Abstract
In the modern medical era, more diverse and effective treatment options have translated into increased life expectancy. With this increased lifespan comes increased age-associated disease and the dire need to understand the underlying causes so that therapies can be designed to mitigate the burden to health and the economy. Aging exacts a seemingly inevitable, multi-system deterioration of function that acts as a risk factor for a variety of age-related disorders, including those that devastate organs of limited regenerative potential such as the brain. Rather than studying the brain and mechanisms that govern its aging in isolation from other organ systems, an emerging approach is to understand the relatively unappreciated communication existing between the brain and the systemic environment. Revisiting classical methods of experimental physiology in animal models has uncovered surprising regenerative activity within young blood with translational implications for aging liver, muscle, brain and other organs. Surprisingly, soluble factors present in young or aged blood are sufficient to improve or impair cognitive function, respectively, suggesting an aging continuum of brain-relevant systemic factors. The age-associated plasma chemokine CCL11 has been shown to impair young brain function while GDF11 has been reported to increase the generation of neurons in aged mice. However, the identities of specific factors mediating memory-enhancing effects of young blood and their mechanisms of action remain enigmatic. Here we review recent brain rejuvenation studies in the broader context of systemic rejuvenation research. We discuss putative mechanisms for blood-borne brain rejuvenation while suggesting promising avenues for future research and development of therapies.
Aging as a multi-system problem
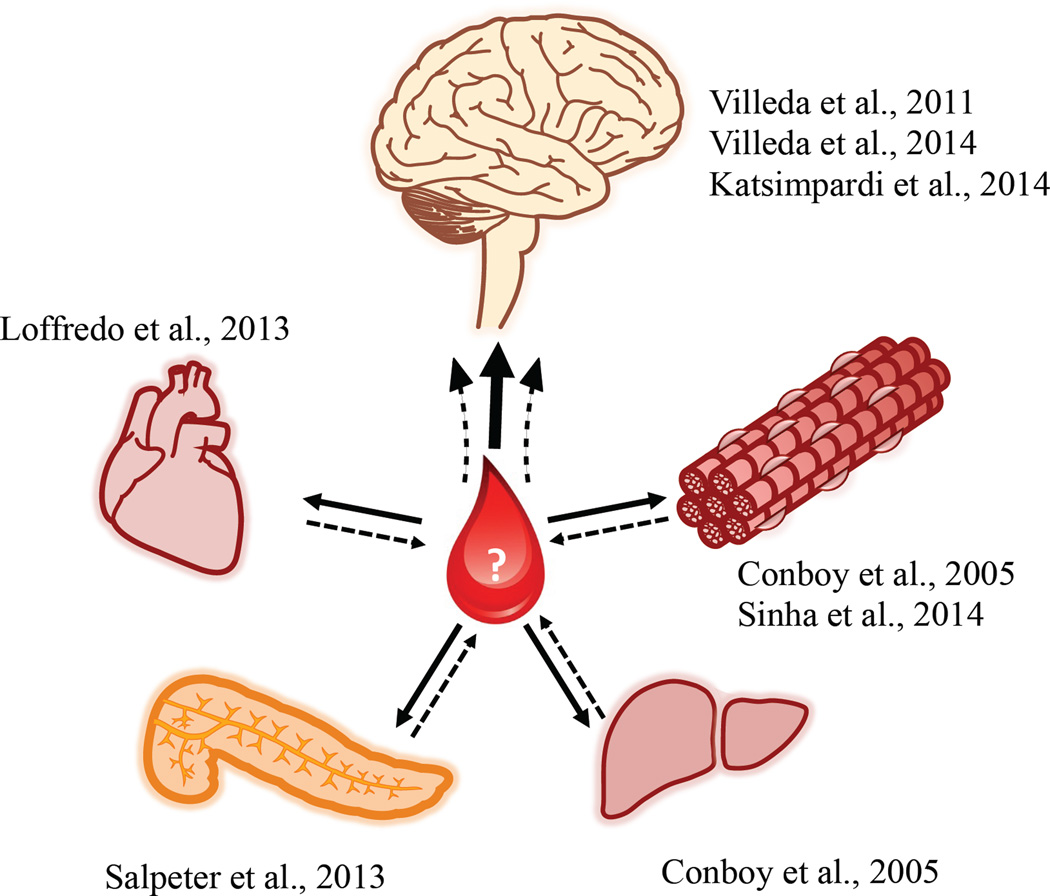
Aging drives a progressive decline in cognitive function that renders the brain susceptible to devastating neurological disorders, including Alzheimer’s disease (AD). In fact, aging is the strongest risk factor for the development of AD, a neurodegenerative disease with no effective treatment that culminates in synaptic dysfunction, neuronal death, and impaired memory and other cognitive function. The number of elderly individuals over 80 is projected to triple globally by 2050, many of whom will suffer from cognitive impairment, AD, or one of several other neurodegenerative diseases for which age increases risk. As the number of aged individuals rises dramatically in the coming decades, widespread social and economic consequences of these and other aging disorders will be felt on an unprecedented scale. One strategy to meet this growing public health threat is to elucidate the mechanisms that underlie cognitive decline to inform therapeutic strategies aimed at ameliorating age-associated disease. As the brain ages, various cell types exhibit hallmark changes, including increased astrogliosis and microgliosis, alterations in the blood-brain barrier, decreased generation of new neurons in specialized areas, and decreased synaptic function, all of which may contribute to impaired cognitive function. The hippocampus within the temporal medial lobe is one region particularly sensitive to the ravages of aging1. Cellular changes within this structure manifest as striking impairments in cognitive tasks ascribed to normal hippocampal function, including spatial and episodic learning and memory function. The brain is not unique in its predisposition to age-associated functional decline. Muscle, liver, pancreas, heart and other organ systems also exhibit declines in function with age, leading to conditions associated with frailty and loss of independence, including sarcopenia, age-associated diabetes, and cardiac hypertrophy or failure. While many cell-autonomous and tissue-intrinsic mediators of aging have been proposed, recent data supports an emerging concept that there is much to learn from potential overlap in the aging process across diverse tissue types. Indeed, a key hallmark shared among age-sensitive organs is a dramatic decline in regenerative potential in specific cell populations. A seminal study published a decade ago uncovered that the loss of proliferative capacity for aged satellite cells and hepatocytes is not immutable – exposure of aged tissue to young blood restored latent regenerative capacity, “rejuvenating” both muscle and liver2. Given the close contact made between vulnerable brain regions such as the hippocampus and the vasculature, it was hypothesized that the systemic environment can influence or drive how the brain ages. We and others recently provided evidence that age-related changes in the blood regulate brain function3–6, raising the possibility that CNS function can be shaped throughout aging by the combined influence of peripheral organ systems via circulatory factors or changes at the interface of communication between these compartments (Figure 1).
Figure 1. Young blood rejuvenates organs of broad diversity.
Various studies using heterochronic parabiosis of young and aged mice have demonstrated rejuvenation effects in muscle, liver, pancreas, heart, and brain (solid lines). Blood-mediated brain rejuvenation may reflect integration of signals from rejuvenation of one or more peripheral organs (dashed lines).
Myriad regenerative properties of young blood
Strides towards understanding systemic influence on the aging process for various tissues began with a key study that took advantage of a classical animal model of physiology – parabiosis. Conboy and colleagues of the Rando laboratory created “heterochronic” pairings (parabionts) in which young and aged mice share blood through the creation of a vascular anastomosis along adjacent, surgically-connected flanks2. Compared to “isochronic” pairings of aged mice, muscle from the aged partner of the heterochronic pairs exhibits youthful levels of regeneration following injury. Consistent with the rejuvenation of skeletal muscle stem cell activation, Notch ligand Delta is upregulated in aged satellite cells exposed to young serum, suggesting that molecular machinery responsible for progenitor cell proliferation can be restored by soluble factors in young blood. Furthermore, exposure to young blood substantially reduces the age-dependent increase in tissue fibrosis that accompanies loss of muscle regeneration with concomitant enhancements in Wnt signaling7. More recently, it was shown that satellite cells isolated from muscle of aged heterochronic parabionts are more myogenic and accumulate less DNA damage than aged isochronic counterparts8. Moreover, treatment of aged mice with recombinant growth differentiation factor GDF11, a protein originally linked to rejuvenation through its effects on cardiac function9, appears to recapitulate some of the effects of young blood on muscle stem cells in the context of cryoinjury, cardiotoxin injury or in an uninjured state8. Egerman and colleagues did not observe regenerative properties of GDF11 following cardiotoxin injury of aged muscle;10 treatment with a three-fold higher dose appears to delay muscle regeneration in young mice10. Further investigation is needed to clarify the extent to which GDF11 signaling regulates tissue regeneration over a range of concentrations and across the lifespan of the organism.
In a separate regenerative peripheral organ, the liver, Conboy and colleagues tested whether young blood reverses the age-related decline in proliferating hepatocytes2. Hepatocytes from aged parabionts of heterochronic pairs indeed exhibit youthful proliferation and a reversal of age-related cEBP-α-Brm, a complex that accumulates with liver aging. Given that loss of cardiac function and associated failure can be a feature of aging, Loffredo and colleagues used heterochronic parabiosis to show that sharing young blood reduces cardiac hypertrophy9. In addition to reducing heart weight in aged mice, young blood exposure markedly decreases the size of ventricular myocytes. While molecular hypertrophic markers atrial natriuretic peptide and brain natriuretic peptide are not normalized to youthful levels in cardiomyocytes, alterations in expression may indicate a remodeling process caused by exposure to young blood. Treating aged mice daily for one month with GDF11, a protein elevated in the plasma of aged heterochronic parabionts, leads to a reduction in heart weight, a modest but significant reduction in the size of cardiomyocytes, and a corresponding normalization of molecular markers of cardiac hypertrophy. Salpeter and colleagues joined 1.5-month-old and 8-month-old mice using heterochronic parabiosis to examine whether young blood could influence the age-related decline in pancreatic β-cell proliferation11. Interestingly, after only 2–3 weeks of sharing blood, pancreatic β-cells from aged mice of the heterochronic pairs display higher levels of replication, an effect that is mimicked by transplanting islets from aged mice into young mice. A further study assessed whether age-related systemic changes influence hair follicle stem cell function12. While sharing young blood modestly increases the colony forming efficiency of hair follicle stem cells in skin isolated from aged parabionts, further in vivo experiments argue that age-related decline in hair follicle stem cells is determined to a greater extent by cell- and tissue-intrinsic factors. The regenerative capacity of aged bone is improved by exposure to young blood13. Following tibial fracture in the aged mice of heterochronic pairs, bone healing improves compared to that normally observed in aged mice, likely through modulation of β-catenin signaling.
Following the revelation that young blood rejuvenates stem cell activity in muscle2, several groups reported that the effects of young blood could extend beyond peripheral organs to mediate rejuvenation within the central nervous system3,5,6. Within the dentate gyrus (DG) subgranular zone of the hippocampus, the cellular niche in which the generation of new neurons occurs is closely associated with blood vessels and thus systemic factors. Together with the Rando laboratory, Villeda and colleagues utilized heterochronic parabiosis to examine the influence of the systemic environment on this neurogenic niche3. Surprisingly, exposure of aged mice to young blood substantially increases the number of proliferating progenitors and newborn neurons in the DG of aged heterochronic parabionts. Aged blood appears to have opposing effects on DG neurogenesis, reminiscent of the inhibitory properties of aged blood on muscle7. Plasma protein profiling revealed age-dependent increases in CCL11 and similar elevations in aged human plasma and CSF. When provided to young mice systemically, CCL11 is sufficient to impair neurogenesis and memory performance. A subsequent study expanded the rejuvenating effects of young blood to that of synaptic plasticity at a cellular, transcriptional, and circuit level6. Exposing aged heterochronic parabionts to young blood strengthens DG synapses and upregulates hippocampal plasticity genes6, including immediate early genes linked to learning and memory1. In a parallel study, the effect of young blood on neurogenesis was examined in a separate neurogenic niche, namely the subventricular zone (SVZ)5. Proliferation of neural stem and progenitor cells within this niche is rescued in aged mice exposed to young blood, with corresponding improvements in olfactory behavior. Sharing young blood rescues age-related blood vessel volume loss in the brain and improves cerebral blood flow, and the plasma factor GDF11 partially restores vessel loss as well as the proliferative progenitor cell population in the SVZ. Young blood-mediated rejuvenation of the CNS has also been observed in the context of injury14. Following focal demyelination of aged spinal cord, young blood exposure restores levels of remyelinating oligodendrocytes to youthful levels and dramatically improves remyelination at the lesion site. Together, these studies demonstrate that young blood restores regenerative activity not only in peripheral organs, but also within the CNS, a site conventionally thought to be primarily regulated by intrinsic factors throughout aging (Figure 1).
Potential mechanisms and clinical applications of young blood
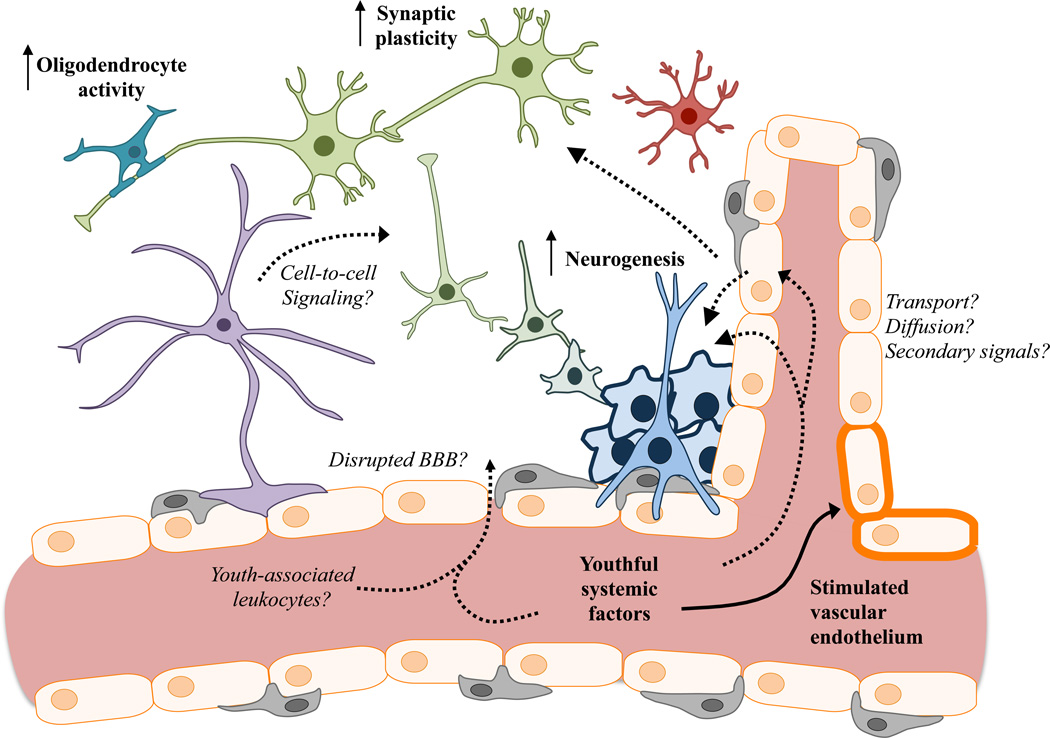
The rejuvenating properties of young blood in different organs may represent reactivation of distinct molecular pathways or shared mechanisms that drive aging via the systemic environment. By virtue of its isolation from the periphery by the blood-brain barrier (BBB), blood-mediated brain rejuvenation may be a reflection of the combined rejuvenation of multiple organs and the transfer of youthful factors into relevant neural niches. Networks of capillaries penetrating memory-relevant niches within the brain may provide opportunity for direct exchange of rejuvenating factor(s), especially in the context of aging or injury when the barriers between CNS and blood are perturbed5,14 (Figure 2). Recent data raise the possibility that endothelial cells but not pericytes are activated by young blood5, indirectly affecting the neurovascular unit to ultimately improve neural function. Still a further mechanism may involve surveillance mediated by immune cells acting at the interface of the blood-cerebrospinal fluid (CSF) barrier, which is supported by the observation that sharing aged blood alters expression of genes involved in leukocyte entry from the choroid plexus epithelium to CSF4. Whether by direct or indirect signaling, factors in young blood mediate cellular changes within the CNS in ways that are yet unclear. Though young blood influences the activity of oligodendrocytes in the injured spinal cord14, it is unknown whether these cells are affected in niches within the brain relevant for cognition, nor is it known to what extent other glia, including astrocytes and microglia, are altered as a result of young blood exposure. A key goal is to understand which cells are affected and whether specific cell types transduce rejuvenating effects to neurons to mediate enhanced plasticity. Young blood activates neural progenitor proliferation, translating to a greater number of mature neurons in the DG and SVZ3,5. While these effects are robust, levels of neurogenesis in the aged rodent brain are markedly lower than in the young brain even after rejuvenation, motivating the need to evaluate the contribution to cognitive benefits. However, a recent study reported much higher levels of neurogenesis in the adult human brain15 than previously appreciated, raising the possibility that restoration of neurogenesis in the human brain could have functionally meaningful benefits. Moreover, computational modeling has suggested that even a small number of newborn neurons can integrate into networks to yield significant functional consequences15. Furthermore, while neurogenesis is clearly affected by young blood exposure, it is unclear at what developmental stages these effects are mediated and what molecular pathways are involved.
Figure 2. Young blood rejuvenates various cell types within the CNS.
Exposure to young blood stimulates plasticity in the aged CNS (bold), particularly in adult neurogenic processes, oligodendrocyte activity (lesioned spinal cord), synaptic plasticity, and vascularization. How youthful blood-borne factors access the aged CNS remains unclear, as does the response of many other cell types that suffer functional decline with age (italics). CNS cells are color-coded as follows: astrocytes (purple); oligodendrocyte (turquoise); microglia (red); neurons (green); neurogenesis stages (blue to green); endothelial cells (orange); pericytes (gray).
In addition to enhancing neurogenesis and synaptic plasticity, young blood has been found to improve olfactory memory5 and other higher-order cognitive function6. Blood-plasma in particular, when administered in mice intravenously up to eight times over several weeks, improves hippocampal-dependent learning and memory6. The key mediators of enhanced plasticity and cognitive function may be proteinaceous based on evidence that heat denaturation ablates positive effects conferred by young plasma6. Indeed, the identification of one or several factors enriched in young blood that can improve learning and memory function remains a key research goal. The advance of using the soluble component of young blood is that it represents a facile approach to probe many unanswered questions of both a fundamental and translational nature, including interrogation of the effects of young blood at a molecular, cellular, circuit, and network level of analysis.
The demonstration that young or aged plasma improves6 or impairs3 brain function, respectively, has many therapeutic implications. With no current treatment for debilitating diseases like Alzheimer’s disease and given the relative safety of blood-plasma products, an appealing approach may be to supply older patients with young plasma to repair damage wrought by the disease on synaptic integrity and function. Both the AMBAR (Alzheimer’s Management by Albumin Replacement) and PLASMA (PLasma for Alzheimer’s SymptoM Amelioration) trials have been initiated to examine symptom improvements in mild-to-moderate Alzheimer’s disease patients following removal or addition of blood factors that impair or improve brain function, respectively (see NCT01561053 and NCT02256306 at clinicaltrials.gov). Given the likelihood that growth-promoting molecules are abundant in young plasma, it will be important to assess safety to limit the potential for cancer. The promise of treating degenerative disorders of the brain by restoring peripheral expression of proteins normally produced by the young body is particularly appealing. Restoring the peripheral expression of a rejuvenating factor in a tissue-specific manner may improve brain function while limiting unexpected side effects associated with systemic supplementation. Other systemic strategies may also hold great promise for the treatment of aging conditions, including the design of small molecules or antibodies that target age-related elevations in proteins that impair plasticity and cognition, including CCL113. In summary, the possibility that one or many proteins in young human blood can rejuvenate a diversity of organs is a tantalizing one that should spur further research, informing both the basic biology of aging as well as the development of novel therapies that target diseases of aging.
Acknowledgments
This work was funded by a Jane Coffin Childs Postdoctoral Fellowship-Simons Foundation (J.M.C), Veterans Affairs (T.W.-C.), NIA (AG045034 (T.W.-C.)) and NINDS (NS083508 (E.D.K.)).
Conflict of Interest Disclosures: T.W.-C. is a co-founder of Alkahest, Inc. T.W.-C. and J.M.C are listed as inventors on a U.S. patent application related to young plasma filed by Stanford University.
References
- 1.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006 Jan;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 2.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005 Feb 17;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 3.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011 Sep 1;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baruch K, Deczkowska A, David E, et al. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014 Oct 3;346(6205):89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014 May 9;344(6184):630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014 Jun;20(6):659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007 Aug 10;317(5839):807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 8.Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014 May 9;344(6184):649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013 May 9;153(4):828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015 May 18; doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salpeter SJ, Khalaileh A, Weinberg-Corem N, et al. Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes. 2013 Aug;62(8):2843–2848. doi: 10.2337/db13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyes BE, Segal JP, Heller E, et al. Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):E4950–E4959. doi: 10.1073/pnas.1320301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baht GS, Silkstone D, Vi L, et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of beta-catenin. Nat Commun. 2015;6:7131. doi: 10.1038/ncomms8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruckh JM, Zhao JW, Shadrach JL, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012 Jan 6;10(1):96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aimone JB, Li Y, Lee SW, et al. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014 Oct;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]