Abstract
Discovering the environmental factors that control microglia is key to understanding and managing brain health. A new study finds that microbiota in the gut are essential for regulating microglia maturation and activation.
The human brain has endowed us with a capacity for self-conciousness and a sense of being independent thinkers. It is easy to forget that we, along with other mammals, in fact share our bodies with trillions of microbes. Could it be that we owe our thoughts, at least in some small part, to these tiny symbionts? The gut microbial communities of mammals have evolved with their hosts to take part in this coexistence1, and are an important environmental factor that contributes to the development of several biological systems. This includes the systemic immune and central nervous systems, though previous studies have mainly examined the influence on hormonal and neuronal function and behavior2–4. In this issue of Nature Neuroscience, Erny, Angelis, and colleagues5, demonstrate that gut microbiota influence the CNS immune system by regulating microglia.
The authors compared microglia from mice with (specific pathogen-free, SPF) and without (germ-free, GF) microbiota colonized guts. RNA-sequencing showed that there was a striking difference in the transcriptional profiles between microglia isolated from young adult animals. Notably, the most amplified gene in microglia from GF mice was DNA damage-inducible transcript 4 (Ddit4), the product of which regulates cell growth, proliferation, and survival. Other genes that were significantly upregulated in microglia from GF mice were Sfp1 (encoding Pu.1) and Csf1r, which are highly expressed in developing microglia6,7, while several genes involved in cell activation were downregulated. Additionally, a greater percentage of microglia from GF mice expressed the surface proteins CSF1R, F4/80, and CD31, which generally decline with cell maturation6. This transcriptional and protein profiling thus suggested that gut microbiota have a role in regulating the maturation and possibly the immune response of microglia.
In his seminal article on microglia, Pio del Rio-Hortega noted that each individual microglia in the mature adult brain seems to occupy a defined territory8. In this study, Erny, Angelis, and colleagues made elegant use of a three-dimensional image reconstruction and automatic quantification technique to compare microglia morphology characteristics. Overall, the processes of GF microglia were more spread out, creating a cellular landscape in which neighboring GF microglia encroached on each others’ territories. This was complemented by the observation that the density of microglia was increased throughout the brains of GF mice. As microglia numbers increase during postnatal development and then begin to decline in early adulthood9, these findings are consistent with immature microglial characteristics in GF mice.
Del Rio-Hortega, and others since, found that in early development microglia have an amoeboid morphology and acquire a highly branched and more complex form during maturation (see for example, ref. 10). The 3D analysis in this study, however, also revealed that microglia from adult GF mice had more complex morphologies, with longer, segmented branches. The phenotypes of these microglia are thus not necessarily fully reminiscent of immature cells, but of course microglial maturation is not a biphasic process. This also brings to question the functional significance of these morphological features, particularly as it relates to microglial development. The authors suggest that the homeostasis of “resting” microglia in these otherwise healthy mice is altered and therefore examined the response of these cells to an immune challenge.
In order to determine whether the immune response of microglia in GF mice was affected, the researchers exposed the animals to either a bacterial (lipopolysaccharide) or viral (lymphocytic choriomeningitis virus) stimulus. After either exposure, gene expression profiling suggested that the innate immune response was reduced or disturbed in the brains of GF mice compared to SPF mice. Though a direct connection remains to be explored, one might imagine that such impaired CNS immune function, particularly at early ages, could gravely impact developing neural circuitry, given the importance of microglia in shaping synapses11.
What specific microbiota-associated factors influence these phenotypes of microglia? To begin addressing this question, the authors examined microglia in GF mice whose intestines were re-colonized with a defined subset of known bacterial species (ASF mice). This limited collection of bacterial strains proved insufficient to re-establish the microglia to their state seen in normal mice. However, normalization of microglial numbers, Ddit4 levels, and morphology was made possible by increasing the microbiota complexity further through co-housing the partially-recolonized, ASF animals with normal SPF animals. Thus, even microglia that have not been exposed to the influence of gut microbiota during development are plastic and remain capable of obtaining mature and homeostatic features given sufficient bacterial conditions.
The researchers probed this mechanistic question further by then focusing on the direct introduction of bacterial-derived factors to GF mice. The GF mice were given a mixture of short-chain fatty acids (SCFA) in their drinking water, which are generated by gut bacteria through fermentation of dietary fiber. Remarkably, this treatment normalized the number of microglia, Ddit4 mRNA levels, microglial morphology, and microglial expression of CSFR1 to those seen in SPF animals. Thus, SCFA appear to be important molecules in the regulation of microglia maturation.
What remains to be discovered, however, is the communication link between bacterial-produced SCFA and brain microglia. Although SCFA are known to travel to various organs via the bloodstream and influence tissue function, including the brain12, the authors of this study did not find microglia, or any other brain cell types, to express the SCFA receptor FFAR2. The strongest expression of FFAR2 was in fact found on myeloid cells in the spleen. One possibility is SCFA act directly on these peripheral myeloid cells via FFAR2, which in turn secrete brain-permissible factors that regulate microglia. Another intriguing hypothesis however, arises from the recent finding that germ-free mice have increased blood-brain barrier permeability beginning in-utero and into adulthood12. Perhaps this could mean that splenic myeloid cells, which do not normally contribute to the resident microglial population7,13, are able to enter the brain parenchyma in the absence of microbiota and SCFA, and there display characteristics of immature and impaired myeloid cells.
The work by Erny, Angelis, and colleagues thus opens several new avenues for future research. These findings clearly have important implications for human conditions in which the constitution of gut bacteria may be altered, such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome14, or in which the bacteria are depleted, as happens during oral antibiotic use15. On this note, the researchers found that depleting the intestinal microbes of SPF mice during adulthood with antibiotics was sufficient to alter the morphology of microglia, such that they resembled the cells found in the brains of the GF mice that had never been exposed to complex microbiota. Though this highlights the sensitivity, and possible dysregulation, of the gut-brain communication system, on a positive note, this work also demonstrates that some treatment may be possible in the form of bacterial reconstitution or SCFA, at least to alleviate the effects on microglia.
As to basic biology, this paper provides a new perspective on the regulation of microglial development and function at a systemic level. Still more generally, this is also an exciting example of “developmental programming”2, showing how early environmental conditions, be they external or, in the special case of the gut microbiome, internal, influence the development of an organ. With studies like this continually demonstrating the link between microbiota and the brain, and the observation that microglia can sculpt synaptic circuits, perhaps there is biological credence to the concept of gut instincts.
Figure 1.
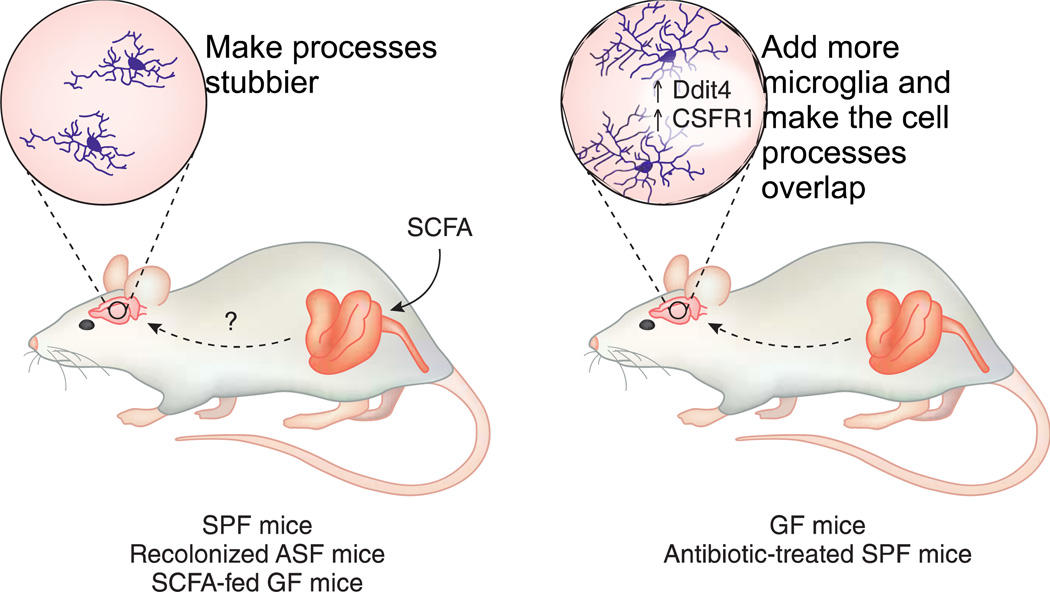
Gut to brain communication regulates microglia. The typical morphology, territorial boundaries, and molecular profile of microglia observed in mice living in standard, clean housing conditions (SPF; mouse on the left) are changed in mice living in a GF environment (mouse on the right). Microglia of GF mice display extended processes that encroach on each others territories and a gene expression profile more akin to immature cells (e.g. upregulation of CSFR1 and Ddit4). Partial ablation of gut microbiota with antibiotics produced a microglia phenotype similar to the one observed in GF mice while recolonization of GF mice with defined ASF bacteria or feeding with SCFA normalized the microglial phenotype. ASF, altered Schaedler Flora; CSFR1, colony stimulating factor 1; Ddit4, DNA damage-inducible transcript 4; GF, germ free; SCFA, short-chain fatty acids; SPF, specific pathogen free.
References
- 1.Ley RE, et al. Evolution of mammals and their gut microbes. Science (New York, NY) 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heijtz RD, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke G, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nature Reviews Neuroscience. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the central nervous system. Nature Neuroscience. 2015 doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kierdorf K, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nature Publishing Group. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 7.Ginhoux F, et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science (New York, NY) 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of microglia. Physiological Reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 9.Nikodemova M, et al. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. Journal of neuroimmunology. 2015;278:280–288. doi: 10.1016/j.jneuroim.2014.11.018. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 11.Schafer Dorothy P, et al. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braniste V, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mildner A, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nature Neuroscience. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 14.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Frontiers in Physiology. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng KM, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]