Abstract
Metabolic engineering of microorganisms such as Escherichia coli and Saccharomyces cerevisiae to produce high-value natural metabolites is often done through functional reconstitution of long metabolic pathways. Problems arise when parts of pathways require specialized environments or compartments for optimal function. Here we solve this problem through co-culture of engineered organisms, each of which contains the part of the pathway that it is best suited to hosting. In one example, we divided the synthetic pathway for the acetylated diol paclitaxel precursor into two modules, expressed in either S. cerevisiae or E. coli, neither of which can produce the paclitaxel precursor on their own. Stable co-culture in the same bioreactor was achieved by designing a mutualistic relationship between the two species in which a metabolic intermediate produced by E. coli was used and functionalized by yeast. This synthetic consortium produced 33 mg/L oxygenated taxanes, including a monoacetylated dioxygenated taxane. The same method was also used to produce tanshinone precursors and functionalized sesquiterpenes.
Introduction
Plants synthesize numerous structurally complex compounds that have important therapeutic properties1–6, e.g. paclitaxel, a potent antitumor agent1. Heterologous production of these molecules in industrial microbes—mainly bacteria and yeasts—could provide a robust and sustainable production process. However, in bacteria it has been challenging to functionally express sophisticated eukaryotic enzymes that are often required in the synthesis of complex compounds7; on the other hand, it has been equally difficult to engineer yeasts for high-yield production of building blocks of natural products, e.g. the isoprenoid biosynthetic pathway of bacteria has higher theoretical yield than that of yeasts1.
In nature, microbes can form interacting communities to accomplish chemically difficult tasks through division of labor among different species8. These natural microbial consortia have been used in food and other industries for decades9. Furthermore, interactions of microbial species in mixed microbial cultures were studied extensively in the 60s and 70s10, 11, aiming to establish operating diagrams for maintaining synthetic co-culture, which has been challenging due to difference in their doubling time and secretion of toxic metabolites11. Recently, a few synthetic consortia comprising genetically engineered microbes have been reported for production of biofuels and chemicals12–14. However, these prior studies were mostly concerned with the stability of microbial consortia while the more recent work focused on utilizing non-conventional biomass, e.g. cellulose12, 13. In these examples, which both involved two different species, the first species only provided the carbon source for the second, which harbored the essential pathway for the final product in its entirety and was able to make the final product on its own. Strictly speaking, none of this prior work examined the potential to use more than one species for the purpose of constructing a long synthetic pathway, which enables production of structurally complex compounds.
In this study, we demonstrate the concept of reconstituting a heterologous metabolic pathway in a microbial partnership in which one microbe is engineered to synthesize a metabolic intermediate that is translocated to another microbe, in which it is further functionalized. In principle, it could be attractive to use synthetic microbial consortia for production of valuable metabolites, especially those with complex structures. One major advantage of this design is that each expression system and pathway module can be constructed and optimized in parallel, so that the time required would be significantly reduced. Other advantages of using synthetic consortia include, (i) taking advantage of unique properties and functions of different microbes, (ii) exploring beneficial interactions among consortium members to enhance productivity, and, (iii) minimizing problems arising from feedback inhibition through spatial pathway module segregation.
We report the use of two model laboratory and industrial microbes, E. coli and S. cerevisiae in a consortium to produce precursors of the anti-cancer drug paclitaxel. E. coli is a fast growing bacterium that can be engineered to overproduce taxadiene, the scaffold molecule of paclitaxel1. S. cerevisiae, having advanced protein expression machinery and abundant intracellular membranes, has been suggested as a preferable host for expressing cytochrome P450s (CYPs), which functionalize taxadiene by catalyzing multiple oxygenation reactions15–17. We find that integration of parts of the whole pathway in separate species cultured together combines dual properties of rapid production of taxadiene in E. coli with efficient oxygenation of taxadiene by S. cerevisiae. This novel approach has overcome the challenges of using E. coli alone—perturbation of the fine-tuned taxadiene production by introducing CYPs and functional expression of these enzymes in E. coli1.
RESULTS
Co-culture design to produce paclitaxel precursors
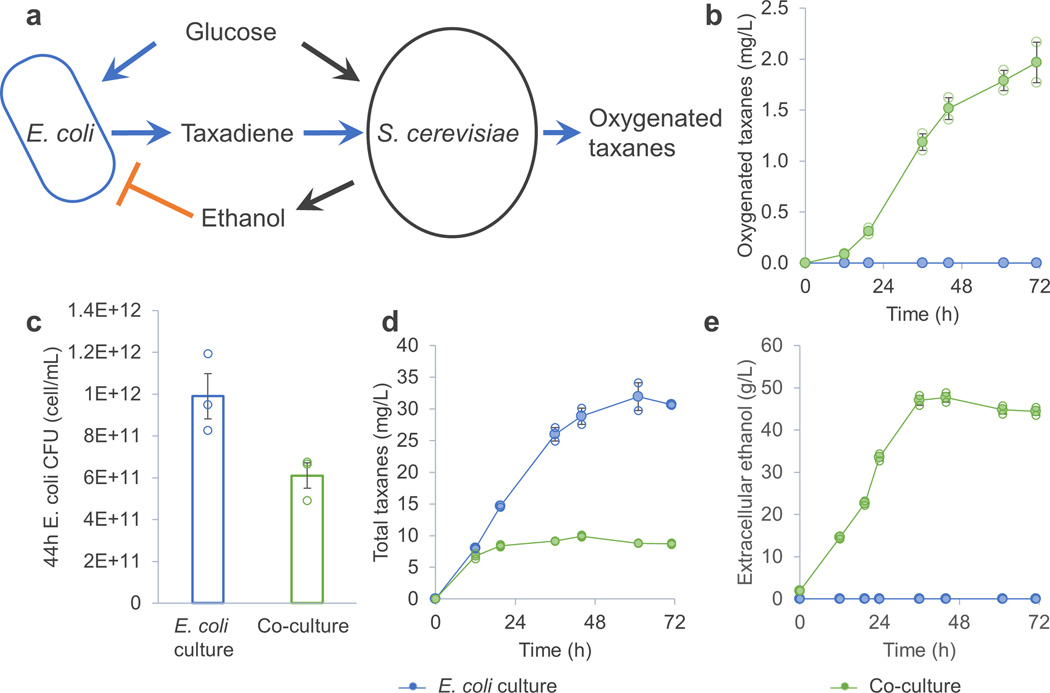
We first engineered S. cerevisiae BY4700 to express taxadiene 5α-hydroxylase and its reductase (5αCYP-CPR, Supplementary Fig.1a), which catalyze the first oxygenation reaction in the pathway of paclitaxel biosynthesis17. Taxadiene was efficiently oxygenated by this yeast (named TaxS1) when taxadiene was externally fed into the culture medium (Supplementary Fig.1b), confirming that the 5aCYP was functional in S. cerevisiae BY4700. Next, we co-cultured this 5αCYP-CPR-expressing yeast with a taxadiene-producing E. coli (named TaxE1) in a fed-batch bioreactor with glucose as the sole carbon and energy source (Figure 1a). The mixed culture produced 2 mg/L of oxygenated taxanes in 72 h (Figure 1b, identification of the oxygenated taxanes is described in online methods, quantification of isoprenoids), whereas in control experiments in which only E. coli TaxE1 (Figure 1b) or S. cerevisiae TaxS1 (data not shown) was cultured, no oxygenated taxanes were produced. These results showed that taxadiene produced by E. coli can diffuse into S. cerevisiae and be subsequently oxygenated. However, the cell density of E. coli (Figure 1c) and the total titer of taxanes (Figure 1d) were significantly reduced in the presence of S. cerevisiae. The cause could be inhibition of E. coli by accumulated ethanol produced by yeast when grown on glucose (Figure 1e). This hypothesis was validated by the fact that ethanol, at the highest concentration observed (50 g/L, Figure 1e), completely inhibited E. coli cell growth and taxadiene production (Supplementary Fig. 2). Similar instances of inhibition have been observed before in natural systems when microbes compete for common resources18.
Figure 1.
A competitive E. coli – S. cerevisiae consortium for production of oxygenated taxanes. (a) Both E. coli TaxE1 and the yeast TaxS1 grew on glucose; E. coli TaxE1 produced taxadiene which can diffuse to the yeast, where it is oxygenated. (b) Only the co-culture produced the oxygenated taxanes. (c) Growth of E. coli TaxE1 was inhibited by the presence of the yeast. (d) The taxane productivity of E. coli TaxE1 was compromised by the presence of the yeast. Total taxanes = Taxadiene + Oxygenated taxanes. (e) These inhibitions could be due to the ethanol produced by the yeast, which was confirmed by follow-up experiments (Supplementary Fig. 2). Error bars, s.e. in all graphs (some error bars are smaller than the plot symbols). All replicates have also been plotted in all graphs (open circle), which indicates the number of replicates for each experiment.
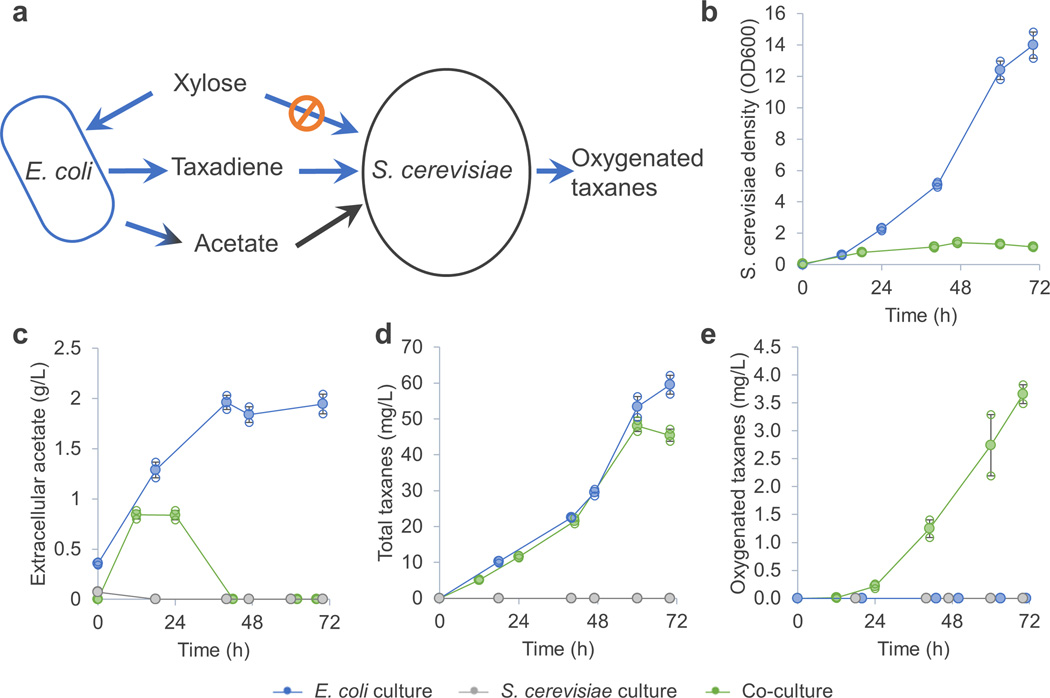
To overcome this problem we designed a mutualistic interaction between the two microorganisms 18. When E. coli metabolizes xylose it excretes acetate, which is inhibitory to its own growth19. S. cerevisiae, on the other hand, cannot metabolize xylose but can use acetate as the sole carbon source for growth without producing ethanol (Figure 2a, Supplementary Table 1). We therefore switched the co-culture carbon source from glucose to xylose. Under these conditions S. cerevisiae only grew in the xylose medium in the presence of E. coli (Figure 2b), and the concentration of extracellular acetate in the co-culture was significantly reduced compared with that observed when E. coli was grown on xylose on its own (Figure 2c). More importantly, this stable co-culture minimised the ethanol concentration to below the limit of detection (0.1 g/L) throughout the experiment. In addition, the titer of total taxanes produced by E. coli was not substantially affected by the presence of S. cerevisiae (Figure 2d), suggesting that ethanol inhibition of E. coli was successfully eliminated and taxadiene production proceeded unabated by the presence of yeast. However, although more oxygenated taxanes were produced in this co-culture (4 mg/L in 72 h, Figure 2e) compared with the previous co-culture (2 mg/L in 72 h, Figure 1b), the taxadiene oxygenation efficiency was still low (only 8% of total taxadiene produced, Figure 2).
Figure 2.
A mutualistic E. coli – S. cerevisiae consortium for production of oxygenated taxanes. (a) E. coli TaxE1 grew on xylose and produced acetate that served as sole carbon source for the yeast to grow. The taxadiene produced by E. coli TaxE1 was oxygenated in yeast TaxS1. (b) Yeast TaxS1 could only grow in presence of the E. coli TaxE1. (c) Yeast TaxS1 removed the acetate produced by E. coli TaxE1. (d) The presence of yeast TaxS1 did not compromise taxane production of E. coli TaxE1. (e) Yeast TaxS1 can only produce oxygenated taxanes when E. coli TaxE1 supplied taxadiene. The taxadiene oxygenation efficiency of this co-culture was 8% (4 mg/L out of 50 mg/L taxadiene was oxygenated). Error bars, s.e. in all graphs (some error bars are smaller than the plot symbols). All replicates have also been plotted in all graphs (open circle), which indicates the number of replicates for each experiment.
Optimization to Improve taxadiene oxygenation
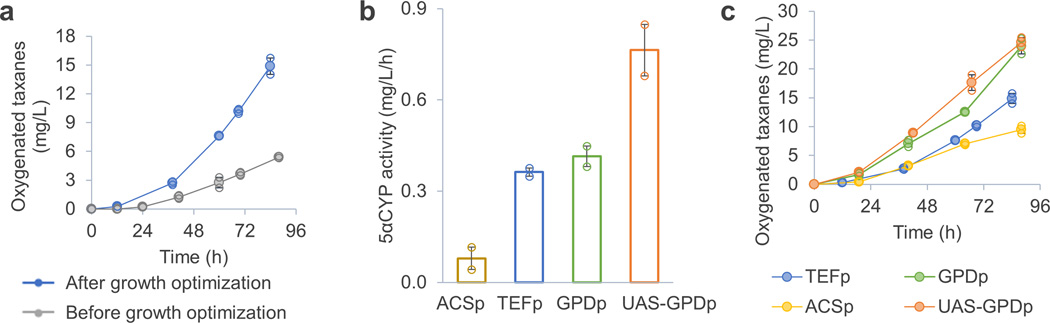
To increase taxadiene oxygenation, we first focused on optimizing the growth of S. cerevisiae, using the rationale that more yeast cells would express more 5αCYP and therefore functionalize more taxadiene. We noted that acetate accumulated in the co-culture during the first 24 h (Figure 2c), indicating that the initial yeast population was insufficient to convert all available substrate in the medium. This was corrected by increasing the initial inoculum of yeast and also periodically feeding additional carbon (xylose), nitrogen (ammonium) and phosphorous (phosphate) sources to ensure that these major nutrients were not limiting yeast growth. After these modifications, no acetate was detected throughout the entire fermentation and the oxygenated taxane titer was improved ~3 fold (16 mg/L in 90 h, Figure 3a). Under these conditions, as growth of S. cerevisiae was strictly limited by the amount of acetate secreted by E. coli, further increase of the relative amount of yeast in the culture relied on engineering the acetate pathway in E. coli (see below). We opted not to feed exogenous acetate in order to preserve the autonomous nature of the co-culture (Supplementary Fig. 3).
Figure 3.
Optimizing the yeast growth and engineering the yeast promoters improved production of the oxygenated taxanes. (a) Growth optimization (increasing the yeast inoculum and feeding additional nutrients) improved production of the oxygenated taxanes by more than two-fold. (b) A stronger promoter (UAS-GPDp), compared to the previously used TEFp, was found in the promoter screening in terms of taxadiene oxygenation. (c) The co-culture using UAS-GPDp also produced significantly (p<0.01, based on Student’s t-test) more oxygenated taxanes than that using TEFp. Error bars, s.e. in all graphs (some error bars are smaller than the plot symbols). All replicates have also been plotted in all graphs (open circle), which indicates the number of replicates for each experiment.
We next improved the specific oxygenation activity of yeast TaxS1. 5αCYP-CPR (fused as a single polypeptide, Supplementary Fig. 1a) was previously expressed under a strong constitutive promoter (TEFp). We replaced TEFp by GPDp (a widely used strong promoter20), UAS-GPDp (an enhanced version of GPDp21) and ACSp (a promoter from the acetate assimilation pathway22, 23 that we hypothesized to be strong in our study since yeast TaxS1 grew on acetate here) and tested taxadiene oxygenation efficiency by the corresponding strains. To this end, yeast strains (TaxS1, TaxS2, TaxS3 and TaxS4) were cultured without E. coli and the oxygenation rate of exogenously supplied taxadiene was measured (Figure 3b). Based on the results of this assay, UAS-GPDp was selected as strongest promoter. Yeast strain TaxS4 was then co-cultured using xylose as a substrate with E. coli TaxE1, and this co-culture produced significantly higher concentrations of oxygenated taxanes (25 mg/L in 90 h) compared with a co-culture in which the TEFp promoter was used (16 mg/L in 90 h, Figure 3c). GPDp and ACSp were also tested in co-culture (Figure 3c), and the results were fairly consistent with those of the feeding experiments (Figure 3b), e.g. ACSp, the promoter characterized to be weaker than TEFp in the feeding experiment, also led to lower production of oxygenate taxanes compared with TEFp in co-cuture (Figure 3c).
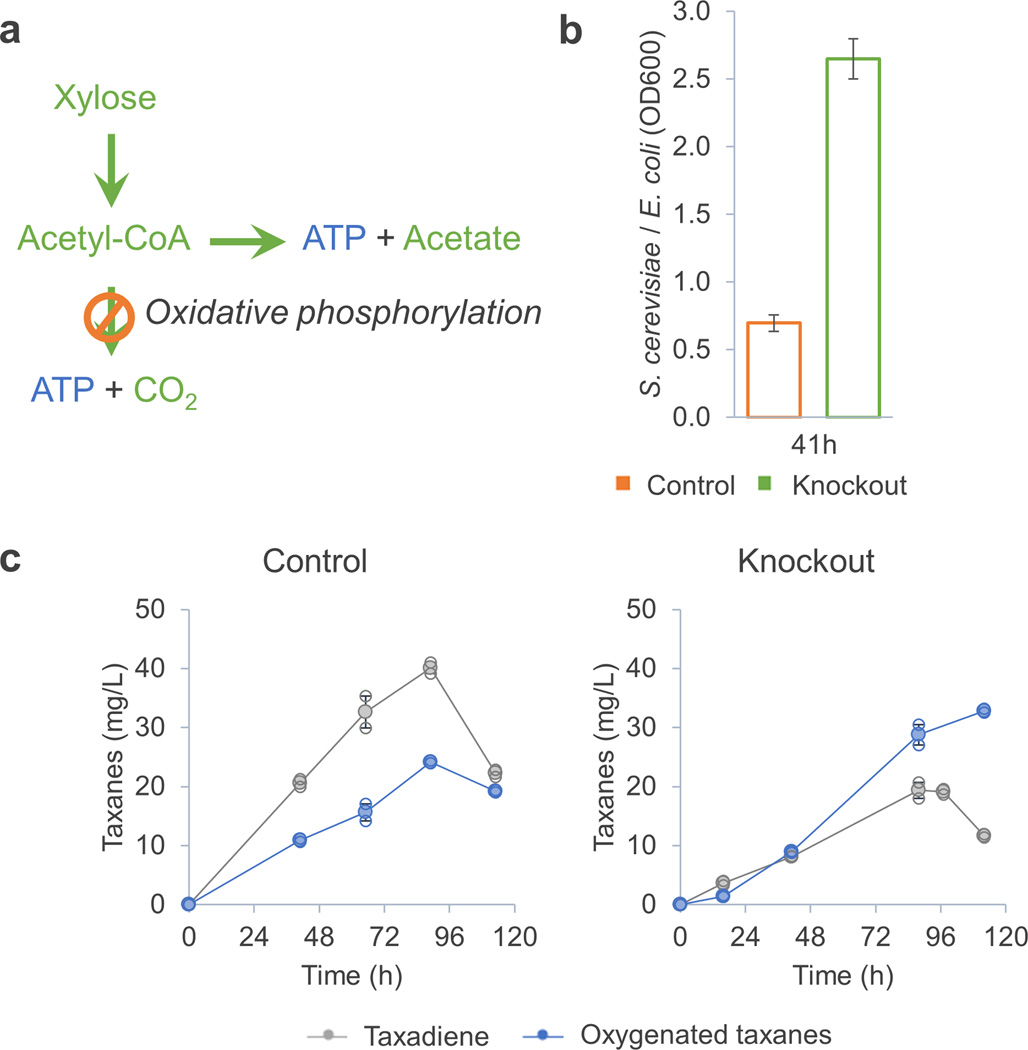
After increasing oxygenation efficiency in yeast, we engineered E. coli to overproduce acetate and thereby further potentially improve the growth rate of S. cerevisiae by increasing the concentration of available substrate. Production of acetate by E. coli is auto-regulated: when acetate accumulates, E. coli growth is inhibited, resulting in lower acetate production rate. First, we overexpressed the genes in the E. coli acetate production pathway (phosphate acetyltransferase, pta, and acetate kinase, ackA), but this neither increased the S. cerevisiae population density nor the oxygenation efficiency substantially (Supplementary Fig. 4). To overcome this problem, we inactivated oxidative phosphorylation by knocking out atpFH24, which is the primary means of ATP production under aerobic conditions. The rationale for this modification was that the atpFH knock-out would force E. coli to produce more acetate, because acetate production would, under these conditions, become the primary ATP generation pathway (Figure 4a). Indeed, this E. coli mutant (named TaxE4) produced up to 5.0±0.1 g/L acetate in test tube, while the parental strain (E. coli TaxE1) only produced 2.3±0.2 g/L acetate. The relative S. cerevisiae population was also much larger when yeast TaxS4 was co-cultured with E. coli TaxE4 compared to that with E. coli TaxE1 (Figure 4b). More importantly, the titer of the oxygenated taxanes was further improved (33 mg/L in 120 h), and the percentage of the taxadiene oxygenated was significantly increased (up to 75%, Figure 4c). Another strategy that could be tested in future to further improve acetate production is the knockout of E. coli ACS, which assimilates extracellular acetate under certain conditions. Such a knockout might make more of the produced acetate available to the yeast strain.
Figure 4.
Inactivating oxidative phosphorylation of the E. coli improved yeast growth and production of the oxygenated taxanes. (a) Inactivation of the E. coli oxidative phosphorylation forces the production of acetate, which became the major pathway of generating ATP in the E. coli. (b) The acetate-overproducing E. coli (TaxE4) improved the yeast growth in the co-culture. Control: TaxE1-TaxS4 co-culture; Knockout: TaxE4-TaxS4 co-culture. (c) The taxadiene oxygenation efficiency was greatly improved when the S. cerevisiae was co-cultured with the acetate-overproducing E. coli. Oxygenation efficiency of the TaxE1-TaxS4 co-culture was ~50% (20 mg/L oxygenated taxanes per 40 mg/L total taxanes), and that of the TaxE4-TaxS4 co-culture was ~75% (30 mg/L oxygenated taxanes per 40 mg/L total taxanes). Error bars, s.e. in all graphs (some error bars are smaller than the plot symbols). All replicates have also been plotted in all graphs (open circle, except b, in which N=4), which indicates the number of replicates for each experiment.
Co-culture to produce monoacetylated dioxygenated taxane
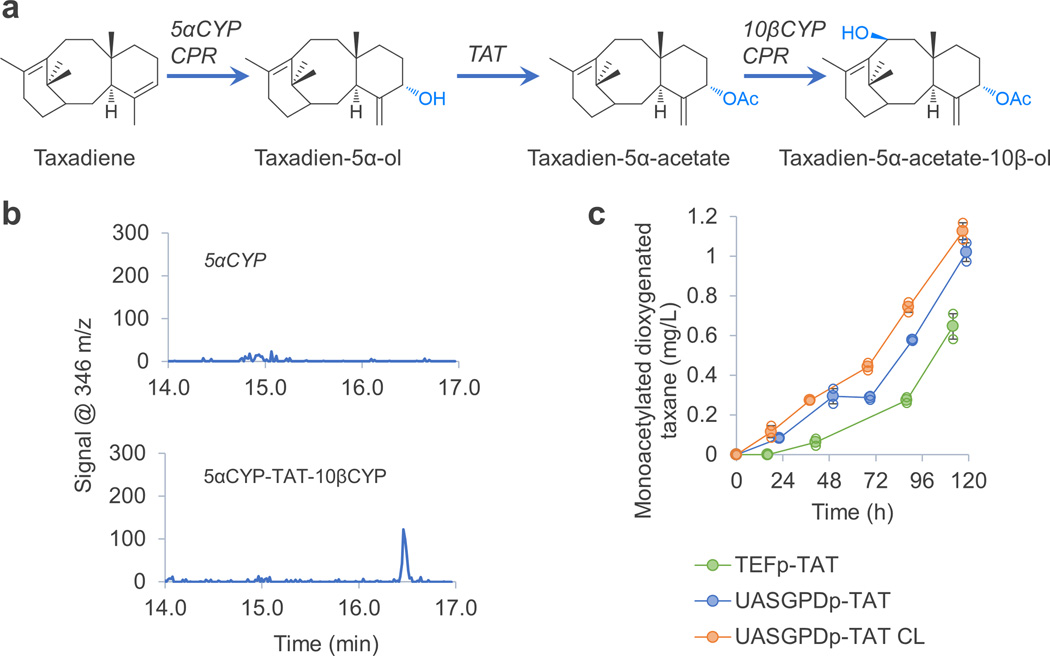
We further engineered the co-culture to produce more complex paclitaxel precursors. A prevailing theory of paclitaxel early-synthesis suggests taxadien-5α-ol is acetylated at its C-5α position, followed by oxygenation at the C-10β position15 (Figure 5a). Because of the modular nature of our microbial consortium, the ability to functionalize taxadien-5α-ol could be achieved by engineering of only the yeast module. Taxadien-5α-ol acetyl-transferase (TAT25) and taxane 10β-hydroxylase (10βCYP26, fused with a CYP reductase1) were co-expressed in yeast TaxS4. When the resulting yeast (named as TaxS6) was co-cultured with E. coli TaxE4, the co-culture produced a monoacetylated dioxygenated taxane (molecular weight 346), which was identified as a single peak on the extracted ion chromatography (346 m/z, GCMS) and was absent from the control co-culture not expressing the TAT and 10βCYP (Figure 5b). A 13C labelling experiment confirmed that the oxygenated diol was indeed derived from taxadiene (Supplementary Fig. 5). The identified compound could be taxadien-5α-acetate-10β-ol, an important intermediate in the paclitaxel synthesis15, because its spectrum contained many of its fragment ions (346, 303, 286, 271 and 243 m/z27, Supplementary Fig. 5). To improve the titer and yield of this compound, we used a stronger promoter for expressing TAT (strain TaxS7), and the change of promoter improved the titer from 0.6 mg/L to 1 mg/L (Figure 5c), confirming the hypothesis that this step was limiting. We then operated the bioreactor under a xylose limited condition, which further increased the titer and also substantially improved the yield, by reducing the xylose consumption (from ~120 g/L to 80 g/L, Figure 5c, Supplementary Fig. 6). This is the first report of producing a monoacetylated dioxygenated taxane from a simple substrate (xylose) in microbes, and it reveals the usefulness of the modularity of a microbial partnership for synthesis of complex metabolites.
Figure 5.
Production of a monoacetylated dioxygenated taxane by the E. coli – S. cerevisiae co-culture. (a) Early paclitaxel biosynthetic pathway. (b) The yeast co-expressing 5αCYP-CPR, TAT and 10βCYP-CPR (TaxS6) produced putative taxadien-5α-acetate-10β-ol when co-cultured with a taxadiene-producing E. coli. Extracted ion chromatograms (346 m/z, molecular weight of monoacetylated dioxygenated taxane) are shown here. 5αCYP: TaxE4/TaxS4 co-culture; 5αCYP-TAT-10βCYP: TaxE4/TaxS6 co-culture. (c) Using a stronger promoter (UASGPDp) to express TAT improved titer of the monoacetylated dioxygenated taxane. Operating the bioreactor at a carbon-limited (CL) condition further improved the production titer and yield (xylose consumption was reduced by 30%). TEFp-TAT: TaxE4/TaxS6 co-culture, where expression of TAT was driven by TEFp; UASGPDp-TAT: TaxE4/TaxS7 co-culture, where UASGPDp was used to express TAT; UASGPDp-TAT CL: TaxE4/TaxS7 co-culture at a carbon limited condition. Error bars, s.e. in all graphs (some error bars are smaller than the plot symbols). All replicates have also been plotted in all graphs (open circle), which indicates the number of replicates for each experiment.
Production of other oxygenated isoprenoids by co-culture
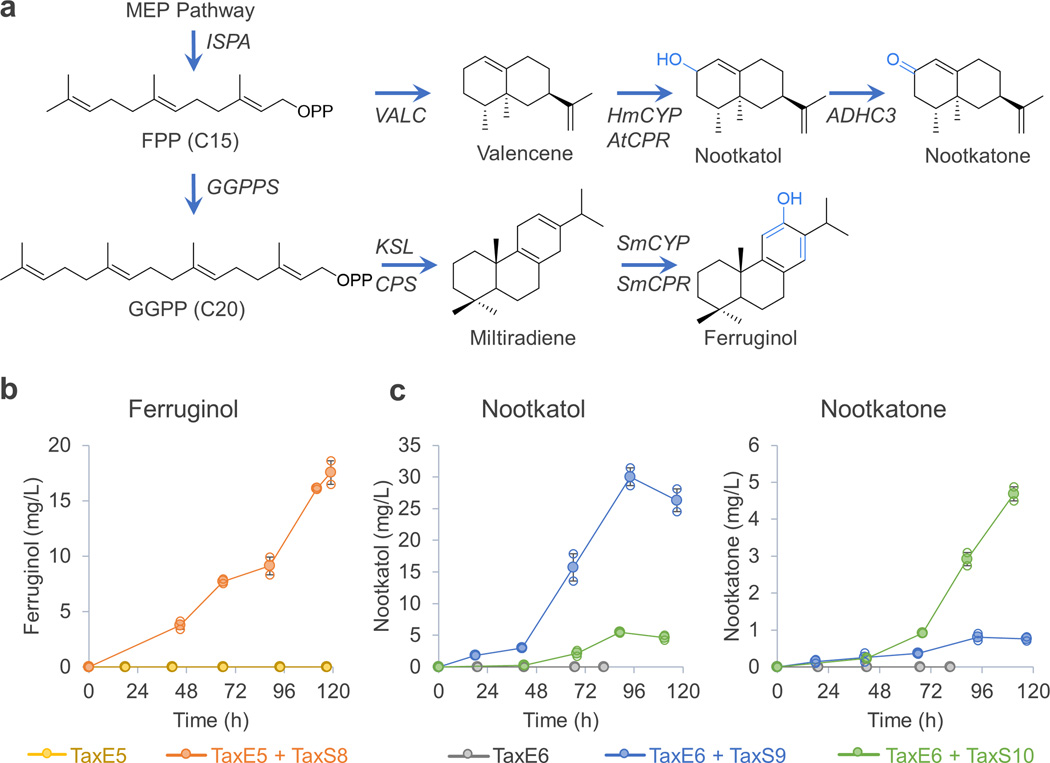
The E. coli-S. cerevisiae co-culture developed in this study could be used for production of any compound if one of the pathway precursors can cross cell membranes. The method should be applicable to most isoprenoids, the largest class of natural products, because their scaffold molecules are generally membrane-permeable. To test this hypothesis, we examined the synthesis of another diterpene, ferruginol, the precursor of tanshinone, which is in clinical trials for treating heart disease28, 29. We replaced taxadiene synthase in E. coli TaxE4 with two enzymes (KSL and CPS, resulting in strain TaxE7) that are required for synthesizing miltiradiene29, a membrane-crossing molecule. At the same time, in S. cerevisiae BY4700 we overexpressed a specific CYP and its reductase (SmCYP and SmCPR, resulting in strain TaxS8), which were reported to oxygenate miltiradiene into ferruginol28 (Figure 6a). When E. coli TaxE5 and yeast TaxS8 were co-cultured in the xylose medium, the co-culture successfully produced 18 mg/L ferruginol (Figure 6b), which exceeds the highest titer reported in the literature (10 mg/L by S. cerevisiae28). This shows that the co-culture concept is generally applicable to diterpenes, and demonstrates the advantages of co-culture over mono-culture, that is, being able to construct parts of the pathway in parallel and achieve higher titers owing to microbial cooperation.
Figure 6.
Use of the E. coli, S. cerevisiae co-culture for production of other oxygenated Isoprenoids. (a) Illustration of biosynthetic pathways of ferruginol and nootkatone. (b) An E. coli was engineered to produce miltiradiene from xylose (TaxE7), which cannot produce ferruginol on its own. When this E. coli was co-cultured with a yeast expressing a specific CYP and its reductase (TaxS8), the co-culture can produce 18 mg/L ferruginol. Mass spectrum of the produced ferruginol was identical to the one in the literature (data not shown). (c) Similarly, an E. coli was engineered to produce valencene (TaxE8); itself cannot produce any oxygenated valencene. When it was co-cultured with a yeast expressing a specific CYP and its reductase (TaxS9), the co-culture can produce 30 mg/L nootkatol and low quantity of nootkatone. When an alcohol dehydrogenase was introduced to TaxS9, the resulting strain TaxS10 can produce 4 mg/L nootkatone in presence of TaxE8. Error bars, s.e. in all graphs. (some error bars are smaller than the plot symbols). All replicates have also been plotted in all graphs (open circle), which indicates the number of replicates for each experiment.
Finally, we synthesized a sesquiterpene—nootkatone, which is a high-end fragrance molecule30. Similarly, we replaced the taxadiene synthase and geranylgeranyl diphosphate synthase in E. coli TaxE4 with a sesquiterpene synthase (VALC, resulting in strain TaxE8) to produce valencene, and in yeast BY4700 we overexpressed a specific CYP and its reductase (HmCYP and AtCPR, resulting in strain TaxS9) that can oxygenate valencene30 (Figure 6a). When these strains (TaxE8 and TaxS9) were co-cultured, they produced 30 mg/L nootkatol and a small quantity of nootkatone (0.8 mg/L, Figure 6c). Recently, a Pichia alcohol dehydrogenase (PpADH3C) was shown to oxidize nootkatol in its native host30. We introduced this enzyme to yeast TaxS9, yielding strain TaxS10 which upon co-culture with E. coli TaxE8, increased the nootkatone titer by a factor of 5 (4 mg/L, Figure 6c). Again, these results supported the hypothesis that the co-culture concept should be widely applicable to production of oxygenated isoprenoids.
DISCUSSION
Our major motivation for using a stable co-culture is the introduction of modularity to the design of pathways for microbial metabolite production by assigning a different part of the metabolic pathway to each member of a partnership or synthetic consortium. In such an experimental set-up pathway modules can be separately optimized and assembled to enable optimal functioning of the complete pathway. The examples in this report demonstrate this modularity: the screening of a better promoter for CYP expression in yeast could be carried out independent of E. coli (Figure 3), and producing the acetylated diol in the co-culture also only required modification of one of its modules (Figure 5). Such modularity should significantly expedite the reconstruction of long biosynthetic pathways in microorganisms as the construction of the cells carrying the pathway modules can be carried out in parallel and the number of genetic modifications per cell is substantially reduced. To achieve this modularity, pathway modules in different cells should not directly interact with each other to minimize possible regulation. For example, CYPs and their reductase involved in taxane oxygenation generate reactive oxygen species31, 32, which inhibit two enzymes (ISPG and ISPH) in the taxadiene biosynthetic pathway containing iron-sulfur clusters that are hyper-sensitive to ROS33. Spatial segregation, in two different microbes, of the pathway of taxadiene production from its oxygenation pathway prevents inactivation of ISPG/ISPH by ROS generated by CYPs.
Because of modularity of a co-culture approach, we were able to exploit advantages of the different species. Before this study, taxadiene could only be overproduced in E. coli1 while most biochemical characterizations of the taxadiene-functionalizing enzymes were carried out in S. cerevisiae16, 26, 34. By using E. coli to synthesize taxadiene and S. cerevisiae to functionalize it, we combined the advantages of the two species for taxane production (fast growth of E. coli and complete protein expression system of S. cerevisiae). Using co-culture, we were able to synthesize a complex taxane (putative taxadiene-5α-acetate-10β-ol) (Figure 5) that has never been produced by microorganisms growing on a simple carbon source in the past, and achieve higher titers of isoprenoid production than has been reported previously (Figure 6b).
As most synthetic microbial consortia are competitive12, 13 (Supplementary Fig. 7), a primary challenge in their design is to avoid the dominance of one species over another, due to a shorter doubling time11, 12 or production of substances that are inhibitory to the other species13. Conventionally, titration of the inoculum ratio13 and optimization of growth conditions (such as pH and temperature11) can be exploited to maintain coexistence. However, these strategies require time-consuming experimental trials or construction of sophisticated mathematical models13, whose parameters also need to be estimated experimentally. In addition, batch-to-batch variability can be high in these competitive co-cultures (data not shown). In this study, we avoided these complications by building a mutualistic co-culture in which S. cerevisiae used as its sole carbon source acetate, which was provided by and inhibitory to E. coli, which in turn grew better in the presence of yeast compared to without the yeast (Supplementary Fig. 8). We applied additional genetic and growth constraints to enforce this cooperation, for instance, the respiration-deficient E. coli was forced to produce acetate as this was its primary way to generate cellular ATP (Figure 4a), and the yeast also had to consume acetate because it cannot utilize xylose (Figure 2a). Under such interdependency, the inoculum ratio of our co-culture can be simply set to over-inoculation of yeast (the inoculum ratio of yeast to E. coli was approximately 40:1, online methods, bioreactor experiments for the E. coli – S. cerevisiae co-culture). This eliminated the inhibitory acetate levels, but did not result in yeast overpopulation, because yeast growth was strictly limited by the concentration of acetate produced by E. coli, leading to a balanced ratio of the two species (the ratio of yeast to E. coli was 1:2 at 41 h, Figure 4b). Furthermore, this ratio was controllable through altering the specific acetate productivity (Figure 4b). Because of this ability to alter the consortium composition by increasing the relative yeast population, we managed to minimize accumulation of the pathway intermediate (taxadiene) and increase the titer of oxygenated taxanes (Figure 4c and Supplementary Fig. 9).
In addition to the mutualistic design, we also explored other strategies to avoid microbial competition. The first was a two-stage culture, in which E. coli was cultured separately for a few days before mixing with an active S. cerevisiae culture. This approach allowed both microbes to grow at their preferred conditions and taxadiene to be efficiently oxygenated (Supplementary Fig. 10). However, this process required a longer cultivation time (180 h) and, additionally, it is more complicated than that of the mutualistic co-culture. We also explored a two-carbon-source strategy, in which xylose can only be utilized by E. coli and ethanol (manually added at low concentration, <2 g/L) was exclusively used by yeast (Supplementary Fig. 11). A stable co-culture could be maintained under these conditions by controlling the ethanol addition, and oxygenated taxanes were also produced at a relatively high titer (8 mg/L in 130 h, Supplementary Fig. 12). However, both E. coli and S. cerevisiae produced acetate under this scheme leading to microbial inhibition (Supplementary Fig. 12), which was eliminated in the mutualistic design.
The co-culture concept is not restricted to the pairing E. coli with S. cerevisiae. We have briefly explored the use of two different E. coli strains for production of oxygenated taxanes (Supplementary Fig. 13), which worked, although the titer was low, mainly due to lack of the mutualistic interactions present in the E. coli-S. cerevisiae co-culture. As a general guideline, a target pathway should be divided into modules, each of which should be assigned to a specific host strain so that the combined genetic traits of the consortium strains are favorable for pathway completion. These microorganisms should rely on each other for supply of an essential nutrient or detoxification of an inhibitory substance, ensuring a stable and controllable microbial composition.
A necessary condition for co-culture is that the pathway intermediate (taxadiene) can cross cell membranes and is secreted to the extracellular medium. This property was first confirmed for taxadiene in prior studies where organic solvent mixed with E. coli cell culture was found to efficiently extract taxadiene (C20) from the cells in a bioreactor1. We also measured distribution of taxadiene in E. coli, medium and yeast in this study, which confirmed that taxadiene can cross cell membranes efficiently even in absence of an organic solvent (Supplementary Fig. 14). This physiochemical property is shared by many isoprenoids ranging from C5 to C40, including isoprene35, limonene3, amorphadiene36 and canthaxanthin37. Hence, the co-culture concept should be generally applicable to the production of most isoprenoids (in this study, we have experimentally validated production of sesquiterpene and diterpene, Figure 6).
The experiments reported here provide evidence that a secondary metabolite pathway can be reconstructed in a microbial consortium, paving the way for engineering the microbial synthesis of natural compounds with complex structures that currently cannot be efficiently synthesized in a single microbe such as alkaloids and flavonoids (including >10,000 molecules), which all derived from aromatic amino acids that can be high-titer produced and excreted by E. coli38 and functionalized by S. cerevisiae39. The co-culture can also benefit producing short chain dicarboxylic acids (C6-C10), whose precursors are short chain fatty acids that can be easily produced in engineered E. coli40, 41 and efficiently oxidized in the yeast expressing CYPs42.
ONLINE METHODS
E. coli strains
E. coli TaxE1 was previously constructed by Chin Giaw Lim in our lab (unpublished works). In brief, the MEP operon1 (dxs-idi-ispDF controlled by T7 promoter) and the TG operon1 (ts-ggpps controlled by T7 promoter) were integrated into locus araA and locus lacY of E. coli MG1655_ΔrecA_ΔendA_DE31 respectively. Strains used in this study are summarized in Supplementary Table 2.
To engineer E. coli TaxE1 to overproduce acetate, we overexpressed pta or pta-ackA operon by using a pSC101 based plasmid containing trc promoter (p5trc1). pta or ackA amplified from E. coli MG1655 chromosome was assembled with part of p5trc by using the recently developed Cross-Lapping In Vitro Assembly (CLIVA) method43 (primer P1-P6 used), yielding plasmid p5trc-pta and p5trc-ackA respectively. Primers used in this study are summarized in Supplementary Table 3. All the plasmids constructed in this study were validated via sequencing. Plasmid p5trc-pta was transformed into E. coli TaxE1, yielding E. coli TaxE2. ackA with trc promoter and terminator was amplified from p5trc-ackA and cloned into p5trc-pta via CLIVA (primer P7-P10 used), yielding plasmid p5trc-pta-trc-ackA. This plasmid was transformed into E. coli TaxE1, yielding E. coli TaxE3. After overexpression of pta and pta-ackA, we inactivated oxidative phosphorylation of E. coli TaxE1 by knocking out atpFH as described previously24 (primer P11 and P12 used), yielding E. coli TaxE4.
To construct E. coli to produce miltiradiene, we knocked out atpFH of E. coli TaxE5 (a strain previously constructed by Chin Giaw Lim in our lab, unpublished works) as described previously24 (primer P11 and P12 used), resulting in strain TaxE6. Then we transformed plasmid p5T7-KSL-CPS-GGPPS into E. coli TaxE6, resulting in strain TaxE7. To obtain plasmid p5T7-KSL-CPS-GGPPS, KSL and CPS amplified from synthetic DNA were assembled with part of p5T7TG1 via CLIVA (primer P13-P18 used). To construct E. coli to produce valencene, ispA amplified from E. coli genome and valC amplified from synthetic DNA were assembled with part of p5T7TG via CLIVA (primer P18 – P23 used), yielding plasmid p5T7-ISPA-VALC, which was transformed into E. coli TaxE6, resulting in strain TaxE8.
To engineer E. coli to express taxadiene 5a–hydroxylase with its reductase (5αCYP-CPR, as a fusion protein), plasmid p5trc-5αCYP-CPR was transformed into E. coli MG1655_ΔrecA_ΔendA_DE3, yielding E. coli TaxE9. Plasmid p5trc-5αCYP-CPR was previously constructed by Chin Giaw Lim in our lab (unpublished works). To obtain this plasmid, coding sequence of 5αCYP-CPR amplified from p10At24T5αOH-tTCPR1 was cloned into p5trc. To be compatible with E. coli TaxE9, E. coli EDE3Ch1TrcMEPp5T7TG1 (named as TaxE10 in this study) was used to produce taxadiene in the E. coli – E. coli co-culture, as both strains were resistant to spectinomycin. An E. coli carrying unbalanced taxadiene synthetic pathway was also constructed in this study: plasmid p5T7TG was transformed into E. coli TaxE4, resulting in strain TaxE11.
S. cerevisiae strains
S. cerevisiae BY4700 (ATCC 200866, MATa ura3Δ0) was used to express the 5αCYP-CPR. Its coding gene amplified from plasmid p10At24T5αOH-tTCPR1 was cloned into plasmid p416-TEF (ATCC 87368) by using the restriction enzyme cloning (XbaI and HindIII, primer P24 and P25 used), yielding plasmid p416-TEFp-5αCYP-CPR. The auxotrophic marker and expression cassette of the new plasmid (URA-TEFp-5αCYP-CPR-CYC1t) was via CLIVA cloned into the integration shuttle vector pUC-YPRC15 (primer P26-P29 used), which was constructed by cloning PCR fragment of BY4700 YPRC locus into plasmid pUC19 (New England Biology) via the restriction enzyme cloning (NotI and EcoRI, primer P30-P33 used). The resulting plasmid (pUC-YPRC15-URA-TEFp-17α5αCYP-CPR-CYCt) was linearized by using NotI and transformed into BY4700 (YPRC15 locus44), yielding yeast TaxS1. This construction was illustrated in Supplementary Fig. 15.
To replace the TEFp with GPDp and ACSp, GPDp amplified from plasmid p414-GPD (ATCC 87356) or ACSp amplified from BY4700 chromosome was combined with part of pUC-YPRC15-URA-TEFp-5αCYP-CPR via CLIVA (primer P34-P41 used), yielding plasmid pUC-YPRC15-URA-GPDp-5αCYP-CPR-CYCt and pUC-YPRC15-URA-ACSp-5αCYP-CPR-CYCt respectively. These two plasmids were linearized by using NotI and transformed into BY4700 (YPRC15 locus), yielding yeast TaxS2 and TaxS3 respectively. To add upstream activation sequence (UAS) to GPDp, the UASTEF-UASCIT1-UASCLB221 was synthesized (as gblock gene fragment, Integrated DNA Technologies) and cloned into pUC-YPRC15-URA-GPDp-5αCYP-CPR-CYCt via CLIVA (primer P42-P45 used), yielding pUC-YPRC15-URA-UAS-GPDp-5αCYP-CPR-CYCt. This plasmid was linearized by using NotI and transformed into BY4700 (YPRC15 locus), yielding yeast TaxS4. Sequences of all the synthetic genes used in this study are summarized in Supplementary Table 4.
S. cerevisiae BY4719 (ATCC 200882, MATa trp1Δ63 ura3Δ0) was used to co-express 5aCYP-CPR, taxadien-5α-ol acetyl-transferase (TAT) and taxane 10β-hydroxylase with its reductase (10βCYP-CPR, as a fusion protein). Plasmid pUC-YPRC15-URA-GPDp-5αCYP-CPR-CYCt was linearized by using NotI and first transformed into BY4719 (YPRC15 locus), yielding yeast TaxS5. To further express TAT and 10βCYP-CPR in TaxS5, we constructed an integration vector (pUC-PDC6-TRP) that targeted locus PDC6 and contained TRP marker. First, plasmid pUC19 was combined with PCR fragment of BY4700 PDC6 locus via CLIVA (primer P46-P49 used), yielding integration plasmid pUC-PDC6. The auxotrophic marker (TRP) of plasmid p414-GPD was then cloned into pUC-PDC6 via CLIVA (primer P50-P53 used), yielding integration plasmid pUC-PDC6-TRP. After the construction of the integration vector, coding gene of Taxus cuspidata TAT was synthesized (Genscript) and cloned into plasmid pJA11545 via CLIVA (primer P54-P57 used), yielding p426-TEFp-TAT-ACTt. Coding gene of Taxus cuspidate 10βCYP was synthesized (as gblocks gene fragments, Integrated DNA Technologies) and cloned into pUC-YPRC15-URA-GPDp-5αCYP-CPR to replace the 5αCYP via CLIVA (primer P58-P63 used), yielding pUC-YPRC15-URA-GPDp-10βCYP-CPR-CYCt. The expression cassettes of these two plasmids (TEFp-TAT-ACTt and GPDp-10βCYP-CPR-CYCt) were assembled with part of the integration vector pUC-PDC6-TRP via CLIVA (primer P64-P69 used), yielding pUC-PDC6-TRP-(GPDp-10βCYP-CPR-CYCt)-(TEFp-TAT-ACTt). This plasmid was linearized by using NotI and transformed into TaxS5 (PDC6 locus44), yielding yeast TaxS6.
To replace the promoter of TAT (TEFp) with a stronger promoter (UASGPDp), coding gene of TAT amplified from plasmid p426-TEFp-TAT-ACTt was assembled with part of pUC-YPRC15-URA-UAS-GPDp-5αCYP-CPR-CYCt via CLIVA (primer P59 and P70–72 used), resulting in plasmid pUC-YPRC15-URA-UAS-GPDp-TAT-CYCt; coding gene of 10βCYP-CPR amplified from pUC-YPRC15-URA-GPDp-10βCYP-CPR-CYCt was assembled with part of p426-TEFp-TAT-ACTt via CLIVA (primer P56, P57, P73 and P74 used), resulting in plasmid p426-TEFp-10βCYP-CPR-ACTt. Then expression operon of plasmid pUC-YPRC15-URA-UAS-GPDp-TAT-CYCt and plasmid p426-TEFp-10βCYP-CPR-ACTt was assembled with part of integration plasmid pUC-PDC6-TRP via CLIVA (primer P65-P68, P75 and P76 used), resulting in plasmid pUC-PDC6-TRP-(TEFp-10βCYP-CPR-ACTt)-(UAS-GPDp-TAT-CYCt), which was linearized by NotI and transformed into TaxS5, resulting in strain TaxS7.
To construct the yeast that can oxygenate miltiradiene, coding gene of SmCYP-SmCPR were synthesized and assembled with part of plasmid pUC-YPRC15-URA-UAS-GPDp-5αCYP-CPR-CYCt via CLIVA (primer P77-P82 used), resulting in plasmid pUC-YPRC15-URA-UAS-GPDp-SmCYP-SmCPR-CYCt, which was transformed into S. cerevisiae BY4700, resulting in strain TaxS8. To construct the yeast that can produce nootkatone from valencene, coding gene of HmCYP-AtCPR was synthesized and assembled with part of plasmid pUC-YPRC15-URA-UAS-GPDp-5αCYP-CPR-CYCt via CLIVA (primer P81-P86 used), resulting in plasmid pUC-YPRC15-URA-UAS-GPDp-HmCYP-AtCPR-CYCt, which was linearized by NotI and transformed into S. cerevisiae BY4700, resulting in strain TaxS9. To improve the nootkatone production, coding gene of PpADHC3 was amplified from Pichia pastoris genomic DNA and assembled with part of plasmid p426-TEFp-TAT-ACTt via CLIVA (primer), resulting in plasmid p426-TEFp-PpADHC3-ACTt; expression operon of this plasmid was further assembled with plasmid pUC-YPRC15-URA-UAS-GPDp-HmCYP-AtCPR-CYCt via CLIVA (primer P56, P57, P87 and P88 used), resulting in plasmid pUC-YPRC15-URA-(UAS-GPDp-HmCYP-AtCPR-CYCt)-(TEFp-PpADHC3-ACTt), which was linearized by NotI and transformed into S. cerevisiae BY4700, resulting in strain TaxS10.
Characterization of the yeast cultures by feeding taxadiene
All S. cerevisiae strains were characterized in absence of E. coli prior to co-culture experiment. We used 14 mL glass tubes (Pyrex) for this type of characterizations. A colony of the S. cerevisiae was inoculated into 1 mL YPD medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose) and grown at 30 °C/250 rpm until cell density OD600 reached 2. 10 µL of 6 g/L synthetic taxadiene stock solution (in DMSO) was added to start the experiments, and the cultures were then incubated at 22 °C/250 rpm. To compare yeast growth and activity when growing on glucose or acetate, the same procedure was used except the medium was the one used in bioreactor experiments with indicated carbon source.
Bioreactor experiments for the E. coli – S. cerevisiae co-culture
A 1 L Bioflo bioreactor (New Brunswick) was used for all the bioreactor works in this study. In initial experiments, seed cultures of E. coli and S. cerevisiae were inoculated into 500 mL of defined medium (13.3 g/L KH2PO4, 4 g/L (NH4)2HPO4, 1.7 g/L citric acid, 0.0084 g/L EDTA, 0.0025 g/L CoCl2, 0.015 g/L MnCl2, 0.0015 g/L CuCl2, 0.003 g/L H3BO3, 0.0025 g/L Na2MoO4, 0.008 g/L Zn(CH3COO)2), 0.06 g/L Fe(III) citrate, 0.0045 g/L thiamine, 1.3 g/L MgSO4, pH 7.0) containing 5 g/L yeast extract and 40 g/L glucose (or 20 g/L xylose). To prepare seed culture of E. coli, a colony of the E. coli was inoculated into Luria-Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl, pH=7) and grown at 37 °C/250 rpm overnight. 5 mL of the grown cell suspension (OD of ~6) was inoculated into the bioreactor. To prepare seed culture of S. cerevisiae, a colony of the S. cerevisiae was inoculated into YPD medium and grown at 30 °C/250 rpm until cell density OD600 reached 20. 10 mL of the grown cell suspension were centrifuged at 3,000 g for 2 min, and pellets were resuspended in phosphate buffered saline (PBS) and inoculated into the bioreactor. In the control experiments, only E. coli or S. cerevisiae was inoculated into the bioreactor. To improve growth of the microbes (refer to Figure 3a), ammonium phosphate was co-fed with xylose (1 g (NH4)2HPO4 per 5 g xylose) and more seed culture of the S. cerevisiae were inoculated (pellets of 50 mL of grown cell suspension, OD600=20).
During the fermentation, oxygen was supplied by filtered air at 0.5 L/min and agitation was adjusted to maintain dissolved oxygen levels at 30% (280–800 rpm). The pH of the culture was controlled at 7.0 using 10% NaOH and 0.5 M HCl. The temperature of the culture in the bioreactor was controlled at 30 °C until the dissolved oxygen level dropped below 40%. The temperature of the bioreactor was then reduced to 22 °C and the E. coli was induced with 0.1 mM IPTG. During the course of the fermentation, the concentration of glucose (or xylose), acetate and ethanol was monitored at constant time intervals. As the glucose concentration dropped below 20 g/L, 20 g/L of glucose was introduced into the bioreactor. As the xylose concentration dropped below 10 g/L, 50 g/L of xylose was introduced into the bioreactor.
Bioreactor experiments for the E. coli – E. coli co-culture
Half liter of rich medium (5 g/L yeast extract, 10 g/L tryptone, 10 g/L NaCl, 5 g/L K2HPO4, 8 g/L glycerol, pH7) containing 50 mg/L spectinomycin, was inoculated with 5 mL of grown culture (OD of 4) of E. coli TaxE5 and 5 mL of grown culture (OD of 4) of E. coli TaxE6.
During the fermentation, oxygen was supplied by filtered air at 0.5 L/min and agitation was adjusted to maintain dissolved oxygen levels at 30% (280–800 rpm). The pH of the culture was controlled at 7.0 using 10% NaOH. The temperature of the culture in the bioreactor was controlled at 30 °C until the dissolved oxygen level dropped below 40%. The temperature of the bioreactor was then reduced to 22 °C and the E. coli was induced with 0.1 mM IPTG. During the course of the fermentation, the concentration of glycerol and acetate was monitored at constant time intervals. Glycerol was fed into the bioreactor at the rate of 0.65 g/h.
Test tube experiments for characterizing acetate production of E. coli
A colony of E. coli was inoculated into LB medium, and incubated at 37 °C/250 rpm overnight. 10 µL of grown cells were inoculated into the same medium as the one used in E. coli – S. cerevisiae bioreactors. The cell suspension was incubated at 22 °C/250 rpm for 96 h and samples were taken for extracellular acetate measurement.
Quantification of isoprenoids
At indicated time points, 200 µL of cell suspension was sampled and mixed with 200 µL ethyl acetate and 100 uL 0.5mm glass beads. The mixture was vortexed at room temperature for 20min, and clarified by centrifugation at 18,000 g for 2 min. 1 µL of the ethyl acetate phase was analyzed by GCMS (Varian saturn 3800 GC attached to a Varian 2000 MS). The samples were injected into a HP-5ms column (Agilent Technologies USA). Helium at flow rate 1.0 mL/min was used as the carrier gas. The oven temperature was kept at 100 °C for 1 min, then increased to 175 °C at the increment of 15 °C/min, then increased to 220 °C at the increment of 4 °C/min, then increased to 290 °C at the increment of 50 °C/min and finally held at this temperature for 1 min. The injector and transfer line temperatures were both set at 250 °C. The MS was operated under scan mode (40–400 m/z) and total ion count of taxanes was used for the quantification. Taxadiene, nootkatol and nootkatone were quantified by using the calibration curve (total ion count vs. concentration) constructed with authentic standard.
The 5αCYP was reported to produce multiple oxygenated taxanes in S. cerevisiae34. After analyzing co-culture samples, we also observed many peaks on total ion chromatography (40–400 m/z, GCMS) between 11–18.5 min, where we did not observe any peak when sample of the single cultures was analyzed (Supplementary Fig. 16a). Five of the major peaks contained significant amount of 288 m/z signal (characteristic mass of mono-oxygenated taxane, 272 (taxadiene) + 16 (oxygen), Supplementary Fig. 16b). Among them, two were previously identified as oxa-cyclotaxane (OCT) and taxadien-5α-ol34 (Supplementary Fig. 17), but the other three taxanes have not been identified before (Supplementary Fig. 18). As a conservative estimate, we only considered these five oxygenated taxanes for calculating titer of total oxygenated taxanes. As standards of these five monooxygenated taxanes, the monoacetylated dioxygenated taxane and ferruginol were not available, they were quantified by using the taxadiene calibration curve.
Quantification of extracellular metabolites
At indicated time points, 1.1 mL of cell suspension was sampled and centrifuged at 18,000 g for 1 min. The supernatant was sterilized by using 0.2 µm filter. 1mL of filtered supernatant was analyzed by a HPLC (Waters 2695 separation module coupled to Waters 410 differential refractometer) to measure concentration of extracellular glucose, xylose, acetate and ethanol. Bio-rad HPX-87H column was used and 14 mM sulfuric acid was used as mobile phase at the flow rate of 0.7 mL/min.
Quantification of E. coli and S. cerevisiae cell number
To measure cell number of E. coli in the E. coli – S. cerevisiae co-cultures, 2 µL of cell suspension was diluted in 200 µL sterile PBS, and 2 µL of the diluted cell suspension was further diluted in 200 µL sterile PBS. 50 µL of the repeatedly diluted cell suspension was plated on LB agar plate (1.5% agar) and incubated at 37 °C for 20 h. After the incubation, only E. coli colonies were visible on the plate (the yeast colonies were only visible after at least 48 h at this condition). As such method of measuring colony forming unit was time consuming and low throughput. We later developed a sucrose gradient centrifugation method to quantify cell number of both E. coli and S. cerevisiae in the co-culture. At indicated time points, 0.5 mL of cell suspension was sampled and loaded onto 1 mL of 45% sucrose solution in a 14 mL falcon tube, which was then centrifuged at 2,100 g for 2 min. Microbes in the supernatant were exclusively E. coli and those in the pellets were mostly S. cerevisiae (Supplementary Fig. 19). After this separation, cell number of the two microbes could be quantified by measuring optical density at 600 nm.
Supplementary Material
Acknowledgments
We acknowledge useful discussions and input from Adel Ghaderi, Felix Lam, Haoran Zhang, Jose Avalos and Wen Wang. This work was supported by National Institutes of Health grant 1-R01-GM085323-01A1 and the Singapore MIT Alliance.
Footnotes
AUTHOR CONTRIBUTIONS
K.Z. and G.S. conceived the project. K.Z., K.Q. S.E. and G.S. designed the experiments, analyzed the results and wrote the manuscript. K.Z., K.Q. and S.E. executed all the experiments.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests. A patent on the co-culture concept has been filed.
References
- 1.Ajikumar PK, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paddon CJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 3.Alonso-Gutierrez J, et al. Metabolic Engineering of Escherichia coli for Limonene and Perillyl Alcohol Production. Metabolic engineering. 2013 doi: 10.1016/j.ymben.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Ajikumar PK, et al. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm. 2008;5:167–190. doi: 10.1021/mp700151b. [DOI] [PubMed] [Google Scholar]
- 5.Hefferon K. Plant-derived pharmaceuticals for the developing world. Biotechnol J. 2013;8:1193–1202. doi: 10.1002/biot.201300162. [DOI] [PubMed] [Google Scholar]
- 6.Melnik S, Stoger E. Green factories for biopharmaceuticals. Current medicinal chemistry. 2013;20:1038–1046. [PubMed] [Google Scholar]
- 7.Chang MC, Eachus RA, Trieu W, Ro DK, Keasling JD. Engineering Escherichia coli for production of functionalized terpenoids using plant P450s. Nat Chem Biol. 2007;3:274–277. doi: 10.1038/nchembio875. [DOI] [PubMed] [Google Scholar]
- 8.Agapakis CM, Boyle PM, Silver PA. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat Chem Biol. 2012;8:527–535. doi: 10.1038/nchembio.975. [DOI] [PubMed] [Google Scholar]
- 9.Smid EJ, Lacroix C. Microbe-microbe interactions in mixed culture food fermentations. Current opinion in biotechnology. 2013;24:148–154. doi: 10.1016/j.copbio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Fredrickson AG, Stephanopoulos G. Microbial competition. Science. 1981;213:972–979. doi: 10.1126/science.7268409. [DOI] [PubMed] [Google Scholar]
- 11.Davison BH, Stephanopoulos G. Effect of pH oscillations on a competing mixed culture. Biotechnology and bioengineering. 1986;28:1127–1137. doi: 10.1002/bit.260280802. [DOI] [PubMed] [Google Scholar]
- 12.Bayer TS, et al. Synthesis of methyl halides from biomass using engineered microbes. Journal of the American Chemical Society. 2009;131:6508–6515. doi: 10.1021/ja809461u. [DOI] [PubMed] [Google Scholar]
- 13.Minty JJ, et al. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14592–14597. doi: 10.1073/pnas.1218447110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia T, Eiteman MA, Altman E. Simultaneous utilization of glucose, xylose and arabinose in the presence of acetate by a consortium of Escherichia coli strains. Microbial cell factories. 2012;11:77. doi: 10.1186/1475-2859-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra-Bubb J, Croteau R, Williams RM. The early stages of taxol biosynthesis: an interim report on the synthesis and identification of early pathway metabolites. Natural product reports. 2012;29:683–696. doi: 10.1039/c2np20021j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennewein S, Long RM, Williams RM, Croteau R. Cytochrome p450 taxadiene 5alpha-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of taxol biosynthesis. Chemistry & biology. 2004;11:379–387. doi: 10.1016/j.chembiol.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Hefner J, et al. Cytochrome P450-catalyzed hydroxylation of taxa-4(5),11(12)-diene to taxa-4(20),11(12)-dien-5alpha-ol: the first oxygenation step in taxol biosynthesis. Chemistry & biology. 1996;3:479–489. doi: 10.1016/s1074-5521(96)90096-4. [DOI] [PubMed] [Google Scholar]
- 18.Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314:1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiteman MA, Altman E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends in biotechnology. 2006;24:530–536. doi: 10.1016/j.tibtech.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, et al. Cloning and characterization of a panel of constitutive promoters for applications in pathway engineering in Saccharomyces cerevisiae. Biotechnology and bioengineering. 2012;109:2082–2092. doi: 10.1002/bit.24481. [DOI] [PubMed] [Google Scholar]
- 21.Blazeck J, Garg R, Reed B, Alper HS. Controlling promoter strength and regulation in Saccharomyces cerevisiae using synthetic hybrid promoters. Biotechnology and bioengineering. 2012;109:2884–2895. doi: 10.1002/bit.24552. [DOI] [PubMed] [Google Scholar]
- 22.De Virgilio C, et al. Cloning and disruption of a gene required for growth on acetate but not on ethanol: the acetyl-coenzyme A synthetase gene of Saccharomyces cerevisiae. Yeast. 1992;8:1043–1051. doi: 10.1002/yea.320081207. [DOI] [PubMed] [Google Scholar]
- 23.Kratzer S, Schuller HJ. Transcriptional control of the yeast acetyl-CoA synthetase gene, ACS1, by the positive regulators CAT8 and ADR1 and the pleiotropic repressor UME6. Molecular microbiology. 1997;26:631–641. doi: 10.1046/j.1365-2958.1997.5611937.x. [DOI] [PubMed] [Google Scholar]
- 24.Causey TB, Zhou S, Shanmugam KT, Ingram LO. Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:825–832. doi: 10.1073/pnas.0337684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker K, Schoendorf A, Croteau R. Molecular cloning of a taxa-4(20),11(12)-dien-5alpha-ol-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Archives of biochemistry and biophysics. 2000;374:371–380. doi: 10.1006/abbi.1999.1609. [DOI] [PubMed] [Google Scholar]
- 26.Schoendorf A, Rithner CD, Williams RM, Croteau RB. Molecular cloning of a cytochrome P450 taxane 10 beta-hydroxylase cDNA from Taxus and functional expression in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1501–1506. doi: 10.1073/pnas.98.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler AL, et al. Taxol biosynthesis: differential transformations of taxadien-5 alpha-ol and its acetate ester by cytochrome P450 hydroxylases from Taxus suspension cells. Archives of biochemistry and biophysics. 2001;390:265–278. doi: 10.1006/abbi.2001.2377. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12108–12113. doi: 10.1073/pnas.1218061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou YJ, et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. Journal of the American Chemical Society. 2012;134:3234–3241. doi: 10.1021/ja2114486. [DOI] [PubMed] [Google Scholar]
- 30.Hom EF, Murray AW. Niche engineering demonstrates a latent capacity for fungal-algal mutualism. Science. 2014;345:94–98. doi: 10.1126/science.1253320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillai VC, Snyder RO, Gumaste U, Thekkumkara TJ, Mehvar R. Effects of transient overexpression or knockdown of cytochrome P450 reductase on reactive oxygen species generation and hypoxia reoxygenation injury in liver cells. Clin Exp Pharmacol Physiol. 2011;38:846–853. doi: 10.1111/j.1440-1681.2011.05622.x. [DOI] [PubMed] [Google Scholar]
- 32.Reed JR, Cawley GF, Backes WL. Inhibition of cytochrome P450 1A2-mediated metabolism and production of reactive oxygen species by heme oxygenase-1 in rat liver microsomes. Drug Metab Lett. 2011;5:6–16. doi: 10.2174/187231211794455253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artsatbanov VY, et al. Influence of Oxidative and Nitrosative Stress on Accumulation of Diphosphate Intermediates of the Non-mevalonate Pathway of Isoprenoid Biosynthesis in Corynebacteria and Mycobacteria. Biochemistry. Biokhimiia. 2012;77:362–371. doi: 10.1134/S0006297912040074. [DOI] [PubMed] [Google Scholar]
- 34.Rontein D, et al. CYP725A4 from yew catalyzes complex structural rearrangement of taxa-4(5),11(12)-diene into the cyclic ether 5(12)-oxa-3(11)-cyclotaxane. The Journal of biological chemistry. 2008;283:6067–6075. doi: 10.1074/jbc.M708950200. [DOI] [PubMed] [Google Scholar]
- 35.Xue J, Ahring BK. Enhancing Isoprene Production by Genetic Modification of the 1-Deoxy-D-Xylulose-5-Phosphate Pathway in Bacillus subtilis. Applied and environmental microbiology. 2011;77:2399–2405. doi: 10.1128/AEM.02341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou K, Zou R, Zhang C, Stephanopoulos G, Too HP. Optimization of amorphadiene synthesis in Bacillus subtilis via transcriptional, translational, and media modulation. Biotechnology and bioengineering. 2013 doi: 10.1002/bit.24900. [DOI] [PubMed] [Google Scholar]
- 37.Doshi R, Nguyen T, Chang G. Transporter-mediated biofuel secretion. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7642–7647. doi: 10.1073/pnas.1301358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos CN, Xiao W, Stephanopoulos G. Rational, combinatorial, and genomic approaches for engineering L-tyrosine production in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13538–13543. doi: 10.1073/pnas.1206346109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minami H, et al. Microbial production of plant benzylisoquinoline alkaloids. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7393–7398. doi: 10.1073/pnas.0802981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi YJ, Lee SY. Microbial production of short-chain alkanes. Nature. 2013;502:571–574. doi: 10.1038/nature12536. [DOI] [PubMed] [Google Scholar]
- 41.Leber C, Da Silva NA. Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnology and bioengineering. 2014;111:347–358. doi: 10.1002/bit.25021. [DOI] [PubMed] [Google Scholar]
- 42.Craft DL, Madduri KM, Eshoo M, Wilson CR. Identification and characterization of the CYP52 family of Candida tropicalis ATCC 20336, important for the conversion of fatty acids and alkanes to alpha, omega-dicarboxylic acids. Applied and environmental microbiology. 2003;69:5983–5991. doi: 10.1128/AEM.69.10.5983-5991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou R, Zhou K, Stephanopoulos G, Too HP. Combinatorial Engineering of 1-Deoxy-D-Xylulose 5-Phosphate Pathway Using Cross-Lapping In Vitro Assembly (CLIVA) Method. PloS one. 2013;8:e79557. doi: 10.1371/journal.pone.0079557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flagfeldt DB, Siewers V, Huang L, Nielsen J. Characterization of chromosomal integration sites for heterologous gene expression in Saccharomyces cerevisiae. Yeast. 2009;26:545–551. doi: 10.1002/yea.1705. [DOI] [PubMed] [Google Scholar]
- 45.Avalos JL, Fink GR, Stephanopoulos G. Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols. Nature biotechnology. 2013;31:335–341. doi: 10.1038/nbt.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.