Abstract
Oxidative C–H coupling reactions were conducted using graphene oxide (GO) as an oxidant. GO showed high selectivity compared with commonly used oxidants such as (diacetoxyiodo) benzene and 2,3-dichloro-5,6-dicyano-p-benzoquinone. A mechanistic study revealed that radical species contributed to the reaction. After the oxidative coupling reaction, GO was reduced to form a material that shows electron conductivity and high specific capacitance. Therefore, this system could concurrently achieve two important reactions: C–C bond formation via C–H transformation and production of functionalized graphene.
The carbon–hydrogen (C–H) bond is one of the most common chemical bonds in organic compounds. The C–H bonds of simple molecules may be cleaved to construct new carbon–carbon (C–C) bonds to obtain more sophisticated value-added chemicals1,2,3. C–H bonds are thermodynamically stable and kinetically inert, so activation of C–H bonds requires addition of a stoichiometric amount of a metal complex4,5,6,7,8 or catalytic amount of a metal complex combined with a stoichiometric amount of oxidant9,10,11,12. Recently, metal-free C–H transformation reactions have been achieved using stoichiometric amounts of hypervalent iodine compounds13,14,15,16, peroxides17,18, and quinone derivatives19,20. However, all of these reactions produce unwanted side products derived from the oxidant.
To achieve more efficient oxidative C–H coupling, we focused on graphene oxide (GO), which is produced from graphite via oxidation and exfoliation using KMnO4 in H2SO4 (Hummers’ method). In this process, numerous carbon atoms are transformed to sp3 hybridized C–O bonds (hydroxyl and epoxy groups) and C=O bonds (carbonyl and carboxyl groups). Because of its two-dimensional sheet structure, GO can be used to produce graphene by reduction of sp3 hybridized C–O bonds to recover sp2 hybridized carbons. GO is readily reduced in the presence of metals, alcohols, amines, proteins, and microorganisms, which means that GO has oxidizing ability21,22,23,24,25,26. Inspired by this, GO-promoted oxidation reactions have been investigated27,28,29,30,31,32; however, they have been limited to the oxidation of reactive alcohols, amines, and benzylic C–H bonds to date (Figs S1–S4). To improve the scope of GO as an oxidant, here we investigated additives that can promote C–H coupling reactions.
Results and Discussion
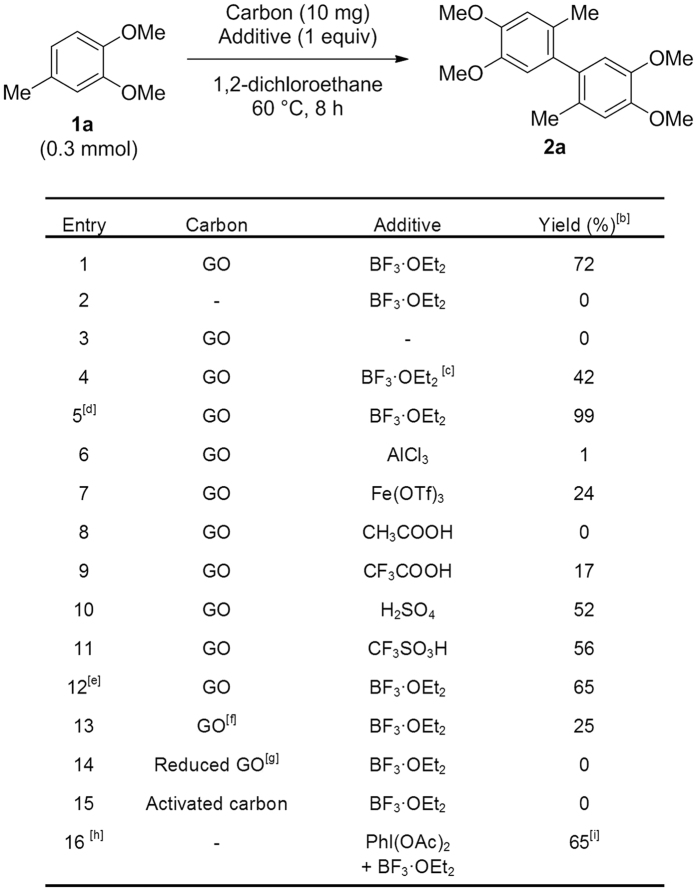
Screening of various additives (Figs S5–S8) revealed that the oxidative C–H homocoupling reaction of 3,4-dimethoxytoluene (1a) proceeded using a combination of GO (41.1 wt% O) and boron trifluoride diethyl etherate (BF3·OEt2) (Fig. 1, Entry 1). The reaction did not proceed without GO or BF3·OEt2 (Fig. 1, Entry 2 and 3). Although BF3·OEt2 worked as a catalyst (Fig. 1, Entry 4), the reaction was slow because of inhibition by water produced during the course of the reaction (Fig. S9). The reaction proceeded quantitatively when the amount of GO was increased (Fig. 1, Entry 5). Metallic Lewis acids AlCl3 and Fe(OTf)3 were not effective in the reaction (Fig. 1, Entry 6 and 7), while Brønsted acids promoted the reaction. The pKa of each Brønsted acid was correlated with the product yield. A lower pKa gave a higher yield; CH3COOH (pKa = 4.75, 0% yield), CF3COOH (pKa = 0.23, 17% yield), H2SO4 (pKa = −3.0, 52% yield), and CF3SO3H (pKa = −12, 56% yield) (Fig. 1, Entry 8–11). The reaction was not affected by oxygen (Fig. 1, Entry 12) or other oxidants (Table S4). GO prepared by Brodie’s method33,34,35,36 gave the product in low yield (Fig. 1, Entry 13). Reduced GO (rGO) and activated carbon did not promote the reaction (Fig. 1, Entry 14 and 15), suggesting that the specific oxygen functional groups of GO contributed to it. For comparison, we employed a hypervalent iodine compound (PhI(OAc)2), which is often used in metal-free oxidative coupling reactions, for this transformation. The reaction proceeded in 65% yield; however, unidentified acetoxylated compounds were also obtained as byproducts (Fig. 1, Entry 16). Therefore, GO was found to be a selective oxidant for the oxidative C–H coupling reaction.
Figure 1. Survey of reaction conditions.
[a](a) Reaction condition: 1a (0.3 mmol), GO (oxygen content: 41.1 wt%, 10 mg), additive (0.3 mmol), 1,2-dichloroethane (0.2 mL) under Ar atomospher. (b) GC yield. (c) BF3·OEt2 (0.2 equiv), 24 h. (d) 20 mg of GO (oxygen content: 41.1 wt%) and 0.5 mL of 1,2-dichloroethane were used. (e) Reaction was performed under O2 atmosphere. (f) GO was prepared by Brodie’s method. (g) Reduced GO was prepared by reduction of GO with hydrazine. (h) Reaction condition: 1a (0.3 mmol), GO (oxygen content: 41.1 wt%, 10 mg), PhI(OAc)2 (0.3 mmol), BF3·OEt2 (0.3 mmol), 1,2-dichloroethane (0.2 mL) under Ar atmosphere. (i) Acetoxylated products of 1a were also formed.
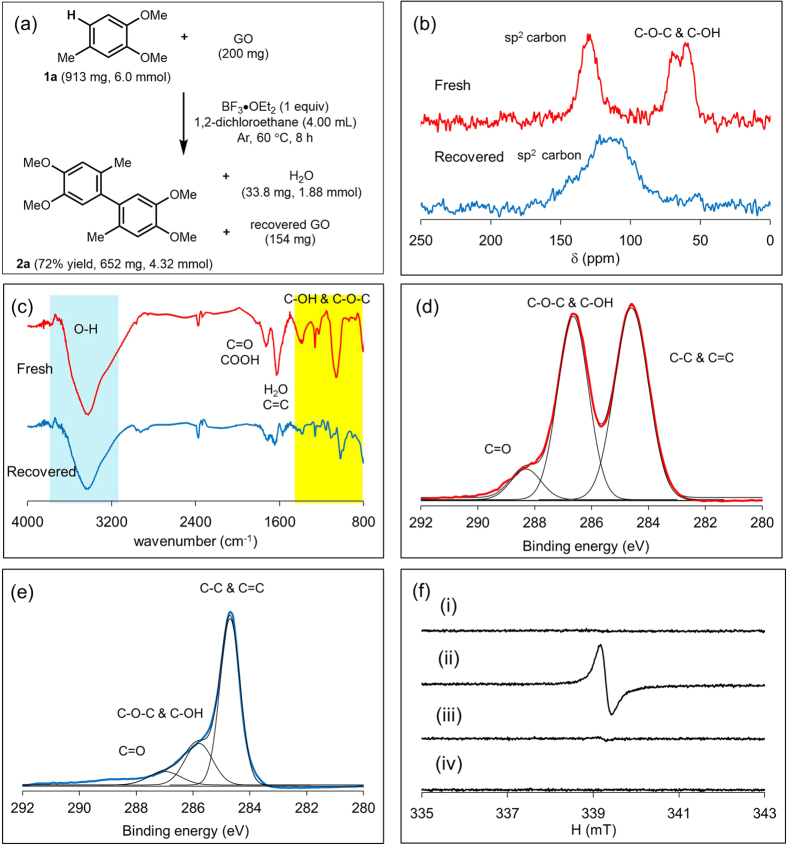
To further clarify the role of GO, the stoichiometry of the reaction was analyzed (Fig. 2a). We reacted 1a (6.00 mmol) and GO (41.1 wt% O, 200 mg) under the conditions described for Entry 1 of Fig. 1. The reaction, which proceeded in 72% yield, was regarded as the removal of 4.32 mmol of hydrogen atoms from 1a, and the weight of recovered GO was 154 mg. Elemental analysis (Table S2) indicated that the carbon content of GO before the reaction was 110 mg (55.0 wt% of 200 mg), and that of recovered GO was 114 mg (74.0 wt% of 154 mg). The hydrogen content of GO before and after the reaction was 4.1 mg, so hydrogenation of GO did not occur. The slight increase in the carbon content of GO could be derived from the adsorption of 1a and/or 2a via π–π interactions. However, we assume that such adsorption is negligible because of the little change in hydrogen content and high mass balance of 1a and 2a. These results suggest that oxygen is exclusively eliminated from GO during the reaction. Supposing that all of the weight loss of GO during the reaction (46 mg) is derived from the elimination of oxygen, 2.88 mmol of oxygen contributed to the oxidation. Based on these observations, one oxygen atom of GO abstracts one or two hydrogen atoms from 1a. To identify the oxygen functional groups involved in the reaction, structural analyses of fresh and recovered GO samples were performed by solid-state 13C nuclear magnetic resonance (NMR) spectroscopy (Fig. 2b), Fourier transform infrared (FT-IR) spectroscopy (Fig. 2c), and X-ray photoelectron spectroscopy (XPS) (Fig. 2d,e). The solid-state 13C NMR spectrum of fresh GO showed three peaks at 60, 70 and 130 ppm (Fig. 2b red line). Meanwhile, the solid-state 13C NMR spectrum of recovered GO (Fig. 2b blue line) revealed a considerable decrease in the content of C–O bonds (60–70 ppm), along with a broad resonance peak at 100–130 ppm. These spectral changes are consistent with the generation of sp2 hybridized carbons accompanied by the loss of oxygen functional groups. The peaks at around 60 and 70 ppm are attributed to hydroxyl and epoxy groups, respectively37,38. However, 13C NMR spectra of model compounds (Fig. S10) suggested that both peaks could originate from epoxy groups. FT-IR spectra of recovered GO also showed the remarkable decrease in the content of oxygen functional groups at 800–1200 cm−1. Unfortunately, it was difficult to identify the actual functional groups because of the complexity of the fingerprint region. There was no marked change in the C–H and O–H stretching vibration peaks at 2800–3600 cm−1, suggesting that C–H bonds are not formed and O–H bonds are not eliminated (Fig. 2c, blue line). XPS analysis of the C 1s region of fresh GO confirmed that the main oxygen functional groups were C–O bonds (Fig. 2d). The decrease in the content of C–O bonds induced by the oxidative coupling reaction was further evidenced by XPS measurement of the recovered GO (Fig. 2e). Considering the above-mentioned stoichiometry calculation and structural analyses, we believe that epoxy groups were the main oxygen functional group of GO39,40,41,42 and acted as an oxidant to form H2O. The formation of water during the reaction was confirmed by the Karl–Fischer method (Table S5).
Figure 2. Comparison of fresh and recovered GO samples from oxidative C–H coupling.
(a) Mass balance of the reaction, and characterization of fresh GO (oxygen content: 41.1 wt%), and recovered GO after the oxidative C–H coupling reaction; (b) solid state 13C NMR spectra, (c) IR spectra, (d) XPS C1s region of fresh GO, and (e) XPS C1s region of recovered GO. (f) ESR spectra of (i) GO (O: 50.7 wt%), (ii) GO with 3,4-dimethoxytoluene and BF3·OEt2, (iii) GO with 3,4-dimethoxytoluene, and (iv) GO with BF3·OEt2.
To obtain mechanistic insights into the reaction41,42, the oxidative C–H coupling reaction with GO was performed in the presence of radical scavenger 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO). TEMPO prevented the reaction from proceeding (see ESI). Furthermore, a kinetic isotope effect was not observed when 6-deuterated 1a was used (see ESI). These results suggest the participation of radical species that can promote rapid hydrogen abstraction from 1a. To prove the formation of radical species in situ, we performed electron spin resonance (ESR) analysis. The ESR spectrum of highly oxidized GO (oxygen content: 50.7 wt%) did not contain a signal (Fig. 2f (i)). When 1a and BF3·OEt2 were added to GO, an ESR signal appeared (Fig. 2f (ii)), whereas negligible signal was observed when only 1a or BF3·OEt2 was added (Fig. 2f (iii) and (iv)). The ESR signal generated by the reaction of 1a, GO, and BF3·OEt2 is consistent with the so-called “carbon radical” stabilized by a resonance effect. A different ESR signal derived from radical cations was observed when 1a, PhI(OAc)2, and BF3·OEt2 were mixed43.
The interaction between GO, 1a, and BF3·OEt2 was further investigated. The FT-IR spectrum of 1a did not change upon addition of BF3·OEt2, while that of GO shifted upon addition of BF3·OEt2 (Fig. S11). 1H NMR spectra showed the same tendency; the signal from the ethyl group of BF3·OEt2 shifted when GO was added, while no change occurred when 1a was added (Fig. S12). These observations suggest that BF3·OEt2 activates GO to form radical species.
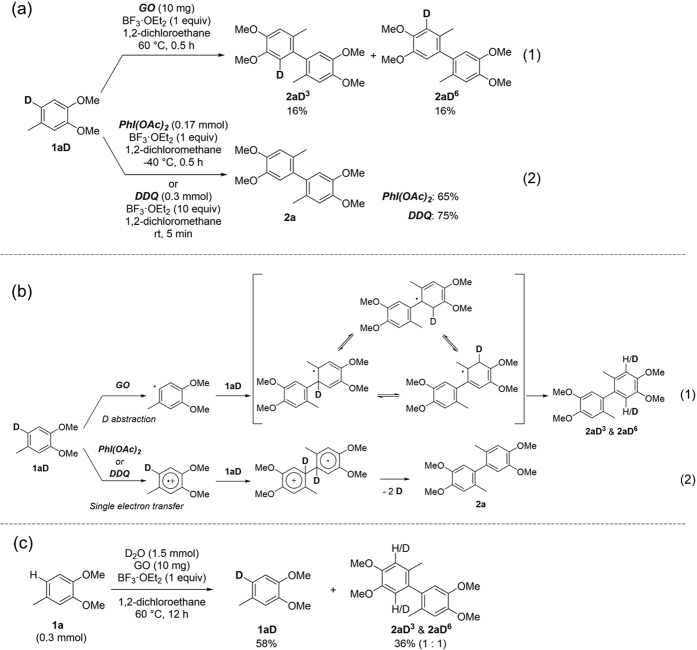
The principal difference between GO and other organic oxidants was determined by deuterium labeling experiments. When 6-deutero-3,4-dimethoxytoluene (1aD) was used, GO promoted the scrambling of D/H to form 2aD3 and 2aD6 (Fig. 3a-1); in contrast, PhI(OAc)2 and 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) produced only 2a (Fig. 3a-2). The proposed reaction mechanisms with GO, PhI(OAc)2 and DDQ are presented in Fig. 3b. When GO is used, deuterium abstraction of 1aD could occur to form an aryl radical, which readily reacts with another 1aD. The formed 3° radical is relatively stable, so scrambling of H/D could occur before elimination of hydrogen or deuterium (Fig. 3b-1). In contrast, PhI(OAc)2 and DDQ are proposed to produce a radical cation via single electron transfer, which was detected by ESR (Fig. 3b-2)20,43. The formation of an aryl radical by GO was proved by deuterium exchange reaction in the presence of D2O. The oxidative coupling reaction proceeded in 36% yield to form 2aD3 and 2aD6 without 2a, and 1a was deuterated to form 1aD (Fig. 3c). For comparison, the H/D exchange was investigated using PhI(OAc)2; however, no deuterium was incorporated in 1a and 2a in this case. These results show that the oxidative coupling reactions using GO and PhI(OAc)2 as the oxidant could pass through different intermediates.
Figure 3. Mechanistic investigation.
(a) Deuterium labelling experiment; (1) GO promoted the formation of 2aD3 and 2aD6 (both in 16% yield). (2) Phl(OAc)2 and DDQ promoted the formation of only 2a in 65% and 75% yield, respectively. (b) Proposed reaction mechanisms promoted by (1) GO, (2) Phl(OAc)2 or DDQ. (c) H/D exchange experiment with D2O. Only 1aD, 2aD3, and 2aD6 were observed in yields of 58%, 18%, and 18%, respectively.
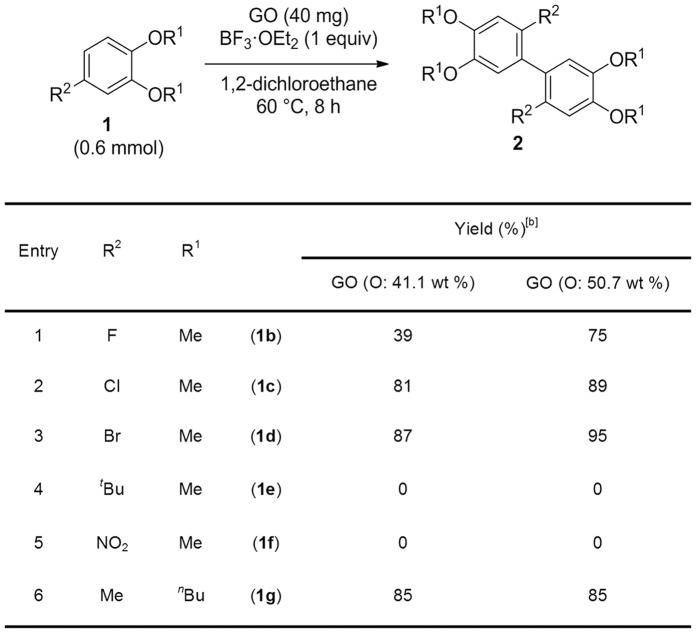
Next, the scope of the reaction was investigated using aromatic compounds with a variety of functional groups (Fig. 4). When halogenated aromatic compounds bearing a fluoro, chloro, or bromo group were used as starting materials, the product yield decreased as the electronegativity of the substituent increased (Fig. 4, Entry 1–3). A bulky or electron-withdrawing group prevented the reaction (Fig. 4, Entry 4, 5), while longer alkoxy groups did not affect the reaction (Fig. 4, Entry 6). Figure 5 shows the reaction scope for various aromatic compounds. The reaction proceeded in moderate yield when 1,3,5-trimethoxybenzene (3a) was used (Fig. 5, Entry 1). Trimerization occurred when 1,2-dimethoxybenzene (3b) was employed (Fig. 5, Entry 2). When another dimethoxybenzene (3c or 3d) was used, oligomerization occurred (Fig. 5, Entry 3, 4) (for mass spectral analysis, see ESI). A naphthalene derivative (3e or 3f) also underwent the oxidative coupling reaction to yield a binaphthyl compound (4e and 4f) (Fig. 5, Entry 5, 6). Intramolecular oxidative coupling reaction of a terphenyl derivative (3g) also proceeded to give 4g in good yield (Fig. 5, Entry 7). An anchored diphenyl compound (3h) underwent intramolecular oxidative coupling and successive dehydrogenative aromatization to give 4h (Fig. 5, Entry 8). Cross-coupling reactions proceeded in moderate to good yields when 1a or 2a and 3e were combined, although small amounts of undesired homocoupling products were detected (Fig. 5, Entry 9, 10). In some cases, highly oxidized GO (oxygen content: 50.7 wt%) showed higher reactivity than less oxidized GO (oxygen content: 41.1 wt%). (Fig. 4, Entry 2, 4, 6).
Figure 4. Substrate scope.
[a](a) Reaction condition: 1 (0.6 mmol), GO (40 mg), BF3·OEt2 (0.6 mmol), 1,2-dichloroethane (1.0 mL) under Ar atmosphere. (b) Isolated yield.
Figure 5. Substrate scope with various aromatic compounds.
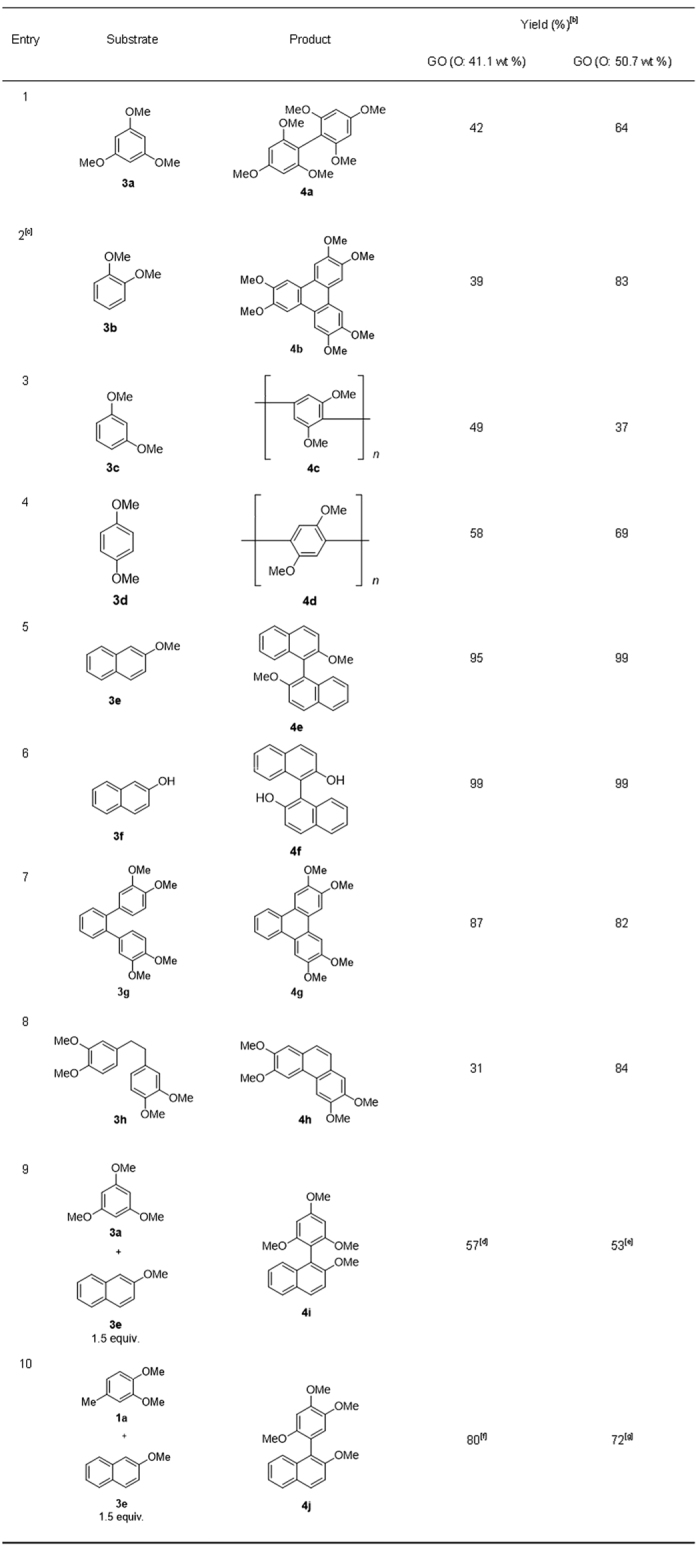
[a](a) Reaction condition: 1 (0.6 mmol), GO (40 mg), BF3·OEt2 (0.6 mmol), 1,2-dichloroethane (1.0 mL) under Ar atmosphere. (b) Isolated yield. (c) Reaction condition: 1,2-dimethoxybenzene (0.6 mmol), GO (100 mg), BF3·OEt2 (2.0 mL) under Ar atmosphere, 60 °C, 8 h. (d) Homocoupling product of 3a and 3e were produced in 1% and 11% yield. (e) Homocoupling product of 3a and 3e were produced in 1% and 6% yield. (f) Homocoupling product of 3a and 3e were produced in 19% and 29% yield. (g) Homocoupling product of 1a and 3e were produced in 19% and 31% yield.
In general oxidation reactions, consumption of an oxidant is wasteful. rGO has great potential for practical use in conductive materials, supercapacitor electrodes, and sensors44,45,46,47. rGO has been produced by reduction with hydrazine48, sodium borohydride49, and alcohols50, and by hydrothermal51, solvothermal52 and thermal annealing53 reactions of GO. The reduction process to form rGO consumes a reductant or energy, and is an inefficient operation. Recovered GO from the oxidative C–H coupling reaction may behave as rGO, and the electrical conductivity and specific capacitance of the recovered GO could be controlled by changing the oxygen content of the original GO54. When GO with the oxygen content of 41.1 wt% was used in the reaction, the recovered GO showed high electron conductivity (40.0 S/cm), because graphene-like structure was readily recovered, however the surface area was low (24.1 m2/g). In contrast, highly oxidized GO with the oxygen content of 50.7 wt% was reduced in the course of the reaction to show high surface area (47.9 m2/g). Because of the high surface area and pseudocapacitance induced by redox reaction of the remained oxygen functional groups on GO, the recovered GO showed high specific capacitance (166 F/g). For comparison, the electron conductivity and specific capacitance of rGO produced by hydrazine reduction were 39.0 S/cm and 80.8 F/g, respectively (Table S6).
Conclusion
Oxidative C–H coupling was performed using GO as an oxidant. Epoxy groups in the GO structure were activated by the acid additive to produce active radical species. The GO recovered after the oxidative C–H coupling reaction showed electrical conductivity, and has potential to work as a supercapacitor electrode. Therefore, this system could achieve two important reactions, C–H transformation and rGO production, in one pot.
Methods
Synthesis of graphene oxide
Graphite (3.0 g) was stirred in 95% H2SO4 (75 mL). The required amount of KMnO4 (6.0 and 15 g) was gradually added to the solution keeping the temperature <10 °C. The mixture was then stirred at 35 °C for 2 h. The resulting mixture was diluted by water (75 mL) under vigorous stirring and cooling so that temperature does not exceed 50 °C. The suspension was further treated by adding 30% aq. H2O2 (7.5 mL). The resulting graphite oxide suspension was purified by centrifugation with water until neutralization. Several GO were analyzed by CHNS elemental analysis to evaluate the oxygen content.
Experimental procedure of mass balance of the reaction
To the solution of 1,2-dichloroethane (4.0 mL), GO (200 mg), 3,4-dimethoxytoluene (913 mg, 6.0 mmol) and BF3·OEt2 (852 mg, 6.0 mmol) were added under Ar atmosphere and stirred at 60 °C for 8 h. After the reaction, reaction mixture was filtrated. Recovered GO was dried under reduced pressure and CHNS elemental analysis was performed. The filtrate was analyzed by GC using n-decane as an internal standard Fig. 2a.
ESR analysis
Spectrometer parameters: Microwave power: 1.0 mW. Microwave Freq.: 9.5 GHz. Scan time: 1 min. Modulation amplitude: 0.01 mT. Modulation Freq.: 100 kHz. Receiver gain: 16. Time constant: 0.01. ESR spectra was measured under ambient pressure at rt. 10.0 mg of GO was added to the quartz sample tube. Then each reactant (0.3 mmol) was added to the tube, and the measurement was performed Fig. 2f.
Kinetic isotope effect
To the solution of 1,2-dichloroethane (0.2 mL), GO (10.0 mg), BF3·OEt2 (42.6 mg, 0.3 mmol) and 3,4-dimethoxytoluene (45.7 mg, 0.3 mmol) or 6-deuterio-3,4-dimethoxytoluene (46.0 mg, 0.3 mmol) were added under Ar atmosphere and stirred at 60 °C for 8 h. After the reaction, reaction mixture was quenched by AcOEt and water. The organic phase was concentrated under reduced pressure and purified by column chromatography. However unseparatable products were obtained when 3,4-dimethoxytoluene-d was used. Structures of product were determined by 1H NMR and EI-MS.
Additional Information
How to cite this article: Morioku, K. et al. Concurrent Formation of Carbon-Carbon Bonds and Functionalized Graphene by Oxidative Carbon-Hydrogen Coupling Reaction. Sci. Rep. 6, 25824; doi: 10.1038/srep25824 (2016).
Supplementary Material
Footnotes
Author Contributions K.M. and N.M. designed experiments, developed techniques, performed experiments, analysed data and wrote the manuscript. Y.T. provided useful advice for reaction mechanism. Y.N. designed experiments and wrote the manuscript.
References
- Kakiuchi F. & Chatani N. Catalytic methods for C–H bond functionalization: Application in organic synthesis. Adv. Synth. Catal. 345, 1077–1101 (2003). [Google Scholar]
- Godula K. & Sames D. C–H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006). [DOI] [PubMed] [Google Scholar]
- Labinger J. A. & Bercaw J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002). [DOI] [PubMed] [Google Scholar]
- Shilov A. E. & Shul’pin G. B. Activation of C–H bonds by metal complexes. Chem. Rev. 97, 2879–2932 (1997). [DOI] [PubMed] [Google Scholar]
- Sarhan A. A. O. & Bolm C. Iron(III) chloride in oxidative C–C coupling reactions. Chem. Soc. Rev. 38, 2730–2744 (2009). [DOI] [PubMed] [Google Scholar]
- Waldvogel S. R. & Trosien S. Oxidative transformation of aryls using molybdenum pentachloride. Chem. Commun. 48, 9109–9119 (2012). [DOI] [PubMed] [Google Scholar]
- Ormsby J. L., Black T. D., Hilton C. L. & King B. B. T. Rearrangements in the Scholl oxidation: Implications for molecular architectures. Tetrahedron 64, 11370–11378 (2008). [Google Scholar]
- Trosien S., Böttger P. & Waldvogel S. R. Versatile oxidative approach to carbazoles and related compounds using MoCl5. Org. Lett. 16, 402−405 (2014). [DOI] [PubMed] [Google Scholar]
- Li C.-J. Cross-dehydrogenative coupling (CDC): Exploring C–C bond formations beyond functional group transformations. Acc. Chem. Res. 42, 335–344 (2009). [DOI] [PubMed] [Google Scholar]
- Satoh T. & Miura M. Oxidative coupling of aromatic substrates with alkynes and alkenes under rhodium catalysis. Chem. Eur. J. 16, 11212–11222 (2010). [DOI] [PubMed] [Google Scholar]
- Chen X., Engle K. M., Wang D.-H. & Yu J.-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: Versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby D. A., Tsai A. S., Bergman R. G. & Ellman J. A. Rhodium catalyzed chelation-assisted C–H bond functionalization reactions. Acc. Chem. Res. 45, 814–825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T. et al. Y. Coupling of quinone monoacetals promoted by sandwiched brønsted acids: Synthesis of oxygenated biaryls. Angew. Chem. Int. Ed. 50, 6142–6146 (2011). [DOI] [PubMed] [Google Scholar]
- Dohi T. et al. Y. Metal-free C–H cross-coupling toward oxygenated naphthalene–benzene linked biaryls. Org. Lett. 13, 6208–6211 (2011). [DOI] [PubMed] [Google Scholar]
- Morimoto K. et al. Metal-free oxidative coupling reactions via σ-iodonium intermediates: The efficient synthesis of bithiophenes using hypervalent iodine reagents. Eur. J. Org. Chem. 31, 6326–6334 (2011). [Google Scholar]
- Hackelöer K., Schnakenburg G. & Waldvogel S. R. Oxidative coupling reactions of 1,3-diarylpropene derivatives to dibenzo[a,c]cycloheptenes by PIFA. Eur. J. Org. Chem. 31, 6314–6319 (2011). [DOI] [PubMed] [Google Scholar]
- Velusamy S. & Punniyamurthy T. Copper (II)-catalyzed C–H oxidation of alkylbenzenes and cyclohexane with hydrogen peroxide. Tetrahedron Lett. 44, 8955–8957 (2003). [Google Scholar]
- Shustov G. V. & Rauk A. Mechanism of dioxirane oxidation of C–H bonds: Application to homo- and heterosubstituted alkanes as a model of the oxidation of peptides. J. Org. Chem. 63, 5413–5422 (1998). [Google Scholar]
- Zhai L., Shukla R. & Rathore R. Oxidative C–C bond formation (Scholl reaction) with DDQ as an efficient and easily recyclable oxidant. Org. Lett. 11, 3474–3477 (2009). [DOI] [PubMed] [Google Scholar]
- Zhai L., Shukla R., Wadumethrige S. H. & Rathore R. Probing the arenium-ion (protontransfer) versus the cation-radical (electron transfer) mechanism of Scholl reaction using DDQ as oxidant. J. Org. Chem. 75, 4748–4760 (2010). [DOI] [PubMed] [Google Scholar]
- Huang X. et al. Graphene-based materials: synthesis, characterization, properties, and applications. Small 7, 1876–1902 (2011). [DOI] [PubMed] [Google Scholar]
- Pei S. & Cheng H.-M. The reduction of graphene oxide. Carbon 50, 3210–3228 (2012). [Google Scholar]
- Chua C. K. & Pumera M. Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem. Soc. Rev. 43, 291–312 (2014). [DOI] [PubMed] [Google Scholar]
- Wang H. & Hu Y. H. Effect of oxygen content on structures of graphite oxides. Ind. Eng. Chem. Res. 50, 6132–6137 (2011). [Google Scholar]
- Wang H. & Hu Y. H. Electrolyte-induced precipitation of graphene oxide in its aqueous solution. J. Colloid Interface Sci. 391, 21–27 (2013). [DOI] [PubMed] [Google Scholar]
- Wang H., Leonard S. C. & Hu Y. H. Promoting effect of graphene on dye-sensitized solar cells. Ind. Eng. Chem. Res. 51, 10613–10620 (2012). [Google Scholar]
- Su C. & Loh K. P. Carbocatalysts: Graphene oxide and its derivatives. Acc. Chem. Res. 46, 2275–2285 (2013). [DOI] [PubMed] [Google Scholar]
- Haag D. & Kung H. H. Metal free graphene based catalysts: A review. Top Catal. 57, 762–773 (2014). [Google Scholar]
- Dreyer D. R., Jia H.-P. & Bielawski C. W. Graphene oxide: A convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew. Chem. Int. Ed. 49, 6813–6816 (2010). [DOI] [PubMed] [Google Scholar]
- Jia H.-P., Dreyer D. R. & Bielawski C. W. Graphite oxide as an auto-tandem oxidation-hydration-aldol coupling catalyst. Adv. Synth. Catal. 353, 528–532 (2011). [Google Scholar]
- Huang H. et al. Graphite oxide as an efficient and durable metal-free catalyst for aerobic oxidative coupling of amines to imines. Green Chem. 14, 930–934 (2012). [Google Scholar]
- Jia H.-P., Dreyer D. R. & Bielawski C. W. C–H oxidation using graphite oxide. Tetrahedron 67, 4431–4434 (2011). [Google Scholar]
- Brodie B. C. On the atomic weight of graphite. Phil. Trans. R. Soc. Lond. 149, 249–259 (1859). [Google Scholar]
- Petit C., Seredych M. & Bandosz T. J. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption, J. Mater. Chem. 19, 9176–9185 (2009). [Google Scholar]
- Botas C. et al. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 65, 156–164 (2013). [Google Scholar]
- Rodriguez-Pastor I. et al. Towards the understanding of the grapheme oxide structure: How to control the formation of humic- and fulvic-like oxidized debris. Carbon 84, 299–309 (2015). [Google Scholar]
- Casabianca L. B. et al. NMR-based structural modeling of graphite oxide using multidimensional 13C solid-state NMR and ab initio chemical shift calculation. J. Am. Chem. Soc. 132, 5672–5676 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 321, 1815–1817 (2008). [DOI] [PubMed] [Google Scholar]
- Taniguchi T. et al. pH-driven, reversible epoxy ring opening/closing in graphene oxide. Carbon 84, 560–566 (2015). [Google Scholar]
- Hatakeyama K. et al. Proton conductivities of graphene oxide nanosheets: single, multilayer, and modified nanosheets. Angew. Chem. Int. Ed. 53, 6997–7000 (2014). [DOI] [PubMed] [Google Scholar]
- Grzybowski M., Skonieczny K., Butenschön H. & Gryko D. T. Comparison of oxidative aromatic coupling and the Scholl reaction. Angew. Chem. Int. Ed. 52, 9900–9930 (2013). [DOI] [PubMed] [Google Scholar]
- King B. T. et al. Controlling the Scholl reaction. J. Org. Chem. 72, 2279–2288 (2007). [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Anilkumar G., Tohma H. & Kita Y. A novel and useful oxidative intramolecular coupling reaction of phenol ether derivatives on treatment with a combination of hypervalent iodine(III) reagent and heteropoly acid. Chem. Eur. J. 8, 5377–5383 (2002). [DOI] [PubMed] [Google Scholar]
- Gilje S. et al. A chemical route to graphene for device applications. Nano Lett. 7, 3394–3398 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou M., Zhai Y. & Dong S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 81, 5603–5613 (2009). [DOI] [PubMed] [Google Scholar]
- Becerril H. A. et al. Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2, 463–470 (2008). [DOI] [PubMed] [Google Scholar]
- Huang X., Qi X., Boey F. & Zhang H. Graphene-based composites. Chem. Soc. Rev. 41, 666–686 (2012). [DOI] [PubMed] [Google Scholar]
- Stankovich S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007). [Google Scholar]
- Shin H.-J. et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 19, 1987–1992 (2009). [Google Scholar]
- Dreyer D. R. et al. Reduction of graphite oxide using alcohols. J. Mater.Chem. 21, 3443–3447 (2011). [Google Scholar]
- Zhou Y. et al. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 21, 2950–2956 (2009). [Google Scholar]
- Dubin S. et al. A one-step, solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano 4, 3845–3852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhi L. & Müllen K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 8, 323–327 (2008). [DOI] [PubMed] [Google Scholar]
- Morimoto N., Kubo T. & Nishina Y. Tailoring the oxygen content of graphite and reduced graphene oxide for specific applications. Sci. Rep. 6, 21715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.