater following cellular acidification in Dahl SS rats (39), we hypothesized that a H+ transport pathway (now Hv1) was more active in SS rats compared with SS.13BN rats. We have not yet directly measured Hv1 activity in mTAL from Dahl SS or salt-resistant SS.13BN rats; however, in the rat, Hv1 is located on chromosome 12, suggesting that any differences that we observed in Hv1-dependent O2·− production in mTAL between SS and SS.13BN rats were mediated by factors other than genetic differences in the coded protein (genes encoded on chromosome 12 should be identical between SS and SS.13BN rats). The most likely explanation for our data then is that differences in mTAL O2·− production observed between SS and SS.13BN rats are mediated by differential expression of other key proteins involved in this pathway. A potential candidate is the NADPH oxidase subunit p67phox, which is located on chromosome 13, and has been demonstrated to have enhanced expression in Dahl SS compared with SS.13BN rats (31). An alternative explanation would be gene-gene interactions originating from coding differences in chromosome 13, which alter the expression or activity of Hv1 in mTAL. This second explanation would more easily explain the difference in pH recovery rate that we observed between SS and SS.13BN rats. Further studies are required to understand the genetic mechanisms resulting in augmented Hv1-dependent O2·− production in mTAL from Dahl SS rats.
When considering the role of Hv1 in O2·− production in mTAL, it may also be premature to exclude other sources of O2·− production other than NADPH oxidase. Furthermore, a number of isoforms of NADPH oxidase are thought to be expressed in mTAL (43), and it remains unknown to which isoform (if any) Hv1 interacts to promote O2·− production or the localization of this interaction. Although our initial studies suggested NADPH oxidase as the source of O2·− production in mTAL following cellular acidification, these studies relied on the NADPH oxidase inhibitor apocynin, which may have nonspecific effects to limit superoxide levels (34, 58). Furthermore, treatment with apocynin only reduced O2·− production by ∼50%, leaving open the possibility that other sources of O2·− may have contributed to the observed response. Given the strong relationship between expression of Hv1 and O2·− production in mTAL, future studies, specifically examining the relationship between various NADPH oxidase isoforms, as well as other potential sources of reactive oxygen species production, are warranted.
Hv1 in the Human Kidney
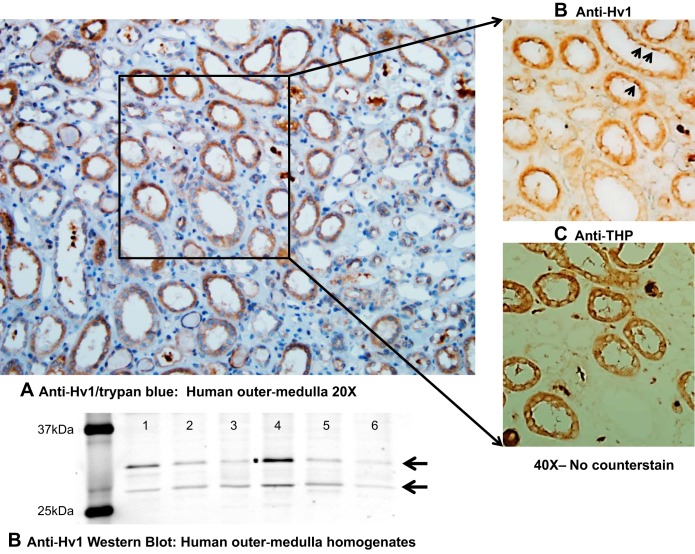
The overall goal of our studies has been to utilize the Dahl salt-sensitive rat model to identify physiological pathways that may contribute to salt-sensitive hypertension and renal injury in humans. Our finding that Hv1−/− Dahl SS rats are markedly protected from the development of pressure-induced renal injury may be particularly important, as novel targets for the treatment of hypertensive renal disease are desperately needed. Translation of preclinical findings in rodents to the treatment of human disease, however, has proven difficult. What evidence is there then that Hv1 is present in the human kidney and could potentially contribute to disease? Our data indicate that Hv1 is expressed in the mTAL of Dahl salt-sensitive rats (39). In contrast, using β-galactosidase expression as an indicator, we found no evidence of Hv1 expression in the renal outer medulla of Hv1 gene knockout first allele (KOMP) C57BL6 mice (O'Connor PM, unpublished observation). These findings indicate potentially important species differences in the expression and physiological importance of renal Hv1. Although data remain limited regarding the expression of Hv1 in human kidneys, importantly, immunohistochemial analysis of human outer-medullary tissue reveals a similar expression profile to that observed in Dahl SS rats, with significant Hv1-positive staining in both mTAL and some circulating cells (presumably immune cells expressing high levels of Hv1) (Fig. 3). Further, Western blot analysis of human tissue kidney homogenates of both renal cortex and renal cortex plus outer medulla indicate the presence of Hv1 (Fig. 3). Interestingly, two bands at molecular weight ∼32 and 27 kDa were identified, corresponding to the long and short for Hv1 recently identified in mice (35), suggesting two splice variants of Hv1 with different properties may be expressed in the human kidney. Together, this evidence suggests that Hv1 may play a similar role in the human kidney as that observed in rats.
Fig. 3.
Hv1 expression in the human outer medulla. A: paraffin sections containing human outer-medullary tissue kidney tissue were obtained from OriGene Technologies (Rockville, MD) and stained with an antibody raised against human Hv1 (Abcam cat. no. ab117520; Abcam, Cambridge, MA) and then counterstained with hematoxylin. Hv1 expression paralleled that observed in rat tissue, with staining localized primarily to mTAL tubular segments and circulating cells within vessels (likely circulating immune cells in which Hv1 expression is known to be high). The identity of medullary thick ascending limb was confirmed via anti-Tamm Horsfall protein staining (THP) [THP (H-135): SC-20631, 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA)]. In the right-hand panels, in which no counterstain was added, sequential tissue sections demonstrate prominent positive Hv1 staining within medullary thick ascending limb tubular segments. Arrowheads highlight enhanced staining on apical side of cell. B: Western blot of human homogenates of renal medulla/cortical tissue. Six normal human kidney protein lysates (lanes 1–6) were purchased from OriGene Technologies, and three protein lysates were 100% cortex (lanes 1, 3, and 5). Lane 2 was 55% cortex and 45% medulla, and lanes 4 and 6 were both 60% cortex and 40% medulla. Fifty micrograms of protein lysate per lane was loaded onto 12% acrylamide gel for electrophoresis before transfer to a Immobilon-FL membrane (Millipore cat. no. IPFL00010). Blot was first incubated with an anti-HVCN1 (Abcam cat. no. ab117520) rabbit polyclonal antibody (1:1,000, overnight at 4°C) followed by secondary antibody (goat anti-rabbit IgG 680 fluorescence, cat. no. A-21076, 1:1,000, Invitrogen, Carlsbad, CA; 1 h at room temperature). Immunoreactivity was detected by scanning the blot using Odyssey 700 channel (Li-Cor, Lincoln, NE).
Is Hv1 Active in mTAL of Dahl SS Rats?
We are confident that we have correctly identified Hv1 as a trigger for augmented O2·− production in mTAL of Dahl SS rats; however, identification of Hv1 raised a number of important questions. Upon finding voltage-gated proton currents in the proximal tubule of the frog, the authors of this study stated that “the role of depolarization-activated GH (Hv1) in proximal tubule is not entirely clear, since proximal tubule cells do not normally experience drastic changes in either pH or membrane potential” (28). Similarly, the conditions in which we characterized O2·− production in response to H+ fluxes in mTAL were contrived to promote maximal activation and were not physiological [0 extracellular NaCl (isotonic with choline chloride) and Ba2+]. These studies highlight the fact that Hv1 opens only under relatively physiological extremes. Therefore, questions remain whether Hv1 in mTAL is active under the physiological conditions in the Dahl SS rat model? Although we are currently unable to definitively answer this question, the conditions normally present in mTAL may give us a clue to the functional state of Hv1 expressed in these cells in vivo. We know that resting apical membrane potential has been reported to be approximately −70–80 mV (5) in mTAL, far more negative than that required to open Hv1 (+20 mV) in the absence of significant pH gradients (21). Importantly, in regard to Hv1 opening, the unique cellular physiology of mTAL results in a large outward pH gradient under normal physiological conditions (69). This large outward pH gradient results from the active reabsortion of NH4+ in this segment (69). While the pKa of NH4+ under biological conditions is ∼9.15, meaning it is a very weak acid and at a physiological pH of 7.4, does not greatly acidify the luminal fluid (only ∼1.7% is in the form of NH3 + H+) (70), under normal physiological conditions, where the tubular perfusate contains ≈4 mmol/l of NH4+, active reabsorption of NH4+ via the NKCC2 cotransporter (NH4+ competes with K+ for binding) results in an outward pH gradient of 0.8 to 0.9 units (69). Using the calculation VTHRESHOLD = 40 mV × (pHo − pHI) + 20 mV for Hv1 opening, where pHo is luminal pH, and pHI is intracellular pH (21), such a pH gradient would be predicted to result in a shift ∼34 mV toward more negative membrane potentials to ∼14 mV negative, still far from resting membrane potential in mTAL. Therefore, it would appear extremely unlikely that apical membrane potential would depolarize enough to surpass the −14-mV threshold, which would represent Hv1 opening, even when the cell pH is acidified relative to lumen, at least under physiological conditions.
One possibility that could promote Hv1 opening in mTAL is that additional factors may reduce the threshold for Hv1 opening to within the physiological parameters observed at the apical membrane. It is known that phosphorylation of Hv1 promotes “enhanced gating mode,” which shifts the opening threshold for Hv1 30 to 40 mV more negative (23, 53). In addition, a short-form variant of Hv1, which lacks the first 20 amino acids, has recently been identified in B-cells that responds more strongly to PKC-dependent phosphorylation, enhancing channel activity further (30). To date, the phosphorylation state, as well as the Hv1 isoforms that predominate in the mTAL remain unknown; however, our data suggest that short-form Hv1 may be expressed in the human kidney. Direct-voltage measurements of membrane Hv1 are required to determine the characteristics of mTAL Hv1 and whether Hv1 localized to the apical membrane of mTAL is open in under physiological conditions. These data will be critical to understanding the physiological function of Hv1 in mTAL.
Opposing the idea that Hv1 channel opening on the apical membrane of mTAL may mediate H+ efflux under physiological conditions, when utilizing a computational model of mTAL transport, greatly enhancing the permeability of the apical membrane of an mTAL cell to H+, results in the net inward flux of H+. This inward movement of H+ occurs, even when a significant outward pH gradient resulting from NH4+ uptake is present and results in further acidification of the cell. In this model, depolarization of the membrane to ∼40 mV negative (highly unlikely to occur under physiological conditions) is required before net H+ flux is outward, (personal communication with Alan M. Weinstein). These data indicate that if the threshold for opening of Hv1 channels is met on the apical membrane of mTAL, under physiological conditions, channel opening is more likely to result in the relatively uncommon scenario of inward H+ flux rather than acid extrusion.
A second possibility that may explain the functional effects of Hv1 on O2·− production in mTAL may be that Hv1 is localized to cellular compartments other than the apical membrane, where the biophysical requirements for channel opening are more easily met. Supporting this possibility, in addition to significant positive staining on the apical membrane, our histological data also indicate positive cytosolic staining for Hv1 in both the human and rat kidney (Figs. 2 and 3). A detailed quantitative analysis of Hv1 expression at the cellular organelle level is required to determine the cellular expression pattern of Hv1 in mTAL. Such an analysis is likely to shed further light on the mechanisms mediating the functional effects of Hv1 on mTAL O2·− production and the stimuli that mediate these.
Hv1, Outer-Medullary Oxidative Stress, and the Dahl Salt-Sensitive Rat: Where Are We Now?
Using a top-down investigative approach, we have identified Hv1 as a novel source of mTAL O2·− production, which appears to be present in both the rat and human kidney. Systemic loss of Hv1 in Dahl SS rats limits lipid oxidation in the renal outer-medullary region (39). Loss of Hv1 also significantly blunted hypertensive renal injury in high-salt diet-fed Dahl SS rats (39). Although these findings are, in part, consistent with our overriding hypothesis that augmented outer-medullary NADPH oxidase activity and consequent oxidative stress lead to renal injury in the Dahl SS rat strain when fed a high-salt diet (14), our data do not support significant involvement of Hv1 in the development of hypertension, and a number of critical questions remain regarding the role of Hv1 in the pathophysiology of the Dahl SS rat model. Systemic loss of Hv1 is much less effective at reducing blood pressure in Dahl SS rats than that reported following deletion of subunits of the NADPH oxidase (19, 31). These data suggest that while Hv1 may modulate O2·− production in mTAL, either 1) opposing the conclusions of earlier studies, this is not critical to the development of hypertension, or 2) other sources of ROS not related to Hv1 remain that mediate these effects. In addition to identifying the pathways through which Hv1 modulates O2·− production, investigations into the effect of Hv1 loss on medullary perfusion or on other markers of oxidative stress in high-salt Dahl SS rats in vivo would likely help resolve these issues.
Our initial ex vivo studies focused on mTAL, as it was thought that Na+ delivery to this segment may link high-salt feeding to renal outer-medullary oxidative stress. Importantly, while we have identified Hv1 as a key modulator of O2·− production in mTAL cells, it remains unclear whether the effects of Hv1 loss in Dahl SS rats are mediated by loss of Hv1 from mTAL. Hv1 is highly expressed in immune cells, and much evidence implicates immune cells in the progression on renal injury. Therefore, systemic loss of Hv1 may have limited renal outer-medullary oxidative stress and injury independently of mTAL Hv1 through changes in immune cell function. Cell-specific manipulation of Hv1 expression will be required to determine the source of the phenotypic differences that renal pathology has revealed.
In regard to the link between high-salt feeding and mTAL O2·− production, our current data do not adequately link tubular O2·− production through Hv1-dependent mechanisms to a high-salt diet. We and others have previously reported that increased Na+ delivery or increased tubular flow stimulates O2·− production in mTAL (1, 37, 60). It remains unknown whether Hv1 is involved in these responses; however, our data indicate that a direct effect of increased Na+ delivery to the apical membrane of mTAL is unlikely to promote O2·− production through Hv1. MTAL is not likely to depolarize in response to increased Na+ delivery. Furthermore, because of the affinities of the primary mTAL Na+ transporters on the apical membrane of mTAL, increased Na+ delivery may have little effect on intracellular Na+ levels (37). Our data, indicating that Hv1-dependent O2·− production is enhanced in 0 Na+ media (58) would also suggest that increased Na+ delivery, if anything, would limit rather than promote Hv1-dependent O2·− production in the mTAL. One possibility that could promote Hv1 opening in mTAL following high-salt feeding is greater NH4+ reabsorption and cellular acidification. Dahl SS rats excrete a greater acid load following high-salt feeding, which likely increases mTAL NH4+ uptake (6, 72). As we have demonstrated that cellular acidification promotes Hv1-dependent O2·− production in mTAL (58), increased NH4+ uptake and mTAL acidification could provide a potential link between high-salt feeding and enhanced mTAL Hv1-dependent O2·− production in Dahl SS rats. Further studies are required to investigate this hypothesis.
Summary and Conclusions
In an effort to identify the source of augmented ROS production in the renal outer medulla of Dahl SS rats, we determined that H+ efflux stimulates the production of O2·− in mTAL through Hv1-dependent mechanisms. These are the first studies to localize Hv1 to the kidney and identify Hv1 as a novel renal channel, which is involved in the regulation of O2·− production in the renal outer medulla. Despite the evidence that Hv1 is the source of augmented ROS production in the renal outer medulla of Dahl SS rats, global loss of Hv1 in this strain has only a small effect on blood pressure. Instead, the primary phenotype of these animals appears to be a marked reduction in the susceptibility to pressure-induced renal injury. Data produced from our laboratory indicate that Hv1 is likely to be a key piece in the Dahl SS rat puzzle, contributing to pathological pathways involved in excess ROS production and renal injury. Further studies are required to determine whether Hv1 may play a similar pathological role in the development of human disease.
GRANTS
The authors would also like to acknowledge financial support from the American Heart Association 10SDG4150061 to Paul O'Connor and National Institutes of Health Grant DK-099548.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.M.O. conception and design of research; P.M.O. drafted manuscript; P.M.O., A.G., C.A.S., and C.J. edited and revised manuscript; P.M.O. approved final version of manuscript; A.G. and C.A.S. prepared figures; J.S. performed experiments; J.S. analyzed data; J.S. interpreted results of experiments.
ACKNOWLEDGMENTS
Alan M. Weinstein for his informative discussions on mTAL transport.
REFERENCES
- 1.Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW Jr. Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Amlal H, Paillard M, Bichara M. NH4+ transport pathways in cells of medullary thick ascending limb of rat kidney. NH4+ conductance and K+/NH4+(H+) antiport. J Biol Chem 269: 21,962–21,971, 1994. [PubMed] [Google Scholar]
- 3.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal 20: 74–101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banfi B, Schrenzel J, Nusse O, Lew DP, Ligeti E, Krause KH, Demaurex N. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J Exp Med 190: 183–194, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Batlle DC, Sharma AM, Alsheikha MW, Sobrero M, Saleh A, Gutterman C. Renal acid excretion and intracellular pH in salt-sensitive genetic hypertension. J Clin Invest 91: 2178–2184, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, Dinsdale D, Pulford K, Khan M, Musset B, Cherny VV, Morgan D, Gascoyne RD, Vigorito E, DeCoursey TE, MacLennan IC, Dyer MJ. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol 11: 265–272, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capasso M, DeCoursey TE, Dyer MJ. pH regulation and beyond: unanticipated functions for the voltage-gated proton channel, HVCN1. Trends Cell Biol 21: 20–28, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherny VV, Markin VS, DeCoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol 105: 861–896, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churchill PC, Churchill MC, Bidani AK. Kidney cross transplants in Dahl salt-sensitive and salt-resistant rats. Am J Physiol Heart Circ Physiol 262: H1809–H1817, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest 124: 2341–2347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet 7: 829–840, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1–R15, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Cowley AW Jr, Abe M, Mori T, O'Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol 308: F179–F197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowley AW Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand 181: 585–592, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Cowley AW Jr., Roman RJ. The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996. [PubMed] [Google Scholar]
- 17.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Cowley AW Jr, Skelton MM, Kurth TM. Effects of long-term vasopressin receptor stimulation on medullary blood flow and arterial pressure. Am J Physiol Regul Integr Comp Physiol 275: R1420–R1424, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Cowley AW Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the importance of Nox4 in production of hypertension in Dahl salt-sensitive rats. Hypertension 67: 440–450, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17,985–17,990, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis JJ, Luke RG, Dustan HP, Kashgarian M, Whelchel JD, Jones P, Diethelm AG. Remission of essential hypertension after renal transplantation. N Engl J Med 309: 1009–1015, 1983. [DOI] [PubMed] [Google Scholar]
- 22.de Bruijn PI, Larsen CK, Frische S, Himmerkus N, Praetorius HA, Bleich M, Leipziger J. Furosemide-induced urinary acidification is caused by pronounced H+ secretion in the thick ascending limb. Am J Physiol Renal Physiol 309: F146–F153, 2015. [DOI] [PubMed] [Google Scholar]
- 23.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeCoursey TE. Hypothesis: do voltage-gated H+ channels in alveolar epithelial cells contribute to CO2 elimination by the lung? Am J Physiol Cell Physiol 278: C1–C10, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Decoursey TE. Voltage-gated proton channels. Compr Physiol 2: 1355–1385, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeCoursey TE, Cherny VV. Na+-H+ antiport detected through hydrogen ion currents in rat alveolar epithelial cells and human neutrophils. J Gen Physiol 103: 755–785, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCoursey TE, Cherny VV, Zhou W, Thomas LL. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci USA 97: 6885–6889, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422: 531–534, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Evans LC, Ryan RP, Broadway E, Skelton MM, Kurth T, Cowley AW Jr. Null mutation of the nicotinamide adenine dinucleotide phosphate-oxidase subunit p67phox protects the Dahl-S rat from salt-induced reductions in medullary blood flow and glomerular filtration rate. Hypertension 65: 561–568, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fellner RC, Cook AK, O'Connor PM, Zhang S, Pollock DM, Inscho EW. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am J Physiol Renal Physiol 307: F33–F40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, Cowley AW Jr. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu X, Sackin H. Effect of pH on potassium and proton conductance in renal proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 269: F289–F308, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr. Arterial pressure regulation overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. [DOI] [PubMed] [Google Scholar]
- 34.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Hondares E, Brown MA, Musset B, Morgan D, Cherny VV, Taubert C, Bhamrah MK, Coe D, Marelli-Berg F, Gribben JG, Dyer MJ, DeCoursey TE, Capasso M. Enhanced activation of an amino-terminally truncated isoform of the voltage-gated proton channel HVCN1 enriched in malignant B cells. Proc Natl Acad Sci USA 111: 18,078–18,083, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong L, Kim IH, Tombola F. Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc Natl Acad Sci USA 111: 9971–9976, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Jans F, Balut C, Ameloot M, Wouters P, Steels P. Investigation of the Ba2+-sensitive NH4+ transport pathways in the apical cell membrane of primary cultured rabbit MTAL cells. Nephron Physiol 106: 45–53, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Jin C, Sun J, Stilphen CA, Smith SM, Ocasio H, Bermingham B, Darji S, Guha A, Patel R, Geurts AM, Jacob HJ, Lambert NA, O'Connor PM. HV1 acts as a sodium sensor and promotes superoxide production in medullary thick ascending limb of Dahl salt-sensitive rats. Hypertension 64: 541–550, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karet FE, Lifton RP. Mutations contributing to human blood pressure variation. Recent Prog Horm Res 52: 263–276; discussion 276-267, 1997. [PubMed] [Google Scholar]
- 41.Kikeri D, Azar S, Sun A, Zeidel ML, Hebert SC. Na+-H+ antiporter and Na+-(HCO3−)n symporter regulate intracellular pH in mouse medullary thick limbs of Henle. Am J Physiol Renal Fluid Electrolyte Physiol 258: F445–F456, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Kikeri D, Sun A, Zeidel ML, Hebert SC. Cellular NH4+/K+ transport pathways in mouse medullary thick limb of Henle. Regulation by intracellular pH. J Gen Physiol 99: 435–461, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140: 327–337, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW Jr. Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Manning RD Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 25: 311–317, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Michel FS, Norton GR, Majane OH, Badenhorst M, Vengethasamy L, Paiker J, Maseko MJ, Sareli P, Woodiwiss AJ. Contribution of circulating angiotensinogen concentrations to variations in aldosterone and blood pressure in a group of African ancestry depends on salt intake. Hypertension 59: 62–69, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Morgan D, Cherny VV, Murphy R, Katz BZ, DeCoursey TE. The pH dependence of NADPH oxidase in human eosinophils. J Physiol 569: 419–431, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan DA, DiBona GF, Mark AL. Effects of interstrain renal transplantation on NaCl-induced hypertension in Dahl rats. Hypertension 15: 436–442, 1990. [DOI] [PubMed] [Google Scholar]
- 53.Mori T, Cowley AW Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 19: 1472–1482, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy R, Cherny VV, Morgan D, DeCoursey TE. Voltage-gated proton channels help regulate pHi in rat alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 288: L398–L408, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Nakanishi K, Mattson DL, Cowley AW Jr. Role of renal medullary blood flow in the development of l-NAME hypertension in rats. Am J Physiol Regul Integr Comp Physiol 268: R317–R323, 1995. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor PM, Cowley AW Jr. Modulation of pressure-natriuresis by renal medullary reactive oxygen species and nitric oxide. Curr Hypertens Rep 12: 86–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Connor PM, Lu L, Liang M, Cowley AW Jr. A novel amiloride-sensitive H+ transport pathway mediates enhanced superoxide production in thick ascending limb of salt-sensitive rats, not Na+/H+ exchange. Hypertension 54: 248–254, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Connor PM, Lu L, Schreck C, Cowley AW Jr. Enhanced amiloride-sensitive superoxide production in renal medullary thick ascending limb of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 295: F726–F733, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohsaki Y, O'Connor P, Mori T, Ryan RP, Dickinson BC, Chang CJ, Lu Y, Ito S, Cowley AW Jr. Increase of sodium delivery stimulates the mitochondrial respiratory chain H2O2 production in rat renal medullary thick ascending limb. Am J Physiol Renal Physiol 302: F95–F102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–R1363, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature 440: 1213–1216, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA 106: 7642–7647, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapp JP, Tan SY, Margolius HS. Plasma mineralocorticoids, plasma renin, and urinary kallikrein in salt-sensitive and salt-resistant rats. Endocr Res Commun 5: 35–41, 1978. [DOI] [PubMed] [Google Scholar]
- 65.Ritter M, Fuerst J, Woll E, Chwatal S, Gschwentner M, Lang F, Deetjen P, Paulmichl M. Na+/H+ exchangers: linking osmotic dysequilibrium to modified cell function. Cell Physiol Biochem 11: 1–18, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science 312: 589–592, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Schreck C, O'Connor PM. NAD(P)H oxidase and renal epithelial ion transport. Am J Physiol Regul Integr Comp Physiol 300: R1023–R1029, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension 17: I61–I68, 1991. [DOI] [PubMed] [Google Scholar]
- 69.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Teiwes J, Toto RD. Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens 20: 109–117, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299: 826–828, 1982. [DOI] [PubMed] [Google Scholar]
- 72.Watts BA 3rd, Good DW. Effects of ammonium on intracellular pH in rat medullary thick ascending limb: mechanisms of apical membrane NH4+ transport. J Gen Physiol 103: 917–936, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu LJ, Wu G, Akhavan Sharif MR, Baker A, Jia Y, Fahey FH, Luo HR, Feener EP, Clapham DE. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci 15: 565–573, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zicha J, Dobesova Z, Vokurkova M, Rauchova H, Hojna S, Kadlecova M, Behuliak M, Vaneckova I, Kunes J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res 61 Suppl 1: S35–S87, 2012. [DOI] [PubMed] [Google Scholar]