Abstract
Mycoplasma pneumoniae was detected in a number of patients with community-acquired pneumonia in a recent prospective study. To assess whether other pathogens were also detected in these patients, TaqMan Array Cards were used to test 216 M pneumoniae-positive respiratory specimens for 25 additional viral and bacterial respiratory pathogens. It is interesting to note that 1 or more codetections, predominantly bacterial, were identified in approximately 60% of specimens, with codetections being more common in children.
Keywords: community-acquired pneumonia, multipathogen detection, Mycoplasma pneumoniae
Mycoplasma pneumoniae was the most commonly detected bacterial pathogen among children and the second most commonly detected bacteria in adults hospitalized with community-acquired pneumonia (CAP) in the recent US Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study [1, 2]. Although we had previously characterized M pneumoniae from 216 polymerase chain reaction (PCR)-positive specimens collected during the EPIC study, including P1 subtyping, multilocus variable-number tandem-repeat analysis, and macrolide susceptibility genotyping [3], the goal of the current study was to determine whether other pathogens were codetected with M pneumoniae in our study samples. To achieve this goal, we tested 216 M pneumoniae-positive specimens from EPIC for 25 additional respiratory viruses and bacteria using the TaqMan Array Card ([TAC] Thermo Fisher Scientific). Few earlier reports have described multipathogen detection including M pneumoniae in the testing algorithm of patients with CAP [4–7], and none included both children and adults.
Children (<18 years old) and adults were enrolled in the EPIC study from January 2010 to June 2012 at 8 hospitals in Chicago, Illinois; Memphis, Tennessee; Nashville, Tennessee; and Salt Lake City, Utah [1, 2]. Informed consent was obtained before enrollment. The study protocol was approved by the institutional review boards at each institution and the CDC. Patients admitted to a study hospital with evidence of acute respiratory infection and radiographic confirmation of pneumonia were included; patients who were recently hospitalized or severely immunocompromised were excluded [1, 2]. For each patient, nasopharyngeal and oropharyngeal (NP/OP) swabs were collected and combined in universal transport media to be tested as a single specimen for respiratory viruses and atypical bacteria, including M pneumoniae, using standardized real-time PCR assays at each study site [1, 2].
Nasopharyngeal and oropharyngeal specimens were stored at −70°C and shipped to the CDC for long-term storage. At the CDC, total nucleic acid was extracted using the MagNA Pure Compact System with Total Nucleic Acid Isolation Kit I (Roche Applied Science) according to the manufacturer's instructions. Of 225 specimens collected within 72 hours of admission from enrolled patients meeting the final CAP case definition [1, 2] and identified as M pneumoniae-positive at the study site, 216 (96%) were confirmed upon repeat testing at the CDC using a validated real-time PCR assay [8] and were included in the current study.
Nucleic acid from each M pneumoniae-positive specimen was tested for the presence of 25 additional bacterial and viral respiratory pathogens (listed in Table 1) using TAC on the ViiA7 Real-Time PCR System (Thermo Fisher Scientific) as previously described [7]. The proportions of codetections of respiratory pathogens determined using TAC were compared between children and adults using χ2 or Fisher's exact test as appropriate. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC); P < .05 was considered significant.
Table 1.
Codetected Respiratory Pathogens in Mycoplasma pneumoniae-Positive Specimens Using TaqMan Array Card
| Codetectiona | Total (n = 209) n (%) | Adults (n = 38) n (%) | Children (n = 171) n (%) | P Valueb |
|---|---|---|---|---|
| Any codetection | 125 (59.8) | 13 (34.2) | 112 (65.5) | <.01 |
| Bacterial only codetections (≥1) | 74 (35.4) | 9 (23.7) | 65 (38.0) | .09 |
| Viral only codetections (≥1) | 17 (8.1) | 4 (10.5) | 13 (7.6) | .5 |
| Bacterial and viral codetections | 34 (16.3) | 0 (0) | 34 (19.9) | <.01 |
| Bordetella pertussis | 0 (0) | 0 (0) | 0 (0) | — |
| Chlamydia pneumoniae | 0 (0) | 0 (0) | 0 (0) | — |
| Haemophilus influenzae | 61 (29.2) | 0 (0) | 61 (35.6) | <.01 |
| Legionella spp | 0 (0) | 0 (0) | 0 (0) | — |
| Moraxella catarrhalis | 30 (14.4) | 3 (7.9) | 27 (15.8) | .3 |
| Staphylococcus aureus | 45 (21.5) | 6 (15.8) | 39 (22.8) | .3 |
| Streptococcus pneumoniae | 50 (23.9) | 3 (7.9) | 47 (27.5) | .01 |
| Streptococcus pyogenes | 12 (5.7) | 0 (0) | 12 (7.0) | .07 |
| Adenoviruses | 3 (1.4) | 0 (0) | 3 (1.8) | 1.0 |
| Human enteroviruses | 12 (5.7) | 0 (0) | 12 (7.0) | .1 |
| Influenza virusc | 2 (1.0) | 0 (0) | 2 (1.2) | 1.0 |
| Human coronavirusd | 16 (7.7) | 2 (5.3) | 14 (8.2) | .7 |
| Human metapneumoviruses | 4 (1.9) | 0 (0) | 4 (2.3) | 1.0 |
| Human parechoviruses | 1 (0.5) | 0 (0) | 1 (0.6) | 1.0 |
| Human parainfluenza viruse | 3 (1.4) | 0 (0) | 3 (1.8) | 1.0 |
| Respiratory syncytial virus | 5 (2.4) | 1 (2.6) | 4 (2.4) | 1.0 |
| Human rhinoviruses | 29 (13.9) | 2 (5.3) | 27 (15.8) | .08 |
a Multiple codetections were identified in a single patient specimen in some cases.
b χ2 or Fisher's exact test as appropriate comparing children with adults.
c Includes influenza A, B, and C viruses.
d Includes human coronaviruses 229E, NL63, OC43, and HKU1.
e Includes human parainfluenza viruses 1–4.
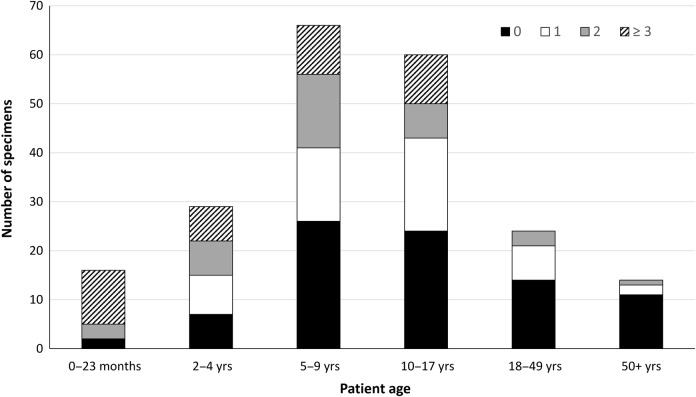
Using TAC, M pneumoniae was detected in 209 (96.8%) of 216 specimens. All 7 specimens that were negative for M pneumoniae by TAC had a Crossing threshold value ≥33 by the original real-time PCR assay, suggesting that the negative result on TAC was most likely due to low quantity of pathogen-specific nucleic acid in the primary specimen. At least 1 other bacterial or viral codetection was identified in 125 of 209 (59.8%) specimens, including 13 of 38 (34.2%) and 112 of 171 (65.5%) specimens from adults and children, respectively (Table 1). The proportion of specimens in which at least 1 codetection was identified was significantly higher among children compared with adults (P < .01). The highest number of codetected organisms was identified in specimens from patients in the 0–23 months and 2–4 years age groups (range, 0–7 codetections per specimen), whereas the highest number of codetections in any specimen from an adult patient was only 2 (Figure 1).
Figure 1.
Number of specimens with 0, 1, 2, or ≥3 codetections in addition to Mycoplasma pneumoniae in each age category. Specimens having 3 or more codetections (range, 3–7) were combined.
One or more bacterial codetections in addition to M pneumoniae was identified in 74 (35.4%) specimens, including 9 (23.7%) adult and 65 (38.0%) pediatric specimens (Table 1). The most frequent bacterial codetections with M pneumoniae were Haemophilus influenzae (n = 61), Streptococcus pneumoniae (n = 50), Staphylococcus aureus (n = 45), and Moraxella catarrhalis (n = 30). The predominant bacterial organisms detected using TAC were not included in the primary study site testing algorithm for PCR of NP/OP specimens, although different methods were used to test other specimen types for some of these bacteria [1, 2]. Viral codetections were less common; 1 or more viruses were found in 4 (10.5%) adults and 13 (7.6%) children; human rhinovirus was the most frequently detected virus (n = 29) and was more common in children (15.8%) compared with adults (5.3%) (Table 1). Mixed bacterial and viral codetections were found in 34 (19.9%) pediatric specimens but no adult specimens (P < .01). The various combinations of codetections are listed in Supplementary Table 1. There were no significant differences in the proportions of specimens in which codetections were identified between sites (data not shown). There were no statistically significant differences in length of stay, intensive care unit admission, invasive mechanical ventilation, or death based on codetection status, although the frequency of these events was low (Supplementary Table 2).
Codetections with M pneumoniae were common, particularly in children. Although most of the codetected organisms have a known pathogenic potential, their contribution to the episodes of CAP in these patients is unclear, given that NP/OP specimens are an indirect measure of what is causing infection in the lung. The proportion of specimens with at least 1 bacterial or viral codetection identified along with M pneumoniae in children hospitalized with CAP is consistent with previous reports, ranging from 50 to >90%, depending on the extent of pathogen testing performed [4–6]. A high prevalence of nasopharyngeal colonization with the most commonly codetected bacteria in the current study, including S pneumoniae and H influenzae, has been reported in children with and without respiratory illness [9–12]. Likewise, human rhinoviruses, commonly codetected with M pneumoniae in pediatric specimens in the current study, were found in similar proportions in children with CAP enrolled in the EPIC study compared with asymptomatic controls [13]. Codetection of influenza virus, human metapneumovirus, human parechovirus, and respiratory syncytial virus was relatively uncommon (<3.0%) in M pneumoniae-positive specimens, similar to previous reports [4–7, 14, 15]. The presence of both viral and bacterial organisms in a single specimen, which was only observed in children and has previously been associated with disease severity [5, 16], warrants further investigation. Additional studies are necessary to understand the mechanisms underlying interactions of codetected organisms with M pneumoniae and the potential impact on the severity of CAP, particularly in children.
This analysis has several shortcomings, including previously identified limitations related to the EPIC study design [1–3]. In particular, the presence of codetected pathogens in upper respiratory specimens may not be clinically relevant. Analysis of lower respiratory specimens may be a preferable specimen type for assessment of the potential contribution of these organisms to CAP; however, collection of lower respiratory specimens is more invasive and difficult to obtain. Furthermore, because M pneumoniae adheres to and replicates in the nasopharynx or oropharynx [17–19], detection in NP/OP swabs likely represents infectious shedding rather than carriage. Tests performed in the TAC format may be less sensitive compared with individual real-time PCR assays, potentially due to the substantially lower reaction volume [20]. Other variables, including the longer duration of storage, additional thawing of frozen specimen, and different extraction method may also impact the ability to detect respiratory pathogens in these specimens. Finally, the TAC design used in the current study was not customized specifically for this patient population, and thus it may not represent the ideal repertoire of assays for testing of upper respiratory tract specimens from both children and adults. Although TAC is a powerful diagnostic tool that could improve patient management and streamline testing decisions for clinicians during CAP, it is currently used for research only, and further validation is needed to support widespread implementation of this technology in clinical laboratories.
CONCLUSIONS
Mycoplasma pneumoniae was rarely detected in NP/OP swab specimens collected from asymptomatic controls in the EPIC study primary analysis, [1, 2], suggesting that M pneumoniae is not a common colonizer of the upper respiratory tract and, when present, indicates a contribution to ongoing disease. However, further investigation is needed to examine potential interactions of codetected organisms with M pneumoniae, including bacterial and viral respiratory pathogens that may be present in a carriage or prolonged shedding state in the upper respiratory tract. Understanding the interplay between M pneumoniae and the respiratory microbiome may lend insight into the transmission and clinical spectrum of M pneumoniae infections.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank the patients who graciously consented to participate in this study and all members of the Etiology of Pneumonia in the Community (EPIC) Study Team for their contributions.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the Influenza Division in the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity.
Potential conflicts of interest. J. D. C. received grants from the CDC during the conduct of the study and has patents licensed to Vanderbilt University (US patent 8 293 498 B2, 23 October 2012; US patent 9 249 195 B2, 2 February 2016). D. J. W. received grants from the CDC during the conduct of the study. S. R. A. received grants from the CDC during the conduct of the study. K. A. received grants from the CDC during the conduct of the study and collaborated with BioFire Diagnostics, Inc. on several National Institutes of Health (NIH) grants. W. H. S. received grants from the CDC during the conduct of the study; received grants from BioMerieux, Affinium Pharmaceuticals, Astute Medical, BRAHMS GmbH/Thermo Fisher, Pfizer, Rapid Pathogen Screening, Venaxis BioAegis Inc, and Sphingotec GmbH; received personal fees from BioFire Diagnostics and Venaxis, Inc. outside the submitted work; and has a utility patent pending (13/632 874) related to sterile blood culture collection system. C. G. G. received grants from the CDC during the conduct of the study and reported consultantship with Pfizer Inc. E. J. A. received grants and nonfinancial support from MedImmune and received personal fees from AbbVie outside the submitted work. J. A. M. received grants from the CDC during the conduct of the study. A. T. P. received grants from the CDC during the conduct of the study and received personal fees from WebMD and Antimicrobial Therapy Inc., and grants from the NIH and BioFire Diagnostics, Inc. outside the submitted work. R. G. W. received grants from the CDC during the conduct of the study and received personal fees from Cempra Pharmaceuticals outside the submitted work. K. M. E. received grants from the CDC during the conduct of the study and received grants from Novartis and reported service on a Data and Safety Monitoring Board from Novartis outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jain S, Self WH, Wunderink RG et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Williams DJ, Arnold SR et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz MH, Benitez AJ, Cross KE et al. Molecular detection and characterization of Mycoplasma pneumoniae among patients hospitalized with community-acquired pneumonia in the United States. Open Forum Infect Dis 2015; 2:ofv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu CY, Chen CJ, Wong KS et al. Impact of bacterial and viral coinfection on Mycoplasmal pneumonia in childhood community-acquired pneumonia. J Microbiol Immunol Infect 2015; 48:51–6. [DOI] [PubMed] [Google Scholar]
- 5.Michelow IC, Olsen K, Lozano J et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 2004; 113:701–7. [DOI] [PubMed] [Google Scholar]
- 6.Peng D, Zhao D, Liu J et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J 2009; 6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waller JL, Diaz MH, Petrone BL et al. Detection and characterization of Mycoplasma pneumoniae during an outbreak of respiratory illness at a university. J Clin Microbiol 2014; 52:849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurman KA, Warner AK, Cowart KC et al. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis 2011; 70:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammitt LL, Bruden DL, Butler JC et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis 2006; 193:1487–94. [DOI] [PubMed] [Google Scholar]
- 10.Roberts AL, Connolly KL, Kirse DJ et al. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr 2012; 12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenenbaum T, Franz A, Neuhausen N et al. Clinical characteristics of children with lower respiratory tract infections are dependent on the carriage of specific pathogens in the nasopharynx. Eur J Clin Microbiol Infect Dis 2012; 31:3173–82. [DOI] [PubMed] [Google Scholar]
- 12.Skevaki CL, Tsialta P, Trochoutsou AI et al. Associations between viral and bacterial potential pathogens in the nasopharynx of children with and without respiratory symptoms. Pediatr Infect Dis J 2015; 34:1296–301. [DOI] [PubMed] [Google Scholar]
- 13.Self WH, Williams DJ, Zhu Y et al. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2015; 213:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalker VJ, Stocki T, Mentasti M et al. Mycoplasma pneumoniae infection in primary care investigated by real-time PCR in England and Wales. Eur J Clin Microbiol Infect Dis 2011; 30:915–21. [DOI] [PubMed] [Google Scholar]
- 15.Chen LL, Cheng YG, Chen ZM et al. [Mixed infections in children with Mycoplasma pneumoniae pneumonia]. Zhonghua Er Ke Za Zhi 2012; 50:211–5. [PubMed] [Google Scholar]
- 16.Cimolai N, Wensley D, Seear M, Thomas ET. Mycoplasma pneumoniae as a cofactor in severe respiratory infections. Clin Infect Dis 1995; 21:1182–5. [DOI] [PubMed] [Google Scholar]
- 17.Waites KB, Atkinson TP. The role of Mycoplasma in upper respiratory infections. Curr Infect Dis Rep 2009; 11:198–206. [DOI] [PubMed] [Google Scholar]
- 18.Waites KB, Balish MF, Atkinson TP. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol 2008; 3:635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004; 17:697–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodani M, Yang G, Conklin LM et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol 2011; 49:2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.