Abstract
Introduction
High-yielding, automated production of a PET tracer that reflects proliferation, 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT), is reported using a modified Bioscan Coincidence FDG reaction module.
Methods
Production of [18F]FLT was implemented through: (1) modification of an original FDG manifold; (2) application of an alternate time sequence; and (3) altered solid-phase extraction (SPE) purification. Quality control testing, including standard radiochemical figures of merit and preclinical positron emission tomography (PET) imaging, was carried out.
Results
High decay-corrected yields of [18F]FLT (16–39%) were reproducibly obtained. The product exhibited very high specific activity (4586.9 TBq/mmol; 123,969 Ci/mmol) and radiochemical purity (499%). Overall, the [18F]FLT produced in this manner was superior to typical productions that utilized a GE TRACERlab FXF-N reaction module. Additionally, purification with SPE cartridges, followed by manual elution, accelerated overall run time and resulted in a two-fold increase in [18F]FLT concentration. PET imaging showed the [18F]FLT produced by this method was highly suitable for non-invasive tumor imaging in mice.
Conclusions
The Bioscan Coincidence GE FDG Reaction Module was readily adapted to reproducibly provide [18F]FLT in high yield, specific activity, and radiochemical purity. The approach was suitable to provide sufficient amounts of material for preclinical studies.
Keywords: [18F]FLT, Automated radiosynthesis, Solid-phase extraction (SPE), Positron emission tomography (PET)
Graphical Abstract

1. Introduction
Non-invasive positron emission tomography (PET) imaging biomarkers of proliferation hold great promise to predict early responses to personalized therapy in oncology and other clinical settings (Manning et al., 2008; McKinley et al., 2013a, 2012a, 2013b; Shah et al., 2009). To this end, 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) was developed as a PET imaging radiopharmaceutical to provide a surrogate biomarker of DNA replication. As an anti-metabolite of the endogenous nucleoside thymidine, [18F]FLT uptake in tissue reflects activity of the thymidine salvage pathway, a mechanism that provides DNA precursors from the extracellular environment to dividing cells (McKinley et al., 2013b). Accordingly, [18F]FLT PET has been used in many preclinical and clinical research studies, especially in oncology (Shields et al., 1998) and other highly proliferative tissue (McKinley et al., 2012).
Classically, production of [18F]FLT suffers from modest yields and time-intensive purification by high-performance liquid chromatography (HPLC) (Grierson and Shields, 2008). These short-comings have been partially addressed through development of improved precursors (nosylate, mesylate, tosylate) (Martin et al., 2002), use of protic solvents (Lee et al., 2012), microfluidic modules (Javed et al., 2014) and double synthesis modules (Niedermoser et al., 2012). Further improvements in synthesis time were recently achieved by performing the radiosynthesis in an automated module in conjunction with purification using a single alumina column, giving the shortest reported synthesis time of 68 min and a radiochemical purity 495% (Nandy and Rajan, 2010). Still, the radiochemical yield (decay corrected) remained less than 10%.
Historically, both preclinical and clinical productions of [18F]FLT at Vanderbilt University have been carried out using a GE TRA-CERlab FXF-N reaction module (hereafter TRACERlab module). These productions typically require more than two hours and give yields less than 8% (decay corrected). Due to the increasing demand of [18F]FLT, especially for preclinical studies, we sought an alternate module-based approach as a potential route for high specific activity and high-yielding production.
The goal of this study was to develop an alternate, automated [18F]FLT production to support these preclinical studies. The Bioscan Coincidence GE FDG reaction module (hereafter Coincidence module) was originally designed for commercial production of 2-deoxy-2-[18F]fluoroglucose ([18F]FDG). In this study, we modified this module to give reliable preclinical productions of [18F]FLT, with a desirable activity-to-volume ratio and high radiochemical purity and specific activity. Results using the new approach were quantitatively compared to production using the TRACERlab module. Preclinical PET imaging of human colon cancer cell-line xenografts with [18F]FLT produced with the Coincidence module demonstrated a high tumor-to-normal tissue uptake ratio of 4:1, which facilitated excellent imaging contrast.
2. Materials and methods
2.1. Reagents and apparatus
3-N-Boc-5′-O-dimethoxytrityl-3′-O-nosyl-thymidine, 2,3′-anhydro-5′-O-benzoyl-2′-deoxythymidine, 3′-deoxy-3′-fluorothymidine, and tetrabutylammonium hydrogen carbonate (TBAHCO3, 75 mM in ethanol) were purchased from ABX (Advanced Biochemical Compounds, Radeberg, Germany). All trapping and purification cartridges were purchased from Waters (Milford, MA) or Grace Davison Discovery Sciences (Deerfield, OR). Anhydrous acetonitrile, potassium carbonate (K2CO3), dimethyl sulfoxide (DMSO), sodium hydroxide (NaOH), hydrochloric acid (HCl), and Kryptofix-222 were purchased from Sigma-Aldrich (St. Louis, MO), and absolute ethanol (200 proof) from Pharmaco Aaper (Brookfield, CT). Membrane filters (0.2-μm) were purchased from Pall (Port Washington, NY). Manifold kits for FDG were purchased from Rotem Industries, Inc. (Arava, Israel). All radiosyntheses were performed using either a Bioscan Coincidence GE FDG reaction module (Fairfield, CT) or a GE TRACERlab FXF-N reaction module. Analytical HPLC was performed using a Hitachi HPLC system (LaChrom Elite) equipped with in-line Hitachi UV (LaChrom Elite model L-2400) and radiometric (Carroll-Ramsey Associates, Berkeley, CA) detectors and a Hitachi LaChrom reversed-phase analytical column (150 mm × 4.6 mm). MicroPET scanning was accomplished using a Concorde Microsystems Focus 220 (Siemens, Berlin, Germany).
2.2. Automated synthesis of [18F]FLT: Coincidence module
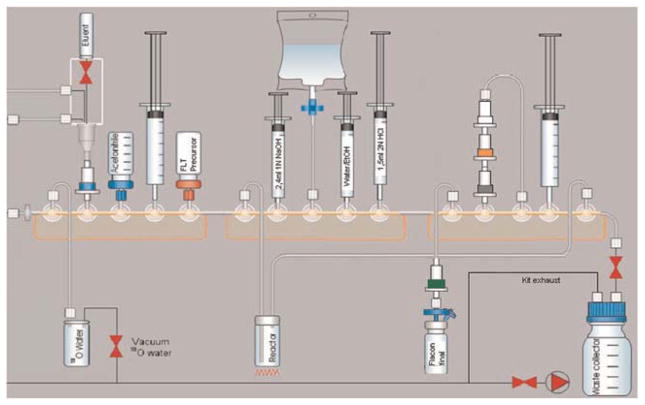
The schematic diagram of the modified automated synthesis is shown in Fig. 1. The following workflow was applied to the unmodified [18F]FLT Coincidence module productions:
Fig. 1.
Schematic of the Bioscan Coincidence GE FDG Reaction Module re-configured for [18F]FLT production.
-
Step 1: Nucleophilic fluorination of 3-N-Boc-5′-O-dimethoxytrityl-3′-O-nosyl-thymidine
[18F]fluoride trapped on a Waters QMA carbonate cartridge with 18O water recovery.
[18F]fluoride eluted from QMA cartridge with 75 mM TBAHCO3 (0.5 mL) into the reaction vessel.
Tetrabutylammonium [18F]fluoride dried by azeotropic distillation with acetonitrile at 95 °C.
Precursor (25 mg), dissolved in acetonitrile (2.0 mL), added to the reaction vessel.
Nucleophilic radiofluorination carried out at 100 °C for 5 min.
-
Step 2: Deprotection
Reaction cooled to 85 °C.
2.0 N HCl (1.5 mL) added to the reaction vessel.
Acid hydrolysis carried at 85 °C for 5 min.
Reaction mixture cooled to 55 °C.
Reaction mixture transferred to 30 mL syringe.
Reaction vessel rinsed with 1.0 N NaOH (2.4 mL) and combined with reaction mixture.
-
Step 3: Purification through solid-phase extraction (SPE) cartridges
Reaction mixture loaded onto three (3) SPE cartridges (PS-H, WAX, HLB).
SPE cartridges washed with 2% EtOH/H2O (60 mL).
[18F]FLT eluted with 10% EtOH/H2O (20 mL) into a final vial containing citrate buffer (3.0 mL).
2.3. Automated synthesis of [18F]FLT: TRACERlab module
[18F]FLT was prepared from [18F]fluoride in a 2-step, 1-pot reaction as previously described using the TRACERlab module (Manning et al., 2008). Briefly, aqueous [18F]fluoride was eluted into the reaction vessel with Kryptofix-222 and K2CO3 in CH3CN/H2O. Three sequences of heating (99 °C) with flowing helium gas resulted in dry [18F]fluoride/Kryptofix-222/K2CO3. To this was added the cyclic precursor 2,3′-anhydro-5′-O-benzoyl-2′-deoxythymidine in DMSO, followed by reaction for 10 min at 160 °C. The benzoyl-protecting group was removed from the labeled intermediate by basic hydrolysis (0.25 M NaOH, 50 °C, 10 min). The reaction mixture was purified on a semipreparative Macherey-Nagel Nucleosil 100-7 C18 column (250 mm × 10 mm) eluting with ethanol/phosphate buffer (10 mM) (8/92, v/v) at a flow rate of 6.0 mL/min. The final product was sterilized by 0.2-μm membrane filtration. Radiochemical identity, purity, and specific activity were determined by analytical HPLC. [18F]FLT was obtained with an average radiochemical purity of 97% and a specific activity of 40 TBq/mmol (1080.5 Ci/mmol).
2.4. Quality control: Coincidence and TRACERlab modules
Analytical HPLC for both Coincidence and TRACERlab module productions was performed using a Hitachi LaChrom Elite HPLC system equipped with in-line LaChrom Elite model L-2400 Hitachi UV and radiometric detectors and a Hitachi LaChrom C18 reversed-phase analytical column (150 mm × 4.6 mm, 5.0 μm). The column was eluted with acetonitrile/water (10/90, v/v) at a flow rate of 1.0 mL/min.
2.5. [18F]FLT PET imaging
HCT-116 human colorectal cancer xenografts were generated in athymic nude mice (n = 10) as previously described (Manning et al., 2008). PET imaging was performed as previously described (McKinley et al., 2013a, 2013b). Mice were administered 7.4–9.3 MBq (200–250 μCi) of [18F]FLT intravenously and allowed free access to food and water during a 50-min uptake period, followed by a 10-min static acquisition in a Concorde Microsystems Focus 220. All PET scans were reconstructed using OSEM3D/MAP. ASIPro software (Siemens) was used to manually draw 3D volumes of interest over the tumors, which were quantified as percent injected dose per gram (%ID/g), as previously described (McKinley et al., 2013a).
3. Results and discussion
3.1. Radiosynthesis of [18F]FLT
Since the Coincidence module was originally designed for [18F]FDG production, we first established that the commercially available ABX FLT reaction kits could be used to consistently deliver [18F]FLT. These kits require three solid-phase extraction (SPE) cartridges for purification (PS-H, WAX, and HLB) that circumvent HPLC purification. Furthermore, we determined that the production of [18F]FLT would meet quality control requirements for preclinical use.
Modification of the module for [18F]FLT production was achieved using an ABX-supplied kit and a modified FDG manifold. The time sequence, developed and supplied through ABX for the production of [18F]FLT, was installed onto the computer console and run without modification. Starting with the protected nosylate precursor, the entire synthesis was completed in 70 min, with a starting activity between 39.2 and 51.1 GBq (1.06–1.38 Ci) that provided [18F]FLT at 0.18–0.23 GBq/mL (4.96–6.13 mCi/mL). Further improvements were made by truncation of the reaction sequence, thereby reducing the production time from 70 to 50 min. This was achieved by pausing the time sequence (Step 3—ii of the workflow) just prior to elution into the final vial, thereby allowing removal of the HLB cartridge with remote manipulators and manual, sequential elution with EtOH (200 proof, 1.0 mL) and saline (0.9%, 9.0 mL) directly into the final vial. This modified step provided [18F]FLT in a total volume of 10 mL, compared to 23 mL if the time sequence was run to completion. This also precluded the requirement for citrate buffer when running the normal reaction sequence to completion.
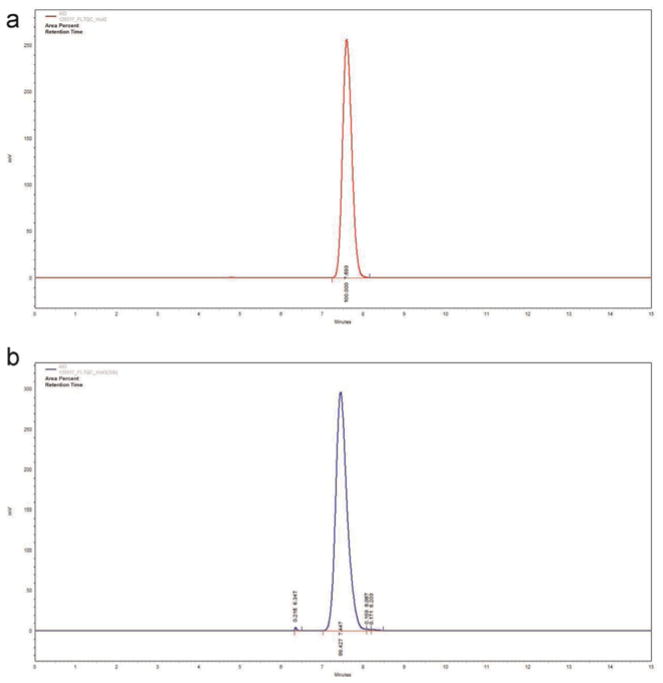
This modification involving the final elution provided the largest improvement to the radiosynthesis, enabling [18F]FLT production at 7.9–19.7 GBq (213–531 mCi) in volumes no greater than 10 mL. The radiochemical purity of the [18F]FLT produced proved consistently 499%, as determined by radio-HPLC analysis (Fig. 2a). Multiple productions were successfully performed in this manner (Table 1). The results show that with a starting activity of [18F]fluoride between 52.5 and 78.8 GBq (1.42–2.13 Ci), we could obtain [18F]FLT in 16–39% decay-corrected radiochemical yield, with specific activities of 1221–4586 TBq/mmol (33,061–123,969 Ci/mmol) and a radiochemical purity 499%. We also compared [18F]FLT prepared with the Coincidence module to a typical sample prepared using the TRACERlab module (Fig. 2b), which revealed that both radiochemical purity and specific activity values were as good, if not better, using the Coincidence module.
Fig. 2.
Radio-HPLC of [18F]FLT synthesized using the: (a) Coincidence module and (b) TRACERlab module.
Table 1.
Production of [18F]FLT with the Coincidence module.
| Run | Starting activity (GBq) | Final activitya (GBq) | Decay-corrected radiochemical yield (%) | Specific activity (TBq/mmol) | Radiochemical purity (%) |
|---|---|---|---|---|---|
| 1 | 68.8 | 13.4 | 29 | 3261.4 | 99.8 |
| 2 | 78.8 | 7.9 | 16 | 1669.4 | 99.5 |
| 3 | 69.9 | 8.8 | 20 | 2302.1 | 99.4 |
| 4 | 56.2 | 11.6 | 31 | 1726.7 | 99.3 |
| 5 | 57.3 | 13.1 | 33 | 1223.3 | 99.5 |
| 6 | 58.1 | 13.8 | 34 | 2235.6 | 99.2 |
| 7 | 58.1 | 13.4 | 35 | 3061.5 | 99.5 |
| 8 | 52.5 | 12.5 | 35 | 1739.8 | 99.9 |
| 9 | 60.1 | 13.7 | 35 | 1590.4 | 99.7 |
| 10 | 77.0 | 19.7 | 37 | 2180.3 | 99.9 |
| 11 | 77.0 | 9.8 | 18 | 4586.9 | 99.4 |
| 12 | 66.2 | 16.9 | 39 | 4360.3 | 99.4 |
| Mean | 65.0 | 12.9 | 30.2 | 2494.8 | 99.54 |
| STD | 9.2 | 3.3 | 7.8 | 1097.3 | 0.23 |
Final activity in 10 mL.
Overall, these [18F]FLT productions with the Coincidence module compared favorably to those performed with the TRA-CERlab module. In our TRACERlab productions, we typically began with twice the starting activity (99.2–139.5 GBq; 2.7–3.8 Ci), which led to decay-corrected radiochemical yields no greater than 8.1%, specific activities of 6.1–95.5 TBq/mmol (165–2581 Ci/mmol), and radiochemical purity 90–99% (Table 2).
Table 2.
Production of [18F]FLT with the TRACERlab module.
| Run | Starting activity (GBq) | Final activitya (GBq) | Decay-corrected radiochemical yield (%) | Specific activity (TBq/mmol) | Radiochemical purity (%) |
|---|---|---|---|---|---|
| 1 | 125.4 | 2.7 | 4.1 | 24.1 | 499 |
| 2 | 99.2 | 2.8 | 5.1 | 36.6 | 97.8 |
| 3 | 139.5 | 2.3 | 3.1 | 26.2 | 97.9 |
| 4 | 128.8 | 3.0 | 4.5 | 6.1 | 499 |
| 5 | 135.4 | 4.5 | 6.6 | 95.5 | 97.8 |
| 6 | 130.6 | 0.5 | 0.7 | 11.3 | 90.3 |
| 7 | 129.9 | 13.4 | 8.1 | 33.9 | 99.7 |
| 8 | 131.0 | 5.3 | 7.7 | 86.2 | 98.7 |
| Mean | 127.5 | 4.3 | 5.0 | 40.0 | 97.0 |
| STD | 12.2 | 4.0 | 2.5 | 33.1 | 3.4 |
Final activity in volume ranging from 6 to 18 mL.
To the best of our knowledge, these Coincidence module productions are the highest specific activities reported to date (up to 4587 TBq/mmol; 123,969 Ci/mmol) and represent a significant improvement from those obtained from the TRACERlab module ( o 100 TBq/mmol; o 2702 Ci/mmol). Reasons for the high specific activity observed through this process, while speculative, could be attributed to the materials used throughout the process. As a standard, [18F]fluoride delivery and transfers are performed using low fluoride burdened materials, such as polymers other than Teflon and Tefzel. Furthermore, since these are strictly preclinical productions, all glassware that comes into contact with the raw [18F]fluoride is typically reused from preparation to preparation, ensuring a low fluoride burden. Interestingly, the measured specific activity has been observed to decrease upon replacement of either delivery lines or glassware.
3.2. [18F]FLT PET imaging
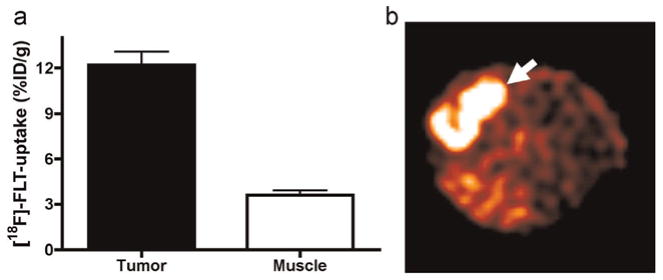
Mice bearing HCT-116 tumors were imaged with PET using [18F]FLT produced on the Coincidence module (67,426.7 Ci/mmol; 2494.8 TBq/mmol). The results show high [18F]FLT uptake within the tumor (Fig. 3a). Additionally, tumor uptake was approximately 3.5-fold higher than in muscle, facilitating excellent imaging contrast (Fig. 3b). These results compare favorably to those obtained using [18F]FLT produced with the TRACERlab module (Manning et al., 2008).
Fig. 3.
(a) Comparison of tracer uptake between tumor and muscle observed in HCT-116 xenografts using high specific activity [18F]FLT. (b) Representative transverse image showing contrast and increased [18F]FLT uptake in the tumor compared to non-tumor tissue.
4. Conclusion
These results show that the Bioscan Coincidence GE FDG reaction module can be modified to provide excellent quality [18F]FLT for preclinical studies. Furthermore, we were able to shorten the run time and more than double the concentration of [18F]FLT by manual elution of the product from the SPE cartridge. The material produced with this module met all required quality control specifications for preclinical use. This approach also takes advantage of SPE cartridges for purification over the traditional and more time-intensive semipreparative HPLC methods typically employed in conjunction with other commercial reaction modules. Moreover, unprecedented, high specific activities were achieved using this approach. In mice bearing colorectal (HCT-116) xenograft tumors, preferential uptake of [18F]FLT was observed within the tumor over muscle. These studies have furthered the capabilities of the Bioscan Coincidence GE FDG reaction module, and as such, we envisaged similar modifications for the production of other [18F]-based PET radiotracers for future molecular imaging studies.
HIGHLIGHTS.
Synthesis of [18F]FLT using a modified Bioscan Coincidence GE FDG reaction module.
Typical decay-corrected yields of 16–39% were obtained (n = 12).
Very high specific activities and radiochemical purity.
High, preferential uptake of [18F]FLT uptake in human colon cancer xenografts.
Acknowledgments
We acknowledge research support from the National Institutes of Health, United States (P50 CA95103; R01 CA140628; RC1 CA145138; P30 DK058404; R25 CA092043; and P50 CA128323), The Kleberg Foundation, United States, and The Lustgarten Foundation.
References
- Grierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[18F]fluorothymidine: [18F]FLT for imaging of cellular proliferation in vivo. Nucl Med Biol. 2008;27:143–156. doi: 10.1016/s0969-8051(99)00104-3. [DOI] [PubMed] [Google Scholar]
- Javed MR, Chen S, Kim HK, Wei L, Czernin J, Kim CJ, Michael VR, Keng PY. Efficient Radiosynthesis of 3′-deoxy-3′-[18F]fluorothymidine using electrowetting-on-dielectric digital microfluidic chip. J Nucl Med. 2014;55:321–328. doi: 10.2967/jnumed.113.121053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Oh SJ, Chi DY, Kil HS, Kim EN, Ryu JS, Moon DH. Simple and highly efficient synthesis of 3′-deoxy-3′-[18 F]fluorothymidine using nucleophilic fluorination catalyzed by protic solvent. Eur J Med Mol Imaging. 2012;34:1406–1409. doi: 10.1007/s00259-007-0391-8. [DOI] [PubMed] [Google Scholar]
- Manning HC, Merchant NB, Foutch C, Virostko JM, Wyatt SK, Shah C, McKinley ET, Xie J, Mutic NJ, Washington MK, LaFleur B, Tantawy MN, Peterson TE, Ansari MS, Baldwin RM, Rothenberg ML, Bornhop DJ, Gore JC, Coffey RJ. Molecular imaging of therapeutic response to epidermal growth factor receptor blockade in colorectal cancer. Clin Cancer Res. 2008;14:7413–7422. doi: 10.1158/1078-0432.CCR-08-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Eisenbarth JA, Mier W, Henze M, Pritzkow H, Haberkorn U, Eisenhut M. A new precursor for the radiosynthesis of [18F]FLT. Nucl Med Biol. 2002;29:263–273. doi: 10.1016/s0969-8051(01)00289-x. [DOI] [PubMed] [Google Scholar]
- McKinley ET, Smith RA, Tanksley JP, Washington MK, Walker R, Coffey RJ, Manning HC. [18F]FLT-PET to predict pharmacodynamic and clinical response to cetuximab therapy in Ménétrier’s disease. Ann Nucl Med. 2012;26:757–763. doi: 10.1007/s12149-012-0636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley ET, Smith RA, Zhao P, Fu A, Saleh SA, Uddin MI, Washington MK, Coffey RJ, Manning HC. 3′-Deoxy-3′-18F-fluorothymidine PET predicts response to V600EBRAF-targeted therapy in preclinical models of colorectal cancer. J Nucl Med. 2013a;54:424–430. doi: 10.2967/jnumed.112.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley ET, Ayers GD, Smith RA, Saleh SA, Zhao P, Washington MK, Coffey RJ, Manning HC. Limits of [18F]-FLT PET as a biomaker of proliferation in oncology. PLoS One. 2013b;8:1–9. doi: 10.1371/journal.pone.0058938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy SK, Rajan MGR. Fully automated and simplified radiosynthesis of [18F]-3′-deoxy-3′-fluorothymidine using anhydro precursor and single alumina purification. J Radioanal Nucl Chem. 2010;283:741–748. [Google Scholar]
- Niedermoser S, Pape M, Gildehaus FJ, Wangler C, Hartenbach M, Schirrmacher R, Bartenstein P, Wangler B. Evaluation of an automated double-synthesis module: efficiency and reliability of subsequent radiosynthesis of FHBG and FLT. Nucl Med Biol. 2012;39:586–592. doi: 10.1016/j.nucmedbio.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Shah C, Miller TW, Wyatt SK, McKinley ET, Olivares MG, Sanchez V, Nolting DD, Buck JR, Zhao P, Ansari MS, Baldwin RM. Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin Cancer Res. 2009;15:4712–4721. doi: 10.1158/1078-0432.CCR-08-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AF, Grierson JR, Dohmen BM, Machulla J, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]