Abstract
Low-grade germinal matrix-intraventricular hemorrhage (GM-IVH) is the most common complication in extremely premature neonates. The occurrence of GM-IVH is highly associated with hemodynamic instability in the premature brain, yet the long-term impact of low-grade GM-IVH on cerebral blood flow and neuronal health have not been fully investigated. We used an innovative combination of frequency-domain near infrared spectroscopy and diffuse correlation spectroscopy (FDNIRS-DCS) to measure cerebral oxygen saturation (SO2) and an index of cerebral blood flow (CBFi) at the infant’s bedside and compute an index of cerebral oxygen metabolism (CMRO2i). We enrolled twenty extremely low gestational age (ELGA) neonates (seven with low-grade GM-IVH) and monitored them weekly until they reached full-term equivalent age. During their hospital stay, we observed consistently lower CBFi and CMRO2i in ELGA neonates with low-grade GM-IVH compared to neonates without hemorrhages. Furthermore, lower CBFi and CMRO2i in the former group persists even after the resolution of the hemorrhage. In contrast, SO2 does not differ between groups. Thus, CBFi and CMRO2i may have better sensitivity than SO2 in detecting GM-IVH-related effects on infant brain development. FDNIRS-DCS methods may have clinical benefit for monitoring the evolution of GM-IVH, evaluating treatment response, and potentially predicting neurodevelopmental outcome.
Twelve percent of live births in the United States are premature, resulting in the need to care for some 500,000 premature infants every year1. This rate is 30 percent greater than it was in the 1980s, and the National Academies estimate that premature births cost the US in excess of $26 billion annually2. Germinal matrix-intraventricular hemorrhage (GM-IVH) is a major complication of prematurity, occurring in about 45 percent of extremely low birth weight infants (weight < 1000 g)3. Even as improved neonatal intensive care and technological advances have increased the survival rate of extremely premature infants, the high incidence of GM-IVH has remained unchanged over the past decade4.
About 50–80 percent of premature infants with GM-IVH are diagnosed as low-grade (Grade I and II), and the majority of these newborns are estimated to be asymptomatic5,6. Diagnosis of GM-IVH in premature infants largely relies on screening via head ultrasound (HUS), even though the reliability in grading mild hemorrhage by HUS is low7,8. Unlike premature infants with severe GM-IVH, the relationship of low-grade GM-IVH to adverse outcomes is less predictable9,10. Moreover, current neuroimaging tools are not adequate surrogates for long-term neurodevelopmental follow-up. Major HUS abnormalities are only moderately predictive of cerebral palsy (CP) at variable ages of follow-up11,12,13,14,15 with still less predictive power where cognitive delay is concerned14,15. Meanwhile, magnetic resonance imaging (MRI) scanning as a predictive tool is still an area of active research16. Thus, there is a need for the discovery of new biomarkers of cerebral health in extremely premature infants. Such biomarkers may ultimately aid in the early detection and/or prevention of low-grade GM-IVH and may similarly have prognostic application.
Immature cerebral vascular anatomy and hemodynamic regulation in premature infants directly contributes to the pathogenesis of GM-IVH. Fluctuations in cerebral blood flow (CBF) associated with prematurity put the highly vascularized germinal matrix (GM) at risk for hemorrhage17,18, In full-term infants the GM regresses before birth19, but in infants born significantly pre-term this is not the case. The special vascular morphology of the GM serves to meet the significant metabolic demands of a wave of cell proliferation and migration from the GM to the cortex prior to term gestational age19. Autopsies of premature infants find hemorrhages in the GM are associated with decreased neuronal migration to the cortex20. The reduction in brain cell proliferation caused by germinal matrix hemorrhage results in a reduction of cerebral myelination21. Thus, although the GM is a transitory deep brain structure, hemorrhage in the GM has the potential to impair normal cortical development in affected infants22. GM-IVH may therefore have an observable impact on cortical physiology.
Cerebral hemoglobin oxygen saturation (SO2) is readily measured by near-infrared spectroscopy-based (NIRS) cerebral oximeters. However, the clinical utility of SO2 is often inconclusive23,24 since changes in SO2 reflect changes in both cerebral oxygen consumption and systemic changes in oxygen supply25. To disentangle the contributions of oxygen supply and demand, cerebral blood flow (CBF) must be measured in order to determine an unambiguous cerebral metabolic rate.
Frequency domain NIRS combined with diffuse correlation spectroscopy (FDNIRS-DCS) is a new technology for non-invasive measurement of cerebral blood flow and metabolic oxygen consumption (CMRO2). DCS measures an index of CBF (CBFi) in units of cm2/s. Although the units of CBFi are unconventional, CBFi values have good agreement with absolute CBF measured by other modalities26,27,28. Due to the difference in units, the subscript i is used to distinguish DCS-measured values from the physiological parameters they estimate. From CBFi and FDNIRS measurements of absolute cerebral hemoglobin concentrations, an estimate of oxygen consumption (CMRO2i) is calculated29. CBFi and CMRO2i from FDNIRS-DCS measurements have been reported to be more sensitive to detect early brain growth in infants than the more commonly measured SO2. CBFi and CMRO2i increase with postmenstrual age (PMA) and can detect differences in brain maturation in neonates30,31. Moreover, FDNIRS-DCS-measured CMRO2i has been used to assess individual treatment response in neonates with hypoxic ischemic encephalopathy32 and congenital heart disease33. In this study, we performed FDNIRS-DCS measurements in extremely low gestational age (ELGA) neonates with and without low-grade GM-IVH on a weekly basis during their hospital stay up until discharge at full-term age. We hypothesized that lower CBF and CMRO2 would be detected and sustained in neonates with low-grade GM-IVH in the first few months of life.
Results
Table 1 summarizes the clinical characteristics of infants in both GM-IVH and control groups. There were no significant inter-group differences with regard to other complications due to prematurity. Premature infants in the GM-IVH group had younger gestational ages at birth than the control group; however, the postmenstrual age (PMA), which is more closely correlated with changes in CBF and CMRO2 during early development31, was the same for both groups for each measurement session.
Table 1. Clinical characteristics of two groups.
| variables | GM-IVH | Control | P-value |
|---|---|---|---|
| N | 7 | 13 | |
| Male | 5 | 9 | 0.918 |
| Gestational age (wk)♭ | 25.2 ± 0.32 | 26.4 ± 0.36 | 0.015* |
| PMA at measurement session(wk)§ | 33.9 (28.7–39.7) | 33.1 (27.5–39.9) | 0.151 |
| Birth weight (g)♭ | 901 ± 60 | 932 ± 71 | 0.363 |
| Apgar at 5 min§ | 8 (6–9) | 8 (7–9) | 0.653 |
| Multiple birth | 3 | 8 | 0.423 |
| PDA | 4 | 8 | 0.848 |
| RDS | 3 | 5 | 0.848 |
| AOP | 1 | 3 | 0.639 |
| #of measurements | 40 | 83 |
AOP (apnea of prematurity), PDA (patent ductus arteriosus) RDS (respiratory distress syndrome).
Data are shown as number (percentage) except when marked as follows:
♭Mean ± SE.
§Median (range).
*Indicated P < 0.05.
Among seven GM-IVH infants, two infants had unilateral grade I GM-IVH and one infant had unilateral grade II. The remaining infants had bilateral GM-IVH with asymmetrical severity. Six infants were identified as having GM-IVH on the first HUS (Day of Life 1–3) and one infant’s diagnosis was made on the second HUS on the sixth day of life. In six of the seven infants, HUS records characterized the hemorrhage as resolved at Day of Life 30, while one infant’s hemorrhage was found to be resolved at Day 60.
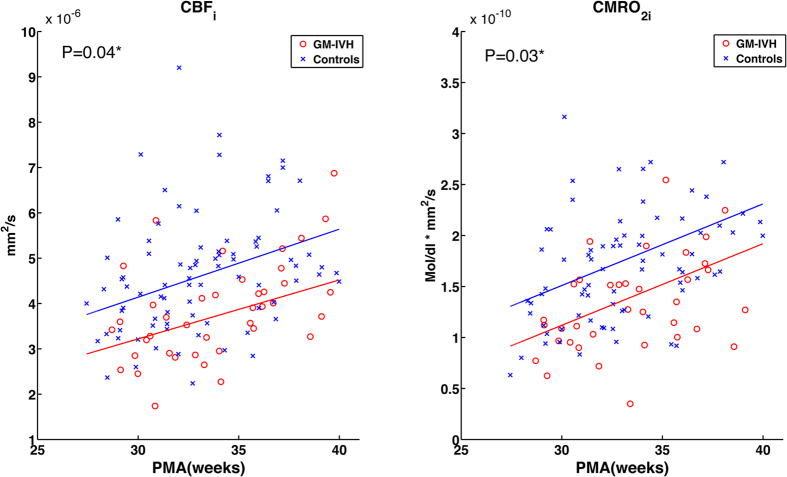
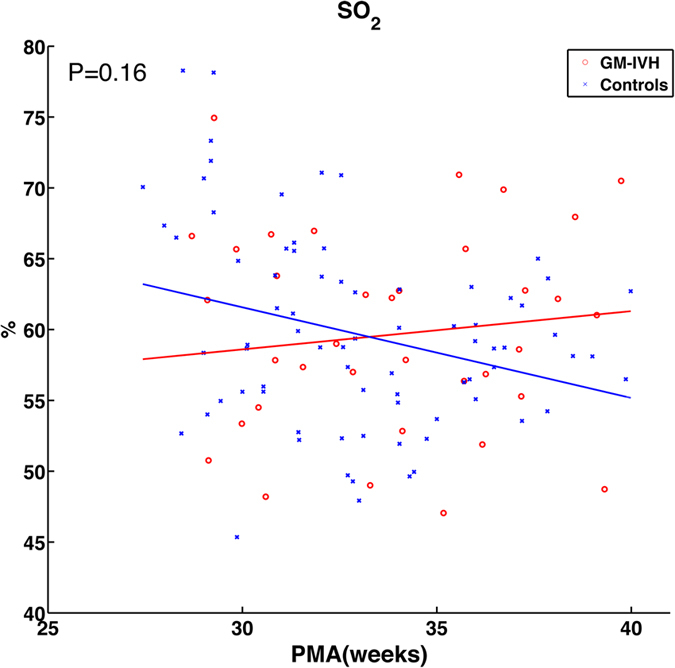
Figure 1 shows CBFi and CMRO2i, as a function of PMA. Slopes of the observed measures were modeled from data taken between 27–40 weeks PMA, the ages at which most of the subjects in the two groups were measured. The CBFi and CMRO2i in both groups increased with time but were statistically lower in the GM-IVH group than in the control group (P = 0.04 and 0.03, respectively). Figure 2 presents the weekly average of SO2 as a function of PMA with standard errors. Unlike CBFi and CMRO2i, SO2 did not show a significant correlation with PMA or a significant difference between groups (P = 0.16).
Figure 1. Scatterplots and estimated time trajectories (solid line) of CBFi and CMRO2i with PMA in premature infants with low-grade GM-IVH (red line) and controls (blue line).
*Indicates significant inter-group difference (P < 0.05).
Figure 2. Scatterplots and estimated time trajectories (solid line) of CBFi and CMRO2i with PMA in premature infants with low-grade GM-IVH (red line) and controls (blue line).
Discussion
This study demonstrated that the ELGA infants with low-grade GM-IVH had lower CBF and CMRO2 than PMA-matched controls, and this difference persists even after the hemorrhages appeared resolved on HUS images. The findings suggest that low-grade GM-IVH in ELGA infants may have a more significant impact on brain development than is currently assumed.
Our study is the first to demonstrate that mild hemorrhage reduces cerebral metabolism in ELGA infants, and this lowered cerebral metabolism is consistent with the hypothesis that GM-IVH decreases neuronal migration to the cortex20. The ganglionic eminence (GE) is a transitory brain region which is the source of critical waves of cell proliferation and migration to the cortex, including a class of neocortical GABAergic interneurons34 as well as neurons for the basal nuclei35. The GE regresses gradually19 with final involution of the GE by 34–36 weeks36. Thus, infants are born prematurely retain the GE through birth and experience a portion of this involution ex utero. Unfortunately, extremely premature infants with immature hemodynamic regulation often put the highly vascularized GE at risk of rupture leading to a germinal matrix hemorrhage. Thus, if the hemorrhage arises from the GE, it is presumed to affect the production of GABAergic interneurons for the neocortex and the coordination and integration of cortical functions22. Del Bigio has reported significant and persistent suppression of cell proliferation following hemorrhage in human GE samples compared to brains without GM-IVH20. There is also no evidence of rebound cell proliferation above normal levels in these GM-IVH brains, suggesting the GE continues to involute following hemorrhages without compensation. Another imaging study using MRI to measure cortical volume in premature infants reported reduced cortical volume in low-grade GM-IVH cases at ages coinciding with those in our study37. Together, these results suggest that small hemorrhages within the germinal matrix at early stages of gestation may have substantial effects on cortical development in the brains of premature infants.
In contrast to previous studies reported in the literature, which mainly examine the impact of severe GM-IVH at an acute stage, we have instead focused on low-grade GM-IVH and have extended the window of observations to cover longer periods, collecting measurements even after hemorrhages were deemed resolved under HUS examination. Because cerebral blood flow and cerebral oxygen metabolism are persistently lower in GM-IVH infants, we hypothesize that the impact of mild GM-IVH may be of clinical significance and associated with poorer neurodevelopmental outcomes9,11,38,39. Indeed, several large retrospective cohort studies report premature infants with low-grade GM-IVH go on at later ages to experience higher rates of CP9,39, major neurologic abnormality39,40, and moderate to severe neurosensory impairment38. Although other studies posit that such correlations may be a consequence of clinical practices at birth41, the higher risks of poor developmental outcomes in premature infants with low-grade GM-IVH suggest that GM-IVH itself may place development of the immature brain at additional risk.
In addition to reduced cerebral metabolism secondary to the presence of hemorrhage, low cerebral blood flow itself may also be a causative factor for the onset of GM-IVH42,43. Prior to our work, indirect measures of CBF fluctuations had been frequently observed before and/or immediately after the hemorrhage occurred in GM-IVH neonates by computed tomography (CT)44, positron emission tomography (PET)45, conventional NIRS46, and Doppler ultrasound on superior vena cava flow47, but these studies were restricted to measurements during the first few days of life. These findings from these other imaging modalities are consistent with the hypothesis that the ischemia-reperfusion cycle drives the pathogenesis of IVH48,49. Our method equips future prospective studies to investigate further the role of impaired CBF regulation in the risk for developing IVH.
Few studies have reported cerebral perfusion or function in preterm infants with mild or uncomplicated GM-IVH and the association of perfusion and function with infants’ later outcomes50. A case-control study using conventional NIRS methods for two hours a day for eight days found persistently lower cerebral rSO2 and higher cerebral oxygen extraction fraction (COEF) in preterm neonates with GM-IVH46. It was suspected that the higher COEF observed in infants with low-grade GM-IVH resulted from low CBF. Increased COEF was also observed after severe hemorrhages occurred and the magnitude of the increase was identical to that observed in lower-grade cases. In the same cohort of infants with low-grade GM-IVH, it was later reported that high and low SO2 on the first day of life was associated with poorer cognitive outcomes among preterm infants at 2 to 3 years old50. This further demonstrates the clinical value of monitoring cerebral physiology in the early postnatal period to predict long-term outcomes51. In the current work, our novel combination of DCS with FDNIRS found that lower CBFi and CMRO2i were consistently observed in premature neonates with GM-IVH throughout the first few months of life, while SO2 did not show a significant difference between affected and unaffected infants. Our results suggest that CBFi and CMRO2i are more robust measures than SO2 for investigating the effects of mild hemorrhages on cortical development and thus may have an important role as an early outcome predictor in this population.
Our results also demonstrate that bedside measures of cerebral blood flow and metabolism measured with FDNIRS-DCS have the potential to detect early abnormal cerebral physiology in preterm infants. Previous NIRS studies using commercial continuous-wave NIRS (CWNIRS) systems use optical absorption to measure changes in hemoglobin concentration and, with some assumptions, estimate SO2 and COEF – a standard measure of oxygen delivery from capillaries to tissue. In contrast, the FDNIRS used in this study measures both the optical absorption and the phase-shift of modulated light to determine separate scattering and absorption coefficients of tissue. Thus, FDNIRS is superior in quantifying absolute cerebral hemoglobin concentrations, eliminating some assumptions required with CWNIRS52. However, the interpretation of COEF values by either method alone is not always straightforward because changes in the COEF represent differences in either oxygen supply or consumption. In order to correctly account for the distinct contributions of supply and demand, we directly measure cerebral blood flow independently so we can unambiguously determine metabolic rate, especially under pathological conditions25. Our FDNIRS-DCS method provides a direct measure of CBF and CMRO2, a more direct measure of neuronal health. Our results also demonstrated that SO2 is not significantly different between the two groups, which is in agreement with our previous findings of smaller variance in SO2 with age and disease than in CBFi, and CMRO2i25,53. Thus, CBFi and CMRO2i detect the impact of GM-IVH more effectively than SO2 alone, and potentially may be a better predictor of outcomes.
The premature neonates in this study did not undergo any clinical MRIs during their hospital stay. Some subtle injuries associated with white matter, brain stem, and cerebellar hemorrhage may not be detected by ultrasound imaging. However, the effects of subtle injuries in cerebral hemodynamics and metabolism have not been fully characterized, and even premature infants without GM-IVH may have a similar chance of hemorrhage occurring in other areas of the brain.
Due to the relatively small sample of infants in this study, there is insufficient statistical power to examine hemispheric differences in hemodynamics. Previously, COEF measured at the left fronto-parietal side showed no statistical difference among infants with unilateral and bilateral GM-IVH46, suggesting hemorrhage-related alterations in CBF may occur globally. On the other hand, Ment et al. found higher blood flow in the side on which the hemorrhages appeared, but these hemisphere differences were only observed in first five days of life54. We plan to conduct larger cohort studies in the future using the FDNIR-DCS method to investigate whether hemispheric differences exist among GM-IVH-associated changes of CBF and/or CMRO2. Our ultimate aim is to identify adequate biomarkers that are predictive of long-term neurodevelopmental outcomes.
Conclusion
Our results demonstrated low-grade GM-IVH-related changes in CBFi and CMRO2i in ELGA infants. Our new technology makes quantification of CBFi and CMRO2i feasible right at the bedside, without the need for radioactive tracers, and with a sensitivity to mild GM-IVH cases that is lacking in existing measures. This method makes it possible for the first time to follow changes in cerebral cortical metabolism as neuronal migration progresses from the GM to the cortex in extremely low gestational age neonates.
Methods
The study protocol was reviewed and approved by the Institutional Review Board for Partners Healthcare, the Partners Human Research Committee (PHRC). The study method was designed and carried out in accordance with PHRC requirements and the regulations that govern human subjects research.
Participants
Seven ELGA infants with low-grade GM-IVH and a control group of 13 ELGA infants without brain injury were enrolled from the neonatal intensive care units (NICUs) of the Massachusetts General Hospital and the Brigham and Women’s Hospital between April 2008 and January 2013. Parents who agreed to participate were asked to read and sign an informed consent form as approved by the Partners Human Research Committee. Infants with gestational age (GA) of 24–28 weeks at birth were eligible for the study. Exclusion criteria included congenital brain malformation; genetic disorders; metabolic or structural abnormality or neoplasm; congenital heart disease; congenital hydrocephalus; imaging evidence of brain lesions other than GM-IVH or white matter abnormalities; or brain infection; Infants with cystic periventricular leukomalacia (PVL) were also not included because the pathogenesis of cystic PVL might affect cerebral oxygenation in ways different from GM-IVH55.
Routine head ultrasound
All of the premature infants had at least three serial HUS for general screening per NICU protocol at three time points: Day of Life (DOL) 1–4, DOL 7–10, and DOL 30. Additional HUS scans were performed as needed, depending on the infants’ clinical conditions. We first screened GM-IVH infants based on the reports of on-site radiologists and/or neonatologists at each hospital. Due to the low agreement in assessments of HUS in detecting low-grade GM-IVH7,8, all the HUS images were examined and interpreted again by an experienced neonatal neuroradiologist (PEG) blinded to the results of FDNIRS-DCS measurements. The Papile classification was used to grade the severity of GM-IVH on ultrasound56.
Near-Infrared Spectroscopy measurements
We used customized frequency-domain near-infrared spectroscopy (FDNIRS) system (Oxiplex, ISS, Inc. Champaign, IL) and an in-house built diffuse correlation spectroscopy (DCS) system to quantify hemoglobin concentrations (HbO & HbR) and direct measures of an index of cerebral blood flow (CBFi). The details of the underlying optical theory and instrumental technology are described in previous work31. In brief, the FDNIRS device has two identical measurement modules consisting of eight laser sources of different wavelengths ranging from 660 to 830 nm and two detectors. Four source-detector distances are used in the multi-distance method to determine both scattering and absorption coefficients52 and thus absolute hemoglobin concentrations and oxygenation are quantified.
Although DCS measures of CBFi are relatively new, these measures have been extensively validated against various conventional CBF measurement modalities26,27,28. DCS measures microvascular blood flow in cortical tissue by quantifying temporal intensity fluctuations after light has been scattered many times by moving red blood cells57,58. DCS is similar to laser Doppler blood flowmetry (they are Fourier transform analogs), but in DCS the source and detectors are spatially separated by a few centimeters. Thus, the DCS signal is averaged over a greater cortical volume and is more weighted towards capillary flow than laser Doppler. Accordingly, DCS has been found to be a more consistent measure of cortical blood flow59. Together, FDNIRS and DCS are combined to make direct quantitative bedside measurement of not only SO2, but also CBFi, from which a cerebral oxygen metabolism index (CMRO2i) can be determined.
We have designed our own FDNIRS-DCS probe specifically for preterm neonates and have fabricated it in house by 3D printing. The source-detector separations of 1 to 2.5 cm yield a depth penetration of about 0.5–1.5 cm, which mostly includes the cerebral cortex in premature neonates60. For measurements, the probe is gently placed on the infant’s scalp in several locations, covering left, middle and right frontal areas32, and measurements are repeated several times to ensure reproducibility of the results. The probe is repositioned in a slightly different area to compensate for local inhomogeneities such as hair and larger superficial vessels, ensuring measurements are representative of the underlying brain region rather than circumstantial artifacts. Once the clinical condition of a participating infant permitted, we would measure infants up to once a week during their hospital stay. In addition, the arterial oxygenation (SaO2) was recorded from the clinical oximeter at the infant’s bedside at the time of the measurement and hemoglobin in the blood (HGB) was obtained from clinical charts in order to estimate CMRO2i31,53. We have adopted this approach61 in over 300 infants in the NICU environment30,31,32.
NIRS Data Analysis
We have developed our own MATLAB scripts which automatically and systematically assess FDNIRS and DCS data quality using previously established statistical criteria29. For each subject, each measurement session, and each location, we calculated absolute hemoglobin concentrations using the FDNIRS multi-distance method62. The total hemoglobin concentration (HbT) was used to calculate SO2 (SO2 = HbO/HbT). We obtained a blood flow index (CBFi) once per second from DCS63. By combining all measured parameters, we can estimate CMRO2i (CMRO2i = CBFi × HGB × (SaO2 − SO2))29.
Statistical analysis
Only data that passed standard data quality assessment were used for the statistical analysis. We averaged SO2, CBFi, and CMRO2i from three locations to represent the frontal region. For group difference analysis, we employed multivariate mixed-model regression analyses with NIRS parameters modeled as a four-dimensional Gaussian random effect with an unstructured covariance matrix and unknown mean vector. We also compared models using Bayesian information criterion (BIC) to best estimate time trajectories of each NIRS parameter as a function of PMA or age. Lastly, for each parameter, PMA or age, group and their interactions, and interactions with NIRS parameters were modeled as fixed effects to assess the statistical significance of between-group differences of time trajectories. This model considered the variances of the unequal number of measurement sessions of each subject as well as the total number of sessions for each group. The analysis was performed in the R statistical package (version 3.1.0), using the “lme4”64 and “lmerTest” libraries65. The clinical data of the two groups were tested by Mann-Whitney U test, X2, or t test (as appropriate).
Additional Information
How to cite this article: Lin, P.-Y. et al. Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix-intraventricular hemorrhage. Sci. Rep. 6, 25903; doi: 10.1038/srep25903 (2016).
Acknowledgments
We thank Dr. Mark Vangel for his assistance in statistical analysis, which is supported by Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and NCATS, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. We thank Drs Erin Buckley and Mathieu Dehaes for their support with the data analysis. We also thank all the families of the participating infants, without whom the study would not be possible, as well as the staff of the participating neonatal intensive care units who contributed their help and support to the project. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. This study was funded by the American Heart Association (P.-Y.L.) K99HD083512 (P.-Y.L.), R21HD072505 (P.E.G.), and R01HD042908 (M.A.F). None of the funders are involved in study design, data collection, data analysis, and manuscript preparation and publication decisions.
Footnotes
Author Contributions P.-Y.L. data collection, data analysis, manuscript writing. K.H. and A.F. subject enrollment, data collection, manuscript editing. P.E.G. clinical imaging justification, data interpretation, manuscript editing. M.A.F. data interpretation, manuscript editing.
References
- Hamilton B. E., Hoyert D. L., Martin J. A., Strobino D. M. & Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics 131, 548–558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman R. E. & Butler A. S. Preterm Birth: Causes, Consequences, and Prevention. (National Academies Press (US), doi: 10.17226/11622 2007). [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D., Friedman H., Minich N., Fanaroff A. A. & Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 115, 997–1003 (2005). [DOI] [PubMed] [Google Scholar]
- Horbar J. D. et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 129, 1019–1026 (2012). [DOI] [PubMed] [Google Scholar]
- Stoll B. J. et al. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrikopoulou M. et al. Perinatal biomarkers in prematurity: Early identification of neurologic injury. Int. J. Dev. Neurosci. 36, 25–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz S. R. et al. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J. Pediatr. 150, 592–6– 596.e1–5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. et al. Low-grade intraventricular hemorrhage: is ultrasound good enough? J. Matern. Fetal Neonatal Med. Suppl 1, 2261–2264 (2015). [DOI] [PubMed] [Google Scholar]
- Bolisetty S. et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133, 55–62 (2014). [DOI] [PubMed] [Google Scholar]
- Payne A. H. et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 167, 451–459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancel P. Y. et al. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: The EPIPAGE cohort study. Pediatrics 117, 828–835 (2006). [DOI] [PubMed] [Google Scholar]
- de Vries L. S., Eken P., Pierrat V., Daniels H. & Casaer P. Prediction of neurodevelopmental outcome in the preterm infant: short latency cortical somatosensory evoked potentials compared with cranial ultrasound. Arch. Dis. Child. 67, 1177–1181 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries L. S., Van Haastert I.-L. C., Rademaker K. J., Koopman C. & Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J. Pediatr. 144, 815–820 (2004). [DOI] [PubMed] [Google Scholar]
- Pinto-Martin J. A., Whitaker A. H., Feldman J. F., Rossem R. & Paneth N. Relation of cranial ultrasound abnormalities in low‐birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Dev. Med. Child. Neurol. 41, 826–833 (1999). [DOI] [PubMed] [Google Scholar]
- Wood N. S. et al. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch. Dis. Child-Fetal. 90, F134–40 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dib M., Massaro A., Bulas D. & Aly H. Neuroimaging and neurodevelopmental outcome of premature infants. Am. J. Perinatol. 27, 803–818 (2010). [DOI] [PubMed] [Google Scholar]
- Bassan H. Intracranial Hemorrhage in the Preterm Infant: Understanding It, Preventing It. Clin. Perinatol. 36, 737–762 (2009). [DOI] [PubMed] [Google Scholar]
- Volpe J. J. In Neurology of the Newborn 517–588 (Elsevier Health Sciences, 2008). [Google Scholar]
- Volpe J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet. Neurol. 8, 110–124 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio M. R. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain 134, 1344–1361 (2011). [DOI] [PubMed] [Google Scholar]
- Jakovcevski I. & Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 49, 480–491 (2005). [DOI] [PubMed] [Google Scholar]
- Petanjek Z., Kostović I. & Esclapez M. Primate-specific origins and Mmgration of cortical GABAergic neurons. Front. Neuroanat. 3, 26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOST-II Australia and United Kingdom Collaborative Groups et al. Outcomes of Two Trials of Oxygen-Saturation Targets in Preterm Infants. New. Eng. J. Med. 374, 749–760 (2016). [DOI] [PubMed] [Google Scholar]
- Hyttel-Sorensen S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 350, g7635–g7635 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas D. A. & Franceschini M. A. Haemoglobin oxygen saturation as a biomarker: the problem and a solution. Philos. Trans. A Math. Phys. Eng. Sci. 369, 4407–4424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V. et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J. Cereb. Blood Flow Metab. 34, 380–388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop M., Verdecchia K., Lee T.-Y. & St Lawrence K. Erratum: Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements: errata. Biomed. Opt. Express 3, 1476–1477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. et al. Validation of diffuse correlation spectroscopy for muscle blood flow with concurrent arterial spin labeled perfusion MRI. Opt. Express. 15, 1064–1075 (2007). [DOI] [PubMed] [Google Scholar]
- Roche-Labarbe N. et al. Noninvasive optical measures of CBV, StO2, CBF index, and rCMRO2 in human premature neonates’ brains in the first six weeks of life. Hum. Brain Mapp. 31, 341–352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.-Y. et al. Regional and hemispheric asymmetries of cerebral hemodynamic and oxygen metabolism in newborns. Cereb. Cortex. 23, 339–348 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche-Labarbe N. et al. Near-infrared spectroscopy assessment of cerebral oxygen metabolism in the developing premature brain. J. Cereb. Blood Flow Metab. 32, 1–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaes M. et al. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J. Cereb. Blood Flow Metab. 34, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaes M. et al. Perioperative cerebral hemodynamics and oxygen metabolism in neonates with single-ventricle physiology. Biomed. Opt. Express 6, 4749–4767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N., Chen Y. & Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J. Comp. Neurol. 491, 109–122 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N. Expression of calbindin and calretinin in the human ganglionic eminence. Pediatric. Neurology. 24, 357–360 (2001). [DOI] [PubMed] [Google Scholar]
- Del Bigio M. Developmental Neuropathology. 150–155 (Wiley-Blackwell, 2006). [Google Scholar]
- Vasileiadis G. T. et al. Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics 114, e367–72 (2004). [DOI] [PubMed] [Google Scholar]
- Klebermass-Schrehof K. et al. Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants. Childs. Nerv. Syst. 28, 2085–2092 (2012). [DOI] [PubMed] [Google Scholar]
- Patra K., Wilson-Costello D., Taylor H. G., Mercuri-Minich N. & Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J. Pediatr. 149, 169–173 (2006). [DOI] [PubMed] [Google Scholar]
- Vavasseur C., Slevin M., Donoghue V. & Murphy J. F. A. Effect of low grade intraventricular hemorrhage on developmental outcome of preterm infants. J. Pediatr. 151, e6–author reply e6–7 (2007). [DOI] [PubMed] [Google Scholar]
- Inder T. E. Neurodevelopmental impact of low-grade intraventricular hemorrhage in very preterm infants. J. Pediatr. 149, 152–154 (2006). [DOI] [PubMed] [Google Scholar]
- Meek J. H., Tyszczuk L., Elwell C. E. & Wyatt J. S. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch. Dis. Child-Fetal. 81, F15–8 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel F., Van de Bor M., Stijnen T., Baan J. & Ruys J. H. Aetiological rôle of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage. Dev. Med. Child. Neurol. 29, 601–614 (1987). [DOI] [PubMed] [Google Scholar]
- Ment L. R., Duncan C. C., Ehrenkranz R. A. & Lange R. C. Intraventricular hemorrhage in the preterm neonate: Timing and cerebral blood flow changes. J. Pediatr. 104, 419–425 (1984). [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Herscovitch P., Perlman J. M. & Raichle M. E. Positron emission tomography in the newborn: extensive impairment of regional cerebral blood flow with intraventricular hemorrhage and hemorrhagic intracerebral involvement. Pediatrics 72, 589–601 (1983). [PubMed] [Google Scholar]
- Verhagen E. A. et al. Cerebral Oxygenation in Preterm Infants With Germinal Matrix-Intraventricular Hemorrhages. Stroke 41, 2901–2907 (2010). [DOI] [PubMed] [Google Scholar]
- Osborn D. A., Evans N. & Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 112, 33–39 (2003). [DOI] [PubMed] [Google Scholar]
- Alderliesten T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698–704.e2 (2013). [DOI] [PubMed] [Google Scholar]
- Noori S., McCoy M., Anderson M. P., Ramji F. & Seri I. Changes in Cardiac Function and Cerebral Blood Flow in Relation to Peri/Intraventricular Hemorrhage in Extremely Preterm Infants. J. Pediatr. 164, 264–270.e3 (2013). [DOI] [PubMed] [Google Scholar]
- Verhagen E. A. et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev. Med. Child. Neurol. 57, 449–455 (2015). [DOI] [PubMed] [Google Scholar]
- Toet M. C. Cerebral Oxygenation and Electrical Activity After Birth Asphyxia: Their Relation to Outcome. Pediatrics 117, 333–339 (2006). [DOI] [PubMed] [Google Scholar]
- Fantini S., Franceschini M. A., Fishkin J. B., Barbieri B. & Gratton E. Quantitative determination of the absorption spectra of chromophores in strongly scattering media: a light-emitting-diode based technique. Appl. Opt., AO 33, 5204–5213 (1994). [DOI] [PubMed] [Google Scholar]
- Franceschini M. A. et al. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatr. Res. 61, 546–551 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment L. R., Ehrenkranz R. A., Lange R. C., Rothstein P. T. & Duncan C. C. Alterations in Cerebral Blood Flow in Preterm Infants with Intraventricular Hemorrhage. Pediatrics 68, 763–769 (1981). [PubMed] [Google Scholar]
- Kissack C. M., Garr R., Wardle S. P. & Weindling A. M. Postnatal Changes in Cerebral Oxygen Extraction in the Preterm Infant Are Associated with Intraventricular Hemorrhage and Hemorrhagic Parenchymal Infarction but Not Periventricular Leukomalacia. Pediatr. Res. 56, 111–116 (2004). [DOI] [PubMed] [Google Scholar]
- Papile L. A., Burstein J., Burstein R. & Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978). [DOI] [PubMed] [Google Scholar]
- Culver J. P. et al. Diffuse optical tomography of cerebral blood flow, oxygenation, and metabolism in rat during focal ischemia. J. Cereb. Blood Flow Metab. 23, 911–924 (2003). [DOI] [PubMed] [Google Scholar]
- Durduran T. et al. Diffuse optical measurement of blood flow, blood oxygenation, and metabolism in a human brain during sensorimotor cortex activation. Opt. Lett. 29, 1766–1768 (2004). [DOI] [PubMed] [Google Scholar]
- Buckley E. M. et al. Validation of diffuse correlation spectroscopic measurement of cerebral blood flow using phase-encoded velocity mapping magnetic resonance imaging. J. Biomed. Opt. 17, 037007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaes M. et al. Assessment of the frequency-domain multi-distance method to evaluate the brain optical properties: Monte Carlo simulations from neonate to adult. Biomed. Opt. Express 2, 552–567 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.-Y. et al. Non-invasive optical measurement of cerebral metabolism and hemodynamics in infants. J. Vis. Exp. e4379, doi: 10.3791/4379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini S. Frequency-domain multichannel optical detector for noninvasive tissue spectroscopy and oximetry. Opt. Eng. 34, 32 (1995). [Google Scholar]
- Boas D., Campbell L. & Yodh A. Scattering and Imaging with Diffusing Temporal Field Correlations. Phys. Rev. Lett. 75, 1855–1858 (1995). [DOI] [PubMed] [Google Scholar]
- me4: Linear mixed-effects models using Eigen and S4. (2014). at http://CRAN.R-project.org/package=lme4.
- lmerTest: Tests for random and fixed effects for linear mixed effect models. (2014). at http://cran.r-project.org/web/packages/lmerTest/index.html.