Background and Purpose
Glucagon‐like peptide‐1 (GLP‐1) analogues improve glycaemic control in type 2 diabetic (T2D) patients and cause weight loss in obese subjects by as yet unknown mechanisms. We recently demonstrated that the GLP‐1 receptor, which is present in adipocytes and the stromal vascular fraction of human adipose tissue (AT), is up‐regulated in AT of insulin‐resistant morbidly obese subjects compared with healthy lean subjects. The aim of this study was to explore the effects of in vitro and in vivo administration of GLP‐1 and its analogues on AT and adipocyte functions from T2D morbidly obese subjects.
Experimental Approach
We analysed the effects of GLP‐1 on human AT and isolated adipocytes in vitro and the effects of GLP‐1 mimetics on AT of morbidly obese T2D subjects in vivo.
Key Results
GLP‐1 down‐regulated the expression of lipogenic genes when administered during in vitro differentiation of human adipocytes from morbidly obese patients. GLP‐1 also decreased the expression of adipogenic/lipogenic genes in AT explants and mature adipocytes, while increasing that of lipolytic markers and adiponectin. In 3T3‐L1 adipocytes, GLP‐1 decreased free cytosolic Ca2 + concentration ([Ca2 +]i). GLP‐1‐induced responses were only partially blocked by GLP‐1 receptor antagonist exendin (9–39). Moreover, administration of exenatide or liraglutide reduced adipogenic and inflammatory marker mRNA in AT of T2D obese subjects.
Conclusions and Implications
Our data suggest that the beneficial effects of GLP‐1 are associated with changes in the adipogenic potential and ability of AT to expand, via activation of the canonical GLP‐1 receptor and an additional, as yet unknown, receptor.
Abbreviations
- ADRP
adipocyte differentiation‐related protein
- AT
adipose tissue
- ATGL
adipose triglyceride lipase
- BMI
body mass index
- FABP4
fatty acid binding protein 4
- FASN
fatty acid synthase
- GLP‐1
glucagon‐like peptide‐1
- HSL
hormone‐sensitive lipase
- LPL
lipoprotein lipase
- MO
morbidly obese
- SAT
subcutaneous AT
- SREBP1
sterol regulatory element‐binding transcription factor 1
- T2D
type 2 diabetic
- VAT
visceral AT
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes d |
| FABP4 | Acetyl CoA carboxylase |
| TNF‐α | Adenylate cyclase |
| GPCRs b | Akt (PKB) |
| GLP‐1 receptor | ERK1 |
| Nuclear hormone receptors c | ERK2 |
| PPARγ | FASN |
| Transporters e | Hormone sensitive lipase (HSL) |
| GLUT4 | PKA |
| LIGANDS | |
|---|---|
| Adiponectin | IBMX |
| cAMP | IL‐6 |
| Dexamethasone | Indomethacin |
| Exenatide (exendin‐4) | Insulin |
| Exendin (9‐39) | Liraglutide |
| GLP‐1 | Metformin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015d, 2015b, 2015e, 2015a, 2015c).
Introduction
Glucagon‐like peptide‐1 (GLP‐1) is a potent physiological regulator of blood glucose that stimulates insulin secretion, which underlies its use for the treatment of type 2 diabetes mellitus (T2D) (Nathan et al., 1992; Nauck et al., 1997). Circulating levels of GLP‐1 rise in association with the postprandial increase in blood glucose concentration. This peptide hormone binds to a specific GPCR, the GLP‐1 receptor, which is expressed in several cell types, including pancreatic beta cells (Baggio and Drucker, 2007; McIntosh et al., 2009). GLP‐1 enhances glucose‐induced insulin synthesis and secretion upon binding to GLP‐1 receptors in beta cells, thus increasing beta cell sensitivity to glucose (Drucker, 2006; Yusta et al., 2006). This effect, referred to as the incretin effect, plays a critical role in the maintenance of systemic glucose homeostasis (Kreyman et al., 1987). Furthermore, GLP‐1 also improves alpha‐cell glucose sensing in patients with T2D and controls food intake by increasing satiety in these patients (Flint et al., 1998; Gutzwiller et al., 1999; Dunning et al., 2005).
The effect of GLP‐1 on adipose tissue (AT) has been poorly studied, and few results have been published. Studies performed in isolated rat and human adipocytes indicate that GLP‐1 may activate both lipogenic and lipolytic mechanisms (Ruiz‐Grande et al., 1992; Perea et al., 1997; Villanueva‐Peñacarrillo et al., 2001a; Azuma et al., 2008; Majumdar and Weber, 2010). Recent studies in murine 3T3‐L1 cells indicate that GLP‐1 promotes pre‐adipocyte differentiation (Challa et al., 2012; Yang et al., 2013). The effects of GLP‐1 in fat, as in the liver or muscle, seem to be exerted through a GLP‐1‐specific receptor that is structurally and/or functionally distinct from that expressed in the pancreas (Montrose‐Rafizadeh et al., 1997; Villanueva‐Peñacarrillo et al., 2001b). Recently, we obtained evidence demonstrating the presence of the GLP‐1 receptor in AT and showed that its mRNA and protein levels are increased in visceral AT (VAT) from morbidly obese (MO) patients with a high degree of insulin resistance (IR) (Montrose‐Rafizadeh et al., 1997; Vendrell et al., 2011) and that, in 3T3‐L1, GLP‐1 enhances lipolysis in a receptor‐dependent manner (Vendrell et al., 2011). Taken together these data support the view that the GLP‐1/GLP‐1 receptor system in AT may play a role in improving insulin sensitivity in obese patients (Vendrell et al., 2011).
Adipocyte differentiation is a tightly regulated process orchestrated by the temporal expression of key transcription factors, such as PPARγ, adipocyte differentiation‐related protein (ADRP) and fatty acid binding protein 4 (FABP4), which result in cytoskeletal changes as well as in the induction of key genes involved in lipogenesis [lipoprotein lipase (LPL), fatty acid synthase (FASN), sterol regulatory element‐binding transcription factor 1 (SREBP1) and forkhead box protein O1(FOXO1)] and lipolysis, such as α‐2‐glycoprotein 1, zinc binding, a potent inducer of lipolysis (Russell et al., 2004); adipose triglyceride lipase (ATGL); hormone‐sensitive lipase (HSL); and perilipin (Hunt et al., 1986; Gregoire et al., 1998; Rosen et al., 1999; Gao et al., 2000; Large et al., 2004; Russell et al., 2004; Farmer, 2006; Kolditz and Langin, 2010). These last two genes are known to be primarily regulated via activation of cAMP/PKA (Kolditz and Langin, 2010). The relative balance of these processes in mature adipocytes is crucial in determining adipocyte size and hence fat mass.
To gain further insights into the effects and mechanisms of GLP‐1 action on AT, herein, we explored the effects of in vitro and in vivo administration of GLP‐1 and its analogues on AT and adipocyte functions from T2D MO subjects.
Methods
Patients and AT collection
All participants gave their informed consent, and the study was reviewed and approved by the Hospital Ethics and Research Committee.
In vitro studies
VAT and subcutaneous AT (SAT) samples were obtained from healthy obese subjects [body mass index (BMI) = 49.09 ± 2.69 kg·m−2] with a low degree of IR [homeostatic model assessment IR (HOMA‐IR) < 4] (n = 27) undergoing elective surgery (cholecystectomy, surgery for abdominal hernia) at the Virgen de la Victoria Hospital (Malaga, Spain). Exclusion criteria were dyslipidaemia, arterial hypertension, cardiovascular diseases and drug treatment.
Transversal pilot study: treatment of T2D MO subjects with the GLP‐1 analogue exenatide
MO subjects (BMI = 48.09 ± 2.69) (n = 18) with T2D who were under treatment with metformin were separated into two groups, receiving (n = 9) or not (n = 9) a daily administration of exenatide (10 μg twice a day) for a duration of 6 months. At the end of the treatment, patients underwent bariatric surgery, and SAT samples were collected at the site of surgical incision from the abdominal wall, while VAT samples were obtained from the omentum. Exclusion criteria were glycated haemoglobin Hba1c > 8%, the use of other treatments apart from metformin and exenatide, a history of cardiovascular disease, and kidney, hepatic or cardiac failure.
The anthropometrical and biochemical characteristics of the patients included in this study are shown in Table 2.
Table 2.
Effects of exenatide in T2D MO subjects treated with metformin
| Metformin‐treated patients (n = 9) | Metformin‐ and exenatide‐treated patients (n = 9) | |
|---|---|---|
| Age (in surgery, years) | 52.33 ± 3.78 | 45.88 ± 3.65 |
| BMI (kg·m−2) | 48.09 ± 2.69 | 49.19 ± 2.31 |
| Waist/hip | 0.95 ± 0.04 | 0.99 ± 0.03 |
| HOMA‐IR | 11.86 ± 2.94 | 7.07 ± 2.051 |
| Glucose (mmol·L−1) | 10.84 ± 1.50 | 7.73 ± 0.77 |
| Triglycerides (mmol·L−1) | 1.61 ± 0.26 | 1.90 ± 0.24 |
| Cholesterol (mmol·L−1) | 4.95 ± 0.28 | 4.89 ± 0.41 |
| SBP | 141.00 ± 5.25 | 133.00 ± 9.82 |
| DBP | 88.88 ± 2.90 | 81.50 ± 9.95 |
| Adiponectin VAT(2−ΔCt) | 3.827 ± 0.780 | 7.021 ± 1.145* |
| Adiponectin SAT (2−ΔCt) | 6.778 ± 1.436 | 7.992 ± 1.302 |
| FABP4 VAT (2−ΔCt) | 13.921 ± 1.094 | 4.532 ± 1.384* |
| FABP4 SAT (2−ΔCt) | 20.379 ± 3.047 | 11.843 ± 2.948* |
| FASN VAT (2−ΔCt) | 0.765 ± 0.174 | 0.404 ± 0.096 |
| FASN SAT (2−ΔCt) | 0.664 ± 0.094 | 0.301 ± 0.064* |
| SREBP1 VAT (2−ΔCt) | 0.221 ± 0.035 | 0.120 ± 0.015* |
| SREBP1 SAT (2−ΔCt) | 0.179 ± 0.179 | 0.122 ± 0.015* |
| Perilipin VAT (2−ΔCt) | 1.885 ± 0.532 | 2.871 ± 0.495* |
| Perilipin SAT (2−ΔCt) | 2.995 ± 0.392 | 2.577 ± 0.157 |
| HSL VAT (2−ΔCt) | 0.121 ± 0.088 | 0.218 ± 0.013 |
| HSL SAT (2−ΔCt) | 0.009 ± 0.002 | 0.008 ± 0.004 |
| ATGL VAT (2−ΔCt) | 0.812 ± 0.177 | 0.575 ± 0.142 |
| ATGL SAT (2−ΔCt) | 0.786 ± 0.106 | 0.519 ± 0.164 |
| TNF‐α VAT (2−ΔCt) | 0.014 ± 0.007 | 0.017 ± 0.006 |
| TNF‐α SAT (2−ΔCt) | 0.010 ± 0.005 | 0.008 ± 0.001 |
| IL‐6 VAT (2−ΔCt) | 0.334 ± 0.271 | 0.056 ± 0.027* |
| IL‐6 SAT (2−ΔCt) | 0.343 ± 0.199 | 0.112 ± 0.697* |
| PPARγ VAT (2−ΔCt) | 0.157 ± 0.036 | 0.111 ± 0.033 |
| PPARγ SAT (2−ΔCt) | 0.132 ± 0.028 | 0.104 ± 0.023 |
MO subjects (n = 9) with T2D being treated with metformin received a supplementary treatment of exenatide (10 μg twice a day) for 6 months, and other MO patients (n = 9) were only treated with metformin. Anthropometric parameters and gene expression of adipogenic, lipogenic, lipolytic and inflammatory markers in VAT and SAT were evaluated in both groups. RNA from patients was isolated from VAT and SAT, and then PPAR γ, FABP4, adiponectin, FASN, SREBP1, acetyl CoA carboxylase, ATGL, perilipin, HSL, GLUT4, aldolase, GADPH, TNF‐α and IL‐6 gene expression was measured by RT‐PCR. In the table, only genes that showed significant changes are presented. Signals were normalized by constitutively expressed cyclophilin using the formula 2−ΔCt. Data are the mean ± SEM. Student's t‐test was used to analyse the association between mRNA expressions. SBP, systolic blood pressure; DBP, diastolic blood pressure.
P < 0.05.
Prospective pilot study: treatment of T2D MO subjects with the GLP‐1 agonist liraglutide
Liraglutide (1.2 mg) was administered daily for 1 month to three T2D patients (BMI > 30 and HA1c > 7.5%) who were under metformin treatment. SAT biopsies were obtained using Tru‐Cut® Soft Tissue Biopsy Needles (CareFusion, Waukegan, IL), with local anaesthesia, before and 1 month after treatment.
The anthropometric and biochemical characteristics are shown in Table 3.
Table 3.
Prospective pilot study for effect of liraglutide treatment on adipogenic, lipogenic and inflammatory marker gene expression in SAT from MO subjects treated with metformin
| Liraglutide treatment | ||||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Age (in surgery, years) | 44 | 44 | 48 | 48 | 51 | 51 |
| Weight (kg) | 137.5 | 136 | 146 | 143 | 158 | 156 |
| BMI (kg·m−2) | 54.5 | 54 | 50.1 | 49.8 | 56 | 55 |
| Waist | 139 | 138 | 132 | 130 | 149 | 147 |
| HOMA‐IR | 9.5 | 9.2 | 9.6 | 9.4 | 9.1 | 9 |
| HbA1c | 8.6 | 8.3 | 7.8 | 7.1 | 8.4 | 7.8 |
| Glucose (mmol·L−1) | 8.60 | 7.66 | 7.99 | 6.66 | 8.44 | 7.77 |
| Triglycerides (mmol·L−1) | 4.59 | 3.39 | 2.58 | 2.26 | 2.14 | 1.99 |
| Cholesterol (mmol·L−1) | 5.44 | 5.23 | 4.82 | 4.66 | 4.45 | 4.66 |
| SBP | 142 | 125 | 138 | 130 | 155 | 140 |
| DBP | 88 | 84 | 80 | 78 | 98 | 92 |
| Adiponectin (2−ΔCt) | 6.037 | 3.715 | 7.853 | 3.995 | 9.229 | 9.050 |
| PPARγ (2−ΔCt) | 0.081 | 0.071 | 0.089 | 0.079 | 0.097 | 0.086 |
| FABP4 (2−ΔCt) | 20.739 | 9.756 | 20.557 | 14.367 | 20.355 | 16.299 |
| ADRP (2−ΔCt) | 1.374 | 0.441 | 1.159 | 0.432 | 0.482 | 0.446 |
| LPL (2−ΔCt) | 1.419 | 0.987 | 1.606 | 1.298 | 1.792 | 1.225 |
| Perilipin (2−ΔCt) | 7.697 | 10.570 | 8.652 | 10.362 | 25.036 | 26.021 |
| TNF‐α (2−ΔCt) | 0.012 | 0.006 | 0.009 | 0.005 | 0.005 | 0.007 |
MO subjects (n = 3) with T2D being treated with metformin received a supplementary treatment of liraglutide for 1 month. Anthropometric parameters and gene expression of adipogenic, lipogenic and inflammatory markers in SAT were evaluated before and after 1 month of liraglutide treatment. RNA from patients was isolated from SAT, and then PPAR γ, FABP4, ADRP, adiponectin, LPL, perilipin and TNF‐α gene expression was measured by RT‐PCR. Signals were normalized to constitutively expressed cyclophilin using the formula 2−ΔCt. SBP, systolic blood pressure; DBP, diastolic blood pressure.
Effect of GLP‐1 on AT explants
VAT and SAT explants (5 mg) from healthy obese subjects (BMI = 49.09 ± 2.69 kg·m−2) with a low degree of IR (HOMA‐IR < 4) were incubated for 30 min in PBS supplemented with 5% BSA (3 mL·g−1) and then in M199‐medium, 10% FBS, 100 U·mL−1 penicillin and 100 μg·mL−1 streptomycin for 1 h at 37°C. GLP‐1 100 nM and/or exendin (9–39) 100 nM were added, and tissue explants were incubated at 37°C for 1, 3 or 6 h for mRNA analysis.
Isolation and expansion of cells derived from the stromal vascular fraction of human VAT and SAT
Isolation and expansion of human cells derived from Stromal Vascular Fraction cells (SVFCs) of AT was carried out using a procedure modified from Moreno‐Navarrete et al. (2012). Briefly, AT samples were incubated in 0.150% collagenase type I (MMP1) and 1.0% BSA for 70 min at 37°C. SVFC was resuspended in DMEM/F12, 10% FBS, 100 μg·mL−1 streptomycin, 100 U·mL−1 penicillin, 2 mM l‐glutamine and 1 μg·mL−1 of amphotericin B. Cells were incubated at 37°C, in 5% CO2 for 7 days until 90% confluence was reached.
Analysis of the effect of GLP‐1 on adipocyte differentiation
SVFCs (20 000 cells cm‐2) were differentiated in adipogenic medium (expansion medium supplemented with 0.5 mM IBMX, 1.0 μM dexamethasone, 10 μM insulin and 200 μM indomethacin) in the absence or presence of 10 nM GLP‐1 for 1, 3, 6, 9, 12 and 15 days. Treatment was renewed every 2 days.
Effects of GLP‐1 and exendin (9–39) on in vitro differentiated human adipocytes
Fourteen‐day differentiated adipocytes were exposed to different doses of GLP‐1 (10, 100 and 1000 nM) in the presence or absence of the GLP‐1 receptor antagonist, exendin (9–39) 100 nM, for 12 h at 37°C. Cells were immediately processed for RNA extraction as described below.
Effect of GLP‐1 on human mature adipocytes
Mature adipocytes were obtained by enzymatic digestion of SAT as indicated above. Adipocytes were resuspended in 400 μL DMEM/F12 containing 10 mg·mL−1 BSA and then placed in an Eppendorf tube containing 600 μL DMEM/F12 supplemented with BSA. Cells were cultured for 4 h in the absence or presence of 10 or 100 nM GLP‐1 at 37°C. Cells were processed for RNA extraction as described below.
Effects of GLP‐1 on differentiated 3T3‐L1 adipocytes
3T3‐L1 cells were differentiated into adipocytes as previously described (Pulido et al., 1999) and processed for [Ca2 +]i, measured by microfluorimetry as previously described (Moreno‐Navarrete et al., 2012). Briefly, 3T3‐L1 cells were cultured in DMEM, 10% FBS, 4 mM glutamine and 1% antibiotic–antimycotic solution. At 100% confluence (day 0), cells were incubated in DMEM containing 10% FBS, 0.5 mM IBMX, 0.25 μM dexamethasone and 10 μg·mL−1 insulin for 72 h (day 3). The culture medium was replaced by DMEM with 10% FBS and 10 μg·mL−1 insulin for an additional 72 h period (day 6) and was then exchanged for DMEM without insulin until days 9–10.
In vitro experiments with 3T3‐L1 were repeated at least three times on different cell preparations, and a minimum of three replicate wells per treatment were tested in each experiment. In another set of experiments, differentiated 3T3‐L1 cells were treated with 100 nM GLP‐1 to assess the effects of the peptide on the activation of signalling intermediates (after 5 and 30 min of GLP‐1 treatment) and on the expression of several lipogenesis and lipolysis markers (after a 24 h treatment with the peptide).
RNA extraction and real‐time qPCR
RNA extraction and real‐time qPCR were carried out as previously described (Vendrell et al., 2011). Briefly, total RNA was isolated from whole AT samples using the TRIzol® RNA isolation method (Invitrogen, Carlsbad, CA, USA) and subsequently purified with the RNeasy® Lipid kit (Qiagen, Valencia, CA, USA). Total RNA from cell cultures was obtained using the RNA‐Stat 60 Reagent (Ams Biotechnology, Abingdon, UK). RT‐qPCR reactions were carried out using specific TaqMan® Gene Expression Assays (Applied Biosystems by Thermofisher Scietific, Spain). During PCR, the Ct values for each amplified product were determined using a threshold value of 0.1. The specific signals were normalized by constitutively expressed cyclophilin signals using the formula 2−ΔΔCt. References for TaqMan® probes are presented in Table S1.
Protein extraction and western blot analysis
Protein extraction from 3T3‐L1 cells was obtained as described previously (Guzmán‐Ruiz et al., 2014). Protein preparations were stored at −80°C until used.
For immunoblotting analysis, 15–30 μg of protein were loaded onto 4–20% precasted SDS‐PAGE gels and transferred onto nitrocellulose membranes. Proteins were detected using antibodies against specific proteins. Antibody references are presented in the figure legends. Anti‐ß‐actin was employed as a loading control. Optical densities of the immunoreactive bands were measured using ImageJ analysis software.
[Ca2 +]i measurements
3T3‐L1 cells were processed as previously described (Moreno‐Navarrete et al., 2012). Briefly, at day 10 of differentiation, cells were loaded with 2.5 μM Fura‐2AM (Molecular Probes, Eugene, OR, USA) and 0.02% Pluronic F127 (Molecular Probes) in phenol red‐free DMEM containing 20 mM NaHCO3 (pH 7.4) for 30 min at 37°C. Cells were then sequentially epi‐illuminated at 340 and 380 nm for 100 ms every 5 s for 8–10 min, and the fluorescent emission was captured at 505/510 nm before (basal line) and after the addition of 100 nM GLP‐1. In another series of coverslips, cells were pre‐incubated for 30 min in the presence of the GLP‐1 receptor antagonist exendin (9–39) (100 nM) and subsequently exposed to 100 nM GLP‐1. Image acquisition was controlled using MetaFluor PC software (Universal Imaging Corp., West Chester, PA, USA), and the fluorescence emission was captured using a back‐thinned CCD cooled digital camera (ORCA II BT; Hamamatsu Photonics, Hamamatsu, Japan) running in 1 bit mode. Changes in [Ca2 +]i were recorded as the ratio of the corresponding excitation wavelengths (F340/F380).
Statistical analyses
The statistical analysis was carried out with the spss software programme (version 15.0 for Windows; SPSS, Chicago, IL, USA). Statistical comparisons of the densitometric data and the differences between the different treatments used were carried out using Student's t‐test. Comparisons between normalized mRNA expression levels of different tissues were performed using the ANOVA test and Duncan's post hoc test. Levene's test was used to assess the equality and homogeneity of variances. No statistical analysis was performed on data from the prospective pilot study due to the small sample size (n = 3). Results are expressed as means ± SEM. For [Ca2 +]i measurements, unpaired t‐tests were used. Statistical significance level was set at P < 0.05. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
Effects of GLP‐1 in differentiated 3T3‐L1 cells
The effects of GLP‐1 were studied in the adipocyte murine cell model, 3T3‐L1 cells. Specifically, we measured the protein content of selected adipogenic and lipolytic markers in differentiated 3T3‐L1 adipocytes after short‐term (4 h) or long‐term (24 h) exposure to 100 nM GLP‐1. No effects were observed at 4 h of treatment (data not shown), while exposure of cells to GLP‐1 for 24 h decreased the protein levels of FABP4, SREBP1 and GAPDH, while increasing those of phosphorylated forms of perilipin and of ATGL. GLUT4 and aldolase protein levels remain unchanged after GLP‐1 treatment (Figure 1A).
Figure 1.
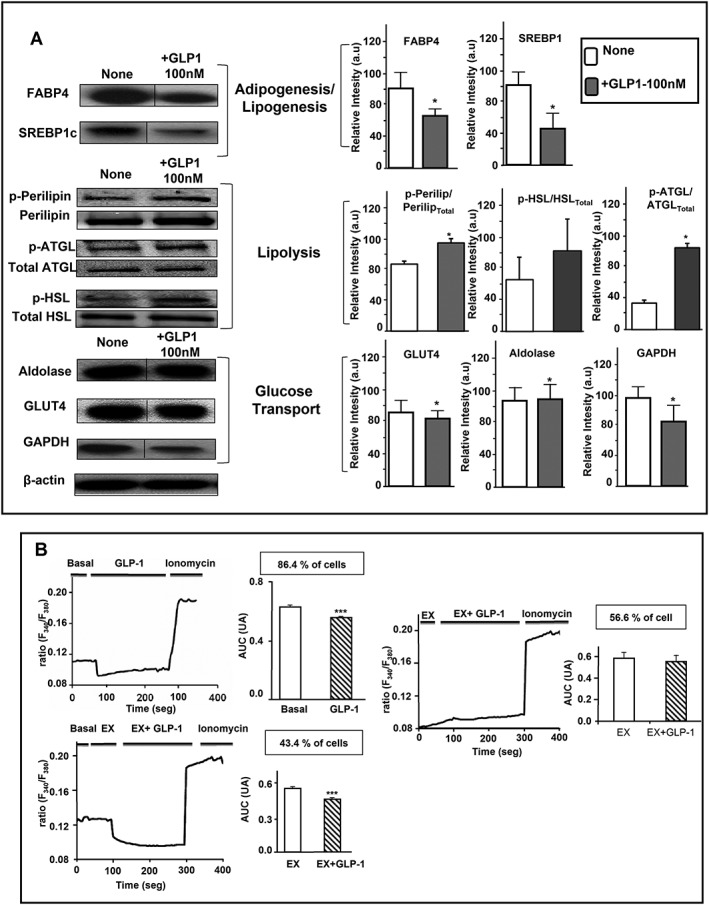
Effects of GLP‐1 on the protein expression levels of adipogenic, lipogenic and lipolytic markers and [Ca2 +]i profile in 3T3‐L1 differentiated cells. (A) 3T3‐L1 (n = 6) were incubated for 24 h in the presence or absence of GLP‐1 100 nM at 37°C. Proteins were extracted and loaded (30 μg) into 4–20% precast SDS‐PAGE gels and transferred to nitrocellulose membranes using the Trans‐Blot Turbo transfer system (Bio‐Rad, Hercules, CA, USA) for western blot analysis. The blots presented are representatives of samples comparing various treatments, which were run on the same blot with their loading controls. Also, these blots are representative of five blots that showed similar results. β‐Actin protein was used as a loading control to ensure that similar quantities of proteins were loaded in each line. Comparisons of the densitometric data were carried out using Student's t‐test. Values for relative intensity obtained after densitometry of the bands are means ± SEM. Student's t‐test was used for comparisons between GLP‐1 treatment and untreated controls. Dividing lines are combined from a single electrophoresis gel, because duplicated results were removed from the figure (lines). (B) Representative profiles of the effects of GLP‐1 (100 nM) on [Ca2 +]i in 3T3‐L1 differentiated cells in basal conditions and in those pre‐incubated with exendin (9–39) 100 nM. A Fura‐2 dual‐wavelength fluorescence imaging system was used to measure [Ca2 +]i as described in Methods. The AUC of the [Ca2 +]i responses elicited by GLP‐1 in responsive 3T3‐L1 cells is presented in arbitrary units. Data are the mean ± SEM of three different experiments for both conditions, basal and pre‐incubated with exendin (9–39) at 100 nM (Student's t‐test).
It has been shown that changes in free cytosolic Ca2 + concentration in adipocytes affect both lipogenesis and basal and stimulated lipolysis (Xue et al., 1998; Gericke et al., 2009). Therefore, we subsequently investigated the effects of GLP‐1 and exendin (9–39) on Ca2 + i dynamics in single, differentiated 3T3‐L1 cells following a protocol optimized by ourselves for this cell type (Moreno‐Navarrete et al., 2012). Exposure of 3T3‐L1 cells to 100 nM GLP‐1 induced a significant decrease in [Ca2 +]i in 86.4% of the cells recorded (45 out of 52 cells; n = 3 independent experiments) (Figure 1B). Specifically, GLP‐1 decreased [Ca2 +]i by 13.3 ± 0.6% as compared with that observed before the administration of the peptide. Maximal [Ca2 +]i inhibition was observed after 17.4 ± 1.5 s of exposure to GLP‐1. Interestingly, when cells were pre‐incubated with exendin (9–39) (100 nM) prior to GLP‐1 exposure, the peptide decreased [Ca2 +]i only in 43.4% of the cells (33 out of 76 cells; n = 3 independent experiments) (Figure 1B). In these cells, GLP‐1 evoked a 17.5 ± 1.1% decrease in [Ca2 +]i, and maximal [Ca2 +]i inhibition was reached later than in cells treated with GLP‐1 alone (Figure S1A). In all the experiments, cells in the coverslips displayed significant [Ca2 +]i increases in response to the Ca2 + ionophore ionomycin.
Finally, we also investigated the effect of GLP‐1 on Akt and ERK, intracellular signalling pathways that have been reported to be activated by GLP‐1 in adipocytes and other cell types (Challa et al., 2012; Yang et al., 2013). Neither Akt nor ERK1/2 phosphorylation rates were modified after 5 or 30 min of exposure of differentiated 3T3‐L1 cells to GLP‐1 (Figure S1B).
Effects of GLP‐1 on expression of adipogenic, lipogenic and lipolytic genes in differentiated human adipocytes in vitro
Differentiated human adipocytes from VAT and SAT were incubated in the presence of 10–1000 nM GLP‐1 for 12 h. Figure 2A shows that exposure of differentiated human adipocytes obtained from SAT samples to GLP‐1 in vitro decreased mRNA levels of a variety of genes involved in adipogenesis (PPARγ and FABP4), lipogenesis (LPL and FASN) and lipolysis (ATGL), as well as adiponectin gene expression. In the in vitro differentiated VAT adipocytes, GLP‐1 caused a significant down‐regulation of adiponectin mRNA when administered at 100 or 1000 nM, while it increased AZGP1 mRNA at the highest dose tested (Figure 2A). Other lipogenic genes (SREBP1, acetyl CoA carboxylase and VLDL‐R), lipolytic genes such as HSL and perilipin as well as genes involved in glucose transport (GLUT4) and metabolism (aldolase) did not show any significant change upon exposure of cells from SAT or VAT to GLP‐1 (Figure S2).
Figure 2.
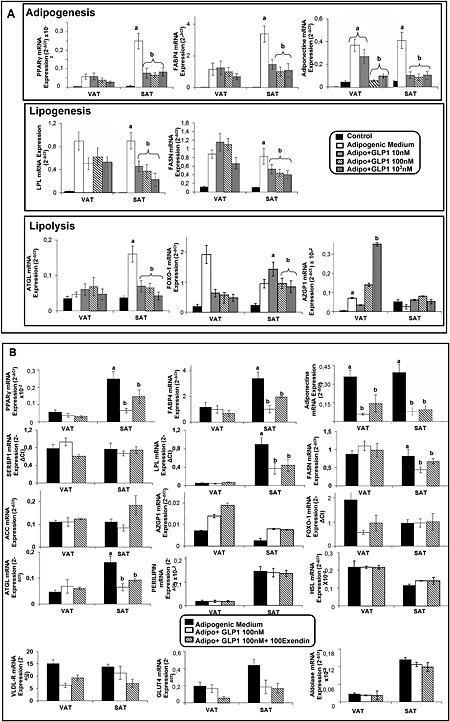
Effects of GLP‐1 and GLP‐1 receptor antagonist exendin (9–39) on GLP‐1‐induced changes in the mRNA expression levels of adipogenic, lipogenic and lipolytic markers in in vitro differentiated human adipocytes from SAT and VAT. (A) SVFCs were isolated from human VAT and SAT samples obtained from obese subjects (n = 6); 14‐day differentiated adipocytes were exposed to GLP‐1 (10, 100 and 1000 nM) for 12 h. PPARγ, FABP4, adiponectin, LPL, FASN, α‐2‐glycoprotein 1, zinc binding (AZGP1), ATGL and forkhead box protein O1 (FOXO‐1) mRNA levels were normalized to cyclophilin mRNA levels. Results were obtained in triplicate for each patient and expressed as the mean ± SEM. Bars with different letters are significantly different according to ANOVA and Duncan's post hoc test. (B) In vitro differentiated adipocytes obtained from VAT and SAT samples of obese patients (n = 6) were incubated in the presence of 100 nM GLP‐1 in combination with 100 nM exendin (9–39) for 12 h at 37°C. The gene expression levels of PPARγ, FABP4, SREBP1, adiponectin, LPL, FASN, acetyl CoA carboxylase (ACC), AZGP1, ATGL, VLDL‐R, FOXO‐1, perilipin, HSL, GLUT4 and aldolase were measured by qRT‐PCR. mRNA levels of the genes investigated were normalized to cyclophilin mRNA levels. Results were obtained in triplicate for each patient and expressed as the mean ± SEM. Levene's test showed the homogeneity of variance. There were differences between treatments according to ANOVA and Duncan's post hoc test, and bars with different letters represent a significant difference according to Student's t‐test for independent samples.
Exendin (9–39) did not reverse the effects induced by GLP‐1, in vitro, on the expression levels of adipogenic, lipogenic or lipolytic genes in differentiated adipocytes obtained from human VAT and SAT (Figure 2B). Exposure of differentiated adipocytes to exendin (9–39) alone caused no effects on the genes examined (data not shown).
Effect of GLP‐1 on expression levels of adipogenic, lipogenic and lipolytic gene markers in human mature adipocytes
Mature adipocytes obtained by enzymatic digestion of SAT were cultured in suspension and treated with GLP‐1 at 10 or 100 nM for 4 h. The mRNA expression levels of ADRP, FABP4, LPL, VLDL‐R and SCD1 were significantly lower in adipocytes exposed to either 10 or 100 nM GLP‐1 than in adipocytes cultured in medium alone (Figure 3A). No significant changes were observed in PPARγ, FASN or SREBP1 mRNA expression in the presence of GLP‐1 (data not shown). In contrast, GLP‐1 treatment increased adiponectin mRNA expression levels in adipocytes. Likewise, this peptide enhanced the mRNA levels of HSL and perilipin at both 10 and 100 nM (Figure 3A). In contrast, exposure to GLP‐1 had no effect on the expression levels of GLUT4 or the glycolytic enzymes aldolase and GAPDH in mature adipocytes (data not shown).
Figure 3.

Effects of GLP‐1 on the mRNA expression levels of adipogenic, lipogenic and lipolytic markers in human mature adipocytes and in human adipocytes during differentiation. (A) Human mature adipocytes, obtained from dispersion of subcutaneous fat (n = 4), were cultured in suspension and exposed to 10 or 100 nM GLP‐1 for 4 h. Gene expression levels of ADRP, adiponectin, PPARγ, FABP4, FASN, LPL, SREBP1, SCD1, VLDL‐R, acetyl CoA carboxylase, perilipin, HSL, ATGL, forkhead box protein O1, GLUT4, aldolase and GADPH mRNA expression levels were measured by RT‐PCR, and signals were normalized to cyclophilin gene expression using the formula 2−ΔCt. ANOVA and Duncan's post hoc test were used to analyse the association between mRNA expressions because Levene's test showed homogeneity of variance. In the figure, only genes that showed significant changes are presented. Data are the mean ± SEM. (B) Effects of GLP‐1 on ADRP, FABP4, perilipin and HSL mRNA expression during human adipocyte differentiation. mRNA expression levels were evaluated in human adipocytes differentiated in vitro from SVFC (n = 3 patients) in the presence or absence of 10 nM GLP‐1 for 1, 3, 6, 9, 12 or 15 days. mRNA levels of test genes were normalized to cyclophilin mRNA levels. Results were obtained in triplicate for each patient and expressed as the mean ± SEM. No statistical analysis was performed due to small sample size.
Effect of GLP‐1 on human adipocyte differentiation
The expression levels of ADRP, FABP4, perilipin and HSL were evaluated at 1, 3, 6, 9, 12 and 15 days of differentiation of SVFCs cultured in the presence or absence of 10 nM GLP‐1. Gene expression levels of the adipogenic markers ADRP and FABP4 were significantly down‐regulated in the presence of GLP‐1 as compared with cells exposed to differentiation medium alone. These effects were already observed at day 6 of differentiation. In contrast, the presence of GLP‐1 (10 nM) in the differentiation medium increased HSL and perilipin mRNA levels in the cell cultures, especially between days 6 and 9 of differentiation, as compared with cells that were differentiated in the absence of this peptide (Figure 3B).
Effect of GLP‐1 on expression of adipogenic, lipogenic and lipolytic genes in VAT and SAT explants from obese subjects
GLP‐1 significantly decreased the expression levels of two key markers of adipocyte differentiation, PPARγ and FABP4, as well as that of adiponectin in VAT explants compared with untreated controls, especially after 3 h of exposure to this peptide. Incubation of AT explants with GLP‐1 receptor antagonist, exendin (9–39), reversed the effect of this peptide on PPARγ and FABP4 mRNA expression levels (Table 1). GLP‐1 decreased mRNA of genes promoting lipogenesis and triglyceride accumulation in adipocytes, including SCD1, LPL and VLDL‐R, although no changes were observed in FASN expression levels (data not shown). In contrast, GLP‐1 increased the expression of several markers of lipolysis in VAT explants, including ATGL, HSL and perilipin, which are the markers responsible for TAG hydrolysis in adipocytes (Smirnova et al., 2006; Kolditz and Langin, 2010) (Table 1). In SAT explants, GLP‐1 had no significant effect on adiponectin and FABP4 expression and only reduced PPARγ transcript content after 6 h of treatment. SCD1 expression decreased at both 3 and 6 h of GLP‐1 treatment, and VLDL‐R was significantly reduced at all the times tested as compared with the corresponding controls. Similar to that observed for VAT explants, GLP‐1 also increased perilipin and HSL. No significant changes were observed in the expression levels of genes involved in glucose metabolism in either the VAT or SAT explants treated with GLP‐1 (data not shown).
Table 1.
Effect of GLP‐1 on adipogenic/lipogenic and lipolytic markers, mRNA expression in human VAT and SAT explants
| 1 h | 3 h | 6 h | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AT | Gene | Control | +GLP‐1 | +Ex(9–39) +GLP‐1 | +GLP‐1 | +Ex(9–39) +GLP‐1 | +Ex (9–39) | +GLP‐1 | +Ex(9–39) +GLP‐1 |
| SAT | AdipQ | 1 | — | — | 1.18 ± 0.21 | 1.10 ± 0.12 | 2.06 ± 1.06# | 1.02 ± 0.22 | 1.12 ± 0.13 |
| PPARγ | 1 | — | — | 0.99 ± 0.33 | 0.96 ± 0.18 | 1.75 ± 0.36# | 0.85 ± 0.009# | 1.04 ± 0.36 | |
| FABP4 | 1 | — | — | 1.10 ± 0.18 | 1.02 ± 0.047 | 1.41 ± 0.63# | 0.94 ± 0.34 | 0.99 ± 0.46 | |
| SCD1 | 1 | — | — | 0.86 ± 0.06* | 1.08 ± 0.13 | 1.01 ± 0.02 | 0.79 ± 0.005# | 0.90 ± 0.001 | |
| VLDL‐R | 1 | — | — | 0.79 ± 0.07* | 0.87 ± 0.19 | 1.00 ± 0.05 | 0.79 ± 0.05# | 0.74 ± 0.008 | |
| Perilipin | 1 | — | — | 1.76 ± 0.19* | 1.53 ± 0.23 | 1.09 ± 0.02# | 1.71 ± 0.11 | 1.41 ± 0.25 | |
| HSL | 1 | — | — | 1.69 ± 0.15* | 1.42 ± 0.28 | 1.01 ± 0.09# | 1.78 ± 0.19# | 1.45 ± 0.15 | |
| VAT | AdipQ | 1 | 0.90 ± 0.23 | 0.88 ± 0.28 | 0.77 ± 0.05* | 0.96 ± 0.10 | 1.7 ± 0.48 | 1.04 ± 0.03 | 1.10 ± 0.09 |
| PPARγ | 1 | 0.98 ± 0.29 | 0.83 ± 0.34 | 0.48 ± 0.07* | 0.90 ± 0.19 # | 0.95 ± 0.20# | 0.83 ± 0.22 | 1.43 ± 0.23 | |
| FABP4 | 1 | 1.16 ± 0.37 | 0.82 ± 0.39 | 0.65 ± 0.007* | 0.80 ± 0.006# | 1.09 ± 0.35# | 0.92 ± 0.10 | 1.07 ± 0.07# | |
| LPL | 1 | 0.67 ± 0.06* | 0.63 ± 0.24 | 0.53 ± 0.12* | 0.56 ± 0.19 | 0.97 ± 0.52# | 0.71 ± 0.08# | 0.78 ± 0.11 | |
| SCD1 | 1 | 0.64 ± 0.09* | 0.81 ± 0.19 | 0.89 ± 0.27 | 1.01 ± 0.11 | 0.93 ± 0.06 | 0.71 ± 0.07# | 0.82 ± 0.13 | |
| VLDL‐R | 1 | 0.84 ± 0.12 | 0.88 ± 0.23 | 0.73 ± 0.08* | 0.82 ± 0.16 | 0.92 ± 0.09 | 0.77 ± 0.25 | 0.81 ± 0.29 | |
| Perilipin | 1 | 1.32 ± 0.03* | 1.14 ± 0.011 | 1.56 ± 0.04* | 1.43 ± 0.22 | 1.11 ± 0.12# | 1.15 ± 0.21 | 1.01 ± 0.35 | |
| HSL | 1 | 1.48 ± 0.012* | 1.51 ± 0.09 | 1.64 ± 0.12* | 1.55 ± 0.08 | 1.06 ± 0.09# | 1.48 ± 0.09# | 1.39 ± 0.12 | |
| ATGL | 1 | 1.21 ± 0.23 | 1.33 ± 0.15 | 1.32 ± 0.11* | 1.24 ± 0.12 | 1.11 ± 0.18 | 1.19 ± 0.18 | 1.23 ± 0.23 | |
VAT and SAT explants (n = 3) were incubated with GLP‐1 (100 nM) and/or exendin fragment (9–39) (100 nM) for 1, 3 or 6 h, and then adiponectin (AdipQ), PPAR γ, FABP4, SREBP1, LPL, SCD1, FASN, VLDL‐R, perilipin, HSL, ATGL, GLUT4 and aldolase mRNA expression was measured by RT‐PCR. Only those genes that showed significant changes are presented in the Table. All mRNA expressions were compared with their respective controls, which represents a positive control of gene expression. GLP‐1 effects at different times were compared with adipogenic controls. Exendin (9–39) effects were compared with GLP‐1. Signals were normalized by constitutively expressed cyclophilin signals using the formula 2−ΔΔCt. Data are the mean ± SEM. Student's t‐test was used to analyse the association between mRNA expressions.
P < 0.05 with respect to control.
P < 0.05 with respect to GLP‐1 effect.
Effect of GLP‐1 supplementation with the GLP‐1 analogues exenatide and liraglutide in metformin‐treated MO T2D patients
T2D subjects with BMI >45 (n = 18) who were being treated with metformin received a supplemental treatment with the GLP‐1 analogue exenatide (10 μg twice a day) for 6 months and were compared with the diabetic/MO metformin‐treated subjects who did not receive any supplementary treatment. As shown in Table 2, the HOMA index and glucose levels tended to decrease albeit without reaching statistical significance in the exenatide‐treated group as compared with patients not treated with this GLP‐1 analogue. No significant differences were found in any other clinical or biochemical variables between the two groups.
qPCR analysis showed that PPARγ gene expression was numerically lower in both SAT and VAT from exenatide‐treated subjects than in non‐treated patients. Similarly, FABP4, FASN and SREBP‐1 expression levels were significantly lower in exenatide‐treated subjects compared with the control group. In contrast, adiponectin gene expression levels were higher in exenatide‐treated subjects than in controls, yet this increase was statistically significant in VAT but not in SAT. Gene expression of the inflammatory cytokine IL‐6 was also lower in the exenatide‐treated group. No changes were observed in the other genes examined, except for perilipin, which was increased in VAT of exenatide‐treated patients (Table 2).
In a prospective pilot study in which three patients were treated for 30 days with liraglutide, no clear effects of this GLP‐1 analogue were apparent on most clinical parameters, although in all three patients their weight decreased by about 2 kg. Expression levels of adipogenic and lipogenic markers in SAT, in particular, ADRP, FABP4 and LPL, showed a clear decrease, while that of perilipin tended to increase in response to liraglutide. In addition, TNF‐α was decreased in AT from liraglutide‐treated patients compared with control values (Table 3).
Discussion
In the present work, we showed that GLP‐1 reduces the expression of both adipogenic and lipogenic genes and enhances those of lipolytic markers in human AT explants. These effects were also observed when the peptide was administered to differentiated human adipocytes or to freshly isolated human mature adipocytes in vitro, thus supporting the view that GLP‐1 impairs adipogenesis and lipogenesis while increasing lipolysis in human AT. Remarkably, these effects were more pronounced in SAT than in VAT. In line with our present results, we previously found that downstream adenylate cyclase/cAMP signalling is involved GLP‐1‐stimulated lipolysis in differentiated 3T3‐L1 adipocytes (Vendrell et al., 2011). These results, together with those obtained in the present study in both human and 3T3‐L1 adipocytes, strongly support the notion that lipolysis is a major target of for the effects of GLP‐1 in this cell type. In our previous study, we demonstrated that GLP‐1 receptor expression showed a different behaviour, depending on AT depot, obesity and extent of IR. In SAT, no differences in GLP‐1 receptor expression were noted in obese subjects with a low degree of IR. In the case of MO, a substantial increase in GLP‐1 receptor expression was observed when compared with the non‐MO cohort. Indeed, functional classification of obese subjects according to IR status revealed that GLP‐1 receptors in VAT depots were markedly up‐regulated when the degree of IR was very high (Vendrell et al., 2011). Moreover, we found that GLP‐1 receptors are expressed in 3T3‐L1, and their mRNA levels were increased after GLP‐1 treatment (Figure S1A).
Notably, a negative role of GLP‐1 in human adipogenesis is also supported by our findings; on continuous exposure of human pre‐adipocytes from obese patients to the peptide during differentiation, the expression levels of several adipogenic/lipogenic factors were reduced in these cells. Other studies have shown that GLP‐1 stimulates adipogenesis in 3T3‐L1 adipocytes (Challa et al., 2012; Yang et al., 2013). GLP‐1 did not exert any significant effect either on adipogenesis or on lipolysis in adipocytes from AT of lean subjects (data not shown), which is in agreement with our previous studies showing that the GLP‐1 receptor is mainly expressed in adipocytes from obese subjects and nearly non‐existent in adipocytes from lean subjects (i.e. those employed in the present study) (Vendrell et al., 2011). Taken together, these findings suggest that the effects of GLP‐1 on adipogenesis may vary depending on the origin of the adipocytes.
It is well known that AT expansion during the development of obesity is initially characterized by fat cell hypertrophy followed by rises in fat cell number (Hausman et al., 2001), and this is involved in the increased adipogenesis and lipogenesis within AT (Kim et al., 2007; Gealekman et al., 2014). In severely obese subjects, body weight loss involves a decrease in adipocyte size and fat mass together with a parallel improvement in circulating adipokine and metabolic profiles (Varady et al., 2009). Likewise, it is well established that GLP‐1 decreases body weight and restores metabolic parameters that are impaired in obesity and T2D (Gutzwiller et al., 2004; Sancho et al., 2006). Our in vitro data suggest that GLP‐1 may reduce fat storage capacity and adiposity by inhibiting both adipocyte differentiation and lipogenesis and stimulating lipolysis in adipocytes, which, together, would contribute to body weight loss and metabolic improvement.
In line with this hypothesis, exenatide and liraglutide, two GLP‐1 analogues that have been found to ameliorate glycaemic concentration, glycosylated haemoglobin and arterial pressure and to reduce body weight in diabetic subjects (Hajer et al., 2008), showed similar effects on adipogenesis, lipogenesis and lipolysis in AT from obese subjects to those observed in vitro with GLP‐1. Specifically, the treatment of T2D MO subjects with exenatide significantly decreased the expression of adipogenic markers as well as that of enzymes involved in fatty acid biosynthesis, in parallel with a concomitant increase in adiponectin mRNA levels. We have to include the caveat that the T2D MO subjects included in our study were receiving metformin therapy. Given that metformin has been shown to regulate lipolysis in AT (Castro Cabezas et al., 2012) and to increase GLP‐1 production in response to food (Mannucci et al., 2001), we cannot exclude the possibility that this drug might have influenced the response observed in exenatide‐treated patients. Notwithstanding, our in vitro data on GLP‐1 support a targeted effect of exenatide on the different AT markers evaluated in this study. Indeed, similar to that observed in human mature adipocytes exposed to GLP‐1, administration of exenatide increased the expression of the insulin‐sensitizing adipokine, adiponectin, in AT of T2D MO subjects. When viewed together, these results suggest that exenatide‐evoked enhancement of adiponectin expression may be responsible, at least in part, to the beneficial effects of this GLP‐1 analogue on the HOMA index observed in these patients. Interestingly, two out of three patients included in the prospective study (i.e. treated with liraglutide for 30 days) showed a marked decrease in adiponectin after treatment. In general, liraglutide treatment showed similar results, although this pilot study must be viewed with caution given the low sample size. Remarkably, exenatide decreased the expression levels of TNF‐α, which is known to interact with adipogenic markers and to promote AT dysfunction (Hajer et al., 2008). These data provide a novel mechanism underlying the beneficial effects of GLP‐1 on AT and further support the crucial role of this peptide in improving lipid metabolism and endocrine function in AT in T2D patients.
We previously showed that GLP‐1 stimulates lipolysis in differentiated 3T3‐L1 in a receptor‐dependent manner that involves downstream adenylate cyclase/cAMP signalling (Vendrell et al., 2011). Recent studies have shown that both Akt and ERK1/2 are activated by GLP‐1 in 3T3‐L1 when the peptide is administered at early stages of adipogenesis, which could account for the increased adipogenic rate observed in these cells (Challa et al., 2012; Yang et al., 2013). In contrast, we observed that this peptide did not evoke the activation of these signalling intermediates in differentiated 3T3‐L1, wherein the peptide did decrease the amount of FABP4 and SREBP1. Taken together, these results suggest that the effects of GLP‐1 may differ according to the differentiation stage of the murine adipocytes. Herein, we have shown that GLP‐1 decreases [Ca2 +]i in differentiated 3T3‐L1 adipocytes. An inhibitory effect of GLP‐1 or its analogues on Ca2 + signalling has also been observed previously in other cell types (Montrose‐Rafizadeh et al., 1997). In contrast, it has been shown that increasing [Ca2 +]i stimulates lipogenesis and inhibits basal and agonist‐stimulated lipolysis in both human and murine adipocytes (Xue et al., 1998; Gericke et al., 2009). Thus, it is tempting to speculate that the down‐regulation of lipogenic markers and the up‐regulation of lipolytic factors caused by GLP‐1 treatment may be related, at least in part, to the inhibitory effect exerted by this peptide on [Ca2 +]i dynamics in adipocytes, although further measurements of [Ca2 +]i in SAT samples and human mature adipocytes are needed to confirm this hypothesis.
Notably, exendin (9–39) only partially prevented GLP‐1‐induced [Ca2 +]i decrease. However, the stimulating effects of GLP‐1 on cAMP production in 3T3‐L1 were fully abolished in the presence of exendin (9–39) (Vendrell et al., 2011). Together, these results are consistent with the presence of an additional, as yet unknown, receptor, distinct from the GLP‐1 receptor, in adipocytes, as has been previously suggested for this and other cell types (Merida et al., 1993; Márquez et al., 1998; 2001; Xie et al., 2006; Wicki et al., 2007; Connolly et al., 2012; Wang et al., 2012). Given the ‘glucagon‐like’ effect of GLP‐1, the glucagon receptor could be a potential receptor for the effects of GLP‐1 in adipocytes, although further experiments are needed to confirm this hypothesis. Identification of other putative GLP‐1 receptors deserves more work and may help in the understanding of the mechanisms by which this peptide facilitates adipocyte function.
Conclusion
In summary, our study provides new clues on the effects and mechanisms activated by GLP‐1 in AT (summarized in Figure 4), which may help elucidate how this peptide improves the metabolic profile of obese patients, thus paving the way for developing novel therapies for the treatment of obesity and T2D.
Figure 4.
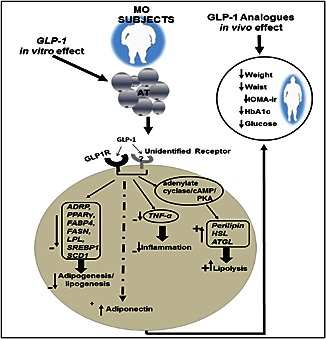
Schematic drawing outlining the main in vivo and in vitro effects evoked by GLP‐1 in AT of obese subjects. The cartoon summarizes data obtained from the analysis (mRNA and protein) of a variety of genes involved in adipogenic/lipogenic, lipolytic and inflammation processes (PPAR γ, FABP4, FASN, LPL, SREBP1, SCD1, ATGL, perilipin, HSL, ATGL, TNF‐α and adiponectin) in human AT and adipocytes in response to GLP‐1. The GLP‐1 receptor antagonist, exendin (9–39), did not fully block GLP‐1‐induced effects, which suggests that GLP‐1 might bind to another receptor different from the specific GPCR, GLP‐1 receptor. Given that HSL and perilipin are known to be primarily regulated via activation of cAMP/PKA (Kolditz and Langin, 2010) and that GLP‐1‐stimulated lipolysis occurs in a receptor‐dependent manner involving downstream adenylate cyclase/cAMP signalling (Vendrell et al., 2011), we propose that GLP‐1 would promote lipolysis by acting on HSL and perilipin via adenylate cyclase/cAMP/PKA signalling. As discussed in the text, a role for Ca2 + signalling in GLP‐1 actions on lipid metabolism in adipocytes is also proposed. All these molecular changes in AT could underlie the clinical improvement observed in morbid subjects treated with GLP‐1 analogues.
Author contributions
R.E.B., F.J.T., D.F.G. and M.M.M. designed the research; L.C.A., W.O.O., R.B.L., M.C.P., J.D.L., A.D.R., R.G.R., J.V., S.H., M.M.R. and R.V.M performed the research; R.E.B., F.J.T. and M.M.M. analysed the data and wrote the paper.
Conflict of interest
There are no conflicts of interest to be declared. F.J.T., M.M.M. and R.E. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Table S1 TaqMan probe's references from Applied Biosystems
Figure S1 Effect of GLP‐1 on GLP‐1 receptor expression, on [Ca2 +]i and on AkT/ERK phosphorylation in 3T3‐L1 differentiated preadipocytes. (A) 3T3‐L1 (n = 6) were incubated for 24 h in the presence or absence of GLP‐1 100 nM at 37°C; mRNA levels were normalized to β‐actin mRNA levels. Results were obtained in triplicate and expressed as the mean ± SEM. * P < 0.05. (B) Data are the mean ± SEM of the AUC, time for maximal response and percentage of [Ca2 +]i inhibition evoked by GLP‐1 (100 nM) alone or in combination with exendin (100 nM) (n = 3 independent experiments). ***P < 0.001 versus basal [Ca2 +]i levels and # P < 0.001 versus GLP‐1 100 nM alone. Student's t‐test. (C) 3T3‐L1 (n = 6) were incubated for 5 and 30 min in the presence or absence of GLP‐1 100 nM at 37°C. Proteins were extracted and loaded (30 μg) into SDS‐PAGE gels and transferred to nitrocellulose membranes using the Trans‐Blot Turbo transfer system (Bio‐Rad) for western blot analysis. Blots are representative of five blots that showed similar results. Total Akt and total ERK proteins were used as loading control to insure that similar quantities of proteins are loaded in each line. Values for relative intensity obtained after densitometry of the bands are means ± SEM.
Figure S2 Effect of GLP‐1 receptor antagonist exendin (9–39) on GLP‐1‐induced changes in the expression of adipogenic, lipogenic and lipolytic markers in human differentiated adipocytes. In vitro differentiated adipocytes obtained from VAT and SAT samples of obese patients (n = 6) were incubated in the presence of 100 nM GLP‐1 alone or in combination with 100 nM exendin (9–39) for 12 h at 37°C. The gene expression levels of PPARγ, FABP4, SERBP1, adiponectin, LPL, FASN, ACC, AZGP1, ATGL, VLDL‐R, FOXO‐1, perilipin, HSL, GLUT4 and aldolase were measured by qRT‐PCR. mRNA levels of test genes were normalized to cyclophilin mRNA levels. Results were obtained in triplicate for each patient and expressed as the mean ± SEM. Bars with different letters have a significant difference (P < 0.05).
Table S1 TaqMan probe's references from Applied Biosystems.
Figure S1 Effect of GLP‐1 on GLP‐1 receptor expression, on [Ca2 +]i and on AkT/ERK phosphorylation in 3T3‐L1 differentiated preadipocytes. (A) 3T3‐L1 (n = 6) were incubated for 24 h in the presence or absence of GLP‐1 100 nM at 37°C; mRNA levels were normalized to β‐actin mRNA levels. Results were obtained in triplicate and expressed as the mean ± SEM. * P < 0.05. (B) Data are the mean ± SEM of the AUC, time for maximal response and percentage of [Ca2 +]i inhibition evoked by GLP‐1 (100 nM) alone or in combination with exendin (100 nM) (n = 3 independent experiments). ***P < 0.001 versus basal [Ca2 +]i levels and # P < 0.001 versus GLP‐1 100 nM alone. Student's t‐test. (C) 3T3‐L1 (n = 6) were incubated for 5 and 30 min in the presence or absence of GLP‐1 100 nM at 37°C. Proteins were extracted and loaded (30 μg) into SDS‐PAGE gels and transferred to nitrocellulose membranes using the Trans‐Blot Turbo transfer system (Bio‐Rad) for western blot analysis. Blots are representative of five blots that showed similar results. Total Akt and total ERK proteins were used as loading control to insure that similar quantities of proteins are loaded in each line. Values for relative intensity obtained after densitometry of the bands are means ± SEM.
Figure S2 Effect of GLP‐1 receptor antagonist exendin (9–39) on GLP‐1‐induced changes in the expression of adipogenic, lipogenic and lipolytic markers in human differentiated adipocytes. In vitro differentiated adipocytes obtained from VAT and SAT samples of obese patients (n = 6) were incubated in the presence of 100 nM GLP‐1 alone or in combination with 100 nM exendin (9–39) for 12 h at 37°C. The gene expression levels of PPARγ, FABP4, SERBP1, adiponectin, LPL, FASN, ACC, AZGP1, ATGL, VLDL‐R, FOXO‐1, perilipin, HSL, GLUT4 and aldolase were measured by qRT‐PCR. mRNA levels of test genes were normalized to cyclophilin mRNA levels. Results were obtained in triplicate for each patient and expressed as the mean ± SEM. Bars with different letters have a significant difference (P < 0.05).
Supporting info item
Acknowledgements
The authors wish to thank all the subjects for their collaboration. The CIBER de Fisiopatología de la Obesidad y Nutrición (CIBEROBN) are part of a Instituto de Salud del Carlos III (ISCIII) Project. The authors thank Juan Alcaide and Alicia Céspedes Vidal (FIMABIS, Laboratorio de Investigación Biomédica, Hospital Virgen de la Victoria) for their technical support in developing our laboratory techniques and D.W.E. Ramsden (Málaga, Spain) for help with the English language version of the text. This work was supported by grants from the Spanish Ministry of Health (FIS) (PI12/02355, PS09/00997 and PI13/02628), the Consejería de Innovación and co‐funded by Fondo Europeo de Desarrollo Regional–FEDER (CTS04369, CTS‐03039, PI11‐CTS‐8181 and PI11‐CTS‐7895) and the Ministerio de Economía y Competitividad and co‐funded by Fondo Europeo de Desarrollo Regional–FEDER (BFU2010–17116). Rajaa El Bekay is recipient of a post‐doctoral grant ‘Miguel Servet II’ (CPII13/00041) from the Spanish Ministry of Health. CIBERobn is an initiative of ISCIII (Instituto de Salud Carlos III), Spain. M.C.P. was recipient of a FPU grant from the Ministry of Education (Spain) (AP2009–4537).
El Bekay, R. , Coín‐Aragüez, L. , Fernández‐García, D. , Oliva‐Olivera, W. , Bernal‐López, R. , Clemente‐Postigo, M. , Delgado‐Lista, J. , Diaz‐Ruiz, A. , Guzman‐Ruiz, R. , Vázquez‐Martínez, R. , Lhamyani, S. , Roca‐Rodríguez, M. M. , Veledo, S. F. , Vendrell, J. , Malagón, M. M. , and Tinahones, F. J. (2016) Effects of glucagon‐like peptide‐1 on the differentiation and metabolism of human adipocytes. British Journal of Pharmacology, 173: 1820–1834. doi: 10.1111/bph.13481.
References
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K, Rádiková Z, Mancino J, Toledo FG, Thomas E, Kangani C et al. (2008). Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 459–464. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ (2007). Biology of incretins: GLP‐1 and GIP. Gastroenterology 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- Castro Cabezas M, van Wijk JP, Elte JW, Klop B (2012). Effects of metformin on the regulation of free fatty acids in insulin resistance: a double‐blind, placebo‐controlled study. J Nutr Metab 2012: 394623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C (2012). Regulation of adipocyte formation by GLP‐1/GLP‐1R signaling. J Biol Chem 287: 6421–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly BM, Vanko A, McQuade P, Guenther I, Meng X, Rubins D et al. (2012). Ex vivo imaging of pancreatic beta cells using a radiolabeled GLP‐1 receptor agonist. Mol Imaging Biol 14: 79–87. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ (2006). The biology of incretin hormones. Cell Metab 3: 153–1657. [DOI] [PubMed] [Google Scholar]
- Dunning BE, Foley JE, Ahrén B (2005). (2005) α‐cell function in health and desease: influence of glucagon‐like pentide‐1. Diabetologia 48: 1700–1713. [DOI] [PubMed] [Google Scholar]
- Farmer SR (2006). Transcriptional control of adipocyte formation. Cell Metab 4: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ (1998). Glucagon‐like peptide‐1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Ye H, Serrero G (2000). Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long‐chain fatty acids. J Cell Physiol 182: 297–302. [DOI] [PubMed] [Google Scholar]
- Gealekman O, Gurav K, Chouinard M, Straubhaar J, Thompson M, Malkani S et al. (2014). Control of adipose tissue expandability in response to high fat diet by the insulin‐like growth factor binding protein‐4. J Biol Chem 289: 18327–18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke MT, Kosacka J, Koch D et al. (2009). Receptors for NPY and PACAP differ in expression and activity during adipogenesis in the murine 3T3‐L1 fibroblast cell line. Br J Pharmacol 157: 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS (1998). Understanding adipocyte differentiation. Physiol Rev 78: 783–809. [DOI] [PubMed] [Google Scholar]
- Gutzwiller P, Drewe J, Goke B (1999). Glucagon‐like peptide‐1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol Regul Integr Comp Physiol 276: R1541–R15447. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Degen L, Heuss L, Beglinger C (2004). Glucagon‐like peptide 1 (GLP‐1) and eating. Physiol Behav 82: 17–19. [DOI] [PubMed] [Google Scholar]
- Guzmán‐Ruiz R, Ortega F, Rodríguez A, Vázquez‐Martínez R, Díaz‐Ruiz A, Garcia‐Navarro S et al. (2014). Alarmin high‐mobility group B1 (HMGB1) is regulated in human adipocytes in insulin resistance and influences insulin secretion in β‐cells. Int J Obes (Lond) 38: 1545–1554. [DOI] [PubMed] [Google Scholar]
- Hajer GR, van Haeften TW, Visseren FL (2008). Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29: 2959–2971. [DOI] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ (2001). The biology of white adipocyte proliferation. Obes Rev 2: 239–254. [DOI] [PubMed] [Google Scholar]
- Hunt CR, Ro JH, Dobson DE, Min HY, Spiegelman BM (1986). Adipocyte P2 gene: developmental expression and homology of 5′‐flanking sequences among fat cell‐specific genes. Proc Natl Acad Sci U S A 83: 3786–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM et al. (2007). Obesity‐associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolditz CI, Langin D (2010). Adipose tissue lipolysis. Curr Opin Clin Nutr Metab Care 13: 377–381. [DOI] [PubMed] [Google Scholar]
- Kreyman B, Williams G, Ghatei MA, Bloom SR (1987). Glucagon‐like peptide‐1 7–36: a physiological incretin in man. Lancet 2: 1300–1303. [DOI] [PubMed] [Google Scholar]
- Large V, Peroni O, Letexier D, Ray H, Beylot M (2004). Review metabolism of lipids in human white adipocyte. Diabetes Metab 30: 294–309. [DOI] [PubMed] [Google Scholar]
- Majumdar ID, Weber HC (2010). Gastrointestinal regulatory peptides and their effects on fat tissue. Curr Opin Endocrinol Diabetes Obes 17: 51–56. [DOI] [PubMed] [Google Scholar]
- Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E et al. (2001). Effect of metformin on glucagon‐like peptide 1 (GLP‐1) and leptin levels in obese nondiabetic subjects. Diabetes Care 24: 489–494. [DOI] [PubMed] [Google Scholar]
- Márquez L, Trapote MA, Luque MA, Alcántara AI, Valverde I, Villanueva‐Peñacarrillo ML (1998). Inositolphosphoglycans possibly mediate the GLP‐1 effects on rat liver and adipose tissue. Cell Biochem and Function 16: 51–56. [DOI] [PubMed] [Google Scholar]
- Márquez L, González N, Puente J, Valverde L, Villanueva‐Peñacarrillo ML (2001). GLP‐1 effect upon the GPI/IPG system in adipocytes and hepatocytes from diabetic rats. Diabetes Nutr Metab 14: 239–244. [PubMed] [Google Scholar]
- McIntosh CH, Widenmaier S, Kim SJ (2009). Glucose‐dependent insulinotropic polypeptide (gastric inhibitory polypeptide; GIP). Vitam Horm 80: 409–471. [DOI] [PubMed] [Google Scholar]
- Mérida E, Delgado E, Molina LM, Villanueva‐Peñacarrillo ML, Valverde I (1993). Presence of glucagon and glucagon‐like peptide‐1(7–36) amide receptors in solubilized membranes of human adipose tissue. J Clin Endocrinol Metab 77: 1654–1657. [DOI] [PubMed] [Google Scholar]
- Montrose‐Rafizadeh C, Yang H, Wang Y, Roth J, Montrose MH, Adams LG (1997). Novel signal transduction and peptide specificity of glucagon‐like peptide receptor in 3T3‐L1 adipocytes. J Cell Physiol 172: 275–283. [DOI] [PubMed] [Google Scholar]
- Moreno‐Navarrete JM et al. (2012). The l‐α‐lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes 61: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Schreiber E, Mojsov S, Habener JF (1992). Insulinotropic action of glucagon like peptide‐I‐(7‐37) in diabetic and nondiabetic subjects. Diabetes Care 15: 270–276. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Holst JJ, Willms B, Schmiegel W (1997). Glucagon‐like peptide 1 (GLP‐1) as a new therapeutic approach for type 2‐diabetes. Exp Clin Endocrinol Diabetes 105: 187–195. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. , NC‐IUPHAR(2014). The IUPHAR/BPS guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42: D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea A, Viñambres C, Clemente F, Villanueva‐Peñacarrillo ML, Valverde I (1997). GLP‐1(7–36) amide effects on glucose transport and metabolism in rat adipose tissue. Horm Metab Res 9: 417–421. [DOI] [PubMed] [Google Scholar]
- Pulido MR, Rabanal‐Ruiz Y, Almabouada F, Díaz‐Ruiz A, Burrell MA, Vázquez MJ et al. (1999). PPARγ is required for the differentiation of adipose tissue in vivo and in‐vitro. Mol Cell 4: 611–617. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS et al. (1999). PPARγ is required for the differentiation of adipose tissue in vivo and in‐vitro. Mol Cell 4: 611–617. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Grande C, Alarcón C, Mérida E, Valverde I (1992). Lipolytic action of glucagon‐like peptides in isolated rat adipocytes. Peptides 13: 13–16. [DOI] [PubMed] [Google Scholar]
- Russell ST, Zimmerman TP, Domin BA, Tisdale MJ (2004). Induction of lipolysis in vitro and loss of body fat in vivo by zinc‐alpha2‐glycoprotein. Biochim Biophys Acta 1636: 59–68. [DOI] [PubMed] [Google Scholar]
- Sancho V, Trigo MV, Martín‐Duce A, Gonz Lez N, Acitores A, Arnés L et al. (2006). Effects of GLP‐1 on d‐glucose transport, lipolysis and lipogenesis in adipocytes of obese subjects. Int J Mol Med 17: 1133–1137. [PubMed] [Google Scholar]
- Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL (2006). ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 7: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Tussing L, Bhutani S, Braunschweig CL (2009). Degree of weight loss required to improve adipokine concentrations and decrease fat cell size in severely obese women. Metabolism 58: 1096–1101. [DOI] [PubMed] [Google Scholar]
- Vendrell J, El Bekay R, Peral B, García‐Fuentes E, Megia A, Macias‐Gonzalez M et al. (2011). Study of the potential association of adipose tissue GLP‐1 receptor with obesity and insulin resistance. Endocrinology 152: 4072–4079. [DOI] [PubMed] [Google Scholar]
- Villanueva‐Peñacarrillo ML, Márquez L, González N, Díaz‐Miguel M, Valverde I (2001a). Effect of GLP‐1 on lipid metabolism in human adipocytes. Horm Metab Res 33: 73–77. [DOI] [PubMed] [Google Scholar]
- Villanueva‐Peñacarrillo ML, Puente J, Redondo A, Clemente F, Valverde I (2001b). Effect of GLP‐1 treatment on GLUT2 and GLUT4 expression in type 1 and type 2 rat diabetic models. Endocrine 15: 241–248. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lim K, Normandin M, Zhao X, Cline GW, Ding YS (2012). Synthesis and evaluation of [(18)F] exendin (9‐39) as a potential biomarker to measure pancreatic beta‐cell mass. Nucl Med Biol 39: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicki A, Wild D, Storch D, Seemayer C, Gotthardt M, Behe M et al. (2007). [Lys40(Ahx‐DTPA‐111In)NH2]‐exendin‐4 is a highly efficient radiotherapeutic for glucagon‐like peptide‐1 receptor‐targeted therapy for insulinoma. Clin Cancer Res 13: 3696–3705. [DOI] [PubMed] [Google Scholar]
- Xie Y, Kang X, Ackerman WE 4th, Belury MA, Koster C, Rovin BH et al. (2006). Differentiation‐dependent regulation of the cyclooxygenase cascade during adipogenesis suggests a complex role for prostaglandins. Diabetes Obes Metab 8: 83–93. [DOI] [PubMed] [Google Scholar]
- Xue BZ, Moustaid N, Wilkison WO, Zemel MB (1998). The agouti gene product inhibits lipolysis in human adipocytes via a Ca2 +‐dependent mechanism. FASEB J 12: 1391–1396. [PubMed] [Google Scholar]
- Yang J, Ren J, Song J, Liu F, Wu C, Wang X et al. (2013). Glucagon‐like peptide 1 regulates adipogenesis in 3T3‐L1 preadipocytes. Int J Mol Med 31: 1429–1435. [DOI] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H et al. (2006). GLP‐1 activation improves β‐cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4: 391–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 TaqMan probe's references from Applied Biosystems
Figure S1 Effect of GLP‐1 on GLP‐1 receptor expression, on [Ca2 +]i and on AkT/ERK phosphorylation in 3T3‐L1 differentiated preadipocytes. (A) 3T3‐L1 (n = 6) were incubated for 24 h in the presence or absence of GLP‐1 100 nM at 37°C; mRNA levels were normalized to β‐actin mRNA levels. Results were obtained in triplicate and expressed as the mean ± SEM. * P < 0.05. (B) Data are the mean ± SEM of the AUC, time for maximal response and percentage of [Ca2 +]i inhibition evoked by GLP‐1 (100 nM) alone or in combination with exendin (100 nM) (n = 3 independent experiments). ***P < 0.001 versus basal [Ca2 +]i levels and # P < 0.001 versus GLP‐1 100 nM alone. Student's t‐test. (C) 3T3‐L1 (n = 6) were incubated for 5 and 30 min in the presence or absence of GLP‐1 100 nM at 37°C. Proteins were extracted and loaded (30 μg) into SDS‐PAGE gels and transferred to nitrocellulose membranes using the Trans‐Blot Turbo transfer system (Bio‐Rad) for western blot analysis. Blots are representative of five blots that showed similar results. Total Akt and total ERK proteins were used as loading control to insure that similar quantities of proteins are loaded in each line. Values for relative intensity obtained after densitometry of the bands are means ± SEM.
Figure S2 Effect of GLP‐1 receptor antagonist exendin (9–39) on GLP‐1‐induced changes in the expression of adipogenic, lipogenic and lipolytic markers in human differentiated adipocytes. In vitro differentiated adipocytes obtained from VAT and SAT samples of obese patients (n = 6) were incubated in the presence of 100 nM GLP‐1 alone or in combination with 100 nM exendin (9–39) for 12 h at 37°C. The gene expression levels of PPARγ, FABP4, SERBP1, adiponectin, LPL, FASN, ACC, AZGP1, ATGL, VLDL‐R, FOXO‐1, perilipin, HSL, GLUT4 and aldolase were measured by qRT‐PCR. mRNA levels of test genes were normalized to cyclophilin mRNA levels. Results were obtained in triplicate for each patient and expressed as the mean ± SEM. Bars with different letters have a significant difference (P < 0.05).
Table S1 TaqMan probe's references from Applied Biosystems.
Figure S1 Effect of GLP‐1 on GLP‐1 receptor expression, on [Ca2 +]i and on AkT/ERK phosphorylation in 3T3‐L1 differentiated preadipocytes. (A) 3T3‐L1 (n = 6) were incubated for 24 h in the presence or absence of GLP‐1 100 nM at 37°C; mRNA levels were normalized to β‐actin mRNA levels. Results were obtained in triplicate and expressed as the mean ± SEM. * P < 0.05. (B) Data are the mean ± SEM of the AUC, time for maximal response and percentage of [Ca2 +]i inhibition evoked by GLP‐1 (100 nM) alone or in combination with exendin (100 nM) (n = 3 independent experiments). ***P < 0.001 versus basal [Ca2 +]i levels and # P < 0.001 versus GLP‐1 100 nM alone. Student's t‐test. (C) 3T3‐L1 (n = 6) were incubated for 5 and 30 min in the presence or absence of GLP‐1 100 nM at 37°C. Proteins were extracted and loaded (30 μg) into SDS‐PAGE gels and transferred to nitrocellulose membranes using the Trans‐Blot Turbo transfer system (Bio‐Rad) for western blot analysis. Blots are representative of five blots that showed similar results. Total Akt and total ERK proteins were used as loading control to insure that similar quantities of proteins are loaded in each line. Values for relative intensity obtained after densitometry of the bands are means ± SEM.
Figure S2 Effect of GLP‐1 receptor antagonist exendin (9–39) on GLP‐1‐induced changes in the expression of adipogenic, lipogenic and lipolytic markers in human differentiated adipocytes. In vitro differentiated adipocytes obtained from VAT and SAT samples of obese patients (n = 6) were incubated in the presence of 100 nM GLP‐1 alone or in combination with 100 nM exendin (9–39) for 12 h at 37°C. The gene expression levels of PPARγ, FABP4, SERBP1, adiponectin, LPL, FASN, ACC, AZGP1, ATGL, VLDL‐R, FOXO‐1, perilipin, HSL, GLUT4 and aldolase were measured by qRT‐PCR. mRNA levels of test genes were normalized to cyclophilin mRNA levels. Results were obtained in triplicate for each patient and expressed as the mean ± SEM. Bars with different letters have a significant difference (P < 0.05).
Supporting info item


