Abstract
Anti‐cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4) and anti‐programmed cell death‐1 (PD‐1) inhibitors have been shown to significantly improve survival in patients with metastatic cutaneous melanoma. However, there was some heterogeneity as well as some variation in the degree of benefit across studies. We reviewed randomized trials and performed a meta‐analysis to determine the efficacy and safety of immune checkpoint inhibitors in comparison with conventional regimens. Eligible studies were limited to randomized controlled trials comparing anti‐CTLA‐4 or anti‐PD‐1 inhibitors to chemotherapy or vaccination treatment in adult patients with unresectable cutaneous metastatic melanoma. Progression‐free survival (PFS) rate at 6 months was 28.5% versus 17.7% (RR: 0.84, 95% CI: 0.76–0.93), overall survival (OS) rate at 1 year was 51.2% versus 38.8% (RR: 0.72, 95% CI: 0.59–0.88), and overall response rate (ORR) at 6 months was 29.6% versus 17.7% (RR: 0.85, 95% CI: 0.76–0.95) favoring immune check point inhibitors over chemotherapies or vaccination. Immune check point inhibitors were associated with more frequent immune‐related adverse events at 13.7% versus 2.4% of treated patients (RR: 6.74, 95% CI: 4.65–9.75). Subgroup analyses demonstrated significant PFS (RR: 0.92 vs. 0.74, P < 0.00001) and ORR (RR: 0.95 vs. 0.76, P = 0.0004) improvement with anti‐PD‐1 treatment compared to anti‐CTLA‐4 when each of them was compared to control treatments. Collectively, these results demonstrate that immune checkpoint inhibitors have superior outcomes compared to conventional chemotherapies or vaccination, and support the results of recent randomized trials that showed superior outcomes with anti‐PD‐1 agents over ipilimumab in unresectable metastatic cutaneous melanoma patients.
Keywords: CTLA‐4, Ipilimumab, Lambrolizumab, metastatic melanoma, nivolumab, PD‐1, pembrolizumab, tremelimumab
Introduction
Metastatic cutaneous melanoma had a poor prognosis with a 2‐year survival rate of <20% with conventional chemotherapies. Targeted therapies such as v‐raf murine sarcoma viral oncogene homolog B1 (BRAF) and MEK inhibitors have shown significant survival advantage in BRAF‐mutant melanoma 1, 2, 3, 4. However, 50–60% of patients with wild‐type BRAF require distinctive therapeutic approaches due to clinical resistance to these agents. Ipilimumab, an anti‐CTLA‐4 (cytotoxic T lymphocyte‐associated antigen‐4) human monoclonal antibody (IgG1) that blocks the T‐cell co‐inhibitory signal 5, 6 demonstrated significant survival benefit in metastatic melanoma patients regardless of BRAF mutation status, whereas tremelimumab, another anti‐CTLA‐4 IgG2 monoclonal antibody, failed to show such benefit 7. Peripheral tissues and tumor cells express PD‐L1, which neutralizes T‐cell antitumor immunity via PD‐1‐mediated co‐inhibitory signal 8. Accordingly, anti‐PD‐1 treatments have been shown to increase T‐cell antitumor activity through independent mechanisms from anti‐CTLA‐4 inhibitor treatment 9, 10. Recent randomized trials with nivolumab and pembrolizumab have demonstrated a survival advantage, including patients who progressed after antecedent ipilimumab treatment 11, 12, 13, 14. However, there was some heterogeneity and the degree of benefit seems to vary across studies. Therefore, we performed a systematic review and meta‐analysis to determine the efficacy and safety of immune checkpoint inhibitors as a category in comparison with conventional chemo‐ or vaccination treatments.
Materials and Methods
Study selection criteria
Eligible studies were (1) randomized controlled trials, (2) assessing patients with unresectable metastatic cutaneous melanoma, (3) treated with either immune check point inhibitors (ipilimumab, tremelimumab, nivolumab, pembrolizumab [previously known as lambrolizumab]) versus chemotherapy or vaccination (dacarbazine, carboplatin, temozolomide, paclitaxel, or gp100), and (4) reporting 6 months PFS and treatment response outcomes. Trials were used only once in the analysis using the most updated available data.
Data sources
Literature search and review of relevant articles were limited to human studies. Key words included metastatic melanoma, CTLA‐4, PD‐1, ipilimumab, tremelimumab, nivolumab, pembrolizumab, and lambrolizumab (Table S1). Relevant studies were identified by searching PubMed, EMBASE, and Cochrane database of systematic review up to Sep 2015. A bibliography of identified articles and additional literatures from relevant references were further investigated manually to identify any relevant studies.
Data extraction and assessment of bias risk
Two reviewers (S.Y. and N.D.V.) independently extracted data with a piloted extraction form and conducted the bias risk assessment using the Cochrane Collaboration tool (Table S3) 15. Any disagreement was resolved by consensus with a third author (M.R.G.). The following information was extracted from individual trial reports: publication year, inclusion/exclusion criteria, sample size, median age, American Joint Committee on Cancer (AJCC) Stage, BRAF mutation status, PD‐L1 positivity, number of prior systemic treatments, response to previous ipilimumab treatment, PFS, OS, ORR (defined as rate of complete remission or partial remission), adverse events, and mortality attributed to disease progression. Extracted from each study report were the number of patients treated with immune check point inhibitors or conventional treatments, number of events (death, treatment response, and treatment‐ or immune‐related adverse events), results from subgroup analyses, risk ratio (RR), odd ratio (OR), hazard ratio (HR), 95% CI, and P values. The primary outcome measures in this meta‐analysis were the 6‐month PFS rate and ORR from treatment. Secondary outcomes included the 1‐year OS rate from treatment and the grade 3/4 immune‐related adverse events rate.
Statistical analysis
Statistical analysis was performed as described in a different meta‐analysis 16. Briefly, meta‐analysis calculations were performed using RevMan Version 5.3 (Copenhagen: The Nordic Cochrane Centre, 2014). We used the Cochran Q statistic to estimate statistical heterogeneity and the I 2 statistic to quantify inconsistency. The assumption of homogeneity was considered invalid if P < 0.10. Treatment effects were calculated with a random effects model. The funnel plot method was applied to assess publication bias. A two‐sided P ≤ 0.05 was considered statistically significant in the RR analysis. We performed an intention‐to‐treat analysis following allocated treatments for the overall response and survival outcomes, and per protocol analyses for the treatment‐related adverse events. Predefined criteria including experimental agent (anti‐CTLA‐4 vs. anti‐PD‐1), response to prior ipilimumab treatment (naïve vs. refractory), BRAF mutation status (wild‐type vs. V600E mutation), and PD‐L1 positivity (expression level >5% vs. ≤5%) were used for subgroup analyses to explore heterogeneity and to identify subgroups with differential benefit from the experimental agents (Table 2). The RR differences between subgroups were evaluated by meta‐regression models.
Table 2.
Subgroup analysis of PFS rate from available data
| Subgroup | No. of Studies | RR (95% CI)‡ | Weight (%) | Heterogeneity within subgroup | ||
|---|---|---|---|---|---|---|
| Criteria | Characteristics | I 2 (%) | P value | |||
| Experimental drug | Anti‐CTLA‐4 | 3 | 0.92 (0.88, 0.97) | 54.7 | 31 | 0.24 |
| Anti‐PD‐1 | 3 | 0.74 (0.69, 0.80) | 45.3 | 0 | 0.52 | |
| Subgroup difference | P < 0.00001b | |||||
| Ipilimumab naïve versus refractory diseasea | Ipilimumab naïve | 1 | 0.70 (0.62, 0.79) | 33.5 | NA | NA |
| Ipilimumab refractory | 2 | 0.77 (0.70, 0.83) | 66.5 | 0 | 0.52 | |
| Subgroup difference | P = 0.27 | |||||
Studies with nivolumab or pembrolizumab were used for the subgroup analyses.
Statistically significant.
CTLA‐4, cytotoxic T lymphocyte‐associated protein‐4; PD‐1, programmed cell death‐1; RR, risk ratio.
Results
Search results
Our initial literature search yielded a total 301 relevant abstracts (Figs 1 and S1). Of these, 266 studies including commentaries, editorials, study protocols, and algorithm were excluded for being irrelevant based on abstract review. The remaining 35 studies were reviewed in full text. Of these, two retrospective studies 17, 18, 18 single arm or randomized studies without control treatment 11, 13, 14, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, one study with sequential treatment with a BRAF inhibitor 34, and one study with resectable melanoma 35 were excluded from the meta‐analysis. Seven duplicate or ad hoc studies 36, 37, 38, 39, 40, 41, 42 were also excluded. Following verification of eligibility, a total of six phase II or III randomized controlled trials (three with anti‐CTLA‐4 7, 43, 44 and three with anti‐PD‐1 12, 45, 46 monoclonal antibodies) were selected for the meta‐analysis. The characteristics of these trials are summarized in Tables 1 and S2.
Figure 1.
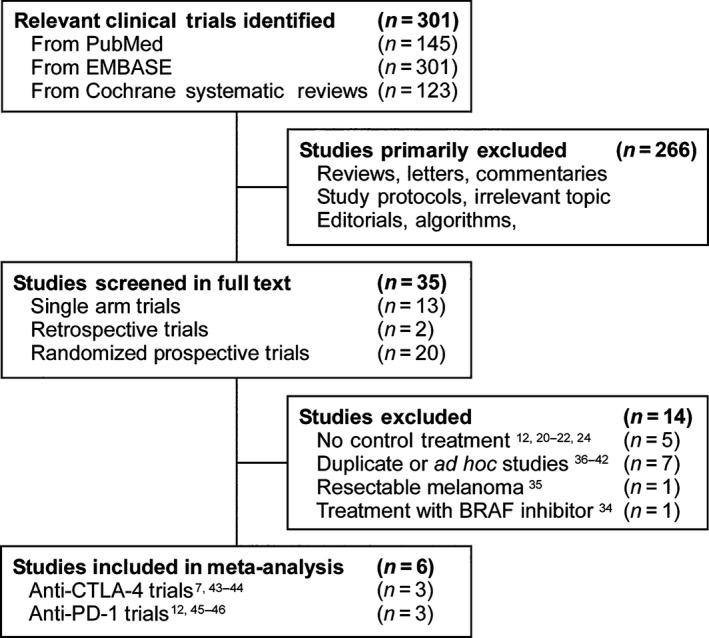
Trials selection process for the meta‐analysis.
Table 1.
Characteristics of trials included in the meta‐analysis
| Study | Exp. drug | Median age (Range) | Pathology | Stage (%)a | No. of prior systemic Tx (%) | BRAF mutant (%) | PD‐L1 positivity (%) | ECOG (%) | Treatment | No. of Patients | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | ||||||||
| Hodi et al. 44NCT00094653 | Ipilimumab | 56 (NR) | 57 (NR) | HLA–A*0201‐positive Cutaneous Melanoma | M0, M1a, or M1b: 193 (29)M1c: 483 (71) | 0: 0 (0)≥1: 676 (100) | NR | NR | 0: 374 (55)1: 291 (43)2: 9 (1)3: 1 (0.1) | Ipilimumab (3 mg/kg) ± gp100 | Gp100 | 540 | 136 |
| Robert et al. 43NCT00324155 | Ipilimumab | 57.5 (NR) | 56.4 (NR) | Cutaneous Melanoma | M0, M1a, or M1b: 220 (44)M1c: 282 (56) | 0: 369 (73)≥1: 133 (27) | NR | NR | 0: 356 (71)1: 146 (29) | Ipilimumab (10 mg/kg) + dacarbazine | Placebo + dacarbazine | 250 | 252 |
| Ribas et al. 7NCT00257205 | Tremelimumab | 56 (22–90) | 57 (22–90) | CutaneousMelanoma | IIIc: 33 (5)M1a: 96 (15)M1b: 144 (22)M1c: 382 (58) | 0: 655 (100) | NR | NR | 0: 449 (69)1: 191 (29) | Tremelimumab (15 mg/kg) | Dacarbazine or temozolimide | 327 | 328 |
| Weber et al. 12NCT01721746 | Nivolumab | 59 (23–88) | 62 (29–85) | Cutaneous Melanoma | III: 13 (3)IV: 392 (97) | 0: 0 (0)1: 111 (27)2: 207 (51) ≥3: 87 (21) | 89 (22) | 259 (65)b | 0: 246 (61)1: 158 (39) | Nivolumab (3 mg/kg) | Dacarbazine or paclitaxel | 272 | 133 |
| Robert et al. 45NCT01721772 | Nivolumab | 64 (18–86) | 66 (26–87) | Cutaneous Melanoma | M0, M1a, or M1b: 163 (39)M1c: 266 (61) | 0: 348 (83)≥1: 70 (17) | 0 (0) | 148 (35)b , c | 0: 269 (64)1: 144 (34)2: 4 (1) | Nivolumab (3 mg/kg) | Dacarbazine | 210 | 208 |
| Ribas et al. 46NCT01704287 | Pembrolizumab | 61 (15–89) | 63 (27–87) | CutaneousMelanoma | M0: 4 (<1)M1a: 37 (7)M1b: 54 (10)M1c: 445 (82) | 0: 1 (<1)1: 143 (26)2: 223 (41)≥3: 173 (32) | 125 (23)d | NR | 0: 295 (55)1: 163 (30) | Pembrolizumab (2 mg/kg or 10 mg/kg) | Carboplatin + paclitaxel, carboplatin, paclitaxel, dacarbazine, or temozolomide | 361 | 179 |
The metastasis stage was defined according to the tumor‐node‐metastasis system of the American Joint Committee on Cancer and the International Union against Cancer 47.
PD‐L1 positivity was defined as at least 5% of tumor cells exhibiting cell surface PD‐L1 staining of any intensity in a section containing at least 100 evaluable cells. PD‐L1 expression was assessed in a central laboratory with an automated Bristol‐Myers Squibb/Dako immunohistochemistry assay using rabbit monoclonal antihuman PD‐L1 antibody (clone 28–8) 21. Antibody specificity was tested with western blot.
PD‐L1 negative and indeterminate groups were calculated together for subgroup analysis in this trial.
BRAF mutation indicates BRAFV600.
HLA, human leukocyte antigen; BRAF, v‐raf murine sarcoma viral oncogene homolog B1; PD‐1, programmed cell death‐1; PD‐L1, PD‐ligand 1; ECOG, Eastern Cooperative Oncology Group; Exp, experimental treatment group; Ctrl, control treatment group; Tx, treatment, NR, not reported.
Patients
All trials included patients with histologically confirmed metastatic cutaneous melanoma. The range of median age of patients across these studies was 56–66 years. A total of 3196 patients were included in the meta‐analysis. Of these, 1960 were treated with either ipilimumab (n = 790), tremelimumab (n = 327), nivolumab (n = 482), or pembrolizumab (n = 361) and 1236 with chemotherapies (dacarbazine, carboplatin, temozolomide, or paclitaxel) (n = 1100) or gp100 (n = 136) (Table 1). Metastasis stage was defined according to AJCC tumor‐node‐metastasis system 47. There were 1858 patients classified as M1c and a total of 911 patients were either M0, M1a, or M1b (Table 1). BRAF mutation status and PD‐L1 positivity were reported in three studies with anti‐PD‐1 treatment 12, 45, 46. The numbers of patients with BRAF mutation, PD‐L1 positivity, no prior systemic treatment, and ipilimumab refractory disease were 89, 407, 1373, and 945, respectively (Table S2). In total, 48% 46, 3.8% 45, and 16.0% 7 of patients in the control groups of three studies were treated with pembrolizumab, nivolumab, and ipilimumab, respectively, while the remaining trials did not have crossover options.
RR of survival and treatment response rate
Immune check point inhibitors were associated with higher 6‐month PFS rate of 28.5% versus 17.7% (RR: 0.84, 95% CI: 0.76–0.93, P = 0.0004), 1‐year OS rate of 51.2% versus 38.8% (RR: 0.72, 95% CI: 0.59–0.88, P = 0.001), and higher ORR of 29.6% versus 17.7% (RR: 0.85, 95% CI: 0.76–0.95, P = 0.005) (Fig. 2). Grade 3/4 immune‐related adverse events were more frequently associated with immune check point inhibitors at 13.7% versus 2.4% (RR: 6.74, 95% CI: 4.65–9.75, P < 0.0001) based on per protocol analysis. There was significant heterogeneity in PFS (I 2 = 85%, P < 0.00001), OS (I 2 = 84%, P = 0.0004), and ORR (I 2 = 89%, P < 0.00001) analyses, but not in immune‐related adverse events (I 2 = 0%, P = 0.48) across studies (Figs. 2 and 2S).
Figure 2.

Survival and treatment response outcomes from available data. Forest plots of risk ratio for PFS (at 6 months), OS (at 1 year), and ORR (at 6 months) from all available data. The size of the data markers (square) corresponds to the weight of the study in the meta‐analysis. The effects of interventions are calculated with the random effects model.
Subgroup analyses
Both anti‐CTLA‐4 and anti‐PD‐1 inhibitor treatments were associated with higher PFS rates when each treatment was compared to control, however, with a significant subgroup difference favoring nivolumab or pembrolizumab over ipilimumab or tremelimumab treatments (RR: 0.92 vs. 0.74, P < 0.00001) (Table 2). The I 2 statistics for the anti‐CTLA and anti‐PD‐1 subgroups were 31% and 0%, explaining the heterogeneity observed in the PFS analysis (Fig. S3A). In trials investigating anti‐PD‐1 treatment, PFS in patients with ipilimumab refractory disease was not different from that of ipilimumab‐naïve patients (RR: 0.77 vs. 0.70, P = 0.27) (Fig. S3B). Similarly, the ORR for anti‐PD‐1 treatment was significantly higher than the ORR for anti‐CTLA‐4 treatment (RR: 0.95 vs. 0.76, P = 0.0004) (Table 3, Fig. S4A and B). In patients treated with nivolumab or pembrolizumab, PD‐L1‐positive and ipilimumab‐naïve patients had better ORR compared to PD‐L1‐negative (RR: 0.57 vs. 0.84, P = 0.001) and ipilimumab‐refractory patients (RR: 0.70 vs. 0.80, P = 0.05) (Tables 2 and 3). BRAF mutation status did not have a statistically significant prognostic impact on ORR (RR: 0.84 vs. 0.85, P = 0.97) (Tables 2 and 3, Figs. S3 and S4).
Table 3.
Subgroup analysis of ORR from available data
| Subgroup | No. of Studies | RR (95% CI) | Weight (%) | Heterogeneity within subgroup | ||
|---|---|---|---|---|---|---|
| Criteria | Characteristics | I 2 (%) | P value | |||
| Experimental drug | Anti‐CTLA‐4 | 3 | 0.95 (0.88, 1.02) | 51.6 | 50 | 0.13 |
| Anti‐PD‐1 | 3 | 0.76 (0.69, 0.84) | 48.4 | 54 | 0.12 | |
| Subgroup difference | P < 0.00001c | |||||
| Ipilimumab naïve versus refractory diseasea | Ipilimumab naïve | 1 | 0.70 (0.62, 0.79) | 30.5 | NA | NA |
| Ipilimumab refractory | 2 | 0.80 (0.75, 0.85) | 69.5 | 0 | 0.78 | |
| Subgroup Difference | P = 0.05c | |||||
| BRAF mutationa | BRAF wild‐type | 2 | 0.84 (0.68, 1.03) | 81.4 | 76 | 0.04 |
| BRAF mutant | 1 | 0.85 (0.64, 1.12) | 18.6 | NA | NA | |
| Subgroup Difference | P = 0.97 | |||||
| PD‐L1 statusa | PD‐L1 positiveb | 2 | 0.57 (0.48, 0.69) | 45.4 | 0 | 0.38 |
| PD‐L1 negative | 2 | 0.84 (0.73, 0.96) | 54.6 | 29 | 0.24 | |
| Subgroup Difference | P = 0.001c | |||||
Data from nivolumab and pembrolizumab trials were used for these subgroup analyses.
PD‐L1 positivity was defined as at least 5% of tumor cells exhibiting cell surface PD‐L1 staining of any intensity in a section containing at least 100 evaluable cells. Patients with indeterminate PD‐L1 expression level were included into PD‐L1‐negative group for the subgroup analysis in study performed by Robert et al 45.
Statistically significant.
CTLA‐4, cytotoxic T lymphocyte‐associated protein‐4; PD‐1, programmed cell death‐1; PD‐L1, PD‐ligand 1; RR, risk ratio; BRAF, v‐raf murine sarcoma viral oncogene homolog B1).
Bias analysis
Four trials were double‐blinded and two were open‐label studies 7, 12. Random sequence generation and allocation concealment were performed adequately in all studies. The adequacy of blinding was judged by whether treatment response was evaluated by a third person who did not know the treatment group of the patients. Four studies 12, 43, 45, 46 performed blinded assessments, but blinding was unclear in two studies 7, 44 (Table S3). The baseline demographic characteristics were balanced in all trials (Tables 1 and S2). Potential sources of bias are described in Table S3. PFS and ORR analyses showed heterogeneity, largely attributable to the experimental agent used (anti‐CTLA‐4 vs. anti‐PD‐1) and the significant subgroup difference observed, but these PFS and ORR subgroup analyses also evidenced intra‐subgroup homogeneity (Tables 2 and 3). The observed funnel plot asymmetry can also be explained as a function of experimental agent used (Fig. S1).
Discussion
Although the benefit of immune checkpoint inhibitors as a class has been observed consistently in previous randomized trials, some of the agents failed to show benefit 7 and the efficacy of immune checkpoint inhibitors seems to be variable. Meta‐analysis, in general, obtains a quantitative synthesis from studies with similar design to estimate the overall effect of interventions and to improve the precision of estimates of treatment effects 48, 49. Therefore, we performed a meta‐analysis comparing the outcomes of immune checkpoint inhibitors as a category to conventional chemotherapies or vaccination in patients with unresectable metastatic cutaneous melanoma, with a focus on subgroup analyses to explain the heterogeneity across studies and to identify subgroups that are associated with better clinical outcomes.
The pooled analyses revealed statistically significant PFS, OS, and ORR benefits with immune check point inhibitors (Fig. 2), suggesting the superiority of immune checkpoint inhibitors over conventional regimens. Both anti‐CTLA‐4 and anti‐PD‐1 treatments were associated with clinical benefit in our meta‐analysis; however, an indirect comparison of these two agents showed superior PFS and ORR in anti‐PD‐1 compared to anti‐CTLA‐4 treatment (Tables 2 and 3). This result is consistent with data from two recent randomized trials that were published while our study was ongoing. The KEYNOTE‐006 trial showed higher PFS, OS, and ORR with two different treatment schedules of pembrolizumab treatment (10 mg/kg every 2 weeks and 3 weeks) compared to ipilimumab 50. The CheckMate 067 trial revealed PFS and ORR improvement with nivolumab (3 mg/kg every 2 weeks) compared to ipilimumab 51. Ipilimumab used to be the standard first‐line treatment for advanced metastatic melanoma based on results from phase II and III trials 22, 23, 43, 44, however, the prevailing guidelines 52 recommend either anti‐PD‐1 monotherapy or nivolumab and ipilimumab combination therapy as the standard first‐line treatment in unresectable metastatic melanoma based on these two randomized trials 50, 51.
Daud et al. showed nominally higher ORRs (38% vs. 29%), 1‐year PFS rates (36% vs. 32%), and 1‐year OS rates (71% vs. 63%) in the ipilimumab naïve versus treated patients in their pooled analysis of 655 patients who were treated with pembrolizumab 53. Similarly, in our subgroup analysis, the ORR of nivolumab treatment in ipilimumab‐refractory patients was lower compared to ipilimumab‐naïve patients, although ORRs in both groups were still better than those in control treatments (Fig. S4B). Collectively, these results suggest that there is a certain patient population that may selectively respond to anti‐PD‐1 treatment and benefit from combination treatment of anti‐CTLA‐4 with anti‐PD‐1 agents. Similar to our results, the CheckMate 067 trial demonstrated ipilimumab and nivolumab combination treatment to have better ORR compared to nivolumab monotherapy, especially in PD‐L1‐positive patients 51, although this study was not designed for statistical comparison of combination treatment versus nivolumab monotherapy. Future randomized trials comparing anti‐CTLA‐4 and anti‐PD‐1 combination versus anti‐PD‐1 monotherapy in ipilimumab‐naïve and ‐refractory patients would provide valuable information to clarify the optimal first‐line treatment.
In prior study of nivolumab, PD‐L1 positivity, defined as more than 5% by immunohistochemistry staining, was associated with better response, although the association disappeared when a cutoff value of 1% was used as the positivity criteria 13. In other studies that used the 5% threshold, PD‐L1‐positive groups had significantly higher ORR in comparison with PD‐L1‐negative groups 20, 54. Similarly, in our meta‐analysis, the subgroup with PD‐L1 positivity (more than 5%) had a better treatment response to anti‐PD‐1 agents compared to the PD‐L1‐negative subgroup (Fig. S4D). However, the PD‐L1‐negative group still had significant ORR improvement in comparison with control treatments. This indicates that PD‐L1 positivity should not be used to select patients for anti‐PD‐1 treatment. Our results are supported by the recent CheckMate 069 trial that showed no ORR difference between PD‐L1 positive versus negative groups when patients received nivolumab and ipilimumab combination therapy 55. On the other hand, the CheckMate 067 trial showed a nominally higher ORR in the PD‐L1‐positive group over the PD‐L1‐negative group when these patients were treated with ipilimumab and nivolumab combination therapy or with nivolumab monotherapy 51. PD‐L1 expression levels were shown to be variable in different metastatic lesions in the same patient and anti‐PD‐1 treatment response also seemed to be affected by tumor mutational load and preexisting intratumor CD8 + T‐cells based on preclinical studies 56, 57, 58. Collectively, the prognostic impact of PD‐L1 status needs further investigation.
Previous studies have suggested that BRAF mutation status does not affect the efficacy of checkpoint inhibitors 21, 59, 60. In our subgroup analysis, the ORR of patients with BRAF WT did not differ from that of BRAF mutation (Fig. S4C). Larkin et al. performed a pooled analysis from four studies including phase I and III trials to compare the clinical outcomes between patients with and without BRAF mutation who were treated with nivolumab 61. Although, this study included data from nonrandomized trials and was retrospectively analyzed, the ORR in BRAF WT versus mutant patients was 34.6% versus 29.7% with no statistically significant difference, which is consistent with our subgroup analysis.
We recognize several limitations of the current meta‐analysis. First, there was significant statistical heterogeneity in PFS and ORR analyses (I 2 = 85%, P < 0.00001 and I 2 = 89%, P = 0.005). However, this was predicted since data from two different classes of immune check point inhibitors were analyzed together. Accordingly, the primary source of the heterogeneity was from experimental agents as evidenced also by the intra‐subgroup homogeneity (I 2 = 31%, P = 0.24 and I 2 = 0%, P = 0.52 in PFS analyses of anti‐CTLA‐4 and anti‐PD‐1 treatment, respectively) (Fig. S3A). In recent studies, an increase in T‐cell receptor (TCR) repertoire was observed in patients treated with anti‐CTLA‐4 agent 62, and anti‐PD‐1 agents were shown to induce intratumoral CD8 + T‐cells proliferation in patients who responded to therapy 57. Also, combination treatment of anti‐CTLA‐4 and anti‐PD‐1 showed distinct immunologic effects in vivo compared to single agent 63. Collectively, the biological differences in mechanisms of action between these two agents may lead to the heterogeneity observed in the clinical outcomes. There was some residual heterogeneity in the anti‐CTLA‐4 subgroup (I 2 = 31%), mainly secondary to the tremelimumab trial 7 that showed no significant benefit from the experimental agent. In this study, patients with lactate dehydrogenase (LDH) higher than two times upper limit were excluded and 16% of patients in the control group were treated with ipilimumab, which may have masked the benefit of tremelimumab treatment. Second, the emergence of irRECIST criteria in 2009 for adjudicating immune‐related treatment response and the interchangeable use of both RECIST and irRECIST may have influenced the outcomes reported in the studies included in our analyses. The primary difference between these two sets of response criteria is the rate of alternative forms of response captured by irRECIST, but coded as progressive disease (PD) by RECIST criteria. The discrepancy in treatment response, estimated to be ~10% for ipilimumab and ~5–10% for anti‐PD‐1 agents, warrant caution in the interpretation of results 64, 65. Third, the PD‐L1 negative subgroup in one study 50 included patients with an indeterminate PD‐L1 level. Fourth, the sample size in each subgroup was relatively small, rendering the prognostic impact of PD‐L1 positivity less conclusive. Fifth, two ipilimumab 43, 44 and one tremelimumab 7 trials did not report BRAF mutation status. Lastly, median survivals of experimental groups in two studies 12, 42 were not reached yet and 1‐year OS rates were not reported. Therefore, the OS result should be interpreted with due caution and needs longer follow‐up.
Several questions remain to be answered. Immune checkpoint inhibitors are associated with significant risk of immune‐related adverse events in a range of 10–40% 22, 24, 43, 66, 67, and our meta‐analysis also demonstrated statistically higher grade 3/4 immune‐mediated adverse events rate in the experimental group (Fig. S2). Recently, nivolumab and ipilimumab combination treatment (which may have better efficacy than monotherapy) was shown to have even higher drug‐related adverse events than ipilimumab monotherapy, which became the most common reason for discontinuation of treatment 51, 55. Most of the immune‐related grade 3/4 adverse events can be effectively managed with either systemic steroid or infliximab therapy, but prophylaxis with budesonide failed to prevent immune‐related adverse events or improve survival outcomes 24. A recent trial showed that sargramostim combined with ipilimumab (10 mg/kg) not only enhanced efficacy but also reduced adverse events, specifically gastrointestinal and pulmonary adverse events, the former of which is the leading cause of treatment disruption and discontinuation 26. Further study is needed to maximize the benefit of immune check point targeting agents by reducing drug‐related adverse events. Second, BRAF inhibitor as a single agent and in combination with a MEK inhibitor was shown to improve survival in patients with BRAF mutation 1, 2, 3, 4, and immune check point inhibitors are effective regardless of BRAF mutation status 21, 59, 60. Therefore, the optimal sequence for treatment, especially in patients with BRAF mutation, needs further investigation. Third, the optimal dose and schedule of immune check point inhibitors remain to be determined. Lastly, the prognostic impact of PD‐L1 expression level as well as independent prognostic factors to anti‐CTLA‐4 and anti‐PD‐1 treatments need further investigation for better patient selection and improved clinical outcomes.
Conclusion
In a meta‐analysis of randomized controlled trials with unresectable cutaneous metastatic melanoma patients, agents targeting immune checkpoints were associated with better PFS, OS, and ORR compared to conventional treatments. Subgroup analyses showed that survival benefit was significantly higher with anti‐PD‐1 treatment regardless of previous response to ipilimumab treatment, suggesting that nivolumab or pembrolizumab is a better choice as the first‐line treatment. Our meta‐analysis also indicates that there is a need for future study to assess the prognostic values of PD‐L1 expression level and optimal sequential treatments for better clinical outcome.
Conflict of Interest
The authors declare that there is no conflict of interest.
Supporting information
Figure S1. Funnel Plot.
Figure S2. Grade 3/4 immune‐related adverse events.
Figure S3. Subgroup analysis of 6‐month PFS rate from available data.
Figure S4. Subgroup analysis of 6‐month ORR from available data.
Table S1. Search detail in PubMed, EMBASE, and Cochrane database.
Table S2. Additional characteristics of included trials.
Table S3. Risk of bias assessment of studies according to Cochrane risk bias assessment tool 8.
Acknowledgments
The authors thank anonymous referees for their helpful comments. This work was supported by predoctoral fellowships to N.D.V. from the Mayo Foundation for Education and Research and Bressler Alpert Society Research Grant to S.Y. from University of Arizona.
Cancer Medicine 2016; 5(7):1481–1491
References
- 1. Chapman, P. B. , Hauschild A., Robert C., Haanen J. B., Ascierto P., Larkin J., et al. 2011. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Long, G. V. , Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., et al. 2014. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371:1877–1888. [DOI] [PubMed] [Google Scholar]
- 3. Long, G. V. , Trefzer U., Davies M. A., Kefford R. F., Ascierto P. A., Chapman P. B., et al. 2012. Dabrafenib in patients with Val600Glu or Val600Lys BRAF‐mutant melanoma metastatic to the brain (BREAK‐MB): a multicentre, open‐label, phase 2 trial. Lancet Oncol. 13:1087–1095. [DOI] [PubMed] [Google Scholar]
- 4. McArthur, G. A. , Chapman P. B., Robert C., Larkin J., Haanen J. B., Dummer R., et al. 2014. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation‐positive melanoma (BRIM‐3): extended follow‐up of a phase 3, randomised, open‐label study. Lancet Oncol. 15:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leach, D. R. , Krummel M. F., and Allison J. P.. 1996. Enhancement of antitumor immunity by CTLA‐4 blockade. Science 271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 6. Pardoll, D. M. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ribas, A. , Kefford R., Marshall M. A., Punt C. J., Haanen J. B., Marmol M., et al. 2013. Phase III randomized clinical trial comparing tremelimumab with standard‐of‐care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong, H. , Strome S. E., Salomao D. R., Tamura H., F. Hirano , Flies D. B., et al. 2002. Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800. [DOI] [PubMed] [Google Scholar]
- 9. Okazaki, T. , Chikuma S., Iwai Y., Fagarasan S., and Honjo T.. 2013. A rheostat for immune responses: the unique properties of PD‐1 and their advantages for clinical application. Nat. Immunol. 14:1212–1218. [DOI] [PubMed] [Google Scholar]
- 10. Curran, M. A. , Montalvo W., Yagita H., and Allison J. P.. 2010. PD‐1 and CTLA‐4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl Acad. Sci. USA 107:4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Topalian, S. L. , Sznol M., McDermott D. F., Kluger H. M., Carvajal R. D., Sharfman W. H., et al. 2014. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber, J. S. , D'Angelo S. P., Minor D., Hodi F. S., R. Gutzmer , Neyns B., et al. 2015. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol. 16:375–384. [DOI] [PubMed] [Google Scholar]
- 13. Weber, J. S. , Kudchadkar R. R., Yu B., Gallenstein D., Horak C. E., Inzunza H. D., et al. 2013. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab‐refractory or ‐naive melanoma. J. Clin. Oncol. 31:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robert, C. , Ribas A., Wolchok J. D., Hodi F. S., O. Hamid , Kefford R., et al. 2014. Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: a randomised dose‐comparison cohort of a phase 1 trial. Lancet 384:1109–1117. [DOI] [PubMed] [Google Scholar]
- 15. Moher, D. , Liberati A., Tetzlaff J., and Altman D. G.; Prisma Group . 2009. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J. Clin. Epidemiol. 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 16. Yun, S. , Vincelette N. D., and Abraham I.. 2015. Cardioprotective role of β‐blockers and angiotensin antagonists in early‐onset anthracyclines‐induced cardiotoxicity in adult patients: a systematic review and meta‐analysis. Postgrad. Med. J. 91:627–633. [DOI] [PubMed] [Google Scholar]
- 17. Thompson, J. A. , Hamid O., Minor D., Amin A., Ron I. G., Ridolfi R., et al. 2012. Ipilimumab in treatment‐naive and previously treated patients with metastatic melanoma: retrospective analysis of efficacy and safety data from a phase II trial. J. Immunother. 35:73–77. [DOI] [PubMed] [Google Scholar]
- 18. Ackerman, A. , Klein O., McDermott D. F., Wang W., Ibrahim N., Lawrence D. P., et al. 2014. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 120:1695–1701. [DOI] [PubMed] [Google Scholar]
- 19. Hamid, O. , Robert C., Daud A., Hodi F. S., Hwu W. J., Kefford R., et al. 2013. Safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma. N. Engl. J. Med. 369:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Topalian, S. L. , Hodi F. S., Brahmer J. R., Gettinger S. N., Smith D. C., McDermott D. F., et al. 2012. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N. Engl. J. Med. 366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolchok, J. D. , Kluger H., Callahan M. K., Postow M. A., Rizvi N. A., Lesokhin A. M., et al. 2013. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolchok, J. D. , Neyns B., Linette G., Negrier S., J. Lutzky , Thomas L., et al. 2010. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double‐blind, multicentre, phase 2, dose‐ranging study. Lancet Oncol. 11:155–164. [DOI] [PubMed] [Google Scholar]
- 23. Hersh, E. M. , O'Day S. J., Powderly J., Khan K. D., Pavlick A. C., Cranmer L. D., et al. 2011. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy‐naïve patients with advanced melanoma. Invest. New Drugs 29:489–498. [DOI] [PubMed] [Google Scholar]
- 24. Weber, J. , Thompson J. A., Hamid O., Minor D., A. Amin , Ron I., et al. 2009. A randomized, double‐blind, placebo‐controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 15:5591–5598. [DOI] [PubMed] [Google Scholar]
- 25. O'Day, S. J. , Maio M., Chiarion‐Sileni V., Gajewski T. F., Pehamberger H., Bondarenko I. N., et al. 2010. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single‐arm phase II study. Ann. Oncol. 21:1712–1717. [DOI] [PubMed] [Google Scholar]
- 26. Hodi, F. S. , Lee S., McDermott D. F., Rao U. N., Butterfield L. H., Tarhini A. A., et al. 2014. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 312:1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ascierto, P. A. , Simeone E., Sileni V. C., Pigozzo J., M. Maio , Altomonte M., et al. 2014. Clinical experience with ipilimumab 3 mg/kg: real‐world efficacy and safety data from an expanded access programme cohort. J. Transl. Med. 12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiarion Sileni, V. , Pigozzo J., Ascierto P. A., Grimaldi A. M., Maio M., Di Guardo L., et al. 2014. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J. Exp. Clin. Cancer Res. 33:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Giacomo, A. M. , Ascierto P. A., Pilla L., Santinami M., Ferrucci P. F., Giannarelli D., et al. 2012. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT‐M1): an open‐label, single‐arm phase 2 trial. Lancet Oncol. 13:879–886. [DOI] [PubMed] [Google Scholar]
- 30. Di Giacomo, A. M. , Ascierto P. A., Queirolo P., L. Pilla , Ridolfi R., Santinami M., et al. 2015. Three‐year follow‐up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)‐M1 phase II study. Ann. Oncol. 26:798–803. [DOI] [PubMed] [Google Scholar]
- 31. Brahmer, J. R. , Drake C. G., Wollner I., Powderly J. D., Picus J., Sharfman W. H., et al. 2010. Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weber, J. S. , O'Day S., Urba W., Powderly J., Nichol G., Yellin M., et al. 2008. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 26:5950–5956. [DOI] [PubMed] [Google Scholar]
- 33. Maker, A. V. , Phan G. Q., Attia P., Yang J. C., Sherry R. M., Topalian S. L., et al. 2005. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte‐associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann. Surg. Oncol. 12:1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ascierto, P. A. , Simeone E., Sileni V. C., Del Vecchio M., Marchetti P., Cappellini G. C., et al. 2014. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest. 32:144–149. [DOI] [PubMed] [Google Scholar]
- 35. Eggermont, A. M. , Chiarion‐Sileni V., Grob J. J., R. Dummer , Wolchok J. D., Schmidt H., et al. 2015. Adjuvant ipilimumab versus placebo after complete resection of high‐risk stage III melanoma (EORTC 18071): a randomised, double‐blind, phase 3 trial. Lancet Oncol. 16:522–530. [DOI] [PubMed] [Google Scholar]
- 36. Maio, M. , Grob J. J., Aamdal S., Bondarenko I., Robert C., Thomas L., et al. 2015. Five‐year survival rates for treatment‐naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J. Clin. Oncol. 33:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDermott, D. , Haanen J., Chen T. T., Lorigan P., S. O'Day , Investigators M‐.. et al. 2013. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010–20). Ann. Oncol. 24:2694–2698. [DOI] [PubMed] [Google Scholar]
- 38. Sherrill, B. , Wang J., Kotapati S., and Chin K.. 2013. Q‐TWiST analysis comparing ipilimumab/dacarbazine vs placebo/dacarbazine for patients with stage III/IV melanoma. Br. J. Cancer 109:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lebbé, C. , Weber J. S., Maio M., Neyns B., Harmankaya K., Hamid O., et al. 2014. Survival follow‐up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann. Oncol. 25:2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolchok, J. D. , Weber J. S., Maio M., Neyns B., K. Harmankaya , Chin K., et al. 2013. Four‐year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann. Oncol. 24:2174–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prieto, P. A. , Yang J. C., Sherry R. M., Hughes M. S., Kammula U. S., White D. E., et al. 2012. CTLA‐4 blockade with ipilimumab: long‐term follow‐up of 177 patients with metastatic melanoma. Clin. Cancer Res. 18:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dummer, R. , Daud A., Puzanov I., Hamid O., D. Schadendorf , Robert C., et al. 2015. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab‐refractory melanoma. J. Transl. Med. 13:2062. [Google Scholar]
- 43. Robert, C. , Thomas L., Bondarenko I., O'Day S., J. Weber , Garbe C., et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 44. Hodi, F. S. , O'Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robert, C. , Long G. V., Brady B., Dutriaux C., Maio M., Mortier L., et al. 2015. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372:320–330. [DOI] [PubMed] [Google Scholar]
- 46. Ribas, A. , Puzanov I., Dummer R., Schadendorf D., O. Hamid , Robert C., et al. 2015. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Balch, C. M. , Gershenwald J. E., Soong S. J., Thompson J. F., Atkins M. B., Byrd D. R., et al. 2009. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 27:6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guyatt, G. H. , Sackett D. L., Sinclair J. C., Hayward R., Cook D. J., and Cook R. J.. 1995. Users' guides to the medical literature. IX. A method for grading health care recommendations. Evidence‐Based Medicine Working Group. JAMA 274:1800–1804. [DOI] [PubMed] [Google Scholar]
- 49. Burns, P. B. , Rohrich R. J., and Chung K. C.. 2011. The levels of evidence and their role in evidence‐based medicine. Plast. Reconstr. Surg. 128:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Robert, C. , Schachter J., Long G. V., Arance A., Grob J. J., Mortier L., et al. 2015. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med.. [DOI] [PubMed] [Google Scholar]
- 51. Larkin, J. , Chiarion‐Sileni V., Gonzalez R., Grob J. J., Cowey C. L., Lao C. D., et al. 2015. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. NCCN Guideline , Melanoma, Version 2.2016: http://www.nccn.org/.
- 53. Daud, A ., Ribas A., Robert C., Hodi S., Wolchok J., A. Joshua , et al. Long‐term efficacy of pembrolizumab (pembro; MK‐3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE‐001. JCO2015. [Google Scholar]
- 54. Taube, J. M. , Klein A., Brahmer J. R., Xu H., Pan X., Kim J. H., et al. 2014. Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti‐PD‐1 therapy. Clin. Cancer Res. 20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Postow, M. A. , Chesney J., Pavlick A. C., Robert C., K. Grossmann , McDermott D., et al. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Madore, J. , Vilain R. E., Menzies A. M., Kakavand H., Wilmott J. S., Hyman J., et al. 2015. PD‐L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti‐PD‐1/PD‐L1 clinical trials. Pigment Cell Melanoma Res 28:245–253. [DOI] [PubMed] [Google Scholar]
- 57. Tumeh, P. C. , Harview C. L., Yearley J. H., Shintaku I. P., Taylor E. J., Robert L., et al. 2014. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rizvi, N. A. , Hellmann M. D., Snyder A., Kvistborg P., Makarov V., Havel J. J., et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kluger, H. , Sznol M., Callahan M., Postow M. A., Gordon R. A., Segal N. H., et al. 2014. Survival, response duration, and activity by BRAF mutation (MT) status in a phase 1 trial of nivolumab (anti‐PD‐1, BMS‐936558, ONO‐4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). Ann Oncol. [Google Scholar]
- 60. Sznol, M. , Kluger H. M., Callahan M. K., Postow M. A., Gordon R. A., Segal N. H., et al. 2014. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti‐PD‐1, BMS‐936558, ONO‐4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). J Clin Oncol. [Google Scholar]
- 61. Larkin, J. , Lao C. D., Urba W. J., McDermott D. F., C. Horak , Jiang J., et al. 2015. Efficacy and safety of nivolumab in patients With BRAF V600 mutant and BRAF wild‐type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 1:433–440. [DOI] [PubMed] [Google Scholar]
- 62. Robert, L. , Tsoi J., Wang X., Emerson R., Homet B., T. Chodon , et al. 2014. CTLA4 blockade broadens the peripheral T‐cell receptor repertoire. Clin. Cancer Res. 20:2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Das, R. , Verma R., Sznol M., Boddupalli C. S., Gettinger S. N., Kluger H., et al. 2015. Combination therapy with anti‐CTLA‐4 and anti‐PD‐1 leads to distinct immunologic changes in vivo. J. Immunol. 194:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoos, A. , Wolchok J. D., Humphrey R. W., and Hodi F. S.. 2015. CCR 20th anniversary commentary: immune‐related response criteria‐capturing clinical activity in immuno‐oncology. Clin. Cancer Res. 21:4989–4991. [DOI] [PubMed] [Google Scholar]
- 65. Wolchok, J. D. , Hoos A., O'Day S., Weber J. S., O. Hamid , Lebbé C., et al. 2009. Guidelines for the evaluation of immune therapy activity in solid tumors: immune‐related response criteria. Clin. Cancer Res. 15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 66. Fellner, C. 2012. Ipilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P. T. 37:503–530. [PMC free article] [PubMed] [Google Scholar]
- 67. Yun, S. , Vincelette N. D., Mansour I., Hariri D., and Motamed S.. 2015. Late onset ipilimumab‐induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep. Oncol. Med. 2015:794842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Funnel Plot.
Figure S2. Grade 3/4 immune‐related adverse events.
Figure S3. Subgroup analysis of 6‐month PFS rate from available data.
Figure S4. Subgroup analysis of 6‐month ORR from available data.
Table S1. Search detail in PubMed, EMBASE, and Cochrane database.
Table S2. Additional characteristics of included trials.
Table S3. Risk of bias assessment of studies according to Cochrane risk bias assessment tool 8.


