Abstract
Viral infections of the central nervous system are often associated with seizures, and while patients usually recover from the infection and the seizures cease, there is an increased lifetime incidence of epilepsy. These viral infections can result in mesial temporal sclerosis, and, subsequently, a type of epilepsy that is difficult to treat. In previous work, we have shown that Theiler’s murine encephalomyelitis virus (TMEV) infections in C57B/6 mice, an animal model of virus-induced epilepsy, results in changes in excitatory currents of CA3 neurons both during the acute infection and two months later, at a time when seizure thresholds are reduced and when spontaneous seizures can occur. The changes in the excitatory system differ at these two time points, suggesting different mechanisms for seizure generation. In the present paper, we examine GABAergic mediated inhibition in CA3 pyramidal cells at these two time points following TMEV infection. We found that amplitudes of sIPSCs and mIPSCs were reduced during the acute infection, but recovered at the two-month time point. These observations are consistent with previous measurements of excitatory currents suggesting different mechanisms of seizure generation during the acute infection and during chronic epilepsy.
Keywords: Viral encephalitis, IPSCs, CA3, Mouse model, Mesial temporal sclerosis, Epilepsy
1. Introduction
Viral encephalitis is often associated with seizures and results in an increased risk of developing chronic epilepsy after recovery from infection [2,7,8,27]. The magnitude of the increased risk of chronic epilepsy is dependent on the occurrence of seizures during the acute infection. The 20-year risk of developing epilepsy is 22% in patients that suffered seizures during infection and 10% in patients that exhibited no seizures [2,27]. Viral encephalitis can cause mesial temporal sclerosis [27] and results in epilepsies that are particularly difficult to treat with traditional pharmacological therapies [18]. In order to better understand and to explore novel pharmacological therapies for virus-induced epilepsies, our laboratory uses the Theiler’s murine encephalomyelitis virus (TMEV) C57Bl/6 mouse model [15,22,24]. TMEV infected mice exhibit seizures during the acute period [15], develop mesial temporal sclerosis [26], and have an increased risk of chronic epilepsy following a latent period [24]. This constellation of symptoms closely mimics the human disease, making this model potentially useful for studying this often intractable form of epilepsy.
TMEV exhibits a strong tropism for the limbic region in C57BL/6 mice [15,24,25] causing dramatic cell death in the CA1 region and sparing CA3 [25]. A number of observations including the death of the CA1 region, results from other laboratories indicating CA3 as a potential source of seizures in temporal lobe epilepsies [16,17], increased expression of Fos following acute seizures, and the well known propensity of CA3 to initiate substantial synchronous behavior under normal [5,30] and pathological situations both in vivo and in vitro [10,19,29] led us to initially examine the CA3 region.
In our previous studies of the CA3 hippocampal region, we found increases in excitation as measured by spontaneous EPSCs (sEPSC) and miniature EPSCs (mEPSC) both during the acute infection and two months following infection [22]. However, the distribution of mEPSC amplitudes differed at these two time points with a shift to mEPSC amplitudes consistent with a strengthening of synapses on the recurrent CA3 collaterals [12], a strengthening associated with hyperexcitability in the CA3 region. This observation suggests different mechanisms for the seizures generated during infection and those that occur spontaneously after the latent period [8], and is consistent with studies using animal models of epilepsy that show different pharmacological sensitivities of seizures occurring during the initial insult and those seizures occurring after a latent period [1,11,23]. These results suggest that drugs effective in treating seizures during the acute infection may be quite different than drugs effective for the chronic epilepsy resulting from infection. To further explore network changes in the TMEV mouse model, we evaluated inhibitory currents recorded in CA3 neurons. We found an initial decrease of inhibition as measured by amplitude of spontaneous IPSCSs (sIPSCs) during the acute infection period; however, this reduction was eliminated at the chronic time point. These results further emphasize the differences in two CA3 neural circuits both potentially giving rise to seizures, one associated with the insult of infection and the second giving rise to reduced seizure thresholds and spontaneous seizures.
2. Methods
Male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME), 4–5 weeks old, were used for all experiments. Animals were kept on a 12-h light/dark cycle and allowed free access to food and water. All animal care and experimental manipulations were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Utah Institutional Animal Care and Use Committee.
Under isoflurane anesthesia, mice were injected in the posterior parietal cortex with either 2 × 104 plaque forming units of the DA strain of TMEV in a total volume of 20 μl sterile phosphate-buffered saline (PBS) for the experimental group or PBS alone for the control group as previously described [26]. Injections were performed with an insulin syringe and a 28-gauges needle. The needle was fitted with a plastic William’s collar to limit needle penetration through the skin, skull, and cortex to a total depth of 2 mm. Mice were then sacrificed either 4–7 days (acute) or 2 months post-injection for preparation of hippocampal brain slices for electrophysiology. Mice at the 2 month time point were not monitored for seizures, but are known to have lower seizure thresholds and can develop spontaneous recurrent seizures at this timepoint [24]. For TMEV-injected mice, only animals exhibiting seizures during the acute period were utilized for the electrophysiological experiments. Mice were anesthetized with pentobarbital (Nembutal) (25 mg/kg), brains were extracted, and hippocampal slices were cut in ice-cold sucrose solution to a thickness of 300 μm. After one hour of incubation in Ringer’s solution, whole-cell patch-clamp recordings were made of CA3 hippocampal neurons in Ringer’s solution CNQX (10 μM) and APV (50 μM) for recording IPSCs. Recordings were done at room temperature. The internal pipette solution for IPSCs was composed of (in mM): 130CsCl, 1 NaCl, 5 EGTA, 10HEPES, 1 MgCl2, 1CaCl2, 5 QX314, and 2 ATP. This creates a chloride equilibrium potential of approximately 1 mV; when the neuron is clamped at −70 mV the currents are inward [21]. Series (access) resistance values of <15 MΩ were used as selection criteria for accepting recordings and were not corrected for. Additionally, only recordings that did not exhibit substantial changes (<20%) in either holding current or input resistance after 20 min of time allowing for the internal pipette solution to equilibrate with the neuron were included in the study. After baseline activity was established, TTX (1 uM) was washed on for 15 min. Recordings of 3 min were made before and after TTX application. Spontaneous IPSC and miniature IPSC (mIPSCS) number, amplitude, rise time, and decay time were measured. Identification and measurement of EPSCs were done using the MiniAnalysis 6.0.1 program (Synaptosoft, Decatur, GA). The threshold for event detection was set at three times baseline root-mean-square noise. This threshold setting biased EPSC classification errors to false negatives, and visual inspection of analyzed traces determined the false-negative rate to be less than 3% of all EPSCs.
2.1. Statistical analysis
Cumulative distributions were constructed from the total number of mIPSCs recorded across all experiments for the various groups. The Kolmogorov–Smirnoff (KS) test was used to assess significant differences between cumulative distributions. For comparisons of averages between both injection type and time following infection, 2-way ANOVAs were used. Statistical tests were performed using Prism version 5, MATLAB, and Minianalysis.
3. Results
3.1. TMEV infection decreases sIPSC number acutely but sIPSC number recovers 2 months after infection
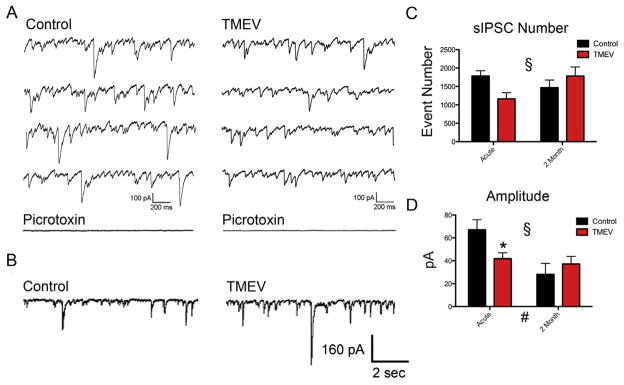
The majority of mice begin to exhibit focal seizures that secondarily generalize on days 3–4 following TMEV infection, after which seizure number and severity increase during the next few days and then resolve after 7–8 days following the injection [26]. Brain slices prepared for study of the acute infection period were prepared from mice 4–7 days post infection. A minimum of two months was allowed to pass post-infection for mice selected for the chronic time point. sIPSCs were isolated by bath application of the glutamate receptor antagonists CNQX (10 μM) and APV (50 μM) for a minimum of 20 min. Following whole-cell patch, an additional 20 min was allowed for the cesium-based internal solution to equilibrate with the CA3 neuron. Fig. 1A shows example traces of isolated sIPSCs recorded from both TMEV-injected and sham-injected animals from the acute period. Application of picrotoxin (50 μM) blocks the currents, verifying that the recordings are inhibitory (Fig. 1A bottom traces). Fig. 1B shows example control and TMEV traces for the chronic period.
Fig. 1.
Amplitudes of spontaneous IPSCs decrease during acute infection, but recover during the time in which seizure thresholds are reduced and spontaneous seizures can occur. (A) Example traces of sIPSCs recorded during acute infection and (bottom) traces verifying picrotoxin blocks IPSCs. (B) Example traces recorded 2 months following infection. (C) There were no significant differences in sIPSC number between control and TMEV-infected animals at either of the two time points examined (ACUTE: control, N = 9; TMEV, N = 12; mean ± SEM: control 1786 ± 145, TMEV 1162 ± 170; t = 2.32, Bonferroni post-hoc test p > 0.05; CHRONIC: control, N = 7; TMEV = 10; chronic mean ± SEM: control 1467 ± 208, TMEV 1784 ± 245; t = 1.06, Bonferroni post-hoc test p > 0.05), however, there was a significant interaction (ANOVA; # p = 0.026). (D) There was a significant reduction of sIPSC amplitudes at the acute time point that recovered chronically (mean ± SEM: control 28.1 ± 9.5 pA, TMEV 37.3 ± 6.5 pA; Bonferroni post-hoc test, t = 0.809, p > 0.05). There was a significant interaction (ANOVA, § p = 0.027) and an overall effect of time (ANOVA, # p = 0.0061).
ANOVA’s were performed on the data sets (sIPSC frequency and sIPSC amplitude) with injection (saline versus TMEV) and time (acute versus 2 months) forming the two groups. Using the Bonferroni post-hoc test, no difference in sIPSC frequency was found at either the acute (Fig. 1C: control, N = 9; TMEV, N = 12; acute mean ± SEM: control 9.92 ± 0.81, TMEV 6.46 ± 0.95; t = 2.32, p > 0.05) or chronic (Fig. 1C: control, N = 7; TMEV, N = 10; chronic mean ± SEM: control 8.15 ± 1.16, TMEV 9.91 ± 1.36; t = 1.06, p > 0.05) time points. However, there was a significant interaction between the two groups (ANOVA; §p = 0.026, Fig. 1C) indicating a change in the effect of TMEV infection depending on the time point (p = 0.025). Examining sIPSC amplitudes, a significant reduction was found at the acute time point (Fig. 1D: acute mean ± SEM: control 67.1 ± 8.9 pA, TMEV 41.9 ± 5.1 pA; Bonferroni post-hoc test, t = 2.579, p < 0.05), but this reduction was rendered invariant at the chronic time point (Fig. 1D: chronic mean ± SEM: control 28.1 ± 9.5 pA, TMEV 37.3 ± 6.5 pA; Bonferroni post-hoc test, t = 0.809, p > 0.05), which as sIPSC events, resulted in a significant interaction (ANOVA, p = 0.027) between the two groups. There was also an overall effect of time (ANOVA #, p = 0.0061, Fig. 1D) meaning a decrease in amplitudes in neurons recorded in slices from both groups as the mice mature; an effect we also saw previously examining EPSCs [22]. Overall, both the significant interaction on sIPSC number between groups and amplitude changes indicate different changes in the CA3 inhibitory system following TMEV infection at these two time points.
3.2. TMEV infection decreases mIPSC amplitude acutely
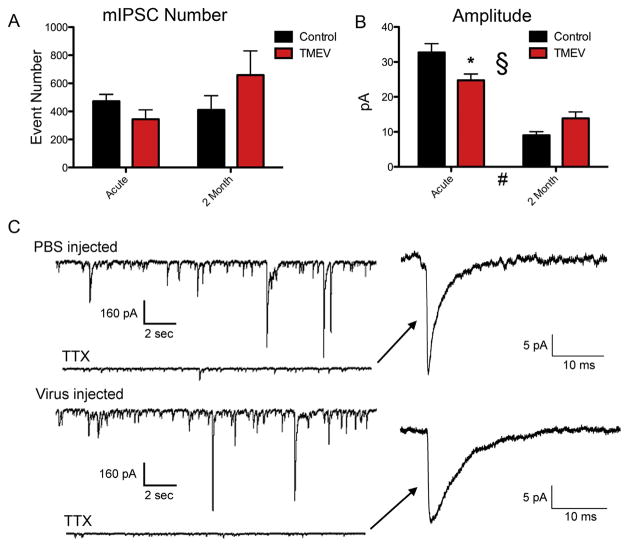
To examine possible changes at the synapse, we isolated mIPSCs with bath application of TTX (1 μM) for 10 min with continued application of APV and CNQX. Fig. 2C shows example traces from the chronic time point before and after application of TTX. Again, ANOVA statistics were applied to amplitudes and number of mIPSCs. Analogously to the results on sIPSCs, no significant differences were found using Bonferroni post-hoc tests in mIPSC frequency (Fig. 2A; acute mean ± SEM: control 2.62 ± 0.28 Hz, TMEV 1.91 ± 0.38 Hz; t = 0.859, p > 0.05; chronic mean ± SEM: control 2.28 Hz ± 0.57 Hz, TMEV 3.66 ± 0.96 Hz; t = 1.50, p > 0.05) but mIPSC amplitudes demonstrated a decrease in amplitude acutely with a recovery at 2 months (Fig. 2B; acute mean ± SEM: control 32.7 ± 2.4 pA, TMEV 24.7 ± 1.8 pA; t = 2.98, p < 0.05; chronic mean ± SEM: control 9.0 ± 1.0 pA, TMEV 13.8 ± 1.9 pA; t = 1.62, p > 0.05). Input resistances between controls and TMEV infected mice were not different at either time point (acute mean ± SEM: control 69.2 ± 8.5 MOhms, TMEV 80.1 ± 6.9 MOhms; p = 0.32; chronic mean ± SEM: control 82.4 ± 10.5 MOhms, TMEV 94.6 ± 14.5 MOhms; p = 0.54). This differing response in mIPSC amplitude at the two time points resulted in a significant interaction between the groups, that is, the effect of TMEV infection on mIPSC amplitude changed depending on the time period after infection, acute or two month (ANOVA, § p = 0.003). Additionally and similarly to the sIPSCs in this study and previous work on mEPSCs [22], there was an overall effect of time on mIPSC amplitude (ANOVA, # p < 0.0001).
Fig. 2.
Miniature IPSC amplitudes are decreased during acute infection, but recover during the time in which seizure thresholds are reduced and spontaneous seizures can occur. (A) There were no significant differences at either time point in mIPSC frequency (B) there was a significant reduction in mIPSC amplitude at the acute time point (mean ± SEM: control 32.7 ± 2.4 pA, TMEV 24.7 ± 1.8 pA; t = 2.98, Bonferroni post-hoc test, p < 0.05) that was no longer observed at the chronic time point (mean ± SEM: control 9.0 ± 1.0 pA, TMEV 13.8 ± 1.9 pA; t = 1.62, Bonferroni post-hoc test, p > 0.05). There was a significant interaction (ANOVA, # p = 0.003) and overall effect of time (ANOVA, § p = 0.0001). (C) Example traces from sham injected and TMEV injected mice at the chronic time point and the traces after application of TTX. (Right) Expanded traces of mIPSCs.
3.3. TMEV infection alters the distribution of mIPSC kinetics
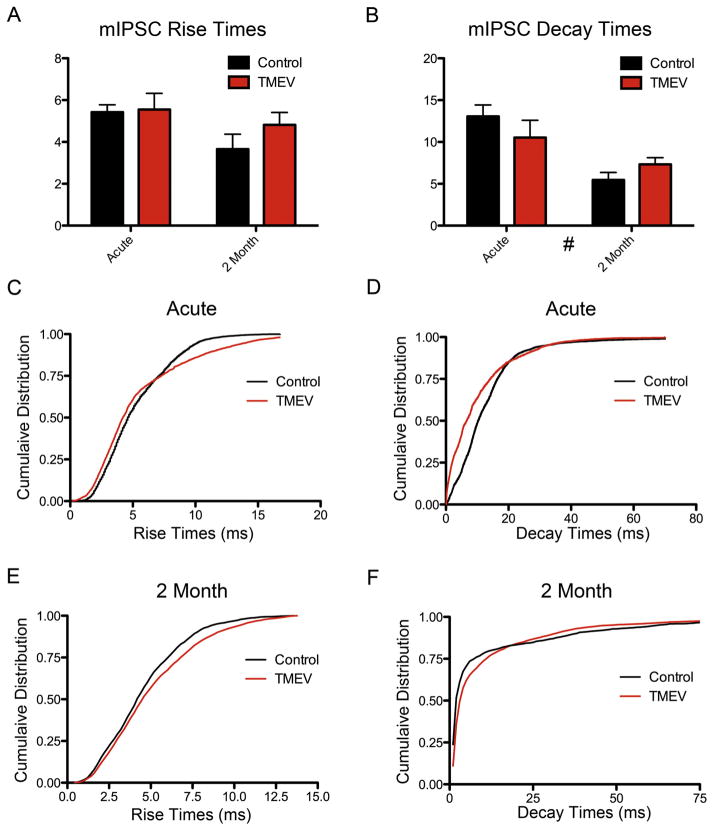
Example traces of mIPSCs suggested a change in current kinetics occurred following infection (Fig. 2C). To test this possibility, ANOVA statistics were applied to average mIPSC rise and decay times, and the Kolmogorov–Smirnoff test was applied to distributions of mIPSCs amplitudes. Bonferroni post-hoc tests following ANOVA revealed no change in mIPSC average amplitudes between control and TMEV-infected animals either acutely (Fig. 3A; rise time mean ± SEM: control 5.42 ± 0.35 ms, TMEV 5.55 ± 0.77 ms; t = 0.142, p > 0.05; decay time mean ± SEM: control 13.1 ms ± 1.36 Hz, TMEV 10.5 ± 2.07 ms; t = 1.19, p > 0.05) or at the 2 month time point (Fig. 3B; rise time mean ± SEM: control 3.66 ± 0.71 ms, TMEV 4.82 ± 0.59 ms; t = 1.16, p > 0.05; decay time mean ± SEM: control 5.45 Hz ± 0.90 ms, TMEV 7.33 ± 0.79 ms; t = 0.79, p > 0.05). There was a statistically significant overall decrease in mIPSC decay times over time (# p = 0.0018), that is, both groups at the two-month time point together had faster decay times than both groups together at the acute time point, coincident with the observed decrease in mIPSC amplitudes in the older mice (Fig. 2).
Fig. 3.
IPSC rise and decay times have altered cumulative distributions following TMEV infection at both time points, but these changes do not result in changes in average rise and decay times. Bonferroni post-hoc tests revealed no change in average (A) rise times or (B) decay times between control and TMEV-infected animals either acutely or at the 2 month time point. Kolmogorov–Smirnoff tests determined significant differences in the cumulative distributions of events in TMEV infected animals compared to controls in (C) rise times during the acute infection, (D) decay times during the acute infection, and (F) decay times at the 2 month time point. The rightward shift in the cumulative distribution at the more frequent amplitudes is consistent with the perceived lengthening of decay times in the average mIPSC trace at the 2 month time point in Fig. 2C. However, no differences in rise times at the 2 month time point were found.
The cumulative distributions of the rise and decay times were also examined using the Kolmogorov–Smirnoff test. Normalized cumulative distributions of rise times at the acute time point were statistically different between controls and TMEV-infected mice (p < 0.0001; Fig. 3C), but were not different at the 2 month time point (p = 0.674; Fig. 3E). Cumulative distributions of decay times between control and TMEV-infected mice are statistically different for both the acute (p < 0.0001; Fig. 3D) and 2 month (p < 0.0001; Fig. 3F) time points.
4. Discussion
TMEV-infected mice exhibit seizures during the acute infection period, as well as spontaneous recurrent seizures after a latent period of 1–2 months [24], analogous to what is seen in patients following CNS infections. In previous work examining excitatory synaptic transmission in CA3 pyramidal cells, we found that these two periods are associated with differential changes in excitability indicated by shifts in mEPSC amplitudes at the two time points, suggesting differences in the CA3 networks contributing to seizures [22]. This same study also showed enhanced numbers and amplitudes of sEPSCs at both time points. In the present study, we examined the inhibitory networks in the CA3 region and found that sIPSC amplitudes were significantly reduced during the acute infection period, but rendered invariant during the period of reduced seizure thresholds, when chronic epilepsy may also be observed. The excitatory and inhibitory work taken together provides a potential explanation for the much greater seizure number seen during the acute period relative to the chronic time point [24,26]. The excitatory/inhibitory imbalance is more severe during the acute period.
There is an overall effect of time on the amplitudes of the mIPSCs, that is mIPSC amplitudes were reduced for both control and TMEV infected mice at the two month time point. We saw a similar overall reduction in mEPSCs amplitudes in a previous study as well [22], which is predicted by the need for homeostatic excitatory/ inhibitory balance [6]. The decrease in amplitudes over time has been observed in normal rats and seems to be a simple consequence of maturing [20]. The continued maturation of the mice after initial infection raises the concern that the mice might respond differently to a later infection, but we have seen the same disease progression when the TMEV infection occurs at three months [14]. There was a reduction of mIPSC amplitude in TMEV infected mice relative to controls at the acute, but not at the two month time point. No change in frequency was observed. This is different from similar studies looking at granule cell mIPSCs in kainate-treated [21] and pilocarpine-treated rats in the week following treatment, a period closer to the acute time point [13]. Both studies saw a decrease in frequency associated with inhibitory cell loss, and there was also a reduction in mIPSC amplitude in the pilocarpine-treated rats. There was no change in frequency of mIPSCs in the TMEV model at either time point, which suggests that inhibitory cell loss may not be as dominant a factor as in the other models of epilepsy. The reduction in mIPSC amplitude could be due to a reduction of receptor number or to changes in GABA receptor subunits [3,9]. While the pattern of mIPSC changes seen in the TMEV animal model are different to varying degrees compared to similar studies in other animal models of epilepsy [13,21], it is analogous to anatomical changes in inhibitory synapses onto granule cells in a pilocarpine treated rat model of epilepsy [28]. This study examined inhibitory synapse at two time points, 5 days and over two months following pilocarpine treatment. At the 5 day time point, a decrease in inhibitory synapse number was measured, but at the later time point inhibitory synapses had rebounded to numbers greater than controls [28]. However, while the pattern of changes in this study is analogous to the pattern seen in the TMEV model, the absence of an increase in sIPSCs or mIPSCs in the TMEV model suggests inhibitory cell death is not a major factor.
The difference in rise and decay time distributions and lack of difference in the averages suggests these changes likely reside in particular time ranges, similar to the changes in mEPSC amplitudes seen in previous work [22]. This can be observed in the cumulative distributions where the control and TMEV curves pull farther apart relative to the rest of the plot. Further work will be needed to determine if these changes result from potential GABA subunit changes [3,4] or alterations in the relative abundance of anatomically different types of synapses onto the CA3 neurons [12,22]. In the latter case, the location of the synapse on the dendritic arbor affects the kinetics of the mIPSCs.
Different animal models of epilepsy can exhibit different patterns of change in inhibitory circuits [21,31] and changes in pharmacological sensitivity of seizures can change over time in the same model [11,22,23]. These observations suggest that attention must be paid to the current state of epileptic networks when determining treatments. The present study also finds changes in the CA3 inhibitory network at two different time points, reduced inhibition during acute infection and recovery during the period in which seizure thresholds are reduced and spontaneous seizures can again be observed. That these two periods where seizures can occur are separated by a lengthy latent period suggests that this period, a period of transition between two excitable states, is deserving of further study.
HIGHLIGHTS.
A mouse model of virus-induced epilepsy is used to explore inhibitory circuit changes.
IPSC amplitudes are reduced during acute infection but recover at the chronic condition.
mIPSC amplitudes are similarly reduced during the acute infection but not chronically.
mIPSC kinetics demonstrate subtle changes following TMEV infection.
Acknowledgments
The authors would like to acknowledge the financial support of Robert and Joyce Rice (Salt Lake City, UT), The Margolis Foundation (Salt Lake City, UT), Citizens United for Research in Epilepsy (HSW and RSF), National Institutes of Health1R01NS065714 (RSF), The Dumke Foundation (KSW) and National Institutes of HealthR01 NS 065434 (KSW & HSW).
Footnotes
Disclosure
Dr. Smeal, Dr. Fujinami, and Dr. Wilcox have no disclosures.
Dr. White reports serving on the Scientific Advisory Board for Upsher-Smith Laboratories and Insero Health. He also reports serving as a consultant for Takeda Pharmaceuticals. Dr. White receives research funding from the NINDS, NIH and is Research Director for Citizen’s United for Research in Epilepsy (CURE). Dr. White is a scientific co-founder for NeuroAdjuvants, Inc.
References
- 1.Anderson WW, Swartzwelder HS, Wilson WA. The NMDA receptor antagonist 2-amino-5-phosphonovalerate blocks stimulus train-induced epileptogenesis but not epileptiform bursting in the rat hippocampal slice. J Neurophysiol. 1987;57:1–21. doi: 10.1152/jn.1987.57.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38:1407. doi: 10.1212/wnl.38.9.1407. [DOI] [PubMed] [Google Scholar]
- 3.Brooks-Kayal A, Shumate M, Jin H, Rikhter T, Coulter D. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Kayal AR, Raol YH, Russek SJ. Alteration of epileptogenesis genes. Neurotherapeutics. 2009;6:312–318. doi: 10.1016/j.nurt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 6.Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 7.Eeg-Olofsson O. Virological and immunological aspects of seizure disorders. Brain Dev. 2003;25:9–13. doi: 10.1016/s0387-7604(02)00162-6. [DOI] [PubMed] [Google Scholar]
- 8.Getts DR, Balcar VJ, Matsumoto I, Muller M, King NJ. Viruses and the immune system: their roles in seizure cascade development. J Neurochem. 2008;104:1167–1176. doi: 10.1111/j.1471-4159.2007.05171.x. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez MI, Brooks-Kayal A. Altered GABA(A) receptor expression during epileptogenesis. Neurosci Lett. 2011;497:218–222. doi: 10.1016/j.neulet.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hablitz JJ. Picrotoxin-induced epileptiform activity in hippocampus: role of endogenous versus synaptic factors. J Neurophysiol. 1984;51:1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- 11.Hellier JL, White A, Williams PA, Dudek FE, Staley KJ. NMDA receptor-mediated long-term alterations in epileptiform activity in experimental chronic epilepsy. Neuropharmacology. 2009;56:414–421. doi: 10.1016/j.neuropharm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henze DA, Card JP, Barrionuevo G, Ben-Ari Y. Large amplitude miniature excitatory postsynaptic currents in hippocampal CA3 pyramidal neurons are of mossy fiber origin. J Neurophysiol. 1997;77:1075–1086. doi: 10.1152/jn.1997.77.3.1075. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, Fujinami RS. Seizures following Picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- 16.Lothman EW. Seizure circuits in the hippocampus and associated structures. Hippocampus. 1994;4:286–290. doi: 10.1002/hipo.450040311. [DOI] [PubMed] [Google Scholar]
- 17.McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misra UK, Kalita J, Nair PP. Status epilepticus in central nervous system infections: an experience from a developing country. Am J Med. 2008;121:618–623. doi: 10.1016/j.amjmed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Schwartzkroin PA, Prince DA. Penicillin-induced epileptiform activity in the hippocampal in vitro prepatation. Ann Neurol. 1977;1:463–469. doi: 10.1002/ana.410010510. [DOI] [PubMed] [Google Scholar]
- 20.Shao LR, Dudek FE. Both synaptic and intrinsic mechanisms underlie the different properties of population bursts in the hippocampal CA3 area of immature versus adult rats. J Physiol. 2009;587:5907–5923. doi: 10.1113/jphysiol.2009.179887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao LR, Dudek FE. Changes in mIPSCs and sIPSCs after kainate treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol. 2005;94:952–960. doi: 10.1152/jn.01342.2004. [DOI] [PubMed] [Google Scholar]
- 22.Smeal RM, Stewart KA, Iacob E, Fujinami RS, White HS, Wilcox KS. The activity within the CA3 excitatory network during Theiler’s virus encephalitis is distinct from that observed during chronic epilepsy. J Neurovirol. 2012;18:30–44. doi: 10.1007/s13365-012-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stasheff SF, Anderson WW, Clark S, Wilson WA. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science. 1989;245:648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- 24.Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69:1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart KA, Wilcox KS, Fujinami RS, White HS. Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia. 2010;51:1418–1428. doi: 10.1111/j.1528-1167.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- 26.Stewart KA, Wilcox KS, Fujinami RS, White HS. Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia. 2009;51(8):1418–1428. doi: 10.1111/j.1528-1167.2009.02405.x. August 2010 (epub 2009) [DOI] [PubMed] [Google Scholar]
- 27.Theodore WH. Epilepsy and viral infections. Epilepsy Curr. 2014;14:35–42. doi: 10.5698/1535-7511-14.s2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol. 2010;518:647–667. doi: 10.1002/cne.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong RK, Traub RD. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2–CA3 region. J Neurophysiol. 1983;49:442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]
- 30.Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]