Abstract
An ecofriendly synthetic pathway for the synthesis of donepezil precursors is described. Alternative energy sources were used for the total synthesis in order to improve yields, regioselectively, and rate of each synthetic step and to reduce the coproduction of waste at the same time. For all products, characterized by an improved structural rigidity respect to donepezil, the inhibitor activity on AChE, the selectivity vs BuChE, the side-activity on BACE-1, and the effect on SHSY-5Y neuroblastoma cells viability were tested. Two potential new lead compounds for a dual therapeutic strategy against Alzheimer’s disease were envisaged.
Keywords: Alzheimer’s disease, donepezil analogues, indanones, aldol condensation, acethylcholinesterase
Alzheimer’s disease (AD) is a neurodegenerative disorder of the central nervous system (CNS) characterized by progressive deterioration of cognitive, memory, and executive functions. The lower levels of acetylcholine (Ach) found in AD patients has been related to an increased activity of acetylcholinesterase (AChE), in the so-called “cholinergic hypotesis”;1vice versa, in the “amyloid hypothesis” the formation and the aggregation of the β-amyloid peptide (Aβ)2 has been associated with the hydrolysis of the amyloid precursor protein (APP) by β-secretase 1 (BACE-1).3 Drugs approved by the FDA-USA for AD clinical treatment (as donepezil, tacrin, etc.), specially addressed to inhibit Ach production, have only a palliative effect. Indeed, the amyloid hypothesis is now the real rationale for AD treatment.4 Moreover, as the anionic site (PAS) of AChE is involved in Aβ aggregation, efforts have been recently devoted to the development of dual inhibitors of AChE and BACE-1.5−9 Nevertheless, among the molecules proposed as dual inhibitors, none has been approved because of their inacceptable pharmacokinetic and CNS penetration profiles.10 For this reason research has moved toward hybrid molecules characterized by a partial similarity to known AD drugs.11
Donepezil is the most effective inhibitor of the AChE currently available on the market.12,13 It has been recently proposed that donepezil analogues with a double bond on the indanone moiety can act as BACE-1 inhibitors thanks to their structural rigidity. According the docking studies performed on these molecules, the amino substituent reached the enzyme active site, while the indanone substituents were exposed to the solvent.8
As reported in literature14−16 the synthesis of donepezil is based on the aldol condensation/dehydratation between the indanone and the piperidine moieties, leading to an unsaturated precursor (Figure 1) that gives rise to donepezil after asymmetric hydrogenation.
Figure 1.
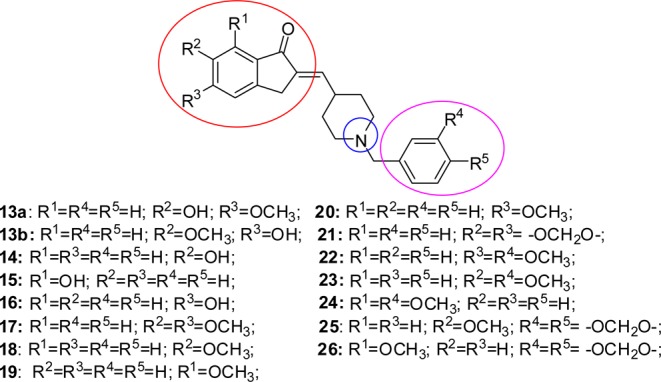
Chemical structure of designed donepezil analogues.
Based on these observations, we supposed that such synthetic donepezil precursor was rigid enough to display a dual activity on AChE and BACE-1. In fact it maintains the N-benzyl piperidine and indanone moieties identified as important interaction binding sites with AChE, but the stereocenter between them was substituted with a double bond. Therefore, we planned a green version of the synthesis of already reported methoxyl-substituted donepezil precursors by aldol condensation. Moreover, some differentiations with respect to the reported precursors were realized in order to test if they could have some positive influence in the dual activity. In particular some hydroxyl substituted derivatives were synthesized, in order to study if a stronger H-donor group can influence the binding on the CAS of AChE, while some dioxole protected derivatives were synthesized in order to bring an additional rigidity on the molecule thus studying the influence on BACE-1 inhibition. The aim of the present work was to find some new lead compounds for AD dual therapy.
In the past decade, our research group has spent a lot of efforts in the synthesis of pharmacologically active molecules through ecofriendly synthetic procedures in agreement with the known principles of green chemistry.17−21
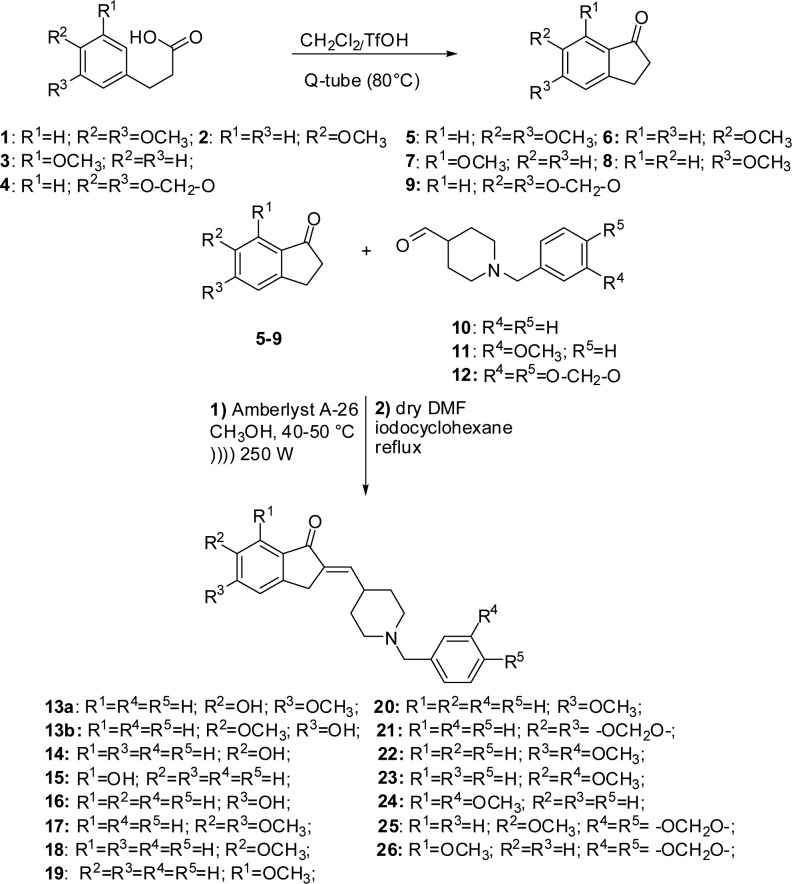
Thus, inspired by this philosophy, we designed the general synthetic pathway for the novel donepezil derivatives depicted in Scheme 1.
Scheme 1. Synthetic Pathway for the Total Synthesis of Donepezil Analogues.
Recently, we have reported the application of three different nonconventional techniques to the synthesis of a library of substituted 1-indanones, via the direct Friedel–Crafts intramolecular cyclization of arylpropionic acids,22 concluding that the Q-tube equipment can be proposed as a valid alternative to monomode MW and US technologies in terms of efficiency, safety, and cleaner reaction profile. Therefore, we applied this methodology to obtain the indanone derivatives 5–8 in quantitative yield and very mild reaction conditions; whereas 6,7-dihydro-5H-indeno [5,6-d][1,3]dioxol-5-one 9 was commercially available. The use of these alternative energy sources made possible to considerably improve the reaction yields and greatly reduce the amount of triflic acid used compared to the protocol reported in the literature (from 10 to 3 equiv in the case of reactions conducted at Q-tube or MW).23
A commercial available N-benzylpiperidine-4-carboxaldehyde 10 was used as N-benzyl piperidine moiety for the synthesis of compounds 13–21, while for the precursors of 22–24 the alkylation of ethyl isopenicotate with 3-metoxybenzyl bromide followed by a serial cascade of reduction and oxidation, affording to N-benzylpiperidin-4-carboxaldehydes 11, was performed according to the protocol reported in the literature.24 The same protocol was applied to the synthesis of N-benzylpiperidine-4-carboxaldehyde 12 from ethyl isopenicotate 5-(bromomethyl) benzo[d](1,3)dioxole, utilized to obtain the final products 25 and 26.24
The methods reported in the literature for the condensation of the indanone nucleus and N-benzylpiperidine-4-carboxaldehyde derivatives do not conform to the commercial and ecosustainable requirements needing hazardous or environmentally unfriendly solvents and tedious step of purification of the product.11−13 Recently, new more ecofriendly methods using alkali metal hydroxides or alkoxides in MeOH under homogeneous conditions were described.25 In that case, hydroxyl impurities occur up to 20% during the formation of the indanonylidenyl compound 17, so the product needs to be further purified before the reduction step. Furthermore, at the best of our knowledge only a report exists for the synthesis of compounds 17 under heterogeneous conditions in a biphasic system H2O/dichlomethane, using NaOH as base in the presence of a phase transfer catalyst.26
Thus, starting from that report, we developed a sustainable US-assisted method to obtain a new class of indanonylidenyl precursors of donepezil by substituting the homogeneous base catalyst with a commercial basic resin Amberlyst A-2627 as efficient and recyclable heterogeneous catalyst to realize a regioselective aldol condensation between the indanones 5–9 and 1-benzyl-piperidine moieties 10–12.
It was known that Amberlyst A-26 catalyzes aldol condensation,27 but as it is shown in Table 1, only few traces of the desired product were obtained when the reaction was catalyzed by the heterogeneous resin (entry 3, Table 1) giving rise to a worse result compared to those registered using classical conditions14,25 (entry 1 and 2, Table 1) even at solvent reflux temperature. When the same reaction was performed under ultrasound (US) assistance, maintaining the temperature lower than 50 °C, we obtained a significant improvement in terms of reaction yield after only 2 h (entry 4 in Table 1), thus demonstrating that ultrasound played here a role of activator, by increasing the interface surface between the dissolved reactants and the resin surface.
Table 1. Condensation Reaction between Indanones 5–9 and 1-Benzylpiperidine-4-carbaldehydes 10–12 in Methanol.
| entry | indanone | aldehyde | product | time (h) | yield (%) |
|---|---|---|---|---|---|
| 1 | 5 | 10 | 17 | 2 | 10a |
| 2 | 5 | 10 | 17 | 2 | 41b |
| 3 | 5 | 10 | 17 | 2 | tracec |
| 4 | 5 | 10 | 17 | 2 | 56 |
| 50d | |||||
| 45e | |||||
| 5 | 6 | 10 | 18 | 1 | 62 |
| 6 | 7 | 10 | 19 | 2.5 | 48 |
| 7 | 8 | 10 | 20 | 1 | 63 |
| 8 | 9 | 10 | 21 | 2 | 64 |
| 9 | 8 | 11 | 22 | 2 | 40 |
| 10 | 6 | 11 | 23 | 2.5 | 45 |
| 11 | 7 | 11 | 24 | 3 | 30 |
| 12 | 6 | 12 | 25 | 2 | 51 |
| 13 | 7 | 12 | 26 | 2 | 35 |
Diisopropylamine, n-butyl Li, DMSO in THF.
MeONa/MeOH.
Reaction conducted without US assistance.
II run reaction.
III run reaction.
Longer reaction time did not improve conversion, probably because of the gradual degradation of the aldehyde, although when it was used in molar excess (1.2 equiv). The pivotal role of the US activation is also confirmed by the cleaner reaction profile and the simplified workup with respect to the conventional conditions.14,25 Indeed, the catalyst was filtered off, washed with ethyl acetate, dried, and reused for three reaction cycles with a slight loss of activity, probably due to the solvation of the basic active sites;28 the product was separated by the reaction mixture simply by recrystallization from methanol; nor was formation of the hydroxyl impurity observed26 or were other purification steps required. Even though the yield was comparable with that reported in literature,26 this method allowed us to obtain the desired product in good yields avoiding the use of dichloromethane or toluene, both during the reaction and the purification steps, and to simplify the workup procedure into a filtration/recrystallization protocol. The registered yields ranged from moderate to good depending on the position of the methoxyl group on the indanone moiety. In particular, the electron-donating effect of the methoxyl group in ortho-position with respect to the carbonyl group, decreased the acidity of the proton in the α-position (entries 3, 8, 10, Table 1) thus lowering the reactivity of the corresponding indanones. The same electronic effect was probably the reason for a sole UV/vis absorption of the products 19, 24, and 26 with respect to the others analogues. Indeed, all products have been detected on the F254 silica gel TLC plates by their good absorbance both at short (254 nm) and long wave (365 nm) UV light, except 19, 24, and 26 products that only absorbed at λ = 254 nm. Taking into account that the reaction was not an oriented cross-aldol reaction, the yields registered in all cases, although moderate, represent an exciting result. Noteworthy, in all cases the regioselectivity was maintained and no autocondesation reactions were observed. The E-stereoselectivity was in all cases confirmed by the chemical shift of the vinyl proton in the 1H NMR spectra, as well as by NOESY experiments performed on selected compounds 17–20 (no signal was detected corresponding to a possible coupling of the olephinic proton around 6.67 ppm with the indanone 3-proton and the piperidine 4-proton, see Supporting Information). Moreover, demethylated compound 15 was selected for NOESY experiment to assess the condensation E-steroselectivity and confirm that the demethylation had no influence on the double bond configuration (see Supporting Information). All the products were obtained pure after recrystallization from methanol, with the only exception of products 20, 23, and 26, isolated after flash chromatography.
The demethylation of monomethylated donepezil analogues 18–20 was realized by a slight modified method reported in literature adopting iodocyclohexane (C6H11I) as demethylating agent, where wet DMF was substituted to dry DMF as reaction solvent (entries 2–4, Table 2).29 Unfortunately, all the attempts to replace DMF with a polar aprotic greener solvent, such as acetone, ethyl acetate, or methyl ethyl ketone, failed even when coupled with nonconventional energy source as heating system (data not shown). Concerning the dimethylated derivatives 17 and 22–24 and the derivatives containing a benzodioxole moiety 21, 25, and 26, the demethylation reactions gave rise to significant results only in the case of compound 17 (entry 1, Table 2), which was transformed in a mix of inseparable monodemethylated derivatives (compounds 13a and 13b, Scheme 1). No products were obtained from the remaining derivatives, even adding NaI as iodine donor to the reaction mixture or changing the demethylation protocol by using a complex system composed by thiols and AlCl3 reported in the literature (data not shown).30 The demethylated compounds 13a/13b–16 were purified by flash chromatography and characterized by HRMS and 1H and 13C NMR.
Table 2. Demethylation Reaction Performed on Compounds 17–26.
| entry | substrate | product | C6H11I (eq) | time (h) | yield (%)a |
|---|---|---|---|---|---|
| 1 | 17 | 13a,b | 45 | 24 | 45b |
| 2 | 18 | 14 | 60 | 10 | 53 |
| 3 | 19 | 15 | 60 | 10 | 65 |
| 4 | 20 | 16 | 45 | 6 | 80 |
| 5 | 21 | 60 | 20 | ||
| 6 | 22 | 60 | 15 | ||
| 7 | 23 | 60 | 15 | ||
| 8 | 24 | 60 | 15 | ||
| 9 | 25 | 60 | 20 | ||
| 10 | 26 | 60 | 20 |
Isolated yield. All the compounds were characterized by 1H and 13C NMR and HRMS.
Monomethylated mixture.
The biological effect of the synthesized compounds has been assessed either on the basis of the inhibitory activity exerted on the in vitro cholinesterase and BACE-1 activities or on the cell viability in SHSY-5Y neuroblastoma cells.
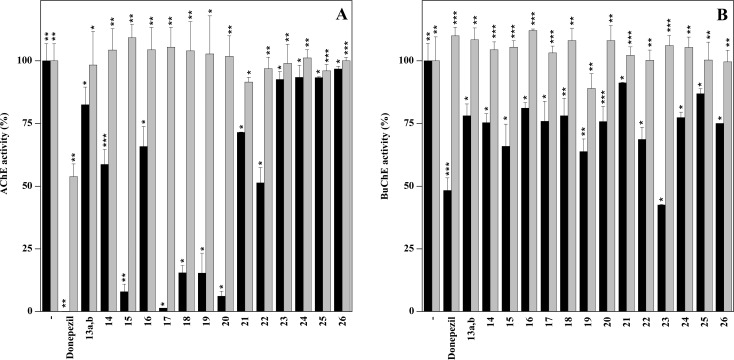
To test the inhibition power, the cholinesterase activity was measured in the absence or in the presence of 1 or 2 μM of each compound for Electrophorus electricus acetylcholinesterase (eeAChE) or horse serum butyrylcholinesterase (BuChE), respectively. The results reported in Figure 2 indicated that, among the tested molecules, 17, 20, 15, 18, and 19, in the order of effectiveness, exhibited the greatest inhibitory activity (black bars) on eeAChE (Panel A); although the inhibitor effect was lower than that exhibited by donepezil, the IC50 still remained below 1 μM. Concerning BuChE activity, the inhibition power of all the synthesized compounds was lower (Panel B), being the estimated IC50 always higher than 1 μM.
Figure 2.
Inhibition and reversibility of the inhibitory activity exerted by synthesized compounds. The cholinesterase activity was determined in the absence or in the presence of the indicated compounds (black bars) or after 1/100 dilution (gray bars). Panel A effect of 1 μM compounds on eeAChE activity; panel B effect of 2 μM compounds on BuChE activity. The data represent the average of at least three different determinations with the indication of the standard error. The significance of the data was evaluated by p value (*, < 0.05; **, < 0.005; ***, < 0.0005).
As demonstrated by the dilution method, the inhibition observed was reversible as the activity was recovered after a 100-fold dilution (gray bars) in all the conditions except for donepezil in the case of eeAChE because 10 nM was a final concentration still inhibiting this enzymatic activity.
Therefore, to determine the IC50 of the synthesized compounds on both human AChE (hAChE) and BuChE, the residual enzymatic activity was tested at different concentration of the inhibitors. The data, besides confirming the data reported in Figure 2 for eeAChE, allowed the calculation of the IC50 that were reported in Table 3. In order to assess the type of inhibition, the effect of different concentration of the compounds was tested at various thiolated substrate concentration, allowing the determination of the Ki. The data (for a representative experiment, see Supporting Information) indicated that among the inhibitors, 21, 25, and 26 acted as mixed inhibitors of hAChE, as donepezil; vice versa, the other synthesized compounds showed a noncompetitive behavior.
Table 3. Inhibition Parameters of Synthesized Compounds on the Activity of hAChE and BuChE.
| human erythrocytes
AChE |
horse serum BuChE |
selectivity |
||||
|---|---|---|---|---|---|---|
| inhibitor | Ki (μM) | IC50 (μM) | Ki (μM) | IC50 (μM) | from Ki | from IC50 |
| donepezil | 0.010 ± 0.006 | 0.011 ± 0.008 | 2.140 ± 1.380 | 1.270 ± 0.410 | 214 | 120 |
| 13a,b | 2.822 ± 0.022 | 1.540 ± 0.090 | 10.611 ± 4.205 | 7.350 ± 0.130 | 4 | 5 |
| 14 | 4.224 ± 1.920 | 1.500 ± 0.23 | 5.613 ± 1.294 | 5.800 ± 0.050 | 1 | 4 |
| 15 | 0.355 ± 0.035 | 0.636 ± 0.209 | 6.480 ± 2.420 | 4.760 ± 0.041 | 18 | 7 |
| 16 | 1.980 ± 0.180 | 2.160 ± 0.760 | 8.460 ± 3.930 | 7.590 ± 0.03 | 4 | 4 |
| 17 | 0.035 ± 0.008 | 0.058 ± 0.033 | 2.440 ± 1.290 | 4.740 ± 0.750 | 71 | 81 |
| 18 | 0.260 ± 0.036 | 0.342 ± 0.029 | 1.140 ± 0.052 | 2.650 ± 0.617 | 4 | 8 |
| 19 | 1.835 ± 0.597 | 1.509 ± 0.510 | 3.099 ± 2.610 | 1.140 ± 0.090 | 2 | 1 |
| 20 | 0.029 ± 0.007 | 0.043 ± 0.007 | 3.140 ± 1.800 | 5.734 ± 0.130 | 110 | 132 |
| 21 | 0.980 ± 0.220 | 0.755 ± 0.025 | 7.254 ± 4.572 | 15.130 ± 0.025 | 7 | 20 |
| 22 | 0.554 ± 0.052 | 0.853 ± 0.052 | 3.490 ± 2.240 | 2.170 ± 1.330 | 6 | 3 |
| 23 | 119.490 ± 61.030 | 34.500 ± 18.470 | 3.888 ± 3.110 | 1.620 ± 0.004 | <1 | <1 |
| 24 | 17.230 ± 6.420 | 12.155 ± 0.225 | 2.378 ± 1.730 | 5.410 ± 0.120 | <1 | <1 |
| 25 | 39.670 ± 12.040 | 25.195 ± 21.720 | 4.328 ± 2.969 | 9.820 ± 0.200 | <1 | <1 |
| 26 | 18.200 ± 7.640 | 11.135 ± 0.054 | 6.437 ± 2.633 | 4.820 ± 0.002 | <1 | <1 |
Concerning the effect on BuChE, 15–19 exhibited a mixed inhibition mechanism, as donepezil did. The remaining compounds acted as noncompetitive.
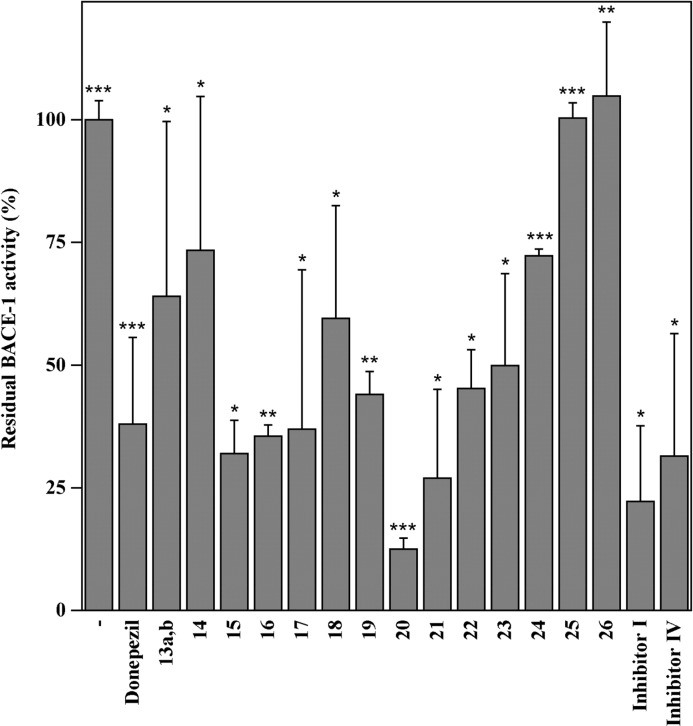
The data on selectivity reported in the Table 3 indicated that, among the compounds synthesized, 17 and 20 were about 2 orders of magnitude more selective for AChE than BuChE, therefore approaching the selectiveness displayed by donepezil. To our knowledge, such traditional donepezil precursors, even if presented as synthetic intermediates, have never been neither separated nor tested as selective AChE inhibitors. Our finding demonstrated that such compounds displays a nondrastic reduction in the inhibiting activity versus an important synthetic simplification, compared to donepezil. The effect of the inhibitors on BACE-1 activity (Figure 3) was compared to that exhibited by donepezil. Among the tested compounds, 15, 16, 17, 20, 21, and 22 exhibited the highest inhibition activity, approaching that of donepezil.31 The values of the IC50 reported in the Supporting Information section confirmed the results reported in Figure 3 and indicated that the above-mentioned compounds have IC50 lower than 1 μM. Such results confirmed our hypothesis on the importance of the double bond, while the presence of a hydroxyl group seemed to have no influence on the inhibitor activity on BACE-1. No special activity was recorded for protected dioxole compounds 21, 25, and 26, thus demonstrating that no more points of rigidity are needed over the double bond. In particular the poor activity of compounds 25 and 26 demonstrated that a big steric hindrance on the N-benzyl piperidine moiety is unfavorable to reach the active site.
Figure 3.
Effect of synthesized compounds on BACE-1 activity. The BACE-1 activity was determined as reported in the experimental section in the Supporting Information, in the absence or in the presence of 1 μM synthesized compounds. The residual activity was compared to that exhibited by donepezil (200 nM) or the known Inhibitor I (1 μM) and IV (100 nM) of BACE-1 activity. The data represent the average of at least three determinations and the significance of the data was evaluated by p value (*, < 0.05; **, < 0.005; ***, < 0.0005).
Moreover, compound 17 and 20 displayed better dual activity and lower IC50 values against both AChE and BACE-1 enzymes compared to those reported in the literature for structurally similar molecules.8
At a concentration up to 30 times higher than the IC50 on AChE (see Table 3) for 48 h, the compounds 17 and 20 did not affect cell viability, in comparison with the untreated control (Figure S3, Supporting Information). Similar results were obtained also for all the other synthesized compounds (not shown).
In conclusion, a sustainable multistep protocol for the synthesis of a set of traditional, as well as differently substituted, donepezil precursors has been presented. The optimization consisted in an alternative Q-tube assisted method for the synthesis of the indanone moieties and in a green US-assisted condensation protocol with the N-benzyl piperidine-4-carboxaldehyde. The enzymatic inhibition tests on AChE and BuChE have been realized on all the synthesized substrates in order to study the influence of the characteristic unsaturation between the two synthons on the donepezil activity and selectivity. Moreover, some dioxole protected derivatives on both aromatic rings were synthesized and a demethylation step was conducted on a set of selected substrates, in order to study the role of such modification on the activity on both enzymes. All the synthesized derivatives were less active than donepezil and did not influence the cell viability in SH-SY5Y neuroblastoma cells. No improvement in the inhibitor activity was registered with the introduction of hydroxyl groups on the molecules, while the substitution of a stereocenter with a double bond, even reducing the inhibition activity on AChE compared to donepezil, opens the possibility to exploit such synthetically simplified donepezil analogues as better inhibitors of BACE-1. Indeed, considering the selectivity in cholinesterase inhibition and the effect on BACE-1 activity, 17 and 20 could represent promising candidates for the development of drugs with dual inhibitor activity for AD treatment.
Acknowledgments
This work was supported by the grant POR Calabria FSE 2007/2013, Asse IV “Capitale Umano”, Obiettivo OperativoM.2., and Piano d’azione 2011–2013- Department 11 “Cultura-Istruzione-Università-Ricerca-Innovazione Tecnologica-AltaFormazione” of Regione Calabria.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00483.
Experimental details for synthetic procedures and biological essays, full compound characterization, HRMS, 1H NMR and 13C NMR, and NOESY NMR spectra of new products (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Martorana A.; Esposito Z.; Koch G. Beyond the cholinergic hypothesis: do current drugs work in Alzheimer’s Disease?. CNS Neurosci. Ther. 2010, 16, 235–245. 10.1111/j.1755-5949.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P.; Veerhuis R.; Scheper W.; Rozemuller A. J.; van Gool W. A.; Hoozemans J. J. The significance of neuroinflammation in understanding Alzheimer’s disease. J. Neural Transm. 2006, 113, 1685–1695. 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- Vassar R. BACE-1: the beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 105–114. 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- Domínguez J. L.; Fernández-Nieto F.; Castro M.; Catto M.; Paleo M. R.; Porto A.; Sardina F. J.; Brea J. M.; Carotti A.; Villaverde M. C.; Sussman F. Computer-Aided Structure-Based Design of Multitarget Leads for Alzheimer’s Disease. J. Chem. Inf. Model. 2015, 55, 135–148. 10.1021/ci500555g. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Xiao K.; Ma L.; Xiong B.; Fu Y.; Yu H.; Wang W.; Wang X.; Hu D.; Peng H.; Li J.; Gong Q.; Chai Q.; Tang X.; Zhang H.; Li J.; Shen J. Design, synthesis and biological evaluation of novel dual inhibitors of acetylcholinesterase and beta-secretase. Bioorg. Med. Chem. 2009, 17, 1600–1613. 10.1016/j.bmc.2008.12.067. [DOI] [PubMed] [Google Scholar]
- Viayna E.; Sabate R.; Muñoz-Torrero D. Dual inhibitors of β-amyloid aggregation and acetylcholinesterase as multi-target anti-Alzheimer drug candidates. Curr. Top. Med. Chem. 2013, 13, 1820–1842. 10.2174/15680266113139990139. [DOI] [PubMed] [Google Scholar]
- OrtegaA; Rincón Á.; Jiménez-Aliaga K. L.; Bermejo-Bescós P.; Martín-Aragón S.; Molina M. T.; Csákÿ A. G. Synthesis and evaluation of arylquinones as BACE1 inhibitors, β-amyloid peptide aggregation inhibitors, and destabilizers of preformed β-amyloid fibrils. Bioorg. Med. Chem. Lett. 2011, 21, 2183–2187. 10.1016/j.bmcl.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Rampa A.; Mancini F.; De Simone A.; Falchi F.; Belluti F.; Di Martino R. M.; Gobbi S.; Andrisano V.; Tarozzi A.; Bartolini M.; Cavalli A.; Bisi A. From AChE to BACE1 inhibitors: The role of the amine on the indanone scaffold. Bioorg. Med. Chem. Lett. 2015, 25, 2804–2808. 10.1016/j.bmcl.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Nguyen V. T.; To D. C.; Tran M. H.; Oh S. H.; Kim J. A.; Ali M. Y.; Woo M. H.; Choi J. S.; Min B. S. Isolation of cholinesterase and β-secretase 1 inhibiting compounds from Lycopodiellacernua. Bioorg. Med. Chem. 2015, 33, 3126–3134. 10.1016/j.bmc.2015.04.080. [DOI] [PubMed] [Google Scholar]
- Stachel S. J. Drug Dev. Res. 2009, 70, 101–110. 10.1002/ddr.20289. [DOI] [Google Scholar]
- Fernández-Bachiller M. I.; Pérez C.; Monjas L.; Rademann J.; Rodríguez-Franco M. I. New Tacrine–4-Oxo-4H-chromene Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease, with Cholinergic, Antioxidant, and β-Amyloid-Reducing Properties. J. Med. Chem. 2012, 55, 1303–1317. 10.1021/jm201460y. [DOI] [PubMed] [Google Scholar]
- Sugimoto H.; Yamanishi Y.; Iimura Y.; Kawakami Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 2000, 7, 303–339. 10.2174/0929867003375191. [DOI] [PubMed] [Google Scholar]
- Van der Zee E. A.; Platt B.; Riedel G. Acetylcholine: future research and perspectives. Behav. Brain Res. 2011, 221, 583–586. 10.1016/j.bbr.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Sugimoto H.; Iimura Y.; Yamanishi Y.; Yamatsu K. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-Benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-yl)methyl]piperidine hydrochloride and related compounds. J. Med. Chem. 1995, 38, 4821–4829. 10.1021/jm00024a009. [DOI] [PubMed] [Google Scholar]
- Lerman O.; Kaspi J.; Arad O.; Alnabari M.; Sery Y.. Process for the preparation of donepezil. Patent US6844440B2, 2005.
- Elati C. R.; Kolla N.; Chalamala S. R.; Vankawala P. J.; Sundaram V.; Vurimidi H.; Mathad V. T. New synthesis of donepezil through palladium-catalyzed hydrogenation approach. Synth. Commun. 2006, 36, 169–174. 10.1080/00397910500334231. [DOI] [Google Scholar]
- Nardi M.; Cozza A.; Maiuolo L.; Oliverio M.; Procopio A. 1,5-Benzoheteroazepines through eco-friendly general condensation. Tetrahedron Lett. 2011, 52, 4827–4834. 10.1016/j.tetlet.2011.06.029. [DOI] [Google Scholar]
- Cravotto G.; Procopio A.; Oliverio M.; Orio L.; Carnaroglio D. Simple sonochemical protocols for fast and repeatable Grignard reactions. Green Chem. 2011, 13, 2806–2809. 10.1039/c1gc15756f. [DOI] [Google Scholar]
- Procopio A.; Cravotto G.; Oliverio M.; Costanzo P.; Nardi M.; Paonessa R. An eco-sustainable erbium(III)-catalysedmethod for formation/cleavage of O-tert-butoxy carbonates. Green Chem. 2011, 13, 436–443. 10.1039/c0gc00728e. [DOI] [Google Scholar]
- Nardi M.; Bonacci S.; De Luca G.; Maiuolo J.; Oliverio M.; Sindona G.; Procopio A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4 -hydroxyphenyl) ethyl (3S, 4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate)]. Food Chem. 2014, 162, 89–93. 10.1016/j.foodchem.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Oliverio M.; Costanzo P.; Nardi M.; Rivalta I.; Procopio A. Facile ecofriendly synthesis of monastrol and its structural isomers via biginelli reaction. ACS Sustainable Chem. Eng. 2014, 2, 1228–1233. 10.1021/sc5000682. [DOI] [Google Scholar]
- Oliverio M.; Nardi M.; Costanzo P.; Cariati L.; Cravotto G.; Giofrè V. S.; Procopio A. Non-conventionalmethodologies in the synthesis of 1-indanones. Molecules 2014, 19, 5599–5610. 10.3390/molecules19055599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakhas G. K. S.; Yang P.; Bela T.; Olah A. G.Superacidtrifluoromethanesulfonic acid induced cycli-acyl alkylation of aromatics. Catal. Lett. 2003, 87, 109–112. 10.1023/A:1023482904174. [DOI] [Google Scholar]
- Caruso A.; Garofalo A.; Grande F.; Aiello F.; Anzini M.; Ortuso F.; Alcaro S.; Panno A.; Saturnino C.; Sinicropi M. S. Synthesis and biological evaluation of 1,3-idandione derivatives as acetylcholinesterase inhibitors. Pharmacologyonline 2009, 1, 264–277. [Google Scholar]
- Aggarval A. K.; Srinivasan C. V.; Wadhwa L.. Process for the preparation of highly pure Donepezil. Patent US2010/0113793Al, 2010.
- Niphade N.; Mali A.; Jagtap K.; Ojha R. C.; Vankawala P. J.; Mathad V. T. An improved and efficient process for the production of donepezil hydrochloride: substitution of sodium hydroxide for n-butyl lithium via phase transfer catalysis. Org. Process Res. Dev. 2008, 12, 731–735. 10.1021/op800066m. [DOI] [Google Scholar]
- Lahyani A.; Chtourou M.; Frikha M. H.; Trabelsi M. Amberlyst-15 and Amberlite-200C: efficient catalysts for aldol and cross-aldol condensation under ultrasound irradiation. Ultrason. Sonochem. 2013, 20, 1296–1301. 10.1016/j.ultsonch.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Chtourou M.; Abdelhédi R.; Frikha M. H.; Trabelsi M. Solvent free synthesis of 1,3-diaryl-2-propenones catalyzed by commercial acid-clays under ultrasound irradiation. Ultrason. Sonochem. 2010, 17, 246–249. 10.1016/j.ultsonch.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Zuo L.; Yao S.; Wei W. An efficient method for demethylation of aril methyl ethers. Tetrahedron Lett. 2008, 49, 4054–4056. 10.1016/j.tetlet.2008.04.070. [DOI] [Google Scholar]
- Node M.; Nishide K.; Fuji K.; Fujita E. Hard acid and soft nucleophile system. 2. Demethylation of methyl ethers of alcohol and phenol with an aluminum halide-thiol system. J. Org. Chem. 1980, 45, 4275–4277. 10.1021/jo01310a003. [DOI] [Google Scholar]
- Mancini F.; Naldi M.; Cavrini V.; Andrisano V. Multiwellfluorometric and colorimetric microassays for the evaluation of beta-secretase (BACE-1) inhibitors. Anal. Bioanal. Chem. 2007, 388, 1175–11831. 10.1007/s00216-007-1356-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.