Abstract
Diarrhea is a leading cause of death among young mammals, especially during weaning. Here, we investigated the effects of Cathelicidin-WA (CWA) on diarrhea, intestinal morphology, inflammatory responses, epithelial barrier and microbiota in the intestine of young mammals during weaning. Piglets with clinical diarrhea were selected and treated with saline (control), CWA or enrofloxacin (Enro) for 4 days. Both CWA and Enro effectively attenuated diarrhea. Compared with the control, CWA decreased IL-6, IL-8 and IL-22 levels and reduced neutrophil infiltration into the jejunum. CWA inhibited inflammation by down-regulating the TLR4-, MyD88- and NF-κB-dependent pathways. Additionally, CWA improved intestinal morphology by increasing villus and microvillus heights and enhancing intestinal barrier function by increasing tight junction (TJ) protein expression and augmenting wound-healing ability in intestinal epithelial cells. CWA also improved microbiota composition and increased short-chain fatty acid (SCFA) levels in feces. By contrast, Enro not only disrupted the intestinal barrier but also negatively affected microbiota composition and SCFA levels in the intestine. In conclusion, CWA effectively attenuated inflammation, enhanced intestinal barrier function, and improved microbiota composition in the intestines of weaned piglets. These results suggest that CWA could be an effective and safe therapy for diarrhea or other intestinal diseases in young mammals.
Diarrhea is a leading cause of death among children under 5 years of age, and causes death in more than one in ten children (approximately 800,000) per year, mainly in developing countries (WHO, 2015). Diarrhea typically occurs during the weaning period, which is a critical and stressful stage for young mammals1,2. Weaning causes reductions in body weight gain, decreases in nutrient absorption, disruptions to immune homeostasis and damage to barrier function3,4,5,6, resulting in susceptibility to pathogen infection and diarrhea7. Antibiotics have been effectively used to treat diarrhea in children and other animal neonates after weaning in past decades. Unfortunately, the widespread use of antibiotics has increased bacterial resistance, leading to delayed administration of effective therapy as well as morbidity and mortality in both humans and animals8,9. As reported, antimicrobial-resistant infections cause more than 700,000 deaths each year globally: at least 23,000 in the USA and 175,000 in the EU10,11. Furthermore, the development of new antibiotics has slowed down, and few antibiotics have been approved by the FDA in recent years10. Moreover, recent studies have reported that therapeutic or sub-therapeutic antibiotic treatments in early life have long-term consequences on intestinal microbiota composition and metabolic homeostasis in the host12,13. All this makes it clear that it is urgent to search effective and safe antimicrobial agents.
Antimicrobial peptides (AMPs) are short cationic molecules that serve as a host defense against microbial infection14,15. It is not easy for bacteria to develop resistance to AMPs because they work through a membrane-disrupting mechanism; therefore, they are considered a promising alternative to traditional antibiotics16. Cathelicidin peptides, such as human LL-37 and mouse CRAMP, exhibit antibacterial, antifungal and antiviral functions17. These peptides not only kill microbes directly but also modulate the immune system of the host9,15. LL-37 was shown to enhance the defenses of rats against pathogen infection, and CRAMP was found to ameliorate colonic colitis in dextran sulfate sodium (DSS)-induced mice18. Cathelicidin-WA (CWA), an AMP derived from the endemic genera Bungarus fascia, is considered a potent alternative to antibiotics19,20. Previous studies have shown that CWA had strong antimicrobial activities against ciprofloxacin-resistant pathogens and enteric pathogens isolated from the feces of piglets with diarrhea21,22. Our studies have revealed that CWA is highly unstable in gastrointestinal tract, but remains substantially intact in serum; the in vivo imaging results showed that intraperitoneal injection with CWA could be absorbed into the systemic circulation and availability to the intestine. Furthermore, CWA showed immunoregulatory capabilities in vivo and in vitro17.
The objective of this study was to investigate whether the CWA could be an effective therapy for diarrhea during weaning and to explore the immunoregulatory and epithelial barrier protective properties of CWA in the intestine of weaned piglets.
Materials and Methods
Peptide synthesis
CWA was synthesized by GL Biochem (Shanghai, China) as previously described23. Peptides were purified at greater than 95% purity via semi-preparative HPLC and characterized by analytical HPLC (Agilent 121 Technologies, CA, USA). The molecular weight of CWA was confirmed using a Thermo Finnigan LCQ ion trap mass spectrometer (Thermo Finnigan, CA, USA). CWA was prepared in saline before injection.
Animals and sample collection
All experiments were approved by the Animal Care Committee of Zhejiang University and were conducted in accordance with the Guidelines for the Care and Use of Agricultural Animals for Research and Teaching at Zhejiang University. A total of 108 piglets with clinical diarrhea were selected from 1260 piglets (Duroc × Landrace × Yorkshire) at 3 d after weaning (25 ± 2 d). The piglets were randomly assigned to 1 of 3 treatments based on body weight, gender and diarrheal index. In total, there were 6 pens for each treatment, with 6 piglets per pen. The piglets were treated by intraperitoneal injection with normal saline (control), 0.6 mg/kg CWA (the dosage was based on results from LPS-induced mice and pre-experiment in diarrheal piglets), or 2.5 mg/kg enrofloxacin (Enro) once a day for 4 d. All piglets were provided with access to the diet (NRC1998) and water ad libitum. The diarrheal index was scored according to a fecal consistency scoring system (0, normal; 1, soft feces; 2, mild diarrhea; and 3, severe diarrhea) as described before24. A fecal score of 2 or 3 was considered clinical diarrhea. Fresh feces from each pen was collected and stored immediately at −20 °C. One piglet from each pen was randomly euthanized (n = 6 per treatment). Blood samples were collected from the anterior vena cava into coagulation accelerator tubes. Serum was obtained after centrifugation at 3000× g for 10 min at 4 °C. Samples of the middle duodenum, middle jejunum, distal ileum and middle colon were collected for analysis.
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-6, IL-8 and IL-22 in serum and the jejunum were determined using ELISA kits (Raybiotech, GA, USA). In addition, the level of D-Lactate (D-Lac) in serum was detected using an ELISA kit (R&D, USA). Samples were measured according to the manufacturers’ instructions.
Analysis of intestinal morphology
Intestinal tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections of 5-μm thickness were stained with hematoxylin and eosin (H&E). Images were acquired using a DM3000 microscope (Leica, Wetzlar, Germany). Villous height and crypt depth were measured using Image-Pro software (MediaCybernetics, MD, USA) as previously described25. Histopathologic damage scores were determined under blinded conditions using a histologic injury scale (0, normal mucosal villi; 1, subepithelial Gruenha-gen’s space; 2, extension of subepithelial space with moderate lifting of epithelial layer from the lamina propria; 3, massive epithelial lifting down the sides of villi; 4, denuded villi with lamina propria and dilated capillaries exposed; and 5, digestion and disintegration of lamina propria) as previously described23.
Scanning electron microscopy (SEM)
Jejunum tissue was fixed with 2.5% glutaraldehyde overnight and then with 1% OsO4 for 1 h. The jejunum specimens were then dehydrated in a graded series of ethanol (30%, 50%, 70%, 80%, 90%, 95% and 100%) for 20 min at each step and then transferred into a mixture of alcohol and iso-amyl acetate (v:v = 1:1) for 30 min and iso-amyl acetate for 1 h. The specimens were then dehydrated in a Hitachi Model HCP-2 critical point dryer with liquid CO2. The dehydrated specimens were coated with gold-palladium and visualized using a Philips Model SU8010 FASEM (HITACHI, Japan).
Transmission electron microscopy (TEM)
Microvilli and epithelial cell junctions were observed by TEM. Jejunum tissue was fixed, dehydrated, and transferred into pure acetone for 20 min. The specimens were placed in a mixture of pure acetone and Spurr resin mixture (1:1 for 1 h and 1:3 for 3 h) and then transferred into Spurr resin mixture overnight. The tissue sections were placed in capsules containing embedding medium and heated at 70 °C for 9 h. The sections were then stained with uranyl acetate and alkaline lead citrate for 15 min and visualized via TEM (Model H-7650, HITACHI, Japan).
Immunohistochemistry
Briefly, sections of jejunum were deparaffinized and rehydrated. The sections were submitted to antigen retrieval using EDTA buffer (pH = 9.0) and a microwave and then incubated in 3% hydrogen dioxide in the dark for 20 min. Following this, the sections were incubated with a primary antibody (1:200 dilution) against myeloperoxidase (MPO; Google Biotechnology Inc., Wuhan, China) to detect neutrophil infiltration. Sections were then incubated with a secondary antibody (1:200 dilution) and treated with DAB substrate. Nuclei were stained with Harris hematoxylin. Images were obtained using a DM3000 microscope.
Real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, USA). RNA quantity and quality were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, MA, USA). cDNA was obtained using 2 μg RNA. Real-time PCR was performed using a StepOne PlusTM system (Applied Biosystems, CA, USA). The primers used for real-time PCR are listed in Table 1. Each reaction included 5 μL FastStart Universal SYBR Green Master Mix (Roche, Switzerland), 0.5 μL forward primer (10 μM), 0.5 μL reverse primer (10 μM) and 4 μL 10-fold diluted cDNA. The thermocycler protocol consisted of 10 min at 95 °C and 40 cycles of 10 s at 95 °C and 35 s at 60 °C, and melt curves were added. GAPDH and 18 S were used as housekeeping genes. mRNA relative expression was calculated using the 2−ΔΔCt method.
Table 1. Primers for real-time PCR in this study.
| Gene | Sequence (5′−3′) | Size(bp) | Accession number |
|---|---|---|---|
| TLR4 | Forward: GCCATCGCTGCTAACATCATCReverse: CTCATACTCAAAGATACACCATCGG | 108 | NM_001113039.1 |
| MyD88 | Forward: TGGTAGTGGTTGTCTCTGATGAReverse: TGGAGAGAGGCTGAGTGCAA | 80 | NM_001099923.1 |
| Mucin-1 | Forward: ACACCCATGGGCGCTATGTReverse: GCCTGCAGAAACCTGCTCAT | 68 | NM_001204296.1 |
| Mucin-2 | Forward: CTGCTCCGGGTCCTGTGGGAReverse: CCCGCTGGCTGGTGCGATAC | 100 | XM_007465997.1 |
| pBD2 | Forward: CCAGAGGTCCGACCACTACAReverse: GGTCCCTTCAATCCTGTTGAA | 88 | AY506573.1 |
| 18 S | Forward: CCCACGGAATCGAGAAAGAGReverse: TTGACGGAAGGGCACCA | 122 | AY265350.1 |
| GAPDH | Forward: ACTCACTCTTCCACTTTTGATGCTReverse: TGTTGCTGTAGCCAAATTCA | 100 | NM_001206359 |
Western blotting
Total protein was extracted with lysis buffer (KeyGEN, Nanjing, China). Protein supernatant was separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking with 5% skimmed milk powder, the membrane was incubated with the appropriate primary antibodies overnight at 4 °C, followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. Bands were detected using ECL (CliNX, Shanghai, China). Band intensity was quantified using ImageJ software. Primary antibodies for β-actin (Abcam, MA, USA), MyD88, phosphorylated NF-κB (Santa Cruz, CA, USA), phosphorylated IκB-α, phosphorylated AKT (Epitomics, USA), TLR4, zonula occluden-1 (ZO-1), occludin, and claudin-1 (Abcam) were used in this study.
Microbial composition analysis
Genomic DNA was extracted from feces using a Fecal DNA Kit (SimGEN, Hangzhou, China). The DNA was quantified using a NanoDrop 2000 spectrophotometer. Quantitative measurements of total bacteria (forward: CGGTGAATACGTTCYCGG; reverse: GGWTACCTTGTTACGACTT), Escherichia coli (forward: CATGCCGCGTGTATGAAGAA; reverse: CGGGTAACGTCAATGA GCAAA), and Lactobacillus (forward: CGATGAGTGCTAGGTGTTGGA; reverse: CAAGATGTCAAGACCTGGTAAG) were performed by real-time PCR using a StepOne PlusTM System as previously described25.
Gas chromatographic analysis
Concentrations of short-chain fatty acids (SCFAs) in feces were determined via gas chromatography (GC-8 A, Shimadzu Corp., Kyoto, Japan). Briefly, 1 g of feces was mixed with 5 mL ddH2O and then centrifuged for 15 min at 10,000× g and 4 °C. Then, 1 mL of supernatant was mixed with 20 μL orthophosphoric acid (85%) for 1 h at 4 °C and centrifuged for 15 min at 12,000× g and 4 °C. The supernatant was transferred into a gas chromatography vial, and 2 μL of supernatant was injected into a 2-m × 3-mm glass column packed with Porapak Q (80 mesh; Agilent Technologies Inc., Santa Clara, CA, USA) as previously described26. SCFA concentrations were normalized to feces weight as μmol/g.
Cell culture
Cells were cultured in RPMI-1640 or DMEM-F12 medium (Invitrogen) supplemented with 10% fetal bovine serum (Gibco) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin sulfate) at 37 °C with 5% CO2 in a humidified incubator before treatment.
Porcine macrophage cells (3D4/2) were incubated for 6 h either with medium only or with 1 μg/mL lipopolysaccharide (LPS, Escherichia coli strain O111:B4, Sigma-Aldrich) after pretreatment with Enro (5, 10, 20, or 40 μg/mL) or CWA (5, 10, 20, or 40 μg/mL) for 12 h. Small-interfering RNA (siRNA) molecules targeting pig TLR4 or MyD88 were designed by Yingrun Biotechnology Co., Ltd. (Changsha, China). The 3D4/2 cells were transfected with 1 μg/mL siRNA using Lipofectamine-2000 (Invitrogen) for 6 h. Then, the transfected cells were incubated with medium only, LPS (1 μg/mL for 6 h), or CWA + LPS (pretreated with 20 μg/mL CWA for 12 h and then 1 μg/mL LPS for 6 h). Culture supernatant was collected for ELISA.
Porcine jejunal epithelial cells (IPEC-J2) were cultured in transwell dishes for at least 21 d until their transepithelial electrical resistance (TER) was stable. Monolayers of IPEC-J2 cells were incubated for 12 h with serum-free medium, LPS (1 μg/mL), or CWA + LPS (pretreated with 20 μg/mL CWA for 12 h and then 1 μg/mL LPS added) in the upper layer. TER was measured using a Millicell ERS-2 epithelial volt-ohm meter (Millipore MERS00002, USA). Cells were collected for Western blotting.
Caco-2 cells were cultured in 35-mm dishes. Caco-2 monolayers were scraped with 200-μL pipette tips as previously described27. The scraped Caco-2 cells were incubated with medium only or 20 μg/mL CWA. Images were obtained at 0 h, 48 h and 96 h. Wound width was measured at 48 h.
Statistical analysis
Statistical analysis was performed using one-way ANOVA or Student’s t-test with SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant at P < 0.05.
Result
CWA effectively attenuated diarrhea and systematic inflammation in weaned piglets
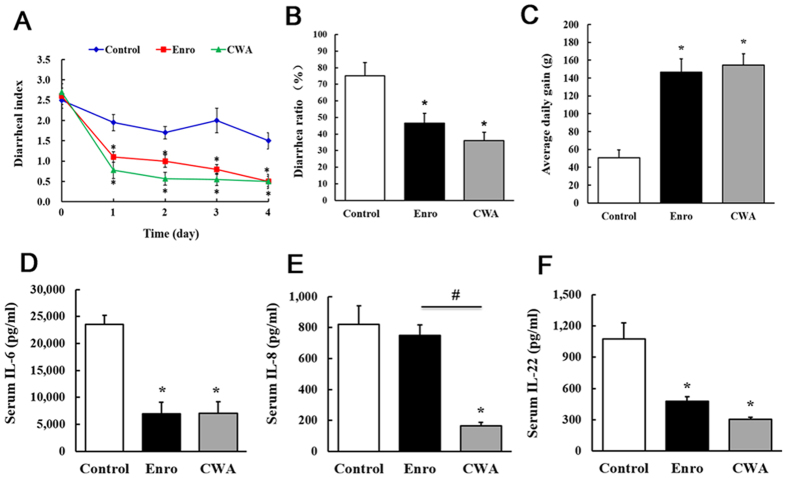
Both CWA and Enro effectively decreased the diarrheal index and the diarrheal ratio and increased body weight gain compared with the control (Fig. 1A–C). Furthermore, CWA and Enro showed no significant differences in the diarrheal index, the diarrhea ratio, or body weight gain. Compared with the control, CWA treatment reduced levels of the pro-inflammatory cytokines IL-6, IL-8 and IL-22 in serum, whereas Enro treatment reduced levels of IL-6 and IL-22 (Fig. 1D–F). These data indicate that the CWA may be an effective alternative to antibiotics for the treatment of diarrhea in weaned piglets.
Figure 1. Cathelicidin-WA attenuated diarrhea and reduced serum pro-inflammatory cytokine levels in weaned piglets.
(A) The diarrheal index was measured using fecal consistency scoring (0, normal; 1, soft feces; 2, mild diarrhea; and 3, severe diarrhea). (B) A fecal score of either 2 or 3 was considered to represent clinical diarrhea, and the diarrhea ratio was calculated. (C) Average daily gain. Serum levels of the pro-inflammatory cytokines IL-6 (D), IL-8 (E), and IL-22 (F) were determined by ELISA. All data are expressed as the mean ± SEM (n = 6). Differences were determined by one-way ANOVA. Enro, Enrofloxacin; CWA, Cathelicidin-WA. *P < 0.05 compared with control; #P < 0.05 CWA compared with Enro.
CWA improved intestinal morphology and integrity in weaned piglets
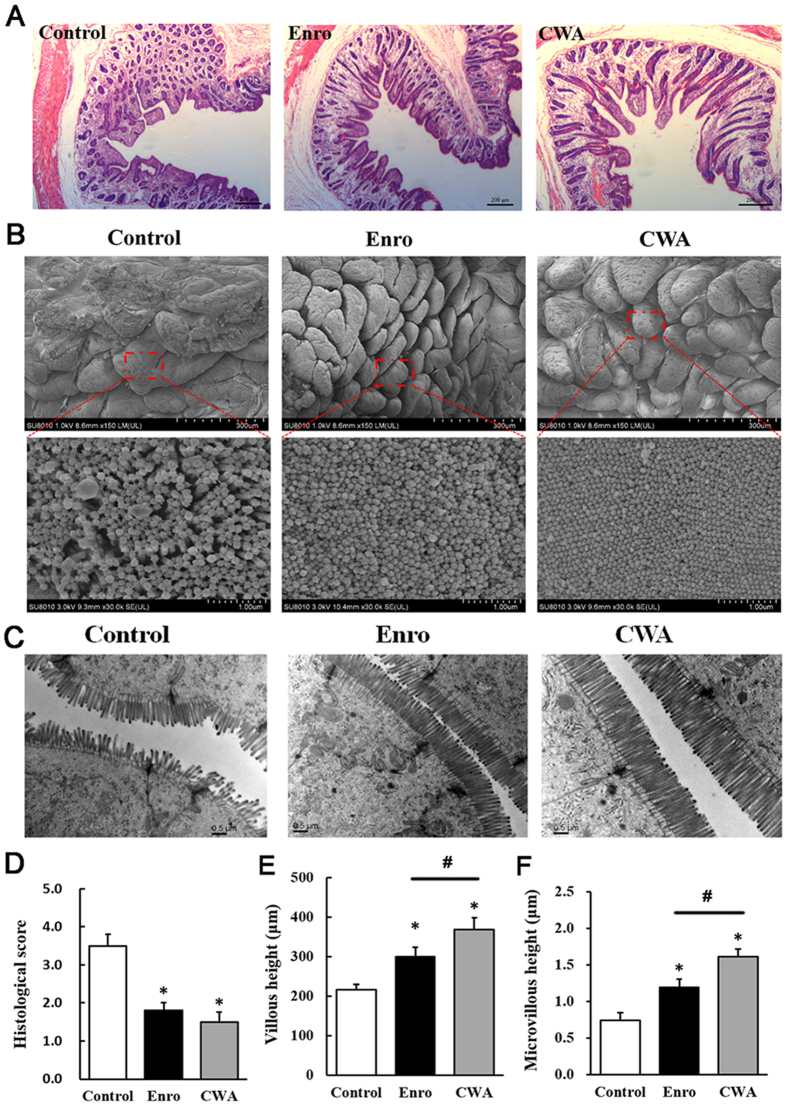
CWA treatment increased villous height in the jejunum compared with control and Enro treatments (Fig. 2A,E); this result was confirmed via SEM at 150× magnification (Fig. 2B). In addition, surface damage to villi in the jejunum was alleviated by CWA and Enro treatments (Fig. 2B, upper). Interestingly, both CWA and Enro increased the quantity of microvilli in the jejunum (Fig. 2B,C). Furthermore, CWA increased the height of microvilli in the jejunum compared with control and Enro (Fig. 2C,F). These results suggest that CWA effectively improved intestinal morphology and integrity in weaned piglets with clinical diarrhea.
Figure 2. Cathelicidin-WA improved intestinal morphology.
Jejunum sections were used to analyze intestinal morphology. (A) Stained with H&E (bars, 200 μm). (B) SEM images (upper, 150×; lower, 30,000×). (C) TEM images (bars, 0.5 μm). (D) Histological scores were determined as described in the Materials and Methods. (E) Villous height in the jejunum. (F) Microvillus height was measured using TEM. All data are expressed as the mean ± SEM (n = 6). Differences were determined by one-way ANOVA. Enro, Enrofloxacin; CWA, Cathelicidin-WA. *P < 0.05 compared with control; #P < 0.05 CWA compared with Enro.
CWA suppressed intestinal inflammation via TLR4-, MyD88-, and NF-κB-dependent pathways
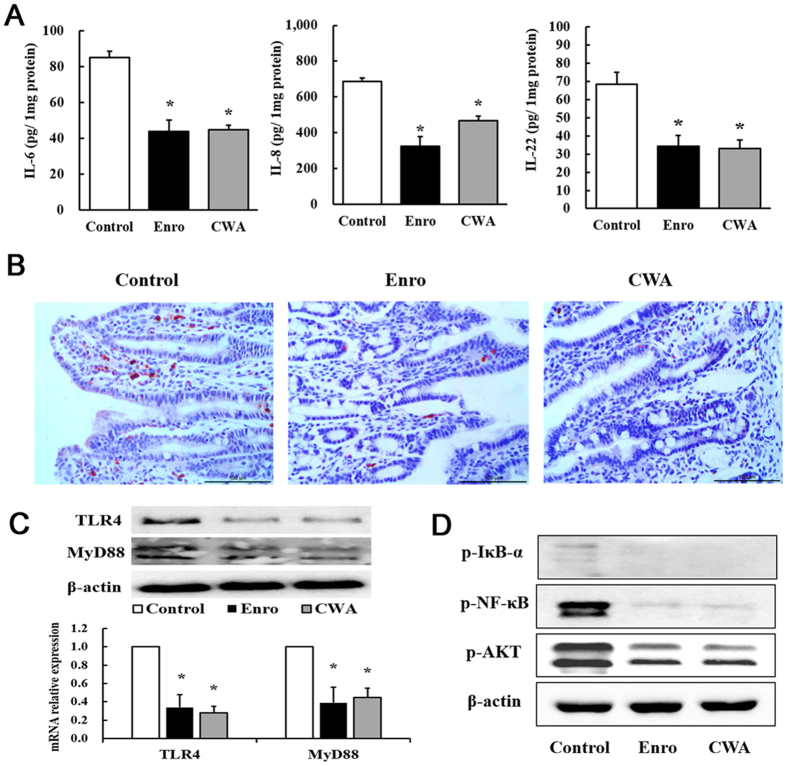
Levels of the pro-inflammatory cytokines IL-6, IL-8, and IL-22 in the jejunum were detected by ELISA. Both CWA and Enro significantly inhibited IL-6, IL-8 and IL-22 production in the jejunum compared with the control (Fig. 3A). In addition, immunohistochemistry results demonstrated that both CWA and Enro effectively decreased neutrophil infiltration in the mucosa of the jejunum compared with the control (Fig. 3B). Compared with the control, both CWA and Enro decreased the gene and protein expressions of TLR4 and MyD88 in the jejunum (Fig. 3C). Consequently, phosphorylation of NF-κB, IκB-α and AKT was suppressed in the jejunum following treatment with CWA and Enro (Fig. 3D). There were no significant differences between CWA and Enro regarding the suppression of intestinal inflammation in vivo in this study. However, in vitro, although Enro had no effects on LPS-induced expression of the pro-inflammatory cytokine IL-6 in porcine macrophages, CWA showed excellent inhibition of IL-6 production in a concentration-dependent manner (Fig. 4A). Furthermore, using siRNA-mediated knockdown, we found that CWA had no significant effects on LPS-induced IL-6 expression in MyD88-silenced or TLR4-silenced macrophages (Fig. 4B). These findings indicate that CWA shows excellent suppression of intestinal inflammation and may be involved in TLR4-, MyD88- and NF-κB-dependent pathways.
Figure 3. Cathelicidin-WA suppressed jejunal inflammation.
(A) Levels of the pro-inflammatory cytokines IL-6, IL-8, and IL-22 in the jejunum. (B) Neutrophil infiltration into the mucosa of the jejunum was determined by immunohistochemical staining with an antibody against MPO (bars, 100 μm). (C) Relative mRNA expression and protein levels of TLR4 and MyD88 in the jejunum. (D) The phosphorylation of NF-κB, IκB-α, and AKT was determined by Western blotting. All data are expressed as the mean ± SEM (n = 6). Differences were determined by Student’s t-test. Enro, Enrofloxacin; CWA, Cathelicidin-WA. *P < 0.05 compared with control.
Figure 4. Cathelicidin-WA ameliorated LPS-induced inflammation via TLR4- and MyD88-dependent signaling pathways.
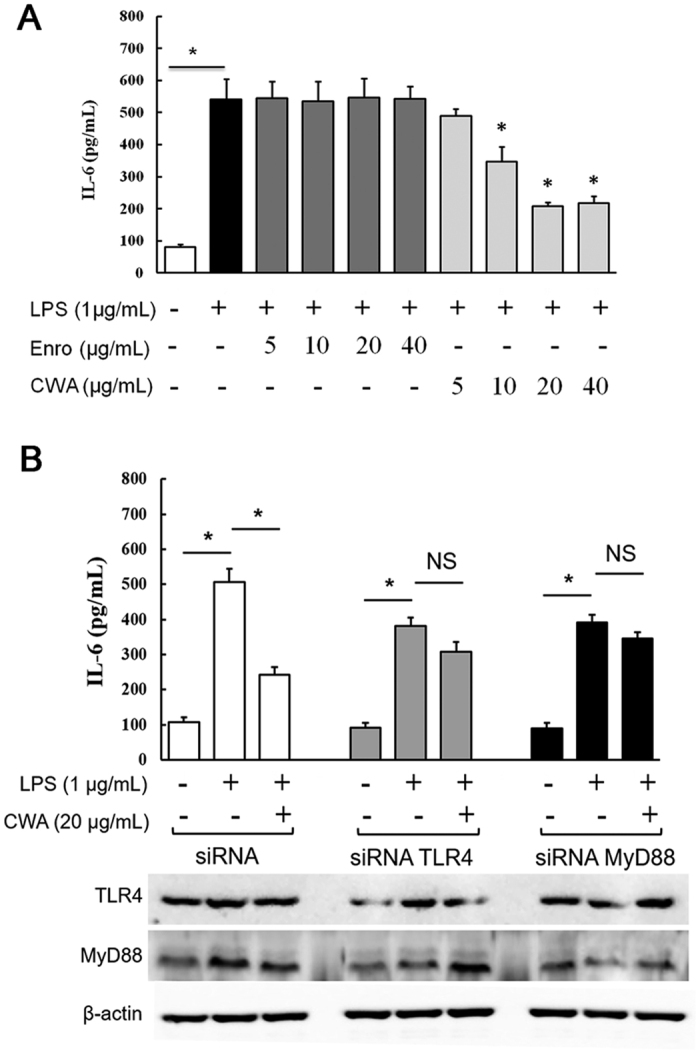
(A) The effects of CWA and Enro on IL-6 expression in LPS-induced macrophages. (B) The effects of CWA on LPS-induced IL-6 expression in TLR4- and MyD88-silenced macrophages. All data are expressed as the mean ± SEM (n = 6). Differences were determined by one-way ANOVA. Enro, Enrofloxacin; CWA, Cathelicidin-WA. *P < 0.05 compared with LPS; NS, not significant, P > 0.05.
CWA enhanced intestinal barrier functions
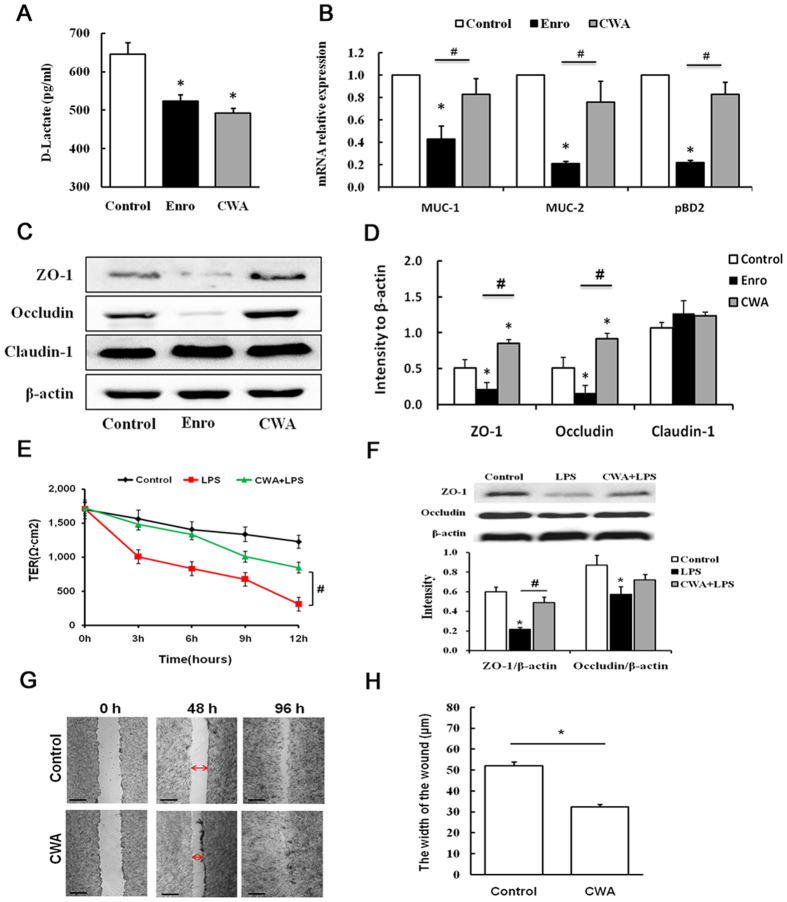
Both CWA and Enro decreased serum D-Lac levels compared with the control (Fig. 5A). Furthermore, Enro significantly reduced the expression of mucin-1 (MUC-1), mucin-2 (MUC-2) and porcine beta-defensin-2 (pBD2) mRNA in the jejunum compared with the control and CWA (Fig. 5B). In addition, we performed Western blot analysis to detect the expression of ZO-1, occludin and claudin-1. Although Enro had no significant effects on claudin-1 expression, ZO-1 and occludin expression levels in the jejunum were significantly reduced by Enro compared with the control (Fig. 5C,D). However, CWA significantly increased ZO-1 and occludin expression in the jejunum compared with the control (Fig. 5C,D). To verify these findings, we tested the effects of CWA on epithelial barriers in vitro. We found that CWA effectively ameliorated LPS-induced decreases in TER in IPEC-J2 cells (Fig. 5E). Furthermore, the expression levels of ZO-1 and occludin proteins in LPS-induced IPEC-J2 cells were increased by CWA (Fig. 5F). Interestingly, CWA significantly reduced would width at 48 h in a scrape assay in Caco-2 cells (Fig. 5G,H), suggesting that CWA could be beneficial for healing damage to intestinal epithelial cells. Collectively, CWA enhanced the intestinal barrier by increasing the expression of TJ proteins and augmenting wound healing ability. By contrast, Enro treatment may disrupt the intestinal barrier by decreasing the expression of TJ proteins and antimicrobial proteins.
Figure 5. Cathelicidin-WA enhanced intestinal barrier function.
(A) D-Lactate level in serum. (B) Relative mRNA expression levels of MUC-1, MUC-2 and pBD2 in the jejunum. (C,D) Protein expression levels of ZO-1, occludin and claudin-1 in the jejunum as determined by Western blotting. The effects of CWA on TER (E) and on the expression of ZO-1 and occludin proteins (F) in IPEC-J2 cell monolayers. (G,H) After being submitting to a scraping assay, Caco-2 cells were incubated with medium only or with 20 μg/mL CWA. Images were obtained at 0 h, 48 h and 96 h. Wound width was measured at 48 h. All data are expressed as the mean ± SEM (n = 6). Differences were determined by Student’s t-test. Enro, Enrofloxacin; CWA, Cathelicidin-WA. *P < 0.05 compared with the control; #P < 0.05 CWA compared with Enro or LPS.
CWA improved microbial composition and SCFA levels in feces
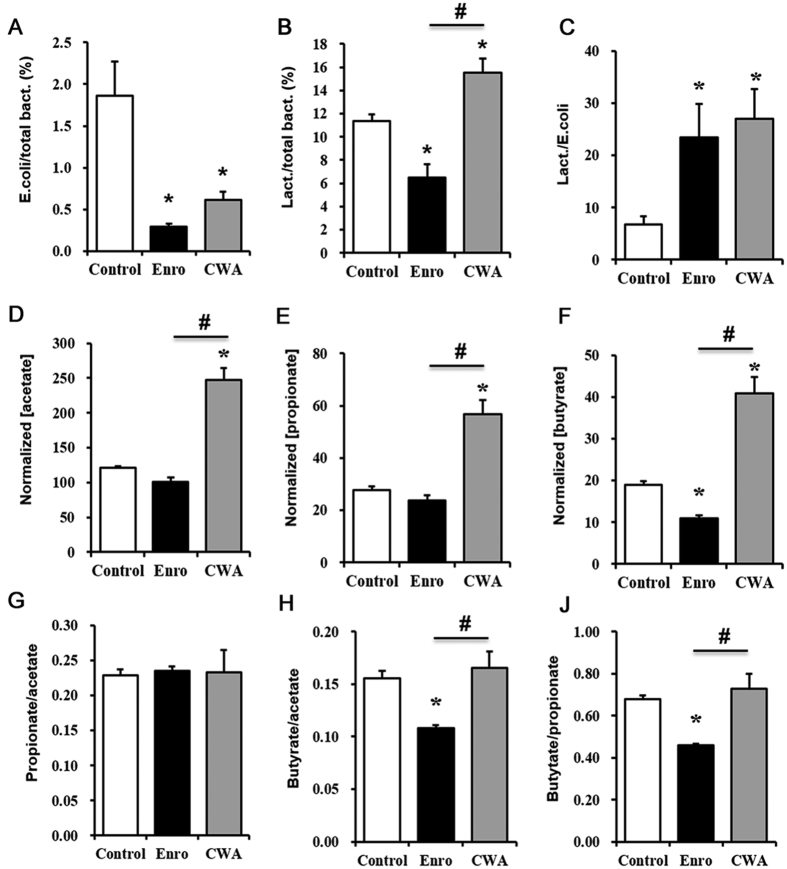
To evaluate CWA’s effects on intestinal epithelial barrier-microbiota interactions, we evaluated microbiota composition and SCFAs in the feces of weaned piglets following CWA treatment. Both CWA and Enro decreased the ratio of Escherichia coli to total bacteria and increased the ratio of Lactobacillus to Escherichia coli in feces compared with the control (Fig. 6A,C). The ratio of Lactobacillus to total bacteria in feces was deceased by Enro but increased by CWA compared with the control (Fig. 6B). Furthermore, CWA increased acetate, propionate and butyrate concentrations, but it did not alter the ratios of propionate/acetate, butyrate/acetate and butyrate/propionate in feces compared with the control (Fig. 6D–J). By contrast, Enro not only decreased butyrate concentration but also reduced the ratios of butyrate/propionate and butyrate/acetate in feces compared with the control and CWA (Fig. 6F,H,J).
Figure 6. Cathelicidin-WA improved microbial composition and SCFA levels in feces.
Ratios of Escherichia coli to total bacteria (A), Lactobacillus to total bacteria (B), and Lactobacillus to Escherichia coli (C) in feces as detected by qPCR. The concentrations of acetate (D), propionate (E), and butyrate (F) in feces were determined by GS, and the data were normalized to feces weight (per gram). Ratios of propionate/acetate (G), butyrate/propionate (H) and butyrate/acetate (J). All data are expressed as the mean ± SEM (n = 6). Differences were determined by one-way ANOVA. Enro, Enrofloxacin; CWA, Cathelicidin-WA. *P < 0.05 compared with the control; #P < 0.05 CWA compared with Enro.
Discussion
In this study, we demonstrated that the CWA can effectively attenuate postweaning diarrhea by suppressing inflammation, enhancing barrier function, and improving microbiota composition and SCFA levels in the intestine of piglets. By contrast, the antibiotic Enro disrupted barrier function, changed microbiota composition and negatively impacted SCFA levels in the intestine. Collectively, these data indicate that CWA could be an effective and safe therapy for diarrhea or other intestinal diseases and may have the potential to reduce antibiotic use in young mammals.
Diarrhea is a leading cause of death in young children, especially during the weaning period. Antibiotics are usually used for the treatment of diarrhea. In this study, we selected weaned piglets with clinical diarrhea as models to compare the effects of CWA and the antibiotic Enro on diarrhea in young mammals. To reduce the influence of bacterial resistance, we chose to evaluate Enro in this study, which is currently approved by the FDA and has strong antibacterial activity against both Gram-negative and Gram-positive bacteria. Surprisingly, we found that CWA attenuated diarrhea in weaned piglets as effectively as Enro. This is the first study to demonstrate that AMPs and antibiotics can produce similar therapeutic efficacy against postweaning diarrhea or other intestinal diseases in young mammals.
Although there are varied causes for diarrhea, intestinal inflammation is usually always present. Pro-inflammatory cytokine levels, such as those of IL-6 and TNF-α, are typically increased in the small intestines of piglets after weaning27. In this study, we demonstrated that CWA suppressed intestinal inflammation by down-regulating the TLR4-, MyD88- and NF-κB-dependent pathways in weaned piglets with diarrhea. Consistent with our results, previous studies have shown that cathelicidin peptides can decrease pro-inflammatory cytokine levels by inhibiting the NF-κB signaling pathway in LPS-induced macrophages and in mice23,28. The anti-inflammatory effects of the human-derived cathelicidin peptide LL-37 have been widely investigated29,30,31. LL-37 regulates inflammatory responses to pathogens, LPS or other TLR agonists by inhibiting NF-κB32. However, cathelicidins appear to possess both anti-inflammatory and pro-inflammatory properties in immunity33. For instance, LL-37 has been shown to promote the expression of the chemokine IL-8 34. In addition, cathelicidins are directly chemotactic for various innate immune cells, such as dendritic cells and macrophages17. Cathelicidins such as mouse CRAMP and human LL-37 play important roles in autoimmune diseases. CRAMP is protective in non-obese diabetes and can convert inflammatory cells into regulatory immune cells via the EGFR and TGF-β signaling pathways35. These data indicate that CWA may also have multiple ways of modulating intestinal inflammation.
As Enro treatment also effectively suppressed intestinal inflammation in weaned piglets, the question arises of whether the anti-inflammatory capacity of CWA is only dependent on its antibacterial activity (such as with antibiotics). Thus, we evaluated the immunomodulatory properties of CWA and Enro in LPS-stimulated porcine macrophages in vitro. Interestingly, Enro had no effects on the LPS-induced expression of the pro-inflammatory cytokine IL-6, whereas CWA effectively suppressed IL-6 expression. The differential regulation of IL-6 expression by Enro and CWA in macrophages may be related to the antibiotic-independent immunomodulatory effects of cathelicidins. Cathelicidins have been reported to suppress LPS-induced inflammation in macrophages via several mechanisms: binding to LPS; perturbation of the MyD88 signaling pathway; and inhibition of NF-κB translocation35,36. In this study, CWA was used to treat macrophages for 12 h and then removed before LPS treatment; thus, any anti-inflammatory activities of CWA were independent of LPS binding activity in macrophages. In addition to inactivating LPS through binding, a lactoferrin peptide was also shown to modulate LPS-induced inflammatory responses by directly affecting the MyD88-NF-κB signaling pathway in macrophages37. Consistent with these results, we found that CWA-mediated suppression of IL-6 expression was a direct result of CWA’s effects on the TLR4 and MyD88 signaling pathways. Collectively, these data suggest that the mechanisms by which CWA inhibits inflammation could be mediated by the down-regulation of the TLR4-, MyD88- and NF-κB-dependent pathways independently of CWA’s antibacterial activity. This effect may explain why CWA (0.6 mg/kg) and Enro (2.5 mg/kg) exhibited similar effects on the suppression of intestinal inflammation in weaned piglets with diarrhea.
Intestinal barrier damage is present in many intestinal diseases, such as diarrhea and inflammatory bowel disease (IBD). The epithelial barrier of the intestine is responsible for restricting the invasion of harmful substances, including microbes, toxins and LPS38. TJs are the most apical intercellular structures in the mucosa of the intestine and create semipermeable barriers that can separate different substances39. ZO-1, occludin and the claudins are the most important components of TJs in intestinal epithelial cells. In this study, we demonstrated that CWA enhanced epithelial barrier function by increasing the expression of ZO-1 and occludin in the intestine of weaned piglets with diarrhea. Consistently, previous studies have shown that AMPs such as pBD2 increase ZO-1, occludin and claudin-1 mRNA expression in the colon of DSS-induced mice17,39. We confirmed this result in vitro by showing that CWA ameliorated decreases in TER and improved the expression of TJ proteins in LPS-induced IPEC-J2 cells. By contrast, Enro treatment reduced the expression of TJ proteins and also inhibited the expression of mucins and pBD2 in the jejunum, suggesting that epithelial barrier function in the intestine is weakened by treatment with antibiotics. Decreased expression of TJ proteins could increase the permeability of the epithelial barrier, exacerbating inflammation40. The mucins and antimicrobial proteins that are secreted by intestinal epithelial cells provide defenses against pathogen invasion into the underlying lamina propria13,41. A previous study demonstrated that administration of the antibiotics neomycin, metronidazole and vancomycin significantly decreased the expression levels of antimicrobial proteins in intestinal epithelial cells42. A recent study also demonstrated that the antibiotic metronidazole compromised goblet cell function and inner mucus layer production and exacerbated Citrobacter rodentium-induced colitis in mice13. These data indicate that CWA treatment could enhance the intestinal barrier, whereas treatment with antibiotics might disrupt the intestinal barrier in young mammals.
Studies using knockout mice and germ-free mice have showed that intestinal mucins and antimicrobial proteins are major mediators of intestinal epithelial cell-commensal interactions, and their functions are largely affected by microbiota41,43. The different effects produced by CWA and Enro on the intestinal barrier may be related to changes in microbiota composition. As reported, dietary supplementation with AMPs can improve microbiota composition in the intestines of weaned pigs44,45,46. However, antibiotic treatments perturb this microbial composition and alter a host’s susceptibility to enteric infection13,47. In the current study, we found that CWA increased the ratio of Lactobacillus to total bacteria and increased SCFA levels in feces, whereas Enro decreased the ratio of Lactobacillus to total bacteria, the concentration of butyrate, and the ratios of butyrate/propionate and butyrate/acetate. These results provide evidence that antibiotics not only perturb microbial composition but also affect the metabolic capabilities of the microbiome in the intestines of a host12.
Given its effects on inflammation, the intestinal barrier and microbiota composition, CWA could be an effective therapy for many diseases linked to imbalances in homeostasis in the intestine, including obesity, IBD and colorectal cancer. The optimal dosage of CWA treatment for different type of diseases needs to be further evaluated. Additionally, the mechanisms underlying how CWA affects the expression of TJ proteins and enhances bacterial resistance need to be determined in the future.
In conclusion, CWA effectively attenuated intestinal inflammation, enhanced intestinal barrier function, and improved microbial composition and metabolic capabilities in the intestines of weaned piglets. Furthermore, our results demonstrated that antibiotic treatment perturbed epithelial barrier function and negatively altered microbiota composition in the intestine. This study indicates that CWA treatment could be an effective therapy for postweaning diarrhea in young mammals as well as a therapeutic strategy for other intestinal diseases, such as IBD and colorectal cancer.
Additional Information
How to cite this article: Yi, H. et al. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 6, 25679; doi: 10.1038/srep25679 (2016).
Acknowledgments
This study was funded by the National Science Fund for Distinguished Young Scholars of China (Grant No. 31025027), the National Natural Science Foundation of China (Grant No. 31572411) and the Modern Agro-Industry Technology Research System of China (CARS-36).
Footnotes
Author Contributions H.Y. and H.X. fed the piglets and performed the qPCR and Western blot experiments. C.Y. performed the ELISA experiments. L.Z. supervised the TEM and SEM experiments. Z.G. and performed the IPEC-J2 and Caco-2 experiments. Y.W., H.D. and H.Y. directed the research and wrote the manuscript. All authors reviewed the manuscript.
References
- Xu J. X. et al. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 30, 584–589, doi: 10.1016/j.nut.2013.10.018 (2014). [DOI] [PubMed] [Google Scholar]
- McLamb B. L., Gibson A. J., Overman E. L., Stahl C. & Moeser A. J. Early Weaning Stress in Pigs Impairs Innate Mucosal Immune Responses to Enterotoxigenic E. coli Challenge and Exacerbates Intestinal Injury and Clinical Disease. Plos One 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. H., Li D. F. & She R. P. Effect of weaning on small intestinal structure and function in the piglet. Archives of Animal Nutrition-Archiv Fur Tierernahrung 56, 275–286, doi: 10.1080/0003942021000019090 (2002). [DOI] [PubMed] [Google Scholar]
- Moeser A. J. et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. American Journal of Physiology-Gastrointestinal and Liver Physiology 292, G173–G181, doi: 10.1152/ajpgi.00197.2006 (2007). [DOI] [PubMed] [Google Scholar]
- Smith F. et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology 298, G352–G363, doi: 10.1152/ajpgi.00081.2009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Glutamine Enhances Tight Junction Protein Expression and Modulates Corticotropin-Releasing Factor Signaling in the Jejunum of Wean ling Piglets. Journal of Nutrition 145, 25–31, doi: 10.3945/jn.114.202515 (2015). [DOI] [PubMed] [Google Scholar]
- Einterz E. M. & Bates M. E. Early-Childhood Feeding Practices in Northern Cameroon. T Roy Soc Trop Med H 88, 575–576 (1994). [DOI] [PubMed] [Google Scholar]
- Yin X. X. et al. A systematic review of antibiotic utilization in China. Journal of Antimicrobial Chemotherapy 68, 2445–2452, doi: 10.1093/Jac/Dkt223 (2013). [DOI] [PubMed] [Google Scholar]
- Gill E. E., Franco O. L. & Hancock R. E. W. Antibiotic Adjuvants: Diverse Strategies for Controlling Drug-Resistant Pathogens. Chem Biol Drug Des 85, 56–78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabanal F. et al. A bioinspired peptide scaffold with high antibiotic activity and low in vivo toxicity. Sci Rep 5, 10058, doi: 10.1038/srep10558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashiru-Oredope D. S. H. Antimicrobial resistance: moving from professional engagement to public action. Journal of Antimicrobial Chemotherapy 70, 4, doi: 10.1093/jac/dkv297 (2015). [DOI] [PubMed] [Google Scholar]
- Cho I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626, doi: 10.1038/nature11400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M. et al. Antibiotic Treatment Alters the Colonic Mucus Layer and Predisposes the Host to Exacerbated Citrobacter rodentium-Induced Colitis. Infection and immunity 79, 1536–1545 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti M., Schmidtchen A. & Malmsten M. Antimicrobial peptides: key components of the innate immune system. Crit Rev Biotechnol 32, 143–171, doi: 10.3109/07388551.2011.594423 (2012). [DOI] [PubMed] [Google Scholar]
- Brogden K. A., Ackermann M., McCray P. B. & Tack B. F. Antimicrobial peptides in animals and their role in host defences. International Journal of Antimicrobial Agents 22, 465–478 (2003). [DOI] [PubMed] [Google Scholar]
- Giuliani A., Pirri G. & Nicoletto S. F. Antimicrobial peptides: an overview of a promising class of therapeutics. Central European Journal of Biology 2, 1–33, doi: 10.2478/s11535-007-0010-5 (2007). [DOI] [Google Scholar]
- Zhang H. W. et al. Cathelicidin-BF, a Novel Antimicrobial Peptide from Bungarus fasciatus, Attenuates Disease in a Dextran Sulfate Sodium Model of Colitis. Mol Pharmaceut 12, 1648–1661 (2015). [DOI] [PubMed] [Google Scholar]
- Tai E. K. K. et al. Intrarectal administration of mCRAMP-encoding plasmid reverses exacerbated colitis in Cnlp(−/−)Imice. Gene Ther 20, 187–193 (2013). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PloS one 3, e3217, doi: 10.1371/journal.pone.0003217 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. P. et al. Cathelicidin-BF, a Snake Cathelicidin-Derived Antimicrobial Peptide, Could Be an Excellent Therapeutic Agent for Acne Vulgaris. Plos One 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. BF-30 effectively inhibits ciprofloxacin-resistant bacteria in vitro and in a rat model of vaginosis. Arch Pharm Res 37, 927–936, doi: 10.1007/s12272-013-0248-6 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Y., Luan C., Xia X., An S. & Wang Y. Antibacterial Activity, Cytotoxicity and Mechanisms of action of Cathelicidin Peptides against Enteric Pathogens in Weaning Piglets. International Journal of Peptide Research and Therapeutics 17, 175–184, doi: 10.1007/s10989-011-9255-y (2011). [DOI] [Google Scholar]
- Song D. G. et al. Antimicrobial peptide Cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia. Int Immunopharmacol 25, 141–147 (2015). [DOI] [PubMed] [Google Scholar]
- Bhandari S. K., Xu B., Nyachoti C. M., Giesting D. W. & Krause D. O. Evaluation of alternatives to antibiotics using an Escherichia coli K88( + ) model of piglet diarrhea: Effects on gut microbial ecology. Journal of animal science 86, 836–847, doi: 10.2527/Jas.2006-822 (2008). [DOI] [PubMed] [Google Scholar]
- Gao Y. et al. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: A comparative study. J Anim Sci 91, 5614–5625, doi: 10.2527/jas.2013-6528 (2013). [DOI] [PubMed] [Google Scholar]
- Wang B. et al. Effects of alfalfa and cereal straw as a forage source on nutrient digestibility and lactation performance in lactating dairy cows. J Dairy Sci 97, 7706–7715 (2014). [DOI] [PubMed] [Google Scholar]
- Pie S. et al. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. Journal of Nutrition 134, 641–647 (2004). [DOI] [PubMed] [Google Scholar]
- Yi H. B. et al. Cathelicidin-BF suppresses intestinal inflammation by inhibiting the nuclear factor-kappa B signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Int Immunopharmacol 28, 61–69 (2015). [DOI] [PubMed] [Google Scholar]
- Nijnik A. & Hancock R. E. W. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Current Opinion in Hematology 16, 41–47, doi: 10.1097/MOH.0b013e32831ac517 (2009). [DOI] [PubMed] [Google Scholar]
- Brown K. L. et al. Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J Immunol 186, 5497–5505, doi: 10.4049/jimmunol.1002508 (2011). [DOI] [PubMed] [Google Scholar]
- Scott A. et al. Evaluation of the ability of LL-37 to neutralise LPS in vitro and ex vivo. PloS one 6, e26525, doi: 10.1371/journal.pone.0026525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Does A. M., Bergman P., Agerberth B. & Lindbom L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. Journal of leukocyte biology 92, 735–742, doi: 10.1189/jlb.0412178 (2012). [DOI] [PubMed] [Google Scholar]
- Hilchie A. L., Wuerth K. & Hancock R. E. W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9, 761–768, doi: 10.1038/Nchembio.1393 (2013). [DOI] [PubMed] [Google Scholar]
- Scott M. G., Davidson D. J., Gold M. R., Bowdish D. & Hancock R. E. W. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. Journal of Immunology 169, 3883–3891 (2002). [DOI] [PubMed] [Google Scholar]
- Sun J. et al. Pancreatic beta-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 43, 304–317, doi: 10.1016/j.immuni.2015.07.013 (2015). [DOI] [PubMed] [Google Scholar]
- Bucki R., Leszczynska K., Namiot A. & Sokolowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Archivum immunologiae et therapiae experimentalis 58, 15–25, doi: 10.1007/s00005-009-0057-2 (2010). [DOI] [PubMed] [Google Scholar]
- Zong X. et al. LFP-20, a porcine lactoferrin peptide, ameliorates LPS-induced inflammation via the MyD88/NF-kappa B and MyD88/MAPK signaling pathways. Developmental and comparative immunology 52, 123–131 (2015). [DOI] [PubMed] [Google Scholar]
- Farhadi A., Banan A., Fields J. & Keshavarzian A. Intestinal barrier: An interface between health and disease. J Gastroen Hepatol 18, 479–497 (2003). [DOI] [PubMed] [Google Scholar]
- Han F. F. et al. Porcine beta-Defensin 2 Attenuates Inflammation and Mucosal Lesions in Dextran Sodium Sulfate-Induced Colitis. Journal of Immunology 194, 1882–1893 (2015). [DOI] [PubMed] [Google Scholar]
- Mennigen R. et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 296, G1140–G1149 (2009). [DOI] [PubMed] [Google Scholar]
- Gallo R. L. & Hooper L. V. Epithelial antimicrobial defence of the skin and intestine. Nature reviews. Immunology 12, 503–516, doi: 10.1038/nri3228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K. et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–U808 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M. & Finlay B. B. Host immune response to antibiotic perturbation of the microbiota. Mucosal immunology 3, 100–103 (2010). [DOI] [PubMed] [Google Scholar]
- Wu S. D. et al. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 35, 225–230, doi: 10.1016/j.peptides.2012.03.030 (2012). [DOI] [PubMed] [Google Scholar]
- Xiao H. et al. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J Anim Sci 91, 4772–4780 (2013). [DOI] [PubMed] [Google Scholar]
- Yoon J. H. et al. Effects of dietary supplementation of synthetic antimicrobial peptide-A3 and P5 on growth performance, apparent total tract digestibility of nutrients, fecal and intestinal microflora and intestinal morphology in weanling pigs. Livest Sci 159, 53–60, doi: 10.1016/j.livsci.2013.10.025 (2014). [DOI] [PubMed] [Google Scholar]
- Sekirov I. et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infection and immunity 76, 4726–4736 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]