Abstract
The orally bioavailable 1-deoxy-sphingosine analog, Enigmol, has demonstrated anticancer activity in numerous in vivo settings. However, as no Enigmol analog with enhanced potency in vitro has been identified, a new strategy to improve efficacy in vivo by increasing tumor uptake was adopted. Herein, synthesis and biological evaluation of two novel fluorinated Enigmol analogs, CF3-Enigmol and CF2-Enigmol, are reported. Each analog was equipotent to Enigmol in vitro, but achieved higher plasma and tissue levels than Enigmol in vivo. Although plasma and tissue exposures were anticipated to trend with fluorine content, CF2-Enigmol absorbed into tissue at strikingly higher concentrations than CF3-Enigmol. Using mouse xenograft models of prostate cancer, we also show that CF3-Enigmol underperformed Enigmol-mediated inhibition of tumor growth and elicited systemic toxicity. By contrast, CF2-Enigmol was not systemically toxic and demonstrated significantly enhanced antitumor activity as compared to Enigmol.
Keywords: Sphingolipids, Enigmol, fluorination, prostate cancer
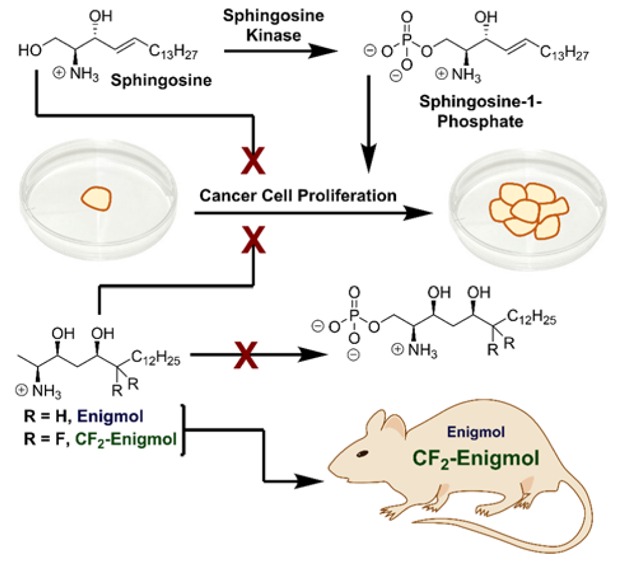
Sphingolipids are a structurally diverse1 class of lipids containing a common scaffold that is exemplified by sphingosine (Figure 1a), a ubiquitous intermediate in the biosynthesis of a wide array of sphingolipids, many of which are second messengers.2 For example, amino group acylation by ceramide synthase (CS) produces ceramides,3 which facilitate signaling events from lipid rafts. Alternatively, phosphorylation of the primary hydroxyl group by sphingosine kinase (SK) generates sphingosine-1-phosphate4 (S1P), the endogenous agonist for seven-transmembrane S1P receptors. The sphingolipid biosynthetic and metabolic pathways include numerous pleiotropic signaling molecules involved in complex equilibria. Notable among these are the opposing roles of sphingosine and S1P: S1P promotes cell survival, whereas sphingosine promotes cell death.5 While healthy cells benefit from a proper balance of S1P and sphingosine, many cancer cell types overexpress SK,6 which tips the balance in favor of S1P. Since the cytotoxicity of endogenous sphingoid bases7 is limited by phosphorylation, inhibition of SK represents a promising anticancer strategy.8
Figure 1.
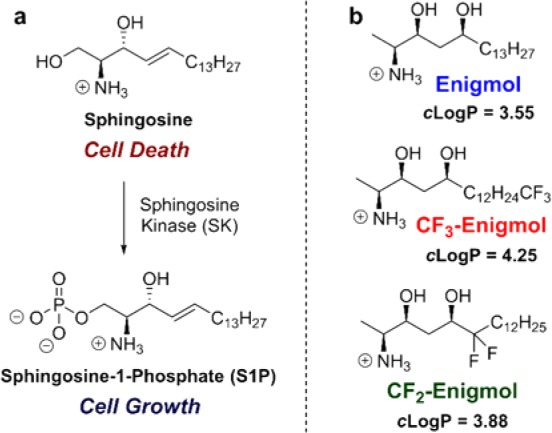
Sphingosine-S1P axis and fluorinated Enigmol analogs. cLogP was calculated using QikProp in Maestro.
By contrast, we envisioned that a sphingosine mimic incapable of phosphorylation could reestablish the proper sphingosine/S1P balance, and thereby, suppress cancer growth. Specifically, sphingoid base analogs lacking the C-1 hydroxyl group were hypothesized to adopt the cytotoxic signaling profile of sphingosine without the counterweight of S1P metabolites. Evaluation of 1-deoxy-sphingoid bases9,10 led to the identification of Enigmol (Figure 1b), which exhibited broad-spectrum cytotoxicity (0.4 μM ≤ IC50 ≤ 14 μM) against 57 human cancer cell lines,11 in addition to LNCaP (IC50 = 9.7 ± 0.3 μM) and PC-3 (IC50 = 10.3 ± 0.2 μM) human prostate cancer cell lines,12in vitro. This activity profile was attributed to (1) inhibition of SK,11 CS,10 and protein kinase C9 (PKC), (2) “immunity” to SK and other kinases,11 and (3) slow CS-mediated N-acylation.10 Sluggish metabolism endows Enigmol with a longer lifetime than its endogenous counterparts, thereby extending the duration of its therapeutic action. While most compounds with micromolar anticancer activity in vitro cannot achieve effective tumor concentrations in vivo, Enigmol robustly partitions into tissue, building up drug reservoirs after multiple oral (p.o.) doses.12 This combination of long lifetime and extensive tissue uptake enabled moderately cytotoxic Enigmol to significantly inhibit tumor growth in multiple cancer models in vivo without causing systemic toxicity.11 In a mouse xenograft model of prostate cancer, for example, Enigmol exhibited comparable efficacy to surgical hormone deprivation and to docetaxel, which are the current clinical standards of care for androgen-dependent and androgen-independent prostate cancer, respectively.12
As evidenced by striking in vivo efficacy and low systemic toxicity, Enigmol’s modest in vitro potency is leveraged by favorable pharmacokinetic (PK) properties in vivo. These results encouraged synthesis and evaluation of various Enigmol analogs,12,13 a process facilitated by two diastereoselective approaches to Enigmol developed in our laboratory.14,12 However, because the maximum potential of these compounds could only be determined with time- and resource-intensive in vivo studies, we decided to increase lipophilicity with the expectation of enhanced anticancer activity due to improved oral absorption and elevated tumor exposure. Specifically, replacing selected hydrogens in Enigmol (cLogP = 3.55) with fluorines was anticipated to increase hydrophobicity without substantially perturbing other physicochemical properties.15,16 Accordingly, we prepared two analogs13 (Figure 1b), CF3-Enigmol (cLogP = 4.25, trifluorination at C-18) and CF2-Enigmol (cLogP = 3.88, difluorination at C-6), and evaluated: (1) cytotoxicity against cultured human prostate cancer cells, (2) plasma and tissue PK properties, and (3) antitumor activity in xenograft models of prostate cancer.
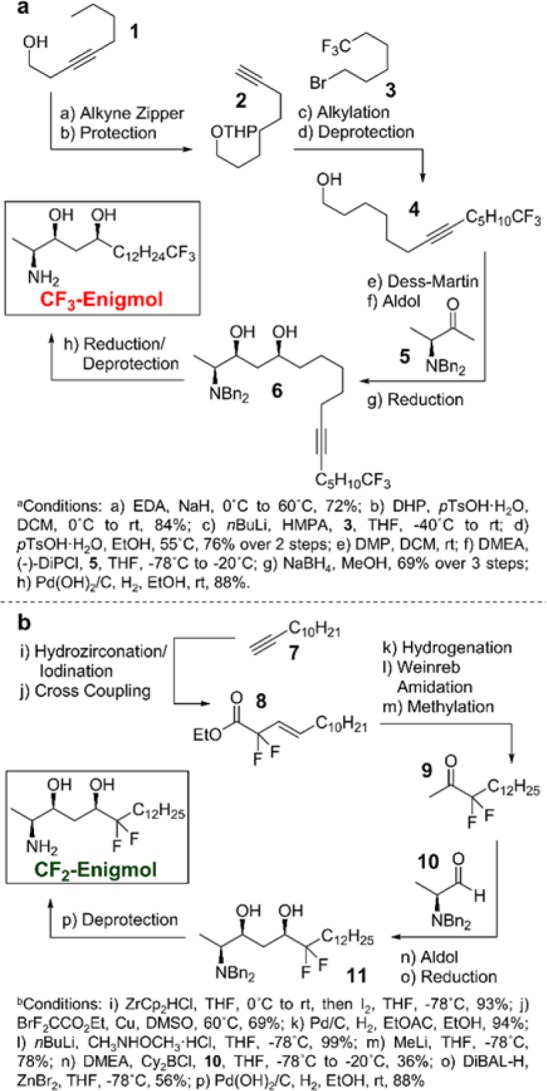
The synthesis of CF3-Enigmol was accomplished using our previously reported diastereoselective aldol route14 (Scheme 1a). Homopropargyllic alcohol 1 was converted to THP-ether 2 via an alkyne zipper reaction,17 followed by protection of the primary alcohol. The resulting terminal alkyne was then alkylated18 with bromide 3, followed by THP group removal and Dess-Martin oxidation to produce the desired aldehyde. Addition of the aldehyde to a preformed, (−)-DiPCl-stabilized enolate of methyl ketone 5(14) produced the aldol adduct, which was immediately subjected to reduction with sodium borohydride, yielding syn-diol 6 in 50% yield over three steps (d.r. = 99:1). Finally, a one-pot alkyne hydrogenation/N,N-dibenzylamino hydrogenolysis furnished CF3-Enigmol. CF2-Enigmol was prepared using a modified aldol approach involving α,α-difluoro methyl ketone 9 and α-aminoaldehyde 10(19) (Scheme 1b). Tandem hydrozirconation/iodination20 of alkyne 7 gave the corresponding terminal vinyl iodide, which was subjected to a copper-mediated coupling21 with ethyl bromodifluoroacetate to give α,α-difluoro ester 8. The resulting olefin was reduced via palladium-catalyzed hydrogenation22 and subsequently converted to ketone 9 using a two-step Weinreb ketone synthesis.23 Achiral borane-mediated aldol reaction with aldehyde 10(19) resulted in a chromatographically separable mixture of diastereomers (d.r. = 2:1, 36% and 20% isolated yields). Although TiCl4 led to improved syn-diastereoselectivity (d.r. = 4:1, Supporting Information Table 1), isolated yield suffered substantially, and the use of either (+)-DiPCl or (−)-DiPCl did not significantly improve syn-stereoinduction. Attempted syn-reduction of the resulting β-hydroxy ketone using Et2BOMe and NaBH424 gave disappointing selectivity as well (Supporting Information Table 2). Interestingly, while reduction with DiBAL-H (d.r. = 1:5) favored the anti-diol, DiBAL-H reduction with additive ZnBr225 (d.r. = 5:2) furnished the desired syn-diol 11 in 56% isolated yield. Finally, deprotection of the N,N-dibenzylamino group via hydrogenolysis produced CF2-Engimol.
Scheme 1. Synthesis of (a) CF3-Enigmol and (b) CF2-Enigmol.
CF3-Enigmol and CF2-Enigmol were assayed for cytotoxicity toward cultured androgen-dependent (LNCaP) and androgen-independent (PC-3) human prostate cancer cells by measuring mitochondrial activity12 (Supporting Information Figure 2). As expected, the IC50 values (Table 1) of CF3-Enigmol and CF2-Enigmol were comparable to Enigmol, indicating that fluorine incorporation did not substantially alter potency, and suggesting that any differences in in vivo activity would stem from altered PK properties. IC90 values were also calculated to indicate therapeutically effective concentrations desired in tumors.
Table 1. Cytotoxicity against Cultured Human Prostate Cancer Cellsa.
|
LNCaP Cells |
PC-3 Cells |
|||
|---|---|---|---|---|
| Drug | IC50(μM) | IC90(μM) | IC50(μM) | IC90(μM) |
| Enigmol | 11.7 ± 1.10 | 19.6 ± 1.10 | 12.1 ± 1.02 | 14.0 ± 1.00 |
| CF3-Enigmol | 11.3 ± 1.10 | 19.2 ± 1.30 | 21.4 ± 1.17 | 24.4 ± 1.20 |
| CF2-Enigmol | 13.4 ± 1.07 | 16.7 ± 1.20 | 11.7 ± 1.05 | 15.3 ± 1.10 |
LNCaP or PC-3 cells were incubated with compound for 24 h (n = 3–4), and viability was assessed using WST-1. Data represent the mean ± SEM.
With therapeutic concentrations established, PK studies were designed to evaluate the effect of fluorination on plasma elimination, oral absorption, and tissue distribution. As rapid intravenous (i.v.) injection effectively normalizes plasma absorption profiles, Sprague–Dawley (SD) rats were injected i.v. with a single 2 mg/kg dose of either Enigmol (n = 3), CF3-Enigmol (n = 4), or CF2-Enigmol (n = 4), facilitating reliable assessment of terminal half-life (t1/2) and rate of clearance (Cl). Analog concentrations in isolated plasma were quantified using liquid chromatography/tandem-mass spectrometry (LC-MS/MS) analysis (Figure 2). Although plasma elimination was anticipated to trend inversely with cLogP due to plasma protein binding, t1/2 and Cl values, as well as the maximum plasma concentration (Cmax) and area under the curve (AUC), indicated that CF3-Enigmol was removed from plasma substantially faster than both Enigmol and CF2-Enigmol. By contrast, CF2-Enigmol followed the expected trend, exhibiting lower Cl and higher t1/2, AUC, and Cmax than Enigmol.
Figure 2.
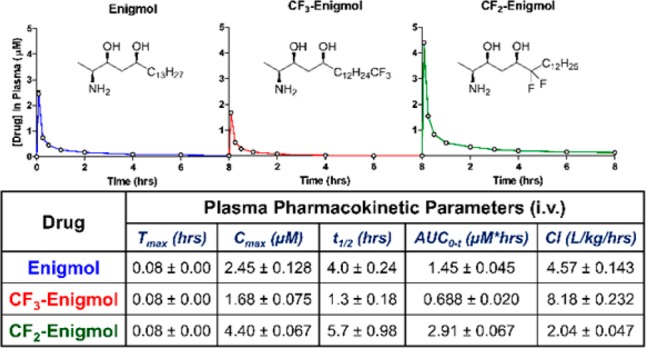
Plasma pharmacokinetics (i.v.). SD rats (n = 3–4 per group) were treated with compound (2 mg/kg) via i.v. injection (vehicle =40:10:3:47 PEG400/EtOH/Tween80/H2O), and plasma concentration was measured periodically using LC-MS/MS. Data represent the mean ± SEM.
Subsequent PK experiments were designed to compare plasma absorption and tissue distribution properties after oral administration. Accordingly, SD rats were dosed once via oral gavage with 10 mg/kg of either Enigmol (n = 4), CF3-Enigmol (n = 4) or CF2-Enigmol (n = 4), and analog concentrations in isolated plasma and various tissues were quantified using LC-MS/MS.26 Counter to results from i.v. experiments, systemic exposure after p.o. dose, as indicated by Cmax and AUC, trended with cLogP (Figure 3a). Furthermore, CF3-Enigmol required the longest amount of time (Tmax) to reach Cmax, and also demonstrated substantially improved oral bioavailability (68%) as compared to Enigmol (11%) and CF2-Enigmol (10%). Exponential decay of CF2-Enigmol plasma concentration after Cmax was likely not observed due to a relatively high t1/2 and a long absorption phase that overlaps with the elimination phase. Notably, Enigmol and CF3-Enigmol were not observed in plasma for >1 h, nor was CF2-Enigmol for >2 h. With plasma elimination virtually complete after 24 h, the accumulation of each compound in red blood cells, brain, lung, liver, kidney, and prostate was measured (Figure 3b). Although tissue distribution was anticipated to correlate with cLogP, CF2-Enigmol (n = 4) achieved substantially higher concentrations than both Enigmol (n = 4) and CF3-Enigmol (n = 4) in all tissues analyzed. While Enigmol never reached its IC90 level (≥14 μM) in any tissue type, CF3-Enigmol did so in lung (≥19 μM). Remarkably, CF2-Enigmol achieved IC90 concentrations (≥15 μM) in lung, brain, and kidney. Also notable are the relative drug levels in prostate, where CF2-Enigmol achieved 2-fold and 9-fold higher concentrations than CF3-Enigmol and Enigmol, respectively.
Figure 3.
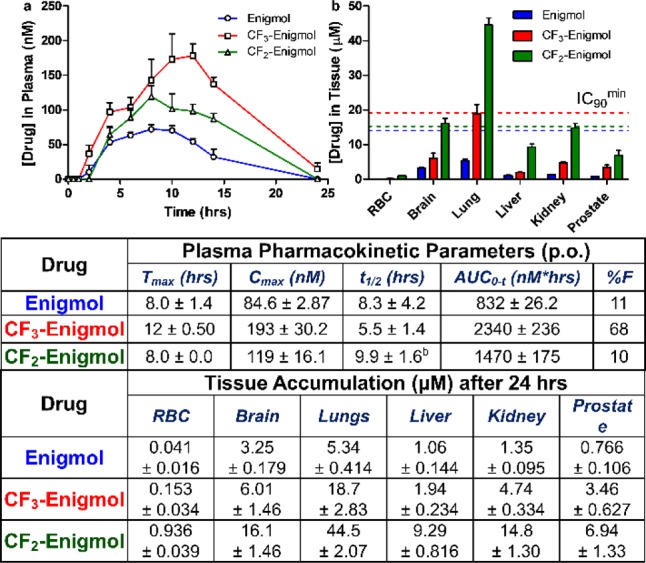
Plasma pharmacokinetics and tissue distribution (p.o.). (a) SD rats (n = 4 per group) were orally administered 10 mg/kg of either Enigmol (vehicle =90:10 olive oil/EtOH), CF3-Enigmol (vehicle =90:10 olive oil/EtOH), or CF2-Enigmol (vehicle =95:5 PEG400/Tween80), and plasma concentration was measured periodically using LC-MS/MS. (b) After 24 h, tissues were harvested, and drug levels were measured by LC-MS/MS. Data represent the mean ± SEM. CF2-Enigmol t1/2 is reported with the exclusion of one rat due to inappropriate semilog plots of the terminal 2, 3, or 4 nonzero data points (positive linear slopes or R2 = 0.038).
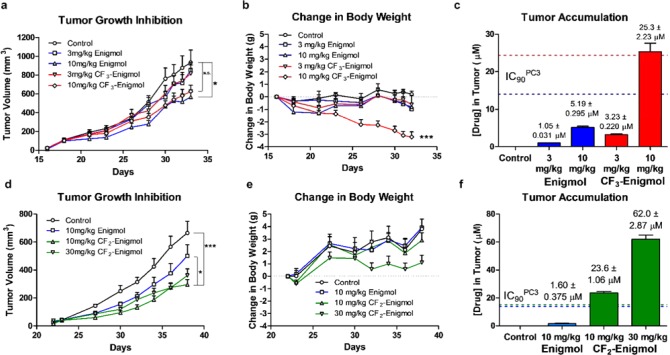
Although tissue accumulation did not trend with cLogP, fluorinated analogs reached higher tissue levels than Enigmol. Therefore, CF3-Enigmol and CF2-Enigmol were expected to achieve elevated tumor concentrations and enhanced antitumor efficacy. To test this hypothesis, nude mice were injected subcutaneously with PC-3 cells, and tumors were established until palpable before beginning once daily oral gavage of either Enigmol (3 mg/kg or 10 mg/kg), CF3-Enigmol (3 mg/kg or 10 mg/kg), or vehicle (95:5 olive oil/EtOH). Tumor volume was measured to quantify drug-induced growth inhibition (Figure 4a). While 3 mg/kg dosing regiments were ineffective for both Enigmol (n = 11) and CF3-Enigmol (n = 10), 10 mg/kg Enigmol (n = 11) significantly inhibited tumor growth (p = 0.02) versus control (n = 11); 10 mg/kg CF3-Enigmol (n = 11) surprisingly did not (p = 0.17), despite substantially elevated tissue concentrations. While no weight loss (Figure 4b) was observed among control, 3 mg/kg Enigmol, 10 mg/kg Enigmol, or 3 mg/kg CF3-Enigmol treatment groups, 10 mg/kg CF3-Enigmol caused significant weight loss (11.6 ± 1.32%) over the treatment course (p = 0.0037). To evaluate the relationship between efficacy and local drug concentrations, tumor accumulation of Enigmol and CF3-Enigmol was quantified using LC-MS/MS at the end of the study (Figure 4c). While 10 mg/kg Enigmol did not achieve IC90PC-3 concentrations (=14 μM) in tumor, 10 mg/kg CF3-Enigmol (IC90PC-3 = 24 μM) did. A related boost in antitumor activity, however, was unexpectedly not observed.
Figure 4.
CF3-Enigmol and CF2-Enigmol in mouse xenograft models of prostate cancer. Nude mice (n = 10–11 per group) with palpable tumors derived from PC-3 human prostate cancer cells were treated once daily via oral gavage with either vehicle (95:5 olive oil/EtOH), Enigmol (3 or 10 mg/kg), or CF3-Enigmol (3 or 10 mg/kg) in the first study (a–c), or with either vehicle (95:5 PEG400/Tween80), Enigmol (10 mg/kg), or CF2-Enigmol (10 or 30 mg/kg) in the second study (d–f). Tumor volume and change in body weight were measured periodically. At the end of each study, tumors were harvested, and accumulation was measured using LC-MS/MS. Data represent the mean ± SEM. Statistical significance (*p < 0.05, **p < 0.01, or ***p < 0.001) of tumor growth inhibition was assessed via a linear mixed model for repeated measurement with the autoregressive covariance structure using Statistical Analysis System (SAS) software. Significance of change in body mass was determined using One-Way ANOVA followed by Sidak’s multiple comparison test.
Thus, focus was shifted to CF2-Enigmol, which was compared head-to-head with Enigmol in a subsequent xenograft study. After tumors were established, once daily oral gavage of vehicle (95:5 PEG400/Tween80, n = 9 or 10, one mouse died on day 32), 10 mg/kg Enigmol (n = 9 or 10, one mouse died on day 36), 10 mg/kg CF2-Enigmol (n = 10), or 30 mg/kg CF2-Enigmol (n = 10) was initiated (Figure 4d). Gratifyingly, 10 mg/kg CF2-Enigmol significantly inhibited tumor growth relative to both control (p = 0.0003) and 10 mg/kg Enigmol (p = 0.04), and neither 10 mg/kg nor 30 mg/kg CF2-Enigmol caused significant weight loss (Figure 4e). Consistent with tissue distribution, 10 mg/kg CF2-Enigmol (IC90PC-3 = 15 μM) achieved strikingly higher tumor concentrations (24 μM) than 10 mg/kg Enigmol (Figure 4f). Although 30 mg/kg CF2-Enigmol reached extraordinary tumor levels (62 μM), it underperformed relative to 10 mg/kg CF2-Enigmol, suggesting that a therapeutically saturating concentration in tumor cells can be achieved after 2 weeks of once daily oral dosing at <10 mg/kg.
CF2-Enigmol exhibited slower plasma elimination kinetics (i.v.), more robust plasma absorption (p.o.), and more pronounced tissue accumulation than Enigmol and CF3-Enigmol. Although oral absorption trended with cLogP, the effects of fluorination on plasma elimination and tissue accumulation followed entirely different trends. Since cLogP can be an insufficient representation of fluorinated small molecule lipophilicity, an astute reviewer suggested that empirical measurements of LogD would help to clarify the role of lipophilicity. Therefore, LogD (pH = 7.4, n = 2, mean ± SEM) of each analog was measured empirically (Absorption Systems, Exton, PA), indicating an unexpected trend in lipophilicity: Enigmol (3.50 ± 0.09) > CF3-Enigmol (3.24 ± 0.06) > CF2-Enigmol (1.73 ± 0.06). Counter to the Hansch hydrophobicity parameters15 and the average increase in LogD upon fluorine incorporation,16 fluorination sometimes actually decreases lipophilicity due to the interplay between change in volume and dipole moment.27 For example, trifluoroethanol15 (LogP = 0.36) is more lipophilic than ethanol (LogP = −0.32) because the volume change upon fluorination overrides the change in dipole moment. Conversely, larger trifluoromethylhexanol (LogP = 1.14) is less lipophilic than hexanol (LogP = 2.03) because the volume change is insignificant compared to the change in dipole moment. In the case of fluorinated Enigmol analogs, an apparent increase in dipole moment upon fluorination appears to override the relatively small increase in volume, leading to decreased lipophilicity. Furthermore, increased acidity of the CF2-Enigmol C-5 alcohol likely leads to improved hydrogen bonding with water,16 combining with the inherent polarity of the difluoromethylene unit28 to decrease LogD. Notably, the conventional wisdom that lipophilicity is a strong determinant of systemic exposure after oral administration did not hold up here: tissue accumulation actually trended inversely with LogD.
Ultimately, the flat in vitro SAR observed with Enigmol analogs was overcome by modulating PK properties via fluorine installation. Since CF3-Enigmol and CF2-Enigmol exhibited similar cytotoxicity to Enigmol against human prostate cancer cells in vitro, we conclude that differences in antitumor efficacy resulted from changes in PK. As no trend between LogD and any PK parameter (other than an inverse trend with tissue accumulation) was observed, the PK results presented herein indicate that physicochemical properties other than lipophilicity contribute to the plasma elimination, oral absorption, and tissue distribution of these compounds. Despite achieving IC90 levels in tumor, CF3-Enigmol did not significantly inhibit tumor growth and caused significant weight loss. Alternatively, CF2-Enigmol statistically suppressed tumor growth better than Enigmol, likely because of elevated tumor concentrations. Since Enigmol was equally efficacious to docetaxel,12 CF2-Enigmol may offer an advantage over taxane-based chemotherapeutics for hormone-refractory prostate cancer, especially given the lack of systemic toxicity. Furthermore, due to enormous CF2-Enigmol concentrations in lung, brain, and kidney, antitumor efficacy could prove to be even more profound for the treatment of solid tumors residing in these tissues.
Acknowledgments
The authors would like to thank Dr. Jinjing Gao (Winship Cancer Institute, Emory University) for conducting statistical analysis for the xenograft studies, as well as Dr. George Painter (Emory Institute for Drug Development), Dr. Jim Snyder, Dr. Bryan Cox, Dr. Terry Moore, Dr. Valerie Truax, Dr. Tim Acker, Dr. John DiRaddo, Cynthia Gaillard (Department of Chemistry, Emory University), and Kristin Jones (Biomarkers Core Laboratory, Yerkes National Primate Research Center) for thoughtful insights and extensive discussion concerning data presented in this manuscript.
Glossary
Abbreviations
- CS
ceramide synthase
- SK
sphingosine kinase
- S1P
sphingosine-1-phosphate
- PK
pharmacokinetic
- i.p.
intraperitoneal
- i.v.
intravenous
- p.o.
per os (oral)
- NOE
Nuclear Overhauser Effect
- WST-1
2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt
- SD
Sprague–Dawley
- LC-MS/MS
liquid chromatography/tandem-mass spectrometry
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00113.
Synthetic procedures utilized for the preparation of CF3-Enigmol and CF2-Enigmol, concentration–response curves of Enigmol, CF2-Enigmol, and CF3-Enigmol, and experimental methods and raw data from pharmacokinetic and xenograft experiments. (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Questions regarding synthesis, pharmacokinetic analysis, and characterization of anticancer activity should be addressed to Eric J. Miller (ejmill2@emory.edu), G. Prabhakar Reddy (pgrudda@emory.edu), or Suzanne G. Mays (smays@emory.edu), respectively.
Work presented herein was funded internally.
The authors declare no competing financial interest.
Supplementary Material
References
- Pruett S. T.; Bushnev A.; Hagedorn K.; Adiga M.; Haynes C. A.; Sullards M. C.; Liotta D. C.; Merrill A. H. Biodiversity of Sphingoid Bases (“Sphingosines”) and Related Amino Alcohols. J. Lipid Res. 2008, 49, 1621–1639. 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A.; Obeid L. M. Principles of Bioactive Lipid Signalling: Lessons from Sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Kollmeyer J.; Symolon H.; Momin A.; Munter E.; Wang E.; Kelly S.; Allegood J. C.; Liu Y.; Peng Q.; Ramaraju H.; Sullards M. C.; Cabot M.; Merrill A. H. Ceramides and Other Bioactive Sphingolipid Backbones in Health and Disease: Lipidomic Analysis, Metabolism, and Roles in Membrane Structure, Dynamics, Signaling and Autophagy. Biochim. Biophys. Acta, Biomembr. 2006, 1758, 1864–1884. 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Spiegel S.; Milstien S. Sphingosine-1-Phosphate: An Enigmatic Signalling Lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Merrill A. H.; Nimkar S.; Menaldino D.; Hannun Y. A.; Loomis C.; Bell R. M.; Tyagi S. R.; Lambeth J. D.; Stevens V. L.; Hunter R.; Liotta D. C. Stuctural Requirements for Long-Chain (Sphingoid) Base Inhibition of Protein Kinase C in Vitro and for the Cellular Effects of These Compounds. Biochemistry 1989, 28, 3138–3145. 10.1021/bi00434a004. [DOI] [PubMed] [Google Scholar]
- Pyne N. J.; Pyne S. Sphingosine 1-Phosphate and Cancer. Nat. Rev. Cancer 2010, 10, 489–503. 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Modrak D. E.; Gold D. V.; Goldenberg D. M. Sphingolipid Targets in Cancer Therapy. Mol. Cancer Ther. 2006, 5, 200–208. 10.1158/1535-7163.MCT-05-0420. [DOI] [PubMed] [Google Scholar]
- Plano D.; Amin S.; Sharma A. K. Importance of Sphingosine Kinase (SphK) as a Target in Developing Cancer Therapeutics and Recent Developments in the Synthesis of Novel SphK Inhibitors. J. Med. Chem. 2014, 57, 5509–5524. 10.1021/jm4011687. [DOI] [PubMed] [Google Scholar]
- Menaldino D. S.; Bushnev A.; Sun A.; Liotta D. C.; Symolon H.; Desai K.; Dillehay D. L.; Peng Q.; Wang E.; Allegood J.; Trotman-Pruett S.; Sullards M. C.; Merrill A. H. Sphingoid Bases and de Novo Ceramide Synthesis: Enzymes Involved, Pharmacology and Mechanisms of Action. Pharmacol. Res. 2003, 47, 373–381. 10.1016/S1043-6618(03)00054-9. [DOI] [PubMed] [Google Scholar]
- Humpf H.-U.; Schmelz E.-M.; Meredith F. I.; Vesper H.; Vales T. R.; Wang E.; Menaldino D. S.; Liotta D. C.; Merrill A. H. Acylation of Naturally Occurring and Synthetic 1-Deoxysphinganines by Ceramide Synthase. J. Biol. Chem. 1998, 273, 19060–19064. 10.1074/jbc.273.30.19060. [DOI] [PubMed] [Google Scholar]
- Symolon H.; Bushnev A.; Peng Q.; Ramaraju H.; Mays S. G.; Allegood J. C.; Pruett S. T.; Sullards M. C.; Dillehay D. L.; Liotta D. C.; Merrill A. H. Enigmol: A Novel Sphingolipid Analogue with Anticancer Activity against Cancer Cell Lines and In Vivo Models for Intestinal and Prostate Cancer. Mol. Cancer Ther. 2011, 10, 648–657. 10.1158/1535-7163.MCT-10-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier-Amblard E. C.; Mays S. G.; Arrendale R. F.; Baillie M. T.; Bushnev A. S.; Culver D. G.; Evers T. J.; Holt J. J.; Howard R. B.; Liebeskind L. S.; Menaldino D. S.; Natchus M. G.; Petros J. A.; Ramaraju H.; Reddy G. P.; Liotta D. C. Novel Synthesis and Biological Evaluation of Enigmols as Therapeutic Agents for Treating Prostate Cancer. ACS Med. Chem. Lett. 2011, 2, 438–443. 10.1021/ml2000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta D. C.; Holt J. J.; Natchus M. G.; Galinski M. R.; Baillie M. T.; Miller E. J.. Sphingosine Analogs, Compositions, and Methods Related Thereto. WO 2013/049280 A2, 2013.
- Bushnev A. S.; Baillie M. T.; Holt J. J.; Menaldino D. S.; Merrill A. H.; Liotta D. C. An Efficient Asymmetric Synthesis of Enigmols (1-Deoxy-5-Hydroxysphingoid Bases), an Important Class of Bioactive Lipid Modulators. ARKIVOC 2010, viii, 263–277. [Google Scholar]
- Yamazaki T.; Taguchi T.; Ojima I.. Fluorine in Medicinal Chemistry and Chemical Biology; Blackwell Publishing, Ltd: 2009. [Google Scholar]
- Böhm H.-J.; Banner D.; Bendels S.; Kansy M.; Kuhn B.; Müller K.; Obst-Sander U.; Stahl M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- Denmark S. E.; Yang S.-M. Intramolecular Silicon-Assisted Cross-Coupling Reactions: General Synthesis of Medium Sized Rings Containing a 1,3-Cis-Cis Diene Unit. J. Am. Chem. Soc. 2002, 124, 2102–2103. 10.1021/ja0178158. [DOI] [PubMed] [Google Scholar]
- Takai K.; Takagi T.; Baba T.; Kanamori T. Synthesis and Characterization of Partially Fluorinated Stearolic Acid Analogs: Effect of Their Fluorine Content on the Monolayer at the Air-Water Interface. J. Fluorine Chem. 2007, 128, 120–126. 10.1016/j.jfluchem.2006.10.014. [DOI] [Google Scholar]
- Reetz M. T. Synthesis and Diastereoselective Reactions of N,N-Dibenzylamino Aldehydes and Related Compounds. Chem. Rev. 1999, 99, 1121–1162. 10.1021/cr980417b. [DOI] [PubMed] [Google Scholar]
- Moreno M.; Murruzzu C.; Riera A. Enantioselective Synthesis of Sphingadienines and Aromatic Ceramide Analogs. Org. Lett. 2011, 13, 5184–5187. 10.1021/ol202064j. [DOI] [PubMed] [Google Scholar]
- Sato K.; Omote M.; Ando A.; Kumadaki I. Reactions of Ethyl Bromodifluoroacetate in the Presence of Copper Powder. J. Fluorine Chem. 2004, 125, 509–515. 10.1016/j.jfluchem.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Morikawa T.; Nishiwaki T.; Nakamura K.; Kobayashi Y. Studies on Organic Fluorine Compounds. LIV. Synthesis of 2,2-Difluoroarachidonic Acid. Chem. Pharm. Bull. 1989, 37, 813–815. 10.1248/cpb.37.813. [DOI] [Google Scholar]
- Iseki K.; Asada D.; Kuroki Y. Preparation of Optically Active α,α-Difluoro-β-Hydroxyketones. J. Fluorine Chem. 1999, 97, 85–89. 10.1016/S0022-1139(99)00033-0. [DOI] [Google Scholar]
- Chen K.-M.; Hardtman G. E.; Prasad K.; Repic O.; Shapiro M. J. 1,3-Syn Diastereoselective Reduction of β-Hydroxyketones Utilizing Alkoxydialkylboranes. Tetrahedron Lett. 1987, 28, 155–158. 10.1016/S0040-4039(00)95673-9. [DOI] [Google Scholar]
- Kuroboshi M.; Ishihara T. Diastereoselective Reduction of α,α-Difluoro β-Hydroxy Ketones to Syn- and Anti-2,2-Difluoro-1,3-Diols. Bull. Chem. Soc. Jpn. 1990, 63, 1185–1190. 10.1246/bcsj.63.1185. [DOI] [Google Scholar]
- Although the CF2-Enigmol Experiment Was Conducted Using a PEG400/Tween80 Vehicle as Opposed to Olive oil/EtOH, These Trends Held When Enigmol and CF3-Enigmol Were Formulated with PEG400/Tween80 (Supporting Information Figure 3).
- Huchet Q. A.; Kuhn B.; Wagner B.; Kratochwil N. A.; Fischer H.; Kansy M.; Zimmerli D.; Carreira E. M.; Muller K. Fluorination Patterning: A Study of Structural Motifs That Impact Physicochemical Properties of Relevance to Drug Discovery. J. Med. Chem. 2015, 58, 9041–9060. 10.1021/acs.jmedchem.5b01455. [DOI] [PubMed] [Google Scholar]
- O’Hagan D.; Wang Y.; Skibiński M.; Slawin A. M. Z. Influence of the Difluoromethylene Group (CF2) on the Conformation and Properties of Selected Organic Compounds. Pure Appl. Chem. 2012, 84, 1587–1595. 10.1351/PAC-CON-11-09-26. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.