Abstract
Low-molecular-weight heparin (LMWH) is part of standard supportive care. We conducted a meta-analysis to investigate the efficacy and safety of LMWH in septic patients. We searched Pubmed, Embase, CKNI and Wanfang database prior to July 2015 for randomized controlled trials investigating treatment with LMWH in septic patients. We identified 11 trials involving 594 septic patients. Meta-analysis showed that LMWH significantly reduced prothrombin time (mean differences [MD] −0.88; 95% CI −1.47 to −0.29), APACHE II score (MD −2.50; 95% CI −3.55 to −1.46), and 28-day mortality (risk ratio [RR] 0.72; 95% CI 0.57–0.91) as well as increased the platelet counts (MD 18.33; 95% CI 0.73–35.93) than the usual treatment. However, LMWH did not reduce D-dimer (MD −0.34; 95% CI −0.85 to 0.18). LMWH also significantly increased the bleeding events (RR 3.82; 95% CI 1.81–8.08). LMWH appears to reduce 28-day mortality and APACHE II score among septic patients. Bleeding complications should be monitored during the LMWH treatment. As for limited data about LMWH and sepsis in the English literature, only trials published in the Chinese were included in the meta-analysis.
Sepsis is a clinical syndrome of microbial infection complicated by systemic inflammation. Sepsis remains a major public health problem. The incidence of severe sepsis is estimated up to 100 cases per 100 000 population1. Despite advances in intensive care technologies, the hospital mortality rate of severe sepsis was 48.7% in China2. Sepsis can induce coagulatory activation, which may contribute to deteriorate organ function3,4. Cross talk exists between inflammatory and coagulation system5. Therefore, targeting the hypercoagulable state should be a promising approach in the treatment of sepsis.
The survival benefits of coagulation inhibitors, recombinant human activated protein C as an adjunctive therapy for sepsis have been demonstrated6,7. However, the high cost and bleeding risk limit its application for the majority of patients. Heparin has the potential role in the treatment of sepsis because of similar anticoagulant actions8. Heparin can be divided into unfractionated and low-molecular-weight heparin(LMWH)9. Unfractionated heparin is the most commonly used anticoagulant worldwide. LMWH is an attractive alternative anticoagulant due to its superior antithrombotic efficacy and fewer reported bleeding risk10. Several studies11,12,13,14,15,16,17,18,19,20,21 have been published in the literature evaluating the effects of LMWH in sepsis. In general, findings from these studies suggested that LMWH might be useful in these septic patients. However, small sample size of each study might lack statistical power to draw definitive conclusions.
Here, we conducted a meta-analysis using the available randomized control trials (RCTs) to evaluate the efficacy and safety of LMWH treatment in septic patients.
Results
Search results and baseline characteristics
Our preliminary literature search yielded 286 potential articles, of which 260 were excluded after screening titles and abstracts. After retrieving for full-text manuscript, 15 citations were excluded for various reasons. Finally, 11 trials11,12,13,14,15,16,17,18,19,20,21 were eligible for the meta-analysis (Fig. 1). Baseline characteristics of the trials are listed in Table 1. All the trials were performed in China and published in Chinese. Eleven trials involved 594 patients with 329 in LMWH groups and 265 in the usual treatment groups. Patients in LMWH groups received two types of heparin: low-molecular weight heparin calcium and low-molecular weight heparin sodium. LMWH treatment durations were 7 and 14 days. Ten trials11,12,13,15,16,17,18,19,20,21 determined the circulating levels of prothrombin time, platelet, d-dimer, pro-inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and clinical acute physiology and chronic health evaluationII (APACHE II) score after 7 days’ LMWH treatment, one trial14 measured above outcomes after 14 days’ treatment. The sample size ranged from 18 to 105. In general, the included trials were usually classified as low to moderate quality. The risk of bias of each trial is summarized in Fig. 2.
Figure 1. Diagram of trials’ selection process.
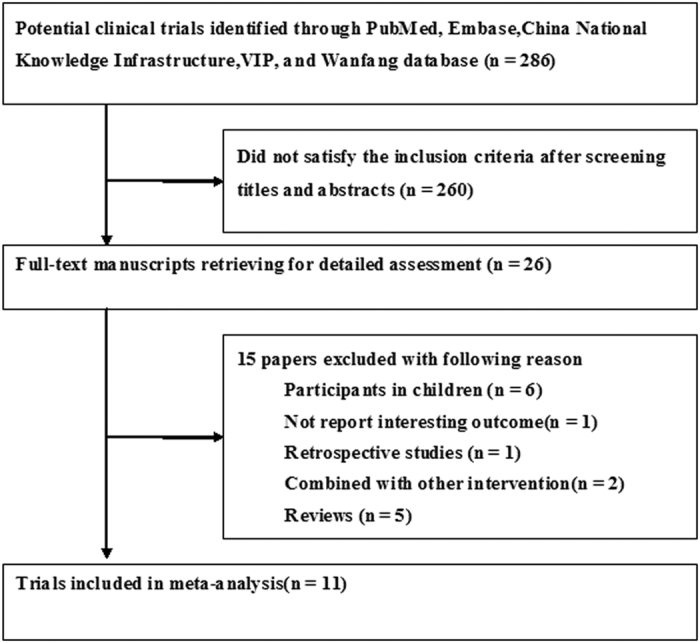
Table 1. Characteristics of the selected trials in the meta-analysis.
| Study/Year | No. patients Exp/Con | Age/Gender (% Female) | Basic APACHE II Exp/Con | Primary diseases distribution | Intervention |
Outcome measures | |
|---|---|---|---|---|---|---|---|
| Experiment Group | UT (control) group | ||||||
| Ai YH et al.11 | 22/18 | 42.0 ± 7.0 44.0% | 14.31 ± 3.71/15.46 ± 4.61 | Severe acute pancreatitis 20, suppurative cholangitis 5, multiple trauma 1, liver abscess 1, respiratory tract infection 3, and urinary tract infection 1. | Low-molecular weight heparin calcium 4100 U/12h × 7 days (SC) + UT. | Anti-infection, supplement blood capacity, stress ulcer prevention, glycemic control, nutritional support, and etiological treatment. | (1)+(2)+(3)+(6)+(7) +(8) |
| Zhang ZC et al.12 | 11/7 | 59.0 ± 9.0 38.9% | 15.87 ± 2.21/16.41 ± 2.15 | Pneumonia 9; Intestinal fistula and peritonitis 5, biliary tract infection 1, severe acute pancreatitis 1, severe trauma 1, catheter-related infection 1. | Low-molecular weight heparin calcium 6150 U/12h × 7 days (SC) + UT. | Broad spectrum antibiotics, supplement blood capacity, glycemic control, nutritional support, and etiological treatment. | (1)+(2)+(3)+(4)+(7) +(8) |
| Ren LB et al.13 | 65/40 | 54.26 ± 18.42 34.3% | 19. 33 ± 8. 27/18. 97 ± 10. 33 | NR. | Low-molecular weight heparin calcium 6150 U/12h × 7 days (SC) + UT. | Broad spectrum antibiotics, supplement blood capacity, glycemic control, nutritional support, and etiological treatment. | (1)+(4)+(5)+(6)+(7)+(8) |
| Huang T et al.14 | 29/30 | 61.1 ± 13.2 28.8% | 24.6 ± 7.5/23.6 ± 6.1 | NR. | Low-molecular weight heparin sodium 4000- 6000 U/12h × 14 days (SC) + UT. | Anti-infection, nutritional support, and etiological treatment. | (1)+(2)+(7)+(8) |
| Han Y et al.15 | 20/20 | 44.0 ± 8.0 45.0% | 14. 80 ± 6. 08/15.15 ± 5. 33 | Respiratory infection 13, suppurative cholangitis 4, multiple trauma 11, acute pancreatitis 9, skin or soft tissue infection 3. | Low-molecular weight heparin calcium 6150 U/12h × 7 days (SC) + UT. | Anti-infection and nutritional support. | (1)+(2)+(4)+(5)+(6)+(7)+(8) |
| Lin YJ et al.16 | 30/22 | 55.0 ± 7.0 44.2% | 22.1 ± 7.0/19.3 ± 5.7 | Severe acute pancreatitis 21, severe pulmonary infection 18, suppurative cholangitis 8, liver abscess 3, bacterial meningitis 1, peritonitis 1. | Low-molecular weight heparin sodium 5000 U/12h × 7 days(SC) + UT. | Broad spectrum antibiotics, fluid resuscitation, stress ulcer prevention, glycemic control, organ support, and etiological treatment. | (1)+(2)+(4)+(7)+(8) |
| Song HZ et al.17 | 30/30 | 73.05 ± 7.25 31.7% | 21.26 ± 5.16/21.78 ± 5.38 | NR. | Low-molecular weight heparin calcium 4100 U/d × 7 days (SC) + UT. | Broad spectrum antibiotics, fluid resuscitation, protecting gastric mucosa, acid suppression. | (1)+(4) |
| Chen P et al.18 | 30/30 | 56.5 ± 12.2 45.0% | 17.52 ± 3.83/19.25 ± 4.57 | Acute lung infection 15, septicemia 10, multiple trauma 8, suppurative cholangitis 7, acute pancreatitis 5, urinary tract infection 3, others 10. | Low-molecular weight heparin calcium 4100 U/d × 7 days (SC) + UT. | Broad spectrum antibiotics, fluid resuscitation, maintaining balance of water and electrolyte, and organ support. | (1)+(2)+(3)+(7)+(8) |
| Liu XF et al.19 | 35/34 | 56 ± 9.2 40.6% | 25.12 ± 5.01/24.88 ± 4.91 | Skin and soft tissue infections, severe acute pancreatitis, suppurative cholangitis, lung infection,, multiple injuries, etc. | Low-molecular weight heparin calcium 4100 U/12h × 14 days (SC)+UT. | Anti-infection, supplement blood capacity, balance of water and electrolyte., inhibition of acid secretion, nutritional support. | (1)+(7)+(8) |
| Zhu XM et al.20 | 22(A)/20(B)/19 | 60.5 ± 9.2 32.8% | 19.8 ± 6.1/18.6 ± 4.6/17.9 ± 5.8 | Lung infection 35, abdominal infection 11, biliary tract infection 8, limbs infection 4, skin and soft tissue infection 3. | A group: Low-molecular weight heparin sodium 5000 U/12h × 7 days (SC); B group 5000 U/d × 7 days (SC) + UT. | Antibiotics, supplement blood capacity, mechanical ventilation, glycemic control, nutritional support, and etiological treatment. | (1)+(2)+(4)+(7)+(8) |
| Chen J et al.21 | 15/15 | 64–94 43.3% | 16.78 ± 5.74/15.37 ± 3.38 | Chronic obstructive pulmonary disease 8, pneumonia 10, cerebral vascular disease 4, after surgery 8. | Low-molecular weight heparin calcium 6150 U/d × 7 days (SC) + UT. | Anti-infection, respiratory support, nutritional support. | (1)+(2) |
Abbreviations: NR, not report; Exp, experiment; Con, control; UT, usual treatment; SC, subcutaneous injection; APACHE II, Acute physiology and chronic health evaluation II.
(1) Acute physiology and chronic health evaluation II; (2) 28-day mortality; (3) length of intensive care unit stay; (4) D-dimer; (5) tumor necrosis factor-α; (6) interleukin-6; (7) platelet; (8) prothrombin time.
Figure 2.
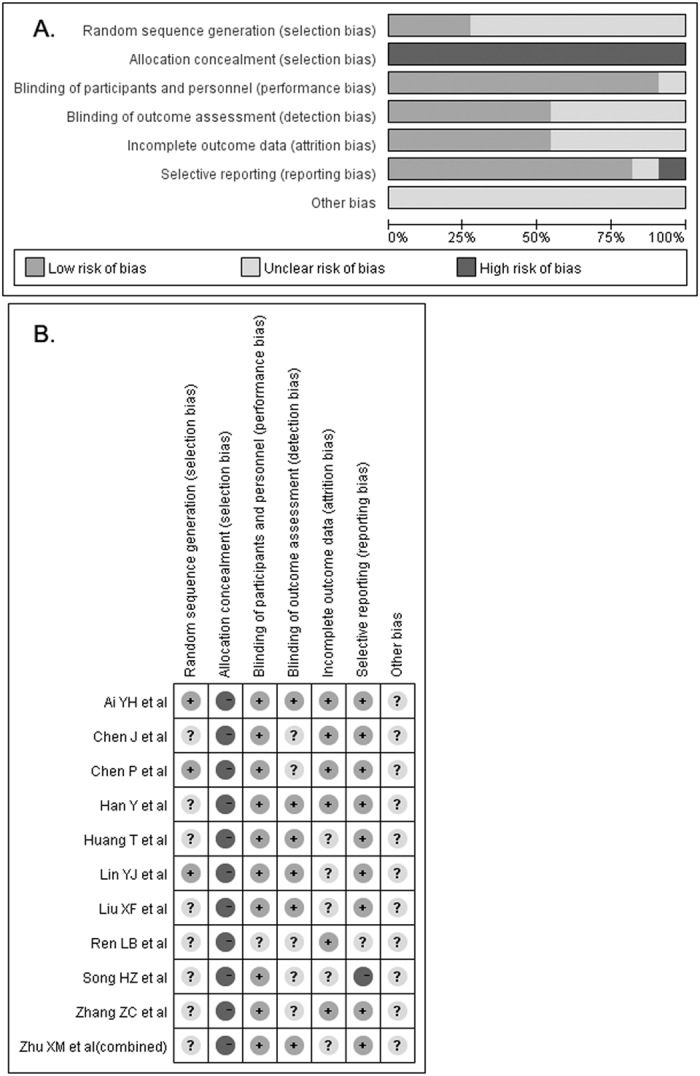
Risk of bias graph (A) and risk of bias summary (B).
Clinical outcomes
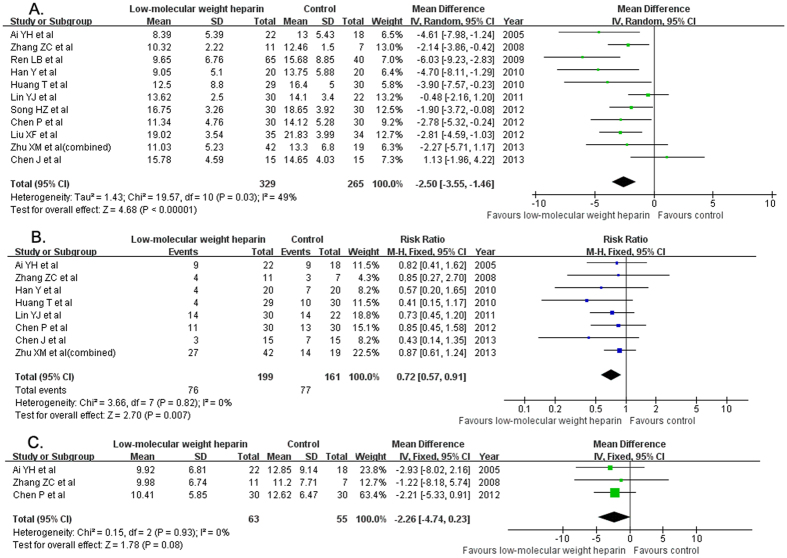
All the included trials provided the changes of APACHE II score. Treatment with LMWH significantly reduced APACHE II score (MD −2.50; 95% CI −3.55 to −1.46; I2 = 49%, P < 0.03) compared with the usual treatment group in a random effect model (Fig. 3A). Eight trials11,12,14,15,16,18,20,21 reported the 28-day mortality outcomes. Treatment with LMWH significantly reduced 28-day mortality (RR 0.72; 95% CI 0.57–0.91; I2 = 0%, P = 0.82) than the usual treatment group in a random effect model (Fig. 3B). There was no significant difference in the length of intensive care unit (ICU) stay (MD −2.26 days; 95% CI −4.74–0.23; I2 = 0%, P = 0.93) between the two groups (Fig. 2C) in three trials11,12,18.
Figure 3. Forest plots showing the clinical outcome of the eligible trials comparing low-molecular weight heparin to the usual treatment.
APACHE II score (A); 28-day mortality (B); length of intensive care unit stay (C).
Changes of serum pro-inflammatory cytokines
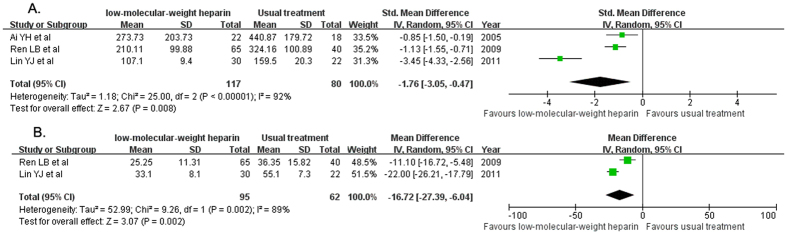
Serum IL-6 level changes were available in three trials11,13,16. Treatment with LMWH significantly reduced serum IL-6 level (SMD −1.76; 95% CI −3.05 to −0.47; I2 = 92%, P < 0.00001) compared with the usual treatment group in a random effect model (Fig. 4A). Serum TNF-α level was reported in two trials13,16. Treatment with LMWH significantly reduced serum TNF-α level (MD −16.72; 95% CI −27.39 to −6.04; I2 = 89%, P = 0.002) compared with the usual treatment group in a random effect model (Fig. 4B).
Figure 4. Forest plots showing changes of serum pro-inflammatory cytokines of the eligible trials comparing low-molecular weight heparin to the usual treatment.
Interleukin-6 (A); tumor necrosis factor-α (B).
Changes of anticoagulant activity
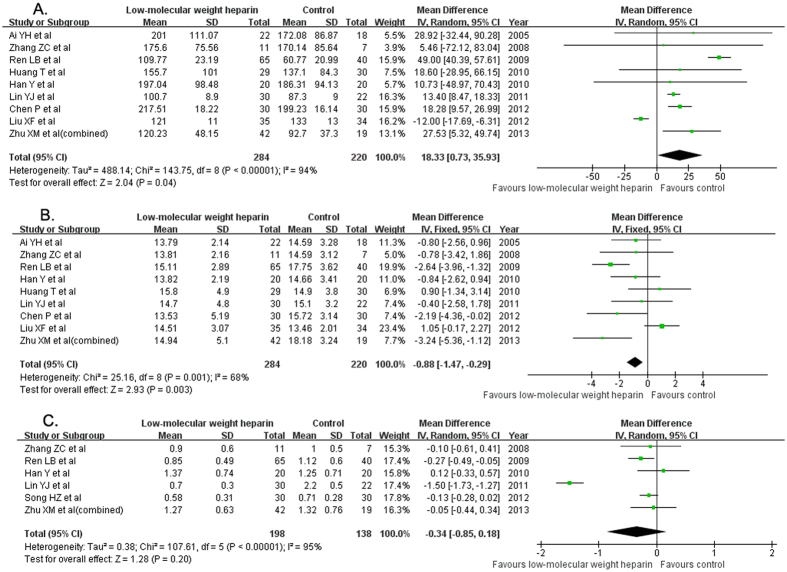
Nine trials11,12,13,14,15,16,18,19,20 provided the changes of platelet counts. Treatment with LMWH significantly increased platelet counts (MD 18.33; 95% CI 0.73–35.93; I2 = 94%, P < 0.001) than the usual treatment group in a random model (Fig. 5A). Nine trials11,12,13,14,15,16,18,19,20 provided the changes of prothrombin time. LMWH treatment significantly reduced PT (MD −0.88; 95% CI −1.47 to −0.29; I2 = 68%, P = 0.003) than the usual treatment group in a random model (Fig. 5B). Six trials12,13,15,16,17,20 reported the changes of serum D-dimer. There was no significant difference in the changes of D-dimer (MD −0.34; 95% CI −0.85 −0.18; I2 = 95%, P < 0.001) than the usual treatment group in a random model (Fig. 5C).
Figure 5. Forest plots showing changes of anticoagulant activity of the eligible trials comparing low-molecular weight heparin to the usual treatment.
Platelet counts (A); prothrombin time (B); D-dimer (C).
Bleeding complications
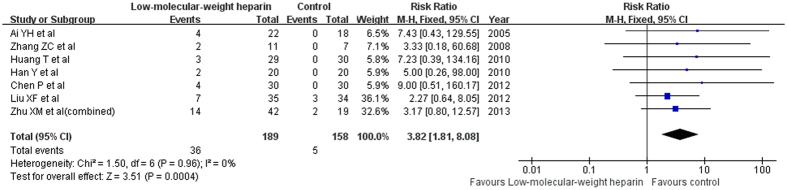
Seven trials11,12,14,15,18,19,20 reported bleeding events. Of the total bleeding events, the frequency of tracheal intubation and deep vein puncture or injection site bleeding occurred 20 (6.07%) of 329 patient receiving LMWH, while frequency in the control group was 3 (1.13%) of 265 patients. Gastrointestinal hemorrhage occurred 14 (4.26%) of 329 patient receiving LMWH, while frequency in the control group was 2 (0.75%) of 265 patients. In addition, one case of small ecchymoses19 and gum bleeding20 was also noted. When we pooled total bleeding events from seven studies, the risk of any bleeding events was significantly increased to 3.82 (95% CI 1.81–8.08; I2 = 0%, P = 0.96) in a fixed effect model (Fig. 6).
Figure 6. Forest plots showing risk of total bleeding complications comparing low-molecular weight heparin to the usual treatment.
Subgroup analyses, sensitivity analyses, and publication bias
Subgroup analyses and publication bias were conducted based on changes in APACHE II score and 28-day mortality. The results of subgroup analyses were presented in Table 2.
Table 2. Subgroup analyses of APACHE II score and 28-day mortality.
| Subgroup | Number of studies | Patient Number LMWH/Control | Pooled effect sizes | 95% CI | Heterogeneity |
|
|---|---|---|---|---|---|---|
| I2statistic | P-value | |||||
| 1. APACHE II | ||||||
| Heparin dose | ||||||
| Once daily | 4 | 117/94 | MD-1.61 | −3.12 to −0.10 | 57.7% | 0.028 |
| Twice daily | 7 | 212/171 | MD-3.09 | −4.51 to −1.67 | 26.1% | 0.255 |
| Heparin type | ||||||
| LMWH calcium | 8 | 228/194 | MD-2.77 | −3.98 to 1.56 | 49.1% | 0.056 |
| LMWH sodium | 3 | 101/71 | MD-1.67 | −3.68 to 0.33 | 36.5% | 0.207 |
| 2. 28-day mortality | ||||||
| Heparin dose | ||||||
| Once daily | 3 | 87/64 | RR 0.78 | 0.57 to 1.08 | 0% | 0.478 |
| Twice daily | 5 | 112/97 | RR 0.67 | 0.47 to 0.95 | 0% | 0.821 |
| Heparin type | ||||||
| LMWH calcium | 5 | 98/90 | RR 0.72 | 0.49 to 1.05 | 0% | 0.838 |
| LMWH sodium | 3 | 101/71 | RR 0.72 | 0.54 to 0.97 | 9.4% | 0.332 |
| Treatment duration | ||||||
| 7 days | 7 | 170/131 | RR 0.76 | 0.60 to 0.97 | 0% | 0.917 |
| 14 days | 1 | 29/30 | RR 0.41 | 0.15 to 1.17 | – | – |
Abbreviations: LMWH, low-molecular-weight heparin; MD, mean differences; RR, risk ratio; CI, confidence interval.
Sensitivity analyses were performed by sequentially omitting one study at each turn. The results showed there was no change in the direction of effect sizes in APACHE II score and 28-day mortality outcomes. Evidence of publication bias for trial reporting the changes of APACHE II score was not observed by the Begg’s test (P = 0.119) and Egger’s test (P = 0.141). Potential publication bias for trial reporting 28-day mortality was observed in the Egger’s test (P = 0.041) but not in the Begg’s test (P = 0.266).
Discussion
Our meta-analysis suggests that treatment with LMWH appears to reduce 28-day mortality and APACHE II score, as well as improvement in pro-inflammatory cytokines and anticoagulant activity among septic patients. Increased any bleeding complications with LMWH administration are identified. However, these findings were concluded by the trials with methodological flaws, and recommendation of these findings should be cautioned.
Sepsis has been grouped into sepsis, severe sepsis, followed by the presence of multiorgan dysfunction, and septic shock22. Sepsis-related inflammation may activate the coagulation system, consume multiple clotting factors and resulting in disseminated intravascular coagulation (DIC)23,24. The Japanese guidelines supported the aggressive treatment of septic DIC25,26. In contrast, the guidelines of the Surviving Sepsis Campaign of the European Union and the USA did not recommend treatment for septic DIC27,28. Two previous systematic reviews and meta-analyses29,30 showed that heparin therapy may be associated with decreased mortality in patients with sepsis. However, these two studies mainly focused on the unfractioned heparin but not LMWH. Therefore, the efficacy and safety of LMWH for sepsis is not well established.
Severe sepsis is associated with higher levels of pro-inflammatory markers. The massive inflammatory activation played a key role in the development of sepsis to septic shock31. Of these proinflammatory cytokines, IL-6 and TNF-α, have got the most attention. Higher serum IL-6 or TNF-α levels indicate an increased risk of mortality32. In the current study, adjuvant treatment with LMWH reduced serum IL-6, TNF-α level to some extent which indicated LMWH had the potential to inhibit the inflammatory process. The potential anti-inflammatory property of LMWH might partly explain its beneficial mechanism.
Cytokine production contributes to the activation of platelets. Coagulation abnormalities are frequent complications of sepsis33. Manifestation coagulation abnormalities in sepsis range from a decrease in platelet counts and subclinical prolongation of prothrombin time to DIC34. The additional benefits of adjunctive use of recombinant human activated protein C in septic patients supported anticoagulation strategy in the treatment of sepsis. LMWH may exert similar beneficial effects as recombinant human activated protein C. Our study found that LMWH could increase platelet counts and reduce prothrombin time. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis35. However, LMWH did not significantly change d-dimer. Therefore, anti-platelet aggregation and anticoagulant properties are another potential pharmacological mechanism.
Benefits of adjunctive use of LMWH have been demonstrated by improvement of APACHE II score. APACHE II score is a widely accepted indicator of disease severity. There was no significant difference in baseline APACHE II score. Adjunctive treatment with LMWH significantly reduced 2.5 points of APACHE II score compared with control, which suggested application of LMWH reduced the disease severity. In addition, adjunctive use LMWH was also associated with 28% lower 28-day mortality. However, there was no significant difference in length of ICU stay. Subgroup analysis indicated high dose LMWH appeared to have more benefits in improvement in APACHE II score and 28-day mortality. Despite the beneficial effect of LMWH in septic patients, a major concern is the occurrence of bleeding complications. Our pooled results indicated that LMWH administration significantly increased bleeding events 3.82 (95% CI 1.81–8.08). LMWH treatment increased both gastrointestinal hemorrhage and invasive operation bleeding events; however, these events were mild and did not need special management. Therefore, monitoring bleeding events in the use of LMWH is recommended.
Several limitations in the current analysis should be noted. First, all the included trials conducted in China and published in Chinese; evidence of publication bias reporting 28-day mortality outcome was observed in the Egger’s test (P= 0.041). Therefore, potential publication bias cannot be ruled out. Second, the methodological quality of individual trials was mainly suboptimal, particularly the sample size of individual trials was too small. Third, significant heterogeneity was noted in pooling the anticoagulant activity and pro-inflammatory cytokines. Type of LMWH, dose or intervention duration and diverse study populations, might partly contribute to the heterogeneity. Finally, all trials included only Chinese patients, the generalization of the current findings to other ethnic population should be cautioned.
In conclusion, this meta-analysis suggests that treatment with LMWH appears to reduce 28-day mortality and APACHE II score among septic patients. The beneficial effect might be related to its anti-inflammatory and anticoagulant/antiaggregant properties. Monitoring bleeding events in the use of LMWH is recommended. More well-designed studies with a larger sample size are required before definitive recommendations can be made.
Methods
Literature search
This study was performed based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) for reporting systematic reviews and meta-analyses of RCT36. We searched for relevant studies using Pubmed, Embase, Cochrane Library, VIP database, China National Knowledge Infrastructure, and Wanfang database up to July 2015. A combination of the following terms was used, including heparin OR low molecular weight heparin AND sepsis OR septic shock OR septicemia OR critically ill OR systemic inflammatory response syndrome AND randomized control trials OR RCTs. The search language was limited to publications that were written in Chinese and English. References lists from eligible studies were manually searched to identify additional trials.
Study selection
We included RCTs that used LMWH therapy in adult septic patients and reported at least prothrombin time, platelet, d-dimer, IL-6, TNF-α, APACHE II score, length of ICU stay, and 28-day mortality as outcome measures. Studies were excluded if the study designs were no RCTs or there were different regimen except for LMWH intervention between LMWH and control group. Studies were also excluded if presented a bleeding tendency because of coagulation disorders (platelet counts <30 × 109), application of anticoagulant <48h prior to enrollment or serious head injury, brain arteriovenous malformation, cerebral aneurysm.
Data extraction and methodological quality
The following items were independently extracted from each eligible studies by 2 authors (Y Fan and ML Jiang): first author name, years of publication, number and age of participant, percentage of female of participant, primary diseases distribution, and outcome measures. Any disagreements between authors were resolved through consensus. The risk of bias of each study was evaluated according to the Cochrane Handbook for Systematic Reviews of Invention37 on the basis of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome data, incomplete outcome data, and selective reporting, and others.
Statistical analysis
All statistical analyses were conducted using the RevMan version 5.1 software. Pooled estimate was presented as mean difference (MD) or standard mean difference (SMD) with 95% confidence interval (CI) for continuous outcomes and risk ratio (RR) with 95% CI for dichotomous outcomes. Comparisons were made as LMWH plus usual treatment versus usual treatment alone. Heterogeneity of the trials was assessed using the Cochrane Q test and I2 statistics38. If I2 value >50% or Cochrane Q test (P < 0.10) suggested significant heterogeneity, a random effects model was selected due to considerable heterogeneity among different trials; otherwise, a fixed-effect model was used. Potential publication bias was assessed by both the Begg’s test and Egger test. Subgroup analyses were conducted according to LMWH dose (once or twice daily) and treatment duration (7 or 14 days).
Additional Information
How to cite this article: Fan, Y. et al. Efficacy and safety of low-molecular-weight heparin in patients with sepsis: a meta-analysis of randomized controlled trials. Sci. Rep. 6, 25984; doi: 10.1038/srep25984 (2016).
Acknowledgments
This work was supported by Jiangsu Provincial Key&D Special Fund (BE2015666).
Footnotes
Author Contributions C.Z. designed the study, performed the data analysis, and revised the manuscript; Y.F. and M.J. conducted literature research and extracted data; D.G. drafted the manuscript; All authors reviewed and approved the final manuscript.
References
- Danai P. & Martin G. S. Epidemiology of sepsis: recent advances. Curr Infect Dis Rep 7, 329–34 (2005). [DOI] [PubMed] [Google Scholar]
- Cheng B. et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med 35, 2538–46 (2007). [DOI] [PubMed] [Google Scholar]
- Knoebl P. Blood coagulation disorders in septic patients. Wien Med Wochenschr 160, 129–38 (2010). [DOI] [PubMed] [Google Scholar]
- Semeraro N., Ammollo C. T., Semeraro F. & Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res 129, 290–5 (2012). [DOI] [PubMed] [Google Scholar]
- O’Brien M. The reciprocal relationship between inflammation and coagulation. Top Companion Anim Med 27, 46–52 (2012). [DOI] [PubMed] [Google Scholar]
- Bernard G. R. et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344, 699–709 (2001). [DOI] [PubMed] [Google Scholar]
- Barie P. S., Hydo L. J., Shou J. & Eachempati S. R. Efficacy of therapy with recombinant human activated protein C of critically ill surgical patients with infection complicated by septic shock and multiple organ dysfunction syndrome. Surg Infect (Larchmt) 12, 443–9 (2011). [DOI] [PubMed] [Google Scholar]
- Cornet A. D., Smit E. G., Beishuizen A. & Groeneveld A. B. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost 98, 579–86 (2007). [PubMed] [Google Scholar]
- Gray E. Standardisation of unfractionated and low-molecular-weight heparin. Handb Exp Pharmacol 207, 65–76, doi: 10.1007/978-3-642-23056-1_4 (2012). [DOI] [PubMed] [Google Scholar]
- Nandurkar H. et al. Low-molecular-weight heparin biosimilars: potential implications for clinical practice. Intern Med J 44, 497–500 (2014). [DOI] [PubMed] [Google Scholar]
- Ai Y. H. et al. Clinical study of low molecular weight heparin therapy for sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 17, 736–9 (2005). [PubMed] [Google Scholar]
- Zhang Z. C. et al. Clinical study of early application of low molecular weight heparin in the treatment of sepsis Shandong Medical Journal 48, 102–3 (2008). [Google Scholar]
- Ren L. B., Zhao L. & Jiang Z. Clinical observation of low molecular weight heparin as adjuvant treatment for 65 cases septic patients with acute kidney injury. Shandong Medical Journal 49, 81–2 (2009). [Google Scholar]
- Huang T. & Guan Q. Clinical observation of low molecular weight heparin in the treatment of severe sepsis. Chinese Medicine Guides 7, 58–9 (2010). [Google Scholar]
- Han Y. et al. Study of changes and clinical significance of P-selcetin in low molecular weight heparin therapy for septic patients. Chi J Critl Care Med 30, 976–9 (2010). [Google Scholar]
- Lin Y. J. & Xiong B. The efficacy of low molecular weight heparin in the treatment of sepsis: a report of 30 clinical cases. Journal of Youjiang Medical College For Nationalities 33, 3–5 (2011). [Google Scholar]
- Song H. Z., Hua W. & Chen Y. Z. Effect of low molecular weight heparin on prognosis of aged patients with sepsis. China Modern Medicine 19, 26–7 (2012). [Google Scholar]
- Chen P. & Qu J. J. Therapeutic Effect of Low Molecular Weight Heparin on Sepsis. Zhejiang JITCWM 22, 336–8 (2012). [Google Scholar]
- Liu X. F. et al. Effect of low-molecular weight heparin calcium on APACHE II scores and blood coagulation function in septic patients. Chinese Journal of Gerontology 32, 3558–9 (2012). [Google Scholar]
- Zhu X. M., Shao B. B., Zhang W. H., Zheng K. & Feng H. B. Therapeutic effects of different doses administration of LMWH in patients with severe sepsis. Practical Pharmacy And Clinical Remedies 16, 1014–7 (2013). [Google Scholar]
- Chen J. et al. Effect of low molecular weight heparin in elderly patients with sepsis with myocardial injury. Prevention and Treatment of Cardio-Cerebral-Vascular Disease 13, 203–5 (2013). [Google Scholar]
- Tang C. H. et al. Non-invasive classification of severe sepsis and systemic inflammatory response syndrome using a nonlinear support vector machine: a preliminary study. Physiol Meas 31, 775–93 (2010). [DOI] [PubMed] [Google Scholar]
- Levi M. & van der Poll T. Inflammation and coagulation. Crit Care Med 38, S26–34 (2010). [DOI] [PubMed] [Google Scholar]
- Okamoto K., Tamura T. & Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care 4, 23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda S. et al. The Japanese guidelines for the management of sepsis. J Intensive Care 2, 55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res 125, 6–11 (2010). [DOI] [PubMed] [Google Scholar]
- Dellinger R. P. et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39, 165–228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger R. P. et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41, 580–637 (2013). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: a systematic review and meta-analysis. Crit Care 18, 563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarychanski R. et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med 43, 511–8 (2015). [DOI] [PubMed] [Google Scholar]
- Cinel I. & Opal S. M. Molecular biology of inflammation and sepsis: a primer. Crit Care Med 37, 291–304 (2009). [DOI] [PubMed] [Google Scholar]
- Hack C. E., Aarden L. A. & Thijs L. G. Role of cytokines in sepsis. Adv Immunol 66, 101–95 (1997). [DOI] [PubMed] [Google Scholar]
- Fourrier F. Severe sepsis, coagulation, and fibrinolysis: dead end or one way? Crit Care Med 40, 2704–8 (2013). [DOI] [PubMed] [Google Scholar]
- Levi M., Schultz M. & van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost 39, 559–66 (2013). [DOI] [PubMed] [Google Scholar]
- Rodelo J. R. et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med 30, 1991–9 (2012). [DOI] [PubMed] [Google Scholar]
- Liberati A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62, e1–34 (2009). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T. & Green S. Assessing risk of bias in included studies. Chapter 8, cochrane handbook for systematic reviews of interventions Version 5.1.0. Available at: www. cochrane.org/handbook/chapter-8-assessing-risk-bias-included-studies. Accessed July 8, 2014.
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–60 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]