Abstract
A series of critical pathways are responsible for the detection, signaling and restart of replication forks that encounter blocks during S-phase progression. Small base lesions may obstruct replication fork progression and processing, but the link between repair of small lesions and replication forks is unclear. In this study, we investigated a hypothesized role for DNA-PK, an important enzyme in DNA repair, in cellular responses to DNA replication stress. The enzyme catalytic subunit DNA-PKcs was phosphorylated on S2056 at sites of stalled replication forks in response to short hydroxyurea treatment. Using DNA fiber experiments, we found that catalytically active DNA-PK was required for efficient replication restart of stalled forks. Further, enzymatically active DNA-PK was also required for PARP-dependent recruitment of XRCC1 to stalled replication forks. This activity was enhanced by preventing Mre11-dependent DNA end resection, suggesting that XRCC1 must be recruited early to an unresected stalled fork. We also found that XRCC1 was required for effective restart of a subset of stalled replication forks. Overall, our work suggested that DNA-PK and PARP-dependent recruitment of XRCC1 is necessary to effectively protect, repair, and restart stalled replication forks, providing new insight into how genomic stability is preserved.
Keywords: DNA-PKcs, XRCC1, stalled replication fork, replication restart
INTRODUCTION
The integrity of DNA replication forks is essential for the maintenance of genomic stability. It is becoming increasingly clear that a variety of lesions can be produced at stalled replication forks, and that these may trigger various responses. Multiple cellular pathways are required to ensure faithful duplication of the genome in an intricate network of cellular events that detect, signal and repair any lesions that will impede the process of DNA replication. Firstly, cells can activate pathways that will utilise low fidelity polymerases that allow replication to bypass the genotoxic stress in a process termed trans-lesion synthesis, for subsequent post replication repair (1,2). Secondly, when the blocking lesion is too great, forks collapse forming a single DNA double-strand end that is generally repaired by homologous recombination (HR) (3–5), but which can also be inappropriately joined to a single DNA end from another collapsed fork resulting in replication stress induced copy number variation as demonstrated by Glover and colleagues (6). Finally, the cell can pause replication while the lesion is repaired and then restart replication from the same place. The molecular mechanisms behind translesion synthesis and replication induced DNA double-strand break repair have been relatively well studied but the processes behind replication restart remain ill-defined (2,7).
Small base lesions are very common spontaneous lesions and may obstruct replication fork progression (8) as well as processing of stalled replication forks. However, to date there is no established link between repair of small lesions and replication forks. In repair of small lesions, PARP1 has been demonstrated to interact with and recruit the DNA repair protein, XRCC1, to sites of DNA single-strand breaks (SSBs) (9,10). XRCC1 has been shown to be a scaffold protein that participates in both the SSB repair and the base excision repair (BER) pathways (11,12), while information on its potential role in S phase cells is lacking.
DNA-PKcs is an early component of the NHEJ pathway which repairs DNA double strand breaks by a relatively rapid and simple mechanism that essentially ligates severed DNA ends with comparatively low fidelity (13,14). While cells deficient in components of the NHEJ pathway display a marked hypersensitivity to agents that cause DNA double-strand breaks, it has also been reported sensitive to fork stalling agents in hamster cell lines (3,5,15). To examine a potential role for DNA-PKcs in mechanisms that alleviate genotoxic stress caused by fork stalling, we assessed the functional role of DNA-PKcs and its phosphorylation in living cells treated with a replication inhibitor, hydroxyurea (HU) (16).
Here, we demonstrate that 1) DNA-PKcs is phosphorylated at S2056 as a consequence of fork stalling; 2) XRCC1 is recruited by PARP1 and DNA-PKcs at stalled replication forks; and 3) XRCC1’s recruitment to stalled forks is important for effective replication restart. This establishes one of the first link between XRCC1-mediated repair and replication repair.
Materials and Methods
Cell Lines and Reagents
V-C8 and V-C8+B2 cell lines were kindly provided by Malgorzata Z. Zdzienicka (N. Copernicus University, Bydgoszcz, Poland)(17). EM9-V, EM9-XH and EM9-CKM cell lines were kindly provided by Keith Caldecott (Sussex). V3 derived cells were grown in α-MEM (gibco) supplemented with 10 % FCS, penicillin-streptomycin, G418 and puromycin. All these Chinese hamster ovary cell lines and a human osteosarcoma cell line U2OS were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FCS at 37°C under an atmosphere containing 5% CO2. All cell lines were authenticated by single tandem repeat analysis and grown up to a maximum of 20 passages and for less than 4 months following resuscitation. All cell lines were routinely tested for morphology, doubling times, as well as being mycoplasma free by verification using the Mycoprobe Mycoplasma Detection Kit (R&D Systems). The GFP-XRCC1 construct was from Valérie Schreiber (Strasbourg). Olaparib (10 µM, otherwise indicated) was purchased from Selleck Chemicals. Mre11 nuclease activity inhibitor Mirin (100 µM, otherwise indicated), CK2 inhibitor TBB (10 µM, otherwise indicated) and DNA-PKcs inhibitor NU7026 (10 µM, otherwise indicated) were purchased from Sigma.
Immunofluorescence
Following the indicated treatment, cells were fixed and stained as previously described (18). The primary antibodies used were XRCC1 (Abcam), phospho-specific DNA-PKcs S2056 (AbCam), Cyclin A (Santa Cruz), γH2AX (Millipore), RPA (Neomarkers), 53BP1 (Bethyl). The secondary antibodies were AlexaFluor 555-conjugated goat anti-mouse IgG (Molecular Probes). DNA was counterstained with DAPI.
High throughput microscopy
For high throughput microscopy, cells were seeded in 96 well plates (10 000 cells / well), fixed in 4% Formaldehyde + 0.5% Triton X-100 for 20 min and permeabilized with 0.5% Triton X-100 for 10 min. For quantitative microscopy, images were taken with an Image Xpress (Molecular Devices) microscope using a 20× lens. Foci numbers per cell were counted using Cell Profiler (Broad Institute) software and plotted using Microsoft Excel.
Western blotting
Cells were lysed in RIPA buffer in the presence of 1 × protease inhibitor cocktail (Sigma). An aliquot of 50 µg total protein was run on an SDS-PAGE gel and transferred to Hybond ECL membrane (Amersham Pharmacia). This membrane was immunoblotted with XRCC1 (Abcam), RAD51 (Santa Cruz), Ku70 (NeoMarkers), Ku80 (NeoMarkers), Tubulin (Sigma), DNA-PK (Calbiochem) and GRB2 (BD Transduction Laboratories) antibodies in 5% Milk overnight. Immunoreactive proteins were visualized using ECL reagents (Roche) following the manufacturer’s instructions.
Toxicity assay
Two hundred cells were plated in duplicate into six-well plates overnight before the addition of indicated treatment. Seven to ten days later, when colonies could be observed, cells were fixed and stained with methylene blue in methanol (4 g/L). Colonies consisting of >50 cells were subsequently counted.
Pull-down of GFP-XRCC1 protein and its interaction partners
Extracts from ~1 × 108 cells were prepared by resuspension and incubation of the cell pellet in 2 ml lysis buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 2 mM PMSF, 0.5% NP40, 1× mammalian protease inhibitor mix) for 30 min on ice. After centrifugation, supernatants were diluted to 5 ml with immunoprecipitation buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA). Extracts were incubated with 5 µg of a GFP-Trap®_A beads (GFP nanotrap; Chromotek, Germany) for 2 h at 4°C with constant mixing (19). GFP-XRCC1 and its interacting proteins were pulled down by centrifugation at 2700g. The beads were washed twice with 5 ml of wash buffer (20 mM Tris–HCl pH 7.5, 300 mM NaCl, 0.5 mM EDTA) and were resuspended in 2× SDS-Sample buffer and boiled at 95 °C for 10 min. Beads was collected by centrifugation and the supernatant were run on an SDS-PAGE gel.
Mass spectrometry analysis
Gel slices were subjected to in-gel trypsinolysis as described (20). The analysis of digested material was performed by LC-MS/MS using an Waters Q-Tof Premier mass spectrometer (Waters) coupled to a nano-UPLC system (NanoAcquity, Waters) using a reversed phase 75 um × 250 mm column as described (21). MS/MS spectra were searched against a UniProt SwissProt Human database (v2011.11.18, 20,326 sequences) in Mascot v2.3.01, allowing two missed cleavage and 50 ppm / 0.1 Da mass deviations in MS / MSMS, respectively. Carbamidomethylation of cysteine was a fixed modification. Oxidation of methionine, deamidation of asparagines and glutamine and phosphorylation of serine and threonine were used as variable modifications.
siRNA treatment
siRNA against DNA-PKcs was purchased from QIAGEN (Hs_PRKDC _5). A non-targeting control “All stars negative control” was used from QIAGEN. Cells were transfected into U2OS cells using Oligofectamine (Invitrogen) according to the manufacturer’s instructions. In brief, cells were double transfected 24 hours apart. Cells were incubated for 72 hours after the initial transfection prior to further analysis.
DNA fibre assay
Cells were pulse-labeled with 25 µM CldU and 250 µM IdU as indicated. Labelled cells were harvested and DNA fibre spreads prepared as described earlier (22). For immunodetection of CldU-labelled tracts, acid-treated fibre spreads were incubated with rat anti-BrdU monoclonal antibody (AbD Serotec) that recognises CldU, but not IdU for 1 hour at room temperature. Slides were fixed with 4% formaldehyde and incubated with an AlexaFluor 555-conjugated goat anti-rat IgG (Molecular Probes) for 1.5 hour at room temperature. IdU-labelled patches were detected using a mouse anti-BrdU monoclonal antibody (Becton Dickinson) that recognises IdU, but not CldU over night at 4 °C, followed by an AlexaFluor 488-conjugated goat anti-mouse F(ab’)2 fragment (Molecular Probes) for 1.5 hour at room temperature. Fibres were examined using a using a Biorad Radiance confocal microscope using a 60 × (1.3NA) lens. The lengths of CldU (AF 555, red) and IdU (AF 488, green) labelled patches were measured using the ImageJ software, and µm values were converted into kb using the conversion factor 1 µm=2.59 kb (22). Replication structures were quantified using the Cell Counter Plug-in for ImageJ (Kurt De Vos, University of Sheffield, UK).
Statistical analysis
The paired one-tailed student’s t test was used for statistical analyses (p ≥ 0.05: n.s.; p < 0.05: *; p < 0.01: **; p < 0.001: ***).
RESULTS
XRCC1 is recruited by PARP to un-resected replication forks to protect fork stability and to promote survival
We have shown previously that PARP plays an important role in repairing replication fork damage by mediating restart and protecting stalled DNA replication forks (18,23). Here, we investigated whether PARP can also recruit XRCC1 to stalled DNA replication forks. As can be seen, XRCC1 nuclear foci are induced following replication stress that results from exposure to hydroxyurea (HU) (Figure 1A–B). Next, cells were labelled with 5-ethynyl-2'-deoxyuridine (EdU) 20 min prior to HU treatment to mark the location of stalled DNA replication forks, and then stained for XRCC1. More than 80% of XRCC1 foci co-localized with sites of DNA replication, as defined by EdU incorporation (Figure 1B). These data indicate that XRCC1 is recruited to sites of stalled replication forks. HU-treated cells were also co-stained for the Cyclin A protein to specifically identify S/G2 phase cells; XRCC1 foci were present only in the Cyclin A-positive cells (Figure 1C), confirming that XRCC1 foci only form in replicating cells after HU treatment.
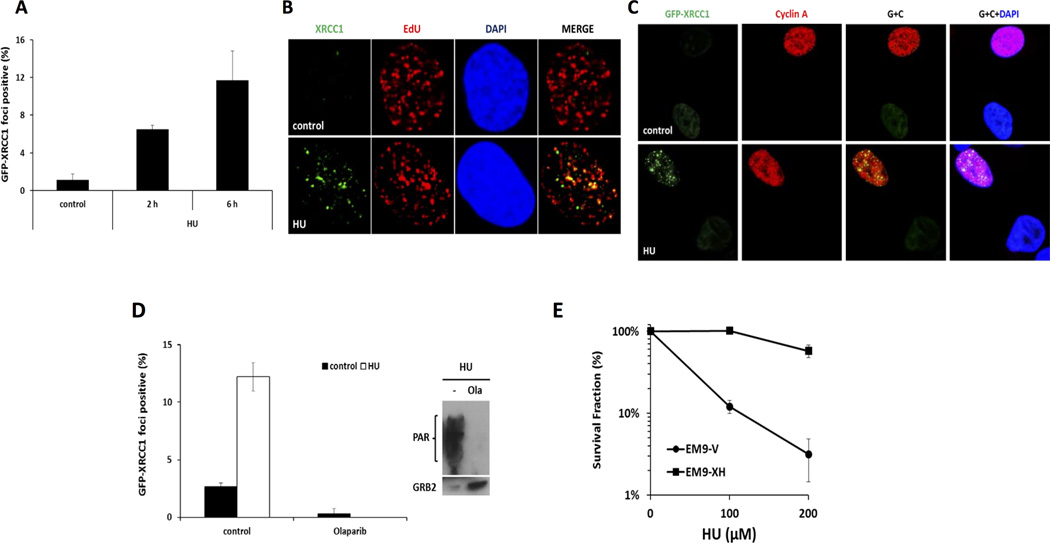
Figure 1. PARP recruits XRCC1 to stalled replication forks.
(A) Quantification of GFP-XRCC1 foci following 2mM HU treatment for 2 or 6 h in U2OS cells. (B) Immunofluorescence staining of endogenous XRCC1 and EdU following treatment of 2 mM HU for 6 h in U2OS cells. (C) Immunofluorescence staining of GFP-XRCC1 and Cyclin A following treatment of 2 mM HU for 6 h in U2OS cells. (D) Left, quantification of GFP-XRCC1 foci following indicated treatment in U2OS cells for 6 h. Right, Western blot analysis of PAR and GRB2 following indicated treatment in U2OS cells for 6 h.. (E) Clonogenic survival following 24 hours treatment with increasing concentrations of HU EM9-V or EM9-XH cells.
GFP-XRCC1 focus formation was analyzed following a combined treatment with HU and the PARP inhibitor olaparib (24). We found that PARP activity was required for HU-induced XRCC1 focus formation (Figure 1D). Furthermore, clonogenic assays were performed with the XRCC1 deficient CHO cell strain EM9. EM9-V cells (vector only transfected) are hyper-sensitive to HU as compared to the EM9 cells complemented with an XRCC1 expression construct (Figure 1E). We conclude that XRCC1 is required for the survival of cells in response to replication stalling.
Next, we asked whether the Mre11 nuclease affects XRCC1’s accumulation or retention at stalled replication forks. When Mre11 nuclease activity was inhibited by the Mre11 inhibitor, Mirin (25), increased numbers of XRCC1 foci were observed (Figure 2A); moreover, induction of HU-induced XRCC1 foci was potentiated by the inhibition of Mre11’s nuclease activity (Figure 2B). These data suggest that Mre11-induced processing of stalled forks can suppress XRCC1 relocalization to replication lesions. The induction of XRCC1 foci following loss of Mre11 activity was fully dependent on PARP activity (Figure 2C).
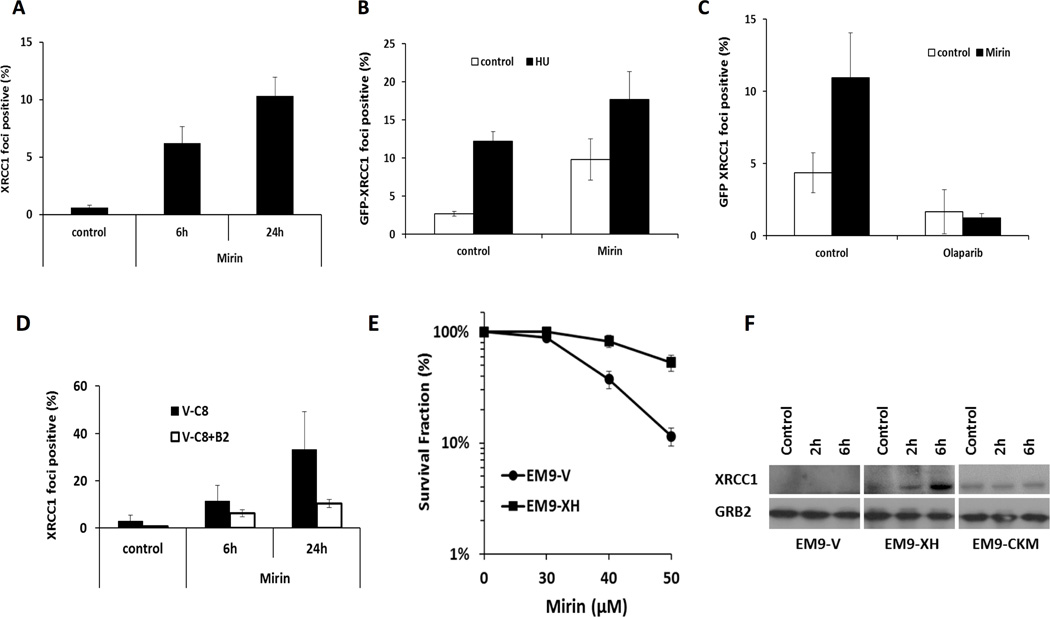
Figure 2. Recruitment of XRCC1 to un-resected forks promotes cell survival.
(A) Quantification of endogenous XRCC1 foci following treatment with 100 µM Mirin for 6 or 24 h in U2OS cells. (B, C) Quantification of GFP-XRCC1 foci following indicated treatment for 6 h in U2OS cells. (D) Quantification of endogenous XRCC1 foci following treatment with 100 µM Mirin for 6 or 24 h in V-C8 or V-C8+B2 cells. (E) Clonogenic survival following continuous treatment with increasing concentrations of Mirin in EM9-V or EM9-XH cells. (F) Western blot analysis of XRCC1 and GRB2 following treatment of 2mM HU in EM9-V, EM9-XH or EM9-CKM cells.
It has been reported that Mre11-mediated resection of stalled replication forks was up-regulated in the absence of BRCA2 (23). Here, the localization of XRCC1 in BRCA2 defective V-C8 and BRCA2 complemented V-C8+B2 cell lines was analyzed. In accordance with the previously reported hyper-activation of PARP activity, XRCC1 foci were observed in BRCA2 defective cells without exposure to DNA damaging agents (Figure 2D). No obvious difference in the level of XRCC1 foci was found in V-C8 and V-C8+B2 cells following HU treatment for 24 hours (Supplementary Figure 1A), suggesting that BRCA2 does not play a direct role in the recruitment of XRCC1 protein to damage in response to replication stalling. However, the induction of XRCC1 by inhibition of Mre11 was dramatically enhanced in the absence of functional BRCA2 (Figure 2D). These data suggest that in the absence of BRCA2 and Mre11’s nuclease activity, there is an increase in un-resected stalled forks; PARP targets XRCC1 to these un-resected stalled replication forks. This notion was further proved by depletion of CtIP in U2OS cells, where increased levels of XRCC1 foci formation was observed (Supplementary Figure 1B, C), suggesting that CtIP-mediated resection may suppress the recruitment of XRCC1 protein to stalled forks.
Given that XRCC1 foci are increased in Mre11-inhibited cells, we reasoned that these two proteins may have compensatory functions in repairing replication damage, and hence be good candidates to have a synthetic lethal relationship (as is the case with PARP and BRCA2). To test this hypothesis, we investigated whether Mre11 activity is required for survival of XRCC1-defective cells. Consistent with this notion, XRCC1 deficient cells (EM9-V) are remarkably sensitive to Mre11 inhibition as compared to isogenic, XRCC1 complemented cells (EM9-XH) (Figure 2E).
CK2 activity is required to recruit XRCC1 to stalled DNA replication forks
Next, we tested whether XRCC1 protein levels are altered in response to replication damage. We found that XRCC1 protein levels increased, especially at early time points following HU treatment, whereas RAD51 protein levels peaked after 24 hours of treatment (Figure 2F, Supplementary Figure 2A). These data are consistent with the findings that XRCC1 foci form early after HU treatment, whereas RAD51 form later (26). This is also consistent with the notion that XRCC1 may be involved in repairing more simple DNA lesions than RAD51, which is required for repair of late forming DSBs following HU treatment (26).
Casein kinase-2 (CK2) phosphorylates and stabilizes XRCC1 to enable the assembly of DNA single-strand break repair protein complexes at sites of DNA damage (27–29). Hence, we tested whether HU-induced stabilization of XRCC1 protein at DNA damage sites is also CK2-dependent. XRCC1 is stabilized following HU exposure in cells expressing wild-type XRCC1 but not in cells expressing the CKM mutant form of XRCC1 that cannot be phosphorylated by CK2 (Figure 2F). To confirm that CK2 activity is needed, cells were treated with the CK2 inhibitor TBB, which also prevented accumulation of XRCC1 (Supplementary Figure 2B). Furthermore, CK2 inhibition prevents HU-induced GFP-XRCC1 focus formation (Supplementary Figure 2C). Taken together, these data reveal a CK2-dependent stabilization of XRCC1 in response to replication stalling.
XRCC1 binds to DNA-PK subunits in response to replication stalling
To better understand how XRCC1 functions at stalled DNA replication forks, a GFP-nanotrap strategy was utilized to “pull down” GFP-XRCC1 and its interacting proteins. Mass spectrometry (MS) analysis was performed to analyse components of the complex. As expected, a number of known XRCC1-interacting partners were identified, including PARP-1, DNA ligase-3, PARP-2, bifunctional polynucleotide phosphatase/kinase (PNKP), DNA polymerase beta, and aprataxin (Figure 3A). Interestingly, DNA-PK subunits, KU70 and KU80 were identified as XRCC1 binding partners (Figure 3A). To quantify the interaction between GFP-XRCC1 and the KU70/KU80 complex, western blots were performed. Although an interaction between XRCC1 and KU70/KU80 was evident even in unperturbed cells, the amount of KU70/KU80 that was pulled-down together with GFP-XRCC1 following HU treatment significantly greater (Figure 3B). The interaction was not DNA/RNA mediated as shown in the presence of Benzonase, a broad-spectrum nuclease (Supplementary Figure 3A). This suggests that binding of KU70/KU80 to XRCC1 was induced following replication damage.
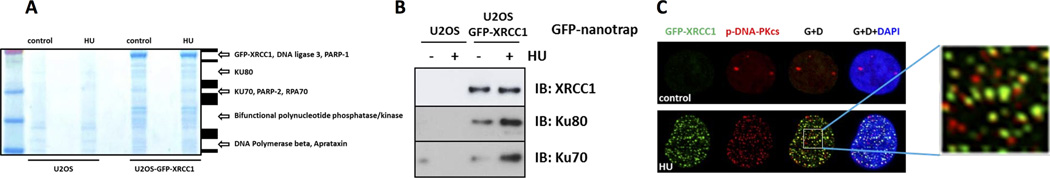
Figure 3. Interaction of XRCC1 with DNA-PK complex under replication stress.
(A) Coomassie staining of GFP-nanotrap pulled down GFP-XRCC1 complexes with treatment or 2mM HU for 6 h in U2OS cells. Specific GFP-XRCC1-interacting protein bands were analyzed by mass spectrometry, with the identified proteins named. (B) Western blot analysis of XRCC1, KU80 and KU70 from A. (C) Immunofluorescence staining of GFP-XRCC1 and phospho-DNA-PKcs following 2mM HU treatment for 6 h in U2OS cells.
HU-induced DNA-PKcs foci are apparent using a phospho-specific DNA-PKcs antibody (anti-pS2056). Moreover, about 50% co-localization of these foci with XRCC1 foci was observed (Figure 3C). Neither depletion of PARP-1 (Supplementary Figure 3B, C) nor loss of XRCC1 (Supplementary Figure 3D) affected the formation of phosphorylated DNA-PKcs foci. However, reducing the expression of DNA-PKcs by siRNA resulted in a decrease in the percentage of cells that displayed HU-induced phosphorylated DNA-PKcs foci, suggesting that this was DNA-PKcs dependent (Supplementary Figure 4A, B).
DNA-PKcs is phosphorylated in response to replication fork stalling and its kinase activity is required for cellular resistance to HU
DNA-PK autophosphorylates itself on numerous residues (likely more than 40) in response to DNA damage (30,31). Phosphorylation within two major clusters of sites [ABCDE and PQR clusters] induce conformational changes that allow DNA-PK to regulate DNA end access; these phosphorylations clearly promote NHEJ. Here, we examined HU-induced DNA-PKcs phosphorylation of serine 2056, within the "PQR" phosphorylation site cluster, in a panel of V3 transfectants expressing wild type and mutant forms of DNA-PKcs. S2056 is strongly phosphorylated in response to 2 mM HU in cells expressing wild type DNA-PKcs; no phosphorylation is observed in cells lacking DNA-PKcs, or in cells expressing the PQR mutant (alanine ablation of 2056 site) confirming the specificity of the phospho-specific reagent. Interestingly in cells expressing a catalytically inactive DNA-PKcs mutant K-R: mutated at K3752R, this phosphorylation event is also observed, albeit at lower levels than in wild type counterpart (Figure 4A, Supplementary Figure 4C). It is important to note that HU-induced 2056 phosphorylation of catalytically inactive DNA-PKcs is more robust than observed in cells (expressing K3752R) treated with either zeocin (a DSB inducing agent) or UV (22) suggesting that another protein kinase can target DNA-PKcs in response to HU.
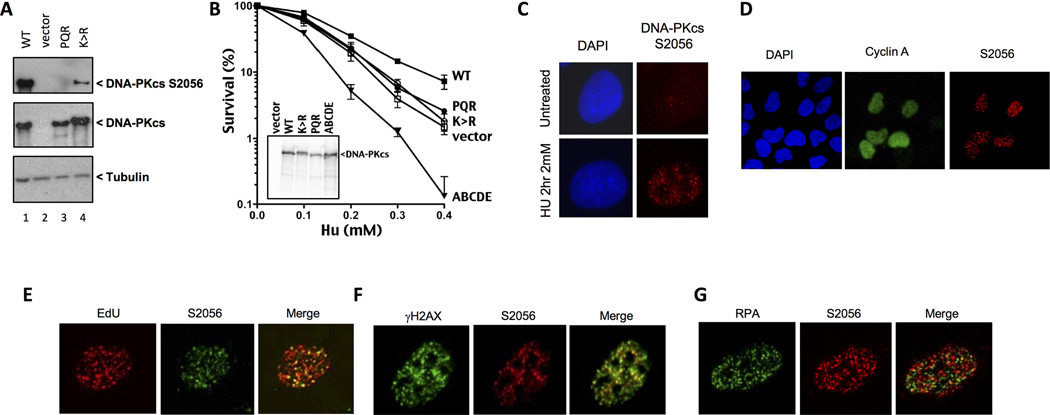
Figure 4. DNA-PKcs is phosphorylated in response to replication fork stalling and is required for cellular resistance to HU.
(A) Cells were treated with 2 mM HU for 2 hours prior to harvesting for polyacrylamide gel electrophoresis. Western blotting of whole cell extracts from the indicated mutant strains was performed and membranes probed with the specified antibodies. (B) Clonogenic survival assays to assess cellular survival of various DNA-PKcs mutant strains after exposure to HU. The indicated cell lines were exposed to increasing doses of HU for 24 hours and then allowed to recover for 7–10 days before staining surviving colonies. Error bars represent the standard error of the mean from at least 3 independent experiments. (C) Representative images of HU induced DNA-PKcs S2056 foci in U2OS cells. (D) Representative images of U2OS cells depicting HU-induced phospho-DNA-PKcs co-staining with cyclin A. (E, F, G) Representative images of U2OS cells depicting phospho-DNA-PKcs co-staining with EdU, γH2AX and RPA in response to a 2-hour treatment of HU (2 mM).
Here, we exploited a panel of DNA-PKcs mutants to assess whether DNA-PK's catalytic activity and phosphorylation affects cellular resistance to HU exposure. Consistent with previous reports, cells lacking DNA-PKcs (vector only) display modest sensitivity to HU compared to wild type controls (Figure 4B). Cells expressing a catalytically inactive mutant are similarly sensitive to HU as are cells that express no DNA-PKcs; cells expressing catalytically inactive DNA-PKcs are also similarly sensitive to agents that induce DSBs as cells that express no DNA-PKcs. In contrast, cells expressing the PQR>ala mutant that are also similarly sensitive to HU as the K>R or vector only expressing cells, are only slightly sensitive to agents that induce DSBs as compared to cells lacking DNA-PKcs or expressing kinase inactive DNA-PKcs. The ABCDE mutant cannot phosphorylate any of 6 sites in the ABCDE cluster, which are important in initiating NHEJ and in promoting end processing; the ABCDE mutant is markedly sensitive, much more so than cells completely lacking DNA-PKcs (Figure 4B). Emerging data suggest that the ABCDE mutant can initate NHEJ and proceed slowly through the process; prolongued occupancy of ABCDE blocked DNA-PK at a DNA end, and resulting slow repair is clearly more detrimental to cell survival than in other mutants, for example catlytically inactive mutants, that can bind DNA ends, but do not initiate NHEJ (15). These data suggest that DNA-PK's functional role at stalled replication forks requires a similar series of phosphorylation events to facilitate repair, perhaps by regulating access to DNA ends.
DNA-PKcs S2056 foci are present in S-phase and represent sites of unresected replication stress
To investigate the role of DNA-PKcs in the cellular response to replication stress we examined the induction of phosphorylation at residue S2056 in response to HU. Asynchronous U2OS cells display punctate p2056 nuclear foci in response to a 2 hour exposure to 2 mM HU presumably reflecting stalled replication forks (Figure 4C). Reducing the expression of DNA-PKcs by siRNA resulted in a decrease in the percentage of cells that displayed HU inducible foci (Supplementary Figure 4A, B). These suggest that DNA-PKcs is phosphorylated at S2056 in response to HU induced genotoxic stress.
Next, we co-stained U2OS cells for cyclin A. Cells which form HU induced DNA-PKcs S2056 foci also display moderate levels of cyclin A staining, confirming that these cells are in S-phase (Figure 4D). We also sought to investigate whether these foci represented sites of replication stress. We determined this by co-staining cells with EdU, and γH2AX. We observed that approximately 80 % of S2056 foci co-localised with EdU confirming that phosphorylated DNA-PKcs responds to regions of DNA that have been recently replicated and undergone subsequent fork stalling (Figure 4E). Furthermore S2056 phosphorylation was also found to strongly co-localise with γ-H2AX confirming its association with replication stress (Figure 4F). Although virtually all p2056 sites co-localize with γH2AX and EdU, this represents only a subset (approximately 50%) of all γH2AX and EdU foci. HU clearly induces γH2AX and EdU that lack p2056. We also examined whether DNA-PKcs was present at ssDNA regions by co-staining for phosphorylated DNA-PKcs with RPA. To our surprise, there was minimal (less than 20%) co-localisation of these proteins suggesting that at least a proportion of these two proteins localise to different DNA structures created by HU induced fork stalling (Figure 4G). These data suggest DNA-PK is phosphorylated at unresected DNA ends during replication stress.
Previously, we observed that HU-induced γ-H2AX foci are quickly recovered after release from a 2 hour HU-induced replication stalling (26). Here, we determined γ-H2AX foci and their subsequent recovery in cells treated with an inhibitor of DNA-PKcs kinase activity (NU7026). Interestingly, we found only a slight defect (but not significantly different) in recovery of γ-H2AX foci in the presence of NU7026 (Supplementary Figure 5A). Similarly, the formation of RPA foci, and their subsequent recovery, after HU induced fork stalling, is normal in the presence of NU7026 (Supplementary Figure 5B). In contrast, 53BP1 foci, a marker for DNA double-strand ends, which have been suggested to form at reversed replication forks (chicken foot 4-way junctions) in response to short exposure to HU treatment, persist in the absence of DNA-PKcs kinase activity (Supplementary Figure 5C). Thus, it seems most likely that DNA-PK binds the double strand end structure (chicken foot) at reversed forks, or in cases of complete fork collapse, the resulting single DNA end. These data are consistent with a previous report showing a requirement for DNA-PK in the repair of 53BP1 foci induced after a 15-hour HU treatment (32).
DNA-PK is required for XRCC1 recruitment to stalled forks and for replication restart
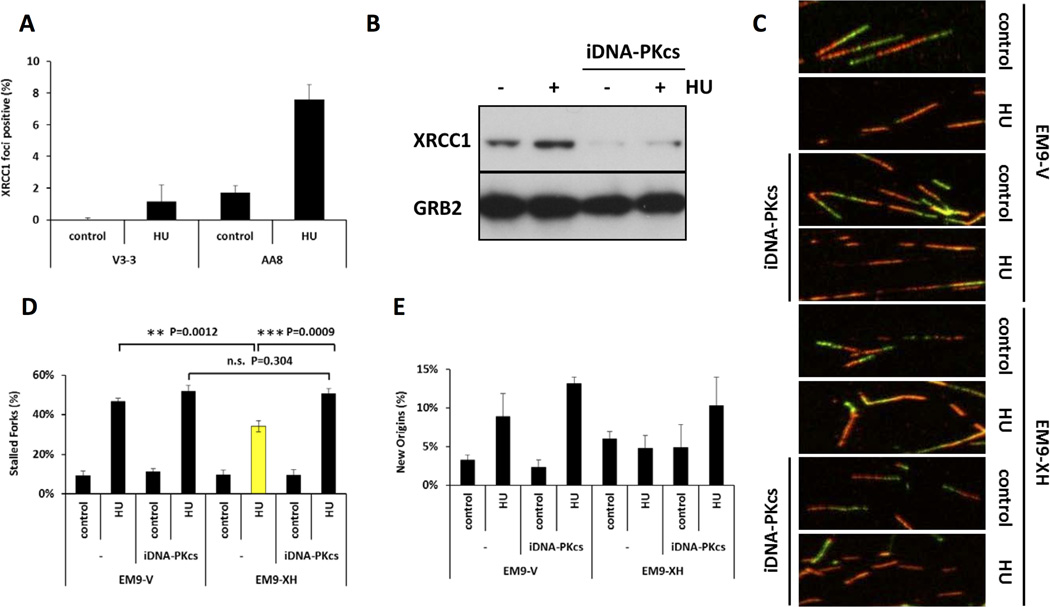
Functional association between DNA-PKcs and XRCC1 was previously shown following ionizing irradiation (33). Next, we tested whether DNA-PKcs was required for XRCC1 recruitment to stalled forks. XRCC1 foci were measured in DNA-PKcs defective V3-3 and wild type AA8 cells following treatment with HU for 6 hours. A dramatic decrease in the level of XRCC1 foci was found in the absence of DNA-PKcs (Figure 5A). Next, we tested various DNA-PKcs mutant cell lines, and found that KR or PQR mutation was also able to reduce the frequency of XRCC1 foci (Supplementary Figure 6). Furthermore, the HU-induced stabilization of XRCC1 protein was also lost by the inhibition of DNA-PKcs activity (Figure 5B). These results collectively suggest that DNA-PKcs activity is required for XRCC1 function and likely acts upstream of XRCC1 at stalled forks. To test whether these activities were also involved in the maintenance of the integrity of stalled forks, we analysed XRCC1 defective EM9-V and the complemented EM9-XH cells, with or without DNA-PKcs inhibitor, for their ability to restart stalled replication forks (Figure 5C–E). We found that, either in the absence of functional XRCC1 or following inhibition of DNA-PKcs activity, more forks failed to resume replication after release from a 2 hour HU block (Figure 5C, D). However, a combination of these two deficiencies did not further decrease the frequency of fork restart (Figure 5C, D). Similar trends were found for new origin firing after release from 2 hours HU block (Figure 5E).
Figure 5. DNA-PK is required for stabilization and recruitment of XRCC1 to stalled forks to promote restart.
(A) Quantification of XRCC1 foci following 2mM HU treatment for 6 hours in V3-3 or AA8 cells. (B) Western blot analysis of XRCC1 and GRB2 following indicated treatment in U2OS cells. (C) Representative images of replication tract, (D) Quantification of fork restart, and (E) Quantification of new origin firing after release from 2 hours HU treatment with or without DNA-PKcs inhibitor.
DNA-PKcs kinase activity is requisite but not sufficient for replication restart
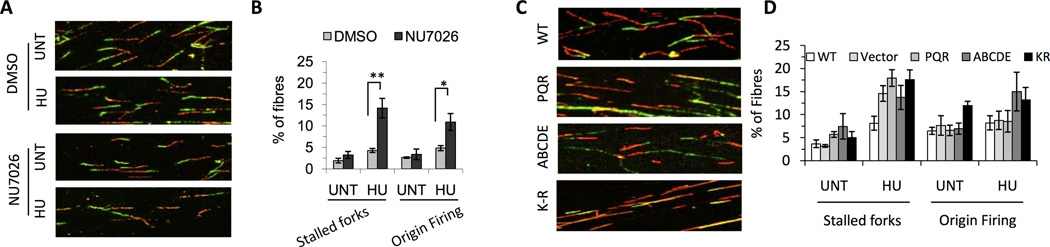
Our data clearly demonstrate that DNA-PK promotes cellular survival after exposure to HU. To explain its role in promoting cell survival after HU exposure, we suggest two possibilities. DNA-PK might either 1) promote inappropriate NHEJ joining of DNA double-strand ends at two adjacent collapsed forks resulting in increased cellular survival, but at the expense of genomic stability; or 2) promote replication restart after binding at the reversed fork (chicken foot structure). To investigate potential effects of DNA-PK on replication restart, the DNA fibre assay was employed. When cells are treated with NU7026 a significant increase in HU induced stalled forks is observed (Figure 6A and 6B), implying that DNA-PK promotes replication restart after HU induced fork arrest. Furthermore, a small but significant increase in the number of new origins firing after HU treatment in NU7026 treated cells was observed, inferring that blocking DNA-PK activity results in increased numbers of complete fork collapse.
Figure 6. DNA-PKcs kinase activity is required for replication restart.
(A, C) Representative images of fibres obtained with the indicated treatment. (B, D) Quantification of stained fibre with the indicated treatment. Error bars represent standard error of the mean from at least 3 independent experiments. Values marked with asterisks are significantly different (Student’s t test, *p<0.05, **p<0.01 and ***p<0.001).
To test whether DNA-PK's kinase activity is important for replication restart, DNA fibre analyses were also performed on V3 cells lacking DNA-PKcs, or expressing a kinase dead mutant of DNA-PKcs K-R (K3752R), as well as the two phosphorylation clusters mutants, PQR and ABCDE (Figure 6C and D). Compared to cells expressing wild type DNA-PKcs, both V3 cells expressing no DNA-PKcs, or catalytically inactive DNA-PKcs displayed elevated levels of replication fork stalling in the presence of HU. These data demonstrate a clear requirement for DNA-PK's catalytic activity in replication restart. However, increased fork stalling was also observed in cells expressing the ABCDE and PQR mutants that both retain full catalytic activity. Thus, we conclude that although DNA-PK's catalytic activity is required for replication restart, it is not sufficient.
Interestingly, cells expressing the catalytically inactive mutant displayed heightened levels of new origin firing, both in untreated cells and in response to HU. Additionally, cells expressing the ABCDE mutant had increased sensitivity in response to HU as compared to cells lacking DNA-PK. These data demonstrate a dominant negative function of the ABCDE mutant DNA-PK. We suggest that this can be explained by DNA-PK being trapped onto replication fork intermediates and may prevent resolution of forks for instance via PARP-mediated pathways.
DISCUSSION
Here, we demonstrate that both PARP and DNA-PK recruit XRCC1 to sites of stalled replication forks upon HU treatment. In support of a novel function for XRCC1 at stalled replication forks, we demonstrate that XRCC1 is required for effective restart and repair of stalled forks.
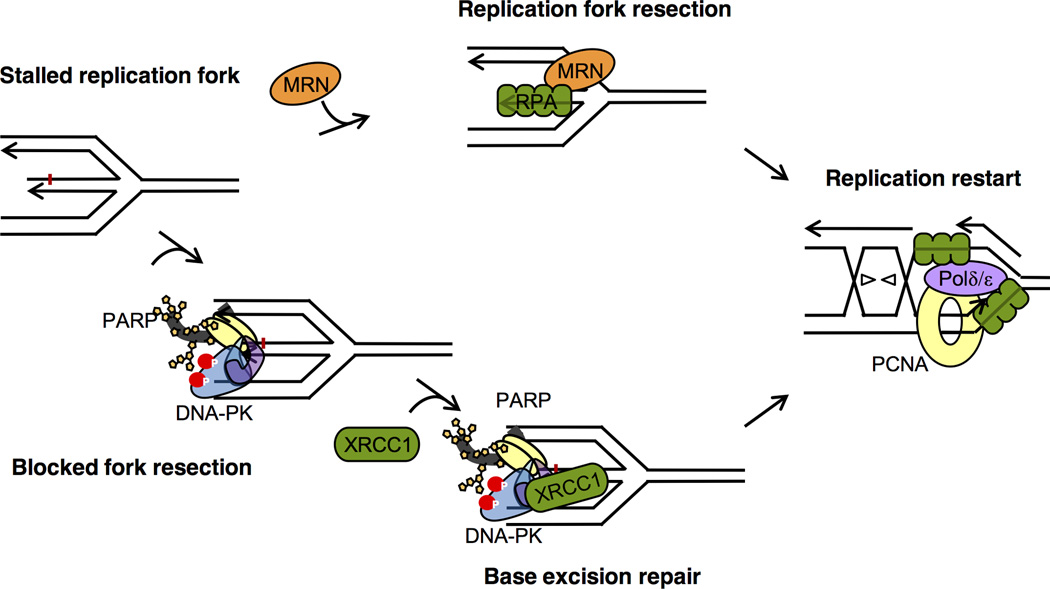
Since we only observed a small defect in the restart of stalled forks, and no absolute requirement for XRCC1 to mediate repair at stalled replication forks, we suggest that the function of XRCC1 is to mediate repair of a subset of stalled forks. There is a possibility that small lesions block a subset of stalled replication forks that otherwise impede re-start of the forks. Indeed, when the DNA double helix is opened up during replication, the unpaired bases in single-stranded DNA (ssDNA) are more prone to be damaged and are not easily repaired when the complementary strand is not based-paired. Furthermore, ligases and topoisomerases are heavily involved in DNA replication, and failed topoisomerase or ligase reactions may trap proteins at sites of stalled replication, which may require XRCC1-mediated repair alongside TDP1 or aprataxin. Alternatively, XRCC1 may also have a more direct role in replication restart and repair. In conclusions, our data support a model where the scaffolding protein XRCC1 is involved in repair of stalled replication forks occurring on unresected DNA ends (Figure 7).
Figure 7. Model for XRCC1 at unresected stalled replication forks to mediate repair and restart.
Resection at a subset of replication forks may be obstructed allowing PARP and DNA-PK binding to these unresected DNA double-stranded ends. In turn, this trigger poly(ADP-ribose) polymerase or kinase activity, respectively. PARP and DNA-PK activities recruit XRCC1 to unresected DNA ends to mediate repair and allow further resection and processing at stalled forks to repair and restart replication to mediate survival.
It is interesting that an increased amount of XRCC1 recruitment to replication forks was observed when Mre11 exonuclease activity is impaired, suggesting that XRCC1 is recruited to either small gaps at replication forks or DNA double-strand ends and not to extended ssDNA regions generated by end resection. This notion is also strengthened by the finding that PARP1 preferentially binds to replication fork structures containing only small ssDNA gaps (18) and that the Ku70/80 subunits of DNA-PK bind to DNA double-strand ends (34).
The HU hypersensitivity of cells expressing DNA-PK that cannot phosphorylate the ABCDE sites is in agreement with our previous observations that cells expressing the ABCDE mutant are more sensitive to IR, zeocin, and mitomycin C than cells completely lacking DNA-PKcs (15,35), in agreement with a dominant negative effect of DNA-PK being trapped to replication intermediates. The HU hypersensitivity of V3 cells expressing the ABCDE mutant are consistent with the hypothesis that this phenotype is the result of an inability of cells with mutations at this phosphorylation cluster to correctly respond to replication stress.
Combining the data presented in this manuscript, we propose a model where DNA-PKcs and PARP1 associate with DNA ends exposed at unresected stalled forks (Figure 7). Early studies suggest that DNA-PK activation occurs in trans (i.e. by interaction with the DNA molecule bound by a second, synaped DNA-PK complex) (36). However, more recent data suggest that DNA dependent, DNA-PK activation can occur at a single isolated, DNA-PK complex, in cis (37). Previously, we demonstrated that Mre11 is recruited to only a portion of stalled replication forks through PARP activity (18). It is very clear that Mre11 is recruited also in the absence of PARP activity and the dependance of PARP may vary between different conditions used to stall replication forks. PARP was also demonstrated to protect stalled replication forks from Mre11-mediated resection (23). We suggest that Mre11 is recruited by PARP to stalled forks that are not easily resected and that may require XRCC1-mediated repair. Under conditions where all stalled replication forks are easily resected, we envision that PARP is not required for Mre11 recruitment, but play a distinctive role in stablizing stalled replication forks.
In conclusion, here we report that PARP and DNA-PKcs are relocating and DNA-PK rapidly phosphorylated at S2056 at unresected stalled replication forks. We demonstrate that PARP and DNA-PK are required for relocation of XRCC1 to stalled replication forks, and we uncovered a novel role for XRCC1, proposed to be for removal of small lesions, to enable effective repair and restart of stalled replication forks.
Supplementary Material
Acknowledgments
We wish to thank Drs Penny Jeggo and Susan Lees-Miller for discussions. This work was supported financially by the National Natural Science Foundation of China (31370901, 81422031), National 1000 Talents Program for Young Scholars, and Zhejiang Provincial Natural Science Foundation of China (LR14H160001), National Institutes of Health (5R01AI048758 – 17) the Swedish Cancer Society, the Swedish Children’s Cancer Foundation, the Swedish Research Council, the Swedish Pain Relief Foundation, the Medical Research Council, and the Torsten and Ragnar Söderberg Foundation.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18(1):134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 2.Hubscher U, Maga G. DNA replication and repair bypass machines. Curr Opin Chem Biol. 2011;15(5):627–635. doi: 10.1016/j.cbpa.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Lundin C, Erixon K, Arnaudeau C, Schultz N, Jenssen D, Meuth M, et al. Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Molecular and cellular biology. 2002;22(16):5869–5878. doi: 10.1128/MCB.22.16.5869-5878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnaudeau C, Rozier L, Cazaux C, Defais M, Jenssen D, Helleday T. RAD51 supports spontaneous non-homologous recombination in mammalian cells, but not the corresponding process induced by topoisomerase inhibitors. Nucleic acids research. 2001;29(3):662–667. doi: 10.1093/nar/29.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saintigny Y, Delacote F, Vares G, Petitot F, Lambert S, Averbeck D, et al. Characterization of homologous recombination induced by replication inhibition in mammalian cells. Embo J. 2001;20(14):3861–3870. doi: 10.1093/emboj/20.14.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlt MF, Mulle JG, Schaibley VM, Ragland RL, Durkin SG, Warren ST, et al. Replication stress induces genome-wide copy number changes in human cells that resemble polymorphic and pathogenic variants. Am J Hum Genet. 2009;84(3):339–350. doi: 10.1016/j.ajhg.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petermann E, Helleday T. Pathways of mammalian replication fork restart. Nat Rev Mol Cell Biol. 2010;11(10):683–687. doi: 10.1038/nrm2974. [DOI] [PubMed] [Google Scholar]
- 8.Groth P, Auslander S, Majumder MM, Schultz N, Johansson F, Petermann E, et al. Methylated DNA causes a physical block to replication forks independently of damage signalling, O(6)-methylguanine or DNA single-strand breaks and results in DNA damage. J Mol Biol. 2010;402(1):70–82. doi: 10.1016/j.jmb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 9.El-Khamisy SF, Masutani M, Suzuki H, Caldecott KW. A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic acids research. 2003;31(19):5526–5533. doi: 10.1093/nar/gkg761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Molecular and cellular biology. 1998;18(6):3563–3571. doi: 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, et al. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell. 2001;104(1):107–117. doi: 10.1016/s0092-8674(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 12.Caldecott KW, Tucker JD, Stanker LH, Thompson LH. Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic acids research. 1995;23(23):4836–4843. doi: 10.1093/nar/23.23.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: From mechanistic understanding to cancer treatment. DNA repair. 2007;6(7):923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434(7033):605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 15.Neal JA, Sugiman-Marangos S, VanderVere-Carozza P, Wagner M, Turchi J, Lees-Miller SP, et al. Unraveling the complexities of DNA-dependent protein kinase autophosphorylation. Molecular and cellular biology. 2014;34(12):2162–2175. doi: 10.1128/MCB.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi V, Pontis E, Reichard P. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. The Journal of biological chemistry. 1986;261(34):16037–16042. [PubMed] [Google Scholar]
- 17.Kraakman-van der Zwet M, Overkamp WJ, van Lange RE, Essers J, van Duijn-Goedhart A, Wiggers I, et al. Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions. Molecular and cellular biology. 2002;22(2):669–679. doi: 10.1128/MCB.22.2.669-679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryant HE, Petermann E, Schultz N, Jemth AS, Loseva O, Issaeva N, et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J. 2009;28(17):2601–2615. doi: 10.1038/emboj.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothbauer U, Zolghadr K, Muyldermans S, Schepers A, Cardoso MC, Leonhardt H. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol Cell Proteomics. 2008;7(2):282–289. doi: 10.1074/mcp.M700342-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Kinter M, Sherman NE. Protein sequencing and identification using tandem mass spectrometry. New York: John Wiley; 2000. p. 301. xvi. [Google Scholar]
- 21.Mackeen MM, Kramer HB, Chang KH, Coleman ML, Hopkinson RJ, Schofield CJ, et al. Small-molecule-based inhibition of histone demethylation in cells assessed by quantitative mass spectrometry. Journal of proteome research. 2010;9(8):4082–4092. doi: 10.1021/pr100269b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry-Mowatt J, Jackson D, Masson JY, Johnson PA, Clements PM, Benson FE, et al. XRCC3 and Rad51 modulate replication fork progression on damaged vertebrate chromosomes. Mol Cell. 2003;11(4):1109–1117. doi: 10.1016/s1097-2765(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 23.Ying S, Hamdy FC, Helleday T. Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res. 2012;72(11):2814–2821. doi: 10.1158/0008-5472.CAN-11-3417. [DOI] [PubMed] [Google Scholar]
- 24.Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(12):3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- 25.Dupre A, Boyer-Chatenet L, Sattler RM, Modi AP, Lee JH, Nicolette ML, et al. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol. 2008;4(2):119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea-Stalled Replication Forks Become Progressively Inactivated and Require Two Different RAD51-Mediated Pathways for Restart and Repair. Mol Cell. 2010;37(4):492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, et al. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117(1):17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- 28.Parsons JL, Dianova II, Finch D, Tait PS, Strom CE, Helleday T, et al. XRCC1 phosphorylation by CK2 is required for its stability and efficient DNA repair. DNA Repair (Amst) 2010;9(7):835–841. doi: 10.1016/j.dnarep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Strom CE, Mortusewicz O, Finch D, Parsons JL, Lagerqvist A, Johansson F, et al. CK2 phosphorylation of XRCC1 facilitates dissociation from DNA and single-strand break formation during base excision repair. DNA repair. 2011 doi: 10.1016/j.dnarep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Dobbs TA, Tainer JA, Lees-Miller SP. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA repair. 2010;9(12):1307–1314. doi: 10.1016/j.dnarep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen BP, Chan DW, Kobayashi J, Burma S, Asaithamby A, Morotomi-Yano K, et al. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. The Journal of biological chemistry. 2005;280(15):14709–14715. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- 32.Orsburn B, Escudero B, Prakash M, Gesheva S, Liu G, Huso DL, et al. Differential requirement for H2AX and 53BP1 in organismal development and genome maintenance in the absence of poly(ADP)ribosyl polymerase 1. Molecular and cellular biology. 2010;30(10):2341–2352. doi: 10.1128/MCB.00091-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy N, Martz A, Bresson A, Spenlehauer C, de Murcia G, Menissier-de Murcia J. XRCC1 is phosphorylated by DNA-dependent protein kinase in response to DNA damage. Nucleic acids research. 2006;34(1):32–41. doi: 10.1093/nar/gkj409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- 35.Douglas P, Cui X, Block WD, Yu Y, Gupta S, Ding Q, et al. The DNA-dependent protein kinase catalytic subunit is phosphorylated in vivo on threonine 3950, a highly conserved amino acid in the protein kinase domain. Molecular and cellular biology. 2007;27(5):1581–1591. doi: 10.1128/MCB.01962-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeFazio LG, Stansel RM, Griffith JD, Chu G. Synapsis of DNA ends by DNA-dependent protein kinase. EMBO J. 2002;21(12):3192–3200. doi: 10.1093/emboj/cdf299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu H, Shimazaki N, Raval P, Gu J, Watanabe G, Schwarz K, et al. A biochemically defined system for coding joint formation in V(D)J recombination. Mol Cell. 2008;31(4):485–497. doi: 10.1016/j.molcel.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.