Abstract
Background:
Rheumatic heart disease (RHD) is a global cause of early heart failure. Early RHD is characterized by valvar regurgitation, leading to ventricular distention and possible elaboration of amino-terminal pro-brain natriuretic peptide (NT-proBNP). We investigated the ability of NT-proBNP to distinguish cases of latent RHD detected by echocardiographic screening from the controls.
Materials and Methods:
Ugandan children (N = 44, 36% males, mean age: 12 ± 2 years) with latent RHD (cases) and siblings (controls) by echocardiography were enrolled. Cases and controls were matched for age and sex, and they had normal hemoglobin (mean: 12.8 mg/dL). Children with congenital heart disease, pregnancy, left ventricular dilation or ejection fraction (EF) below 55%, or other acute or known chronic health conditions were excluded. RHD cases were defined by the World Heart Federation (WHF) 2012 consensus guideline criteria as definite. Controls had no echocardiography (echo) evidence for RHD. At the time of echo, venous blood samples were drawn and stored as serum. NT-proBNP levels were measured using sandwich immunoassay. Paired t-tests were used to compare NT-proBNP concentrations including sex-specific analyses.
Results:
The mean NT-proBNP concentration in the cases was 105.74 ± 67.21 pg/mL while in the controls, it was 86.63 ± 55.77 pg/mL. The cases did not differ from the controls (P = 0.3). In sex-specific analyses, male cases differed significantly from the controls (158.78 ± 68.82 versus 76 ± 42.43, P = 0.008). Female cases did not differ from the controls (75.44 ± 45.03 versus 92.30 ± 62.35 respectively, P = 0.4).
Conclusion:
Serum NT-proBNP did not distinguish between latent RHD cases and the controls. Sex and within-family exposures may confound this result. More investigation into biomarker-based RHD detection is warranted.
Keywords: Biomarkers, echocardiography, global health, mitral regurgitation, rheumatic heart disease
INTRODUCTION
Rheumatic heart disease (RHD) occupies a unique place in pediatric global health. RHD is endemic where 85% of children under 15 years old live, placing nearly 2 billion children at risk.[1,2] RHD is responsible for nearly 345,000 deaths per year, the vast majority of which occur during the 3rd and 4th decades of life.[1,2] As such, it robs persons of the most productive portion of life, deprives their families of breadwinners, and curtails the workforce by death and debilitation. Coupled with the pregnancy-related complications in RHD-affected women, the deleterious impact of RHD can have a multiplier effect echoing across generations.[3,4,5,6] Therefore, arresting RHD may have long-lasting and rippling effects on the affected persons, relatives, and society at large.[7]
Pathophysiologically, repeated bouts of group A streptococcal pharyngitis lead predisposed hosts to progressive left-sided cardiac valvar dysfunction resulting in congestive heart failure (CHF), arrhythmia, stroke, and eventually death.[2,5,8,9] Encouragingly, early detection and assiduous secondary antibiotic prophylaxis of mild RHD cases presents an opportunity for global RHD control.[2,10] A critical period for the provision of prophylaxis would be during the period of mild RHD since in low- and middle-income countries, the majority of patients present in adult life with advanced RHD, which began decades earlier.[11,12] Echocardiographic (echo) screening studies have revealed a large burden of latent RHD, defined as echo findings of RHD in asymptomatic children.[13,14,15,16] Recent Markov modeling work has shown echocardiographic screening to be a cost-effective strategy for RHD control but it is still relatively costly for widespread deployment in resource-limited settings.[17] Alternative population screening modalities for latent RHD should be less resource-intense with respect to staff, equipment, and time and therefore, more scalable. Blood biomarker techniques could be such an approach.
Amino-terminal pro-brain natriuretic peptide (NT-proBNP) is a peptide that sits in preformed vesicles in ventricular cardiomyocytes.[18,19,20,21,22,23,24] NT-proBNP is cleaved from the released pro-form of B-type natriuretic peptide (BNP) with the amino-terminal fragment being stable in the blood for some time.[21] Adult studies demonstrate that in clinical circumstances including cardiomyopathies, valvar regurgitation, and CHF, the BNP system is ubiquitously and sensitively elevated. Even subtle elevation in BNP levels for subclinical, stable, and unstable cardiac disease predict progression including death.[18,25,26,27] Biologically, BNP has numerous effects including inducing diuresis and antifibrotic remodeling, both of which may be adaptive in RHD.[23,24,28] In RHD, BNP is highly elevated in adults with severe mitral disease and the levels diminish after mitral valve surgery.[22] While BNP is known to be elevated in children with primarily moderate-to-severe RHD-related and acute rheumatic fever-related regurgitation,[29] the role of BNP as a marker of latent mild RHD, detectable by echocardiographic visualization, is unknown. This investigation tested the hypothesis that serum NT-proBNP is higher in children with latent, definite mild RHD without left atrial or left ventricular (LV) dilation, compared to matched controls.
MATERIALS AND METHODS
Study participants
Participants were recruited from northern Uganda. Children of 9-17 years old who had previously undergone school-based echocardiographic screening for RHD and were enrolled in a clinical follow-up program were included. Age- and gender-matched controls (within 6 months of the cases) were recruited from the siblings of these children. This recruitment strategy would efficiently equilibrate background features between the cases and controls in terms of living conditions and sociodemographic status. All the participants assented and the parents gave their consent for participation in this study after approval by the local institutional review boards in Uganda and core laboratory partner sites in the USA. Definite RHD was defined by the World Heart Federation (WHF) 2012 guidelines for the echocardiographic diagnosis of RHD.[9] Exclusion criteria for the cases and controls included more than moderate mitral or aortic regurgitation on echo, left atrial (LA) enlargement (>4.2 cm in girls and >4.6 cm in boys),[30] left ventricle (LV) dilation, LV dysfunction defined as ejection fraction (EF) by Simpson's biplane below 55%, congenital heart disease or other acquired heart diseases, pregnancy, anemia [hematocrit (Hct) <30] or polycythemia (Hct > 55) as they may independently elevate BNP levels,[31,32,33] and other known significant acute or chronic health conditions. Of the 30 cases of definite RHD who were initially recruited, six were excluded for disqualifying echocardiographic findings and two for a combination of disqualifying echocardiographic findings and abnormal Hcts. Additionally, one initially matched control child was excluded for polycythemia and replaced with a second matched control.
Assessment
At the research visit, a local cardiologist (TA) or a specialty-trained nurse performed echocardiograms on the participant children using standard portable echo machines (Vivid-Q, General Electric, Milwaukee, WI, USA), The echos were sent to a core laboratory with a single cardiologist with expertise in RHD performing interpretation and RHD diagnosis was confirmed by WHF 2012 criteria [Figure 1]. Mitral and aortic regurgitation were assessed in the parasternal long and apical four-chamber views and were graded according to the published guidelines.[9] Simpson's biplane method was used to assess left ventricular EF. A two-dimensional (2D) still frame at end systole in the parasternal long-axis view was used to measure LA size. Placing further emphasis on excluding patients with severe RHD, further exclusion criteria for cases were applied at this point, including more than moderate mitral or aortic regurgitation, decreased left ventricular systolic function, or left atrial enlargement.
Figure 1.
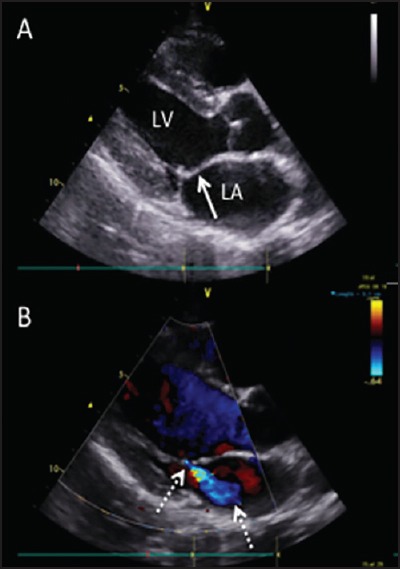
Parasternal long-axis image of a typical case of latent, definite RHD. (a) 2D image highlights the thickened mitral valve leaflet with a characteristic “dog-leg” deformity (solid arrow) (b) Color Doppler image shows characteristic posteriorly-directed mild mitral regurgitation (dashed arrows)
At the time of the research visit, the case children and control children had 10 mL of whole blood divided into two tubes at the same draw. From one tube, hemoglobin was measured locally. From the second tube, serum was separated and frozen to -20°C and then shipped as a batch for analysis. NT- proBNP was measured in the clinical laboratory on the Roche E Modular system (Roche Diagnostics, Indianapolis, IN, USA) by a quantitative sandwich enzyme immunoassay technique. Day-to-day imprecision values at concentrations of 175 pg/mL, 434 pg/mL, and 6781 pg/mL were 3.2%, 2.4%, and 2.2%, respectively. Assays were performed after echocardiographic interpretation, thereby blinding echo readers to the results.
Statistical analysis
Paired Student's t-test was used to compare NT-proBNP concentrations in RHD children versus control and to compare the levels in a sex-specific fashion due to the well-known differences in BNP levels between males and females.[18,21,23] To address concerns about hemoglobin more precisely, cases and controls were combined into a pooled, multivariable adjusted logistic regression analysis. Case status was the dependent outcome, NT-proBNP concentration was the predictor of interest, and adjustment covariates included age, sex, and hemoglobin. Statistical Package for the Social Sciences (SPSS) 21.0 (PASW, Chicago IL, USA) was used for statistical analyses and two-tailed P values <0.05 were considered to be significant.
RESULTS
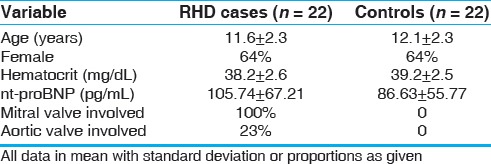
The 22 pairs of matched participants had a mean age of 12 years (range: 6–15 years), and 64% were females [Table 1]. There was no difference in the mean Hct between the cases and controls (P = 0.7). By definition, no case or control child had clinical cardiac symptoms, and all had normal left atrial size, left ventricular size, and left ventricular function. All 22 cases had mitral regurgitation (15 mild, 7 moderate) and at least 2 morphological abnormalities of the mitral valve. Additionally, five had aortic regurgitation (two trivial and three moderate) and three had at least two morphological abnormalities of the aortic valve. No included children had mitral or aortic stenosis.
Table 1.
Primary data
Overall, RHD children had mean NT-proBNP concentration of 105.74 ± 67.21 pg/mL while for matched control children it was 86.63 ± 55.77 pg/mL (paired t-test P = 0.3). In adjusted regression models, RHD status was not a predictor of NT-proBNP level. Dichotomizing NT-proBNP at above versus below 125 pg/mL[34,35] did not affect results. There was no correlation between LA size and NT-proBNP level (Pearson r2 = -0.11, P = 0.46).
Given the well-described sex differences in NT-proBNP, sex stratified analysis shows that RHD boys had mean NT-proBNP of 158.78 ± 68.82 while for the controls, it was 76 ± 42.43 pg/mL (P = 0.008). RHD-affected girls had a mean concentration of 75.44 ± 45.03 while for the controls, it was 92.3 ± 62.35 (P = 0.4). In girls, 8 out of 14 had worse than mild mitral involvement. In boys, four out of eight had worse than mild mitral valve disease, indicating little difference in the distribution in severity of disease.
DISCUSSION
In this case-control study of children, randomly drawn circulating NT-proBNP was not significantly different between RHD-affected and age-sex matched control children. However, in sex-specific analyses, NT-proBNP was significantly higher in RHD-affected boys while several girls in the control group had unexplained higher NT-proBNP levels.
The detection and secondary prophylaxis of early RHD offers great promise in RHD control and mitigation with knock-on effects for the affected person, family, and society, particularly in low-resource settings. Provision of cost-effective, simple antibiotic prophylaxis is of proven efficacy.[8,9,10] Recent studies have demonstrated that echo-based screening for RHD compared to clinical evaluation detects substantially more cases of early latent RHD. To standardize detection, the WHF developed a suggested diagnostic schema for the proper diagnosis of clinically silent RHD, especially for high prevalence areas.[9] Although recent work gives details that echo-based screening may be cost-effective under most conditions,[17] echo screening is resource-intense with respect to equipment and personnel and challenges children's modesty, which is not trivial considering that more than 95% of disrobed children are predictably negative, leading to an ethical dilemma common to most screening programs regarding the burden on healthy persons to identify sick persons.
In response to these challenges, this study was designed to identify a new candidate biomarker for RHD detection. Screening biomarkers of RHD should be sufficiently sensitive to effectively rule out disease in otherwise clinically well persons. Biomarkers also need to identify early stages of the disease so that secondary prophylaxis can be instituted to prevent progression. In order to maximize efficiency, biomarkers would be testable on point of care, portable equipment with easy to interpret, simplified read-outs. A putative marker of RHD that fits these criteria might improve on the current gold standard methods by possibly reducing equipment costs, personnel costs, and localize ultrasonographic resources to specially trained centers. To identify a candidate biomarker, we relied on early RHD characteristically causing valvar regurgitation. Regurgitation volume loads the heart. This volume load can be compensated and even attenuated depending on the RHD progression such that LV dilation does not occur.
One such highly sensitive response to dilation and stretch is the release of BNP. Adult studies well-demonstrate that in clinical circumstances associated with ventricular dilation BNP is ubiquitously and sensitively elevated. BNP is well-documented to have a sexual dimorphism wherein females have a much higher BNP compared to males for an unclear cause.[18,21,23,25,26,32,36,37] In RHD, BNP is highly elevated in adults with severe mitral disease and the levels diminish after mitral valve surgery to remove the volume load.[38,39,40] While BNP is described to be elevated in children with predominantly moderate to severe RHD or acute rheumatic fever,[29] the role of BNP in latent mild RHD was unknown.
We found that NT-proBNP may distinguish mild RHD cases from the controls in boys but not in girls. This conflicting result may reflect the known sex difference in BNP.[21,23,25,26,37] This result alternatively may be due to the controls not truly being unaffected. Since our recruitment strategy was to recruit unaffected siblings of RHD-affected children as controls, we cannot ensure that these children were free of any past history of RHD. Indeed, RHD and even echocardiographically detected RHD are known to regress and heal. If such an improvement occurred in the control children, unaffected siblings may be misclassified from a biological perspective although clinically and ultrasonographically at the time of evaluation they were not affected. Second, while we took care to follow WHF echo screening criteria as per previous studies, the natural history of latent, echo-only findings is not well-understood. In other words, it is possible but not likely that false-positive cases can occur even using WHF criteria if some persons have structural valvar variants, which give mild regurgitation but are physiologic and therefore, do not have pathologic BNP release. Similarly, our study focuses on mild, echocardiographically detected cases of RHD. The utility of NT-proBNP detection in a broader range of RHD severity and regurgitation cannot be inferred from this data. We assiduously attempted to exclude children with other illness and especially, those with confounders such as anemia. However, we may not have been perfectly successful in doing so and therefore, other clinical conditions confounded our results. Likewise, family members may have similar BNP levels and therefore, the distinction in NT-proBNP between a mildly affected child and an unaffected sibling may not have been large enough to detect a statistically significant difference in signal versus noise. We also cannot exclude exercise activity differences between the sexes as playing a role in the observed sex differences. Finally, our data focuses on the Ugandan population, which may not be generalizable to other contexts both in terms of RHD endemicity or biological response to valvar regurgitation.[13]
CONCLUSION
NT-proBNP did not distinguish children with mild, echocardiographically detected RHD without LV dilation from the controls. Boys did appear to differ significantly while girls did not due to higher concentrations in some girls. Nevertheless, the role of biomarkers of latent RHD is unclear and merit continuing scrutiny.
Financial support and sponsorship
Nil.
Conflicts of interest
We declare we have neither any relevant financial conflicts of interest nor relations with industry.
Acknowledgements
This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute K23 HL111335 (JPZ), the National Center for Advancing Translational Sciences UL1TR000075 and KL2TR000076 (AZB), and Boston Children's Research Faculty Council Pilot Award (JPZ). The contents of this paper are the responsibility of the authors and do not necessarily represent the views of the funding agencies.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–64. doi: 10.1016/S0140-6736(11)61171-9. [DOI] [PubMed] [Google Scholar]
- 3.Essop MR, Nkomo VT. Rheumatic and nonrheumatic valvular heart disease: Epidemiology, management, and prevention in Africa. Circulation. 2005;112:3584–91. doi: 10.1161/CIRCULATIONAHA.105.539775. [DOI] [PubMed] [Google Scholar]
- 4.Diao M, Kane A, Ndiaye MB, Mbaye A, Bodian M, Dia MM, et al. Pregnancy in women with heart disease in sub-Saharan Africa. Arch Cardiovasc Dis. 2011;104:370–4. doi: 10.1016/j.acvd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 6.Luijckx GJ, Ukachoke C, Limapichat K, Heuts-van Raak EP, Lodder J. Brain infarct causes under the age of fifty: A comparison between an east-Asian (Thai) and a western (Dutch) hospital series. Clin Neurol Neurosurg. 1993;95:199–203. doi: 10.1016/0303-8467(93)90124-y. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead M, Dahlgren G, Evans T. Equity and health sector reforms: Can low-income countries escape the medical poverty trap? Lancet. 2001;358:833–6. doi: 10.1016/S0140-6736(01)05975-X. [DOI] [PubMed] [Google Scholar]
- 8.Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–41. doi: 10.1056/NEJMp078039. [DOI] [PubMed] [Google Scholar]
- 9.Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease--an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massell BF, Chute CG, Walker AM, Kurland GS. Penicillin and the marked decrease in morbidity and mortality from rheumatic fever in the United States. N Engl J Med. 1988;318:280–6. doi: 10.1056/NEJM198802043180504. [DOI] [PubMed] [Google Scholar]
- 11.Bland EF, Duckett Jones T. Rheumatic fever and rheumatic heart disease; a twenty year report on 1000 patients followed since childhood. Circulation. 1951;4:836–43. doi: 10.1161/01.cir.4.6.836. [DOI] [PubMed] [Google Scholar]
- 12.Jones TD, Lingley JR. Rheumatic heart disease, with mitral and aortic stenosis. N Engl J Med. 1949;240:1018–22. doi: 10.1056/NEJM194906232402506. [DOI] [PubMed] [Google Scholar]
- 13.Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation. 2012;125:3127–32. doi: 10.1161/CIRCULATIONAHA.112.092312. [DOI] [PubMed] [Google Scholar]
- 14.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 15.Saxena A, Ramakrishnan S, Roy A, Seth S, Krishnan A, Misra P, et al. Prevalence and outcome of subclinical rheumatic heart disease in India: The RHEUMATIC (Rheumatic Heart Echo Utilisation and Monitoring Actuarial Trends in Indian Children) study. Heart. 2011;97:2018–22. doi: 10.1136/heartjnl-2011-300792. [DOI] [PubMed] [Google Scholar]
- 16.Webb R, Wilson NJ, Lennon D. Rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:208–9. doi: 10.1056/NEJMc072555. [DOI] [PubMed] [Google Scholar]
- 17.Zachariah JP, Samnaliev M. Echo-based screening of rheumatic heart disease in children: A cost-effectiveness Markov model. J Med Econ. 2015;18:410–9. doi: 10.3111/13696998.2015.1006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maisel A. B-type natriuretic peptide levels: A potential novel “white count” for congestive heart failure. J Card Fail. 2001;7:183–93. doi: 10.1054/jcaf.2001.24609. [DOI] [PubMed] [Google Scholar]
- 19.Mega JL, Morrow DA, De Lemos JA, Sabatine MS, Murphy SA, Rifai N, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: An ENTIRE-TIMI-23 substudy. J Am Coll Cardiol. 2004;44:335–9. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–70. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 21.Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, et al. NT-pro-B-type natriuretic peptide in infants and children: Reference values based on combined data from four studies. Pediatr Cardiol. 2009;30:3–8. doi: 10.1007/s00246-008-9258-4. [DOI] [PubMed] [Google Scholar]
- 22.Sutton TM, Stewart RA, Gerber IL, West TM, Richards AM, Yandle TG, et al. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. 2003;41:2280–7. doi: 10.1016/s0735-1097(03)00486-8. [DOI] [PubMed] [Google Scholar]
- 23.Vuolteenaho O, Ala-Kopsala M, Ruskoaho H. BNP as a biomarker in heart disease. Adv Clin Chem. 2005;40:1–36. [PubMed] [Google Scholar]
- 24.Yap LB, Mukerjee D, Timms PM, Ashrafian H, Coghlan JG. Natriuretic peptides, respiratory disease, and the right heart. Chest. 2004;126:1330–6. doi: 10.1378/chest.126.4.1330. [DOI] [PubMed] [Google Scholar]
- 25.Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, et al. B-type natriuretic peptides and cardiovascular risk: Systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–87. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 26.Macheret F, Boerrigter G, McKie P, Costello-Boerrigter L, Lahr B, Heublein D, et al. Pro-B-type natriuretic peptide (1-108) circulates in the general community: Plasma determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2011;57:1386–95. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, Jarolim P, et al. Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294:2866–71. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 28.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–44. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotby AA, El-Shahed GS, Elmasry OA, El-Hadidi IS, El Shafey RN. N-Terminal proBNP Levels and Tissue Doppler Echocardiography in Acute Rheumatic Carditis. ISRN Pediatr 2013. 2013:970394. doi: 10.1155/2013/970394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Machado RF, Hildesheim M, Mendelsohn L, Remaley AT, Kato GJ, Gladwin MT. NT-pro brain natriuretic peptide levels and the risk of death in the cooperative study of sickle cell disease. Br J Haematol. 2011;154:512–20. doi: 10.1111/j.1365-2141.2011.08777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto M, Tsujino T, Naito Y, Lee-Kawabata M, Ezumi A, Yamamoto K, et al. Anemia as a factor that elevates plasma brain natriuretic peptide concentration in apparently healthy subjects. Int Heart J. 2008;49:577–86. doi: 10.1536/ihj.49.577. [DOI] [PubMed] [Google Scholar]
- 33.Nybo M, Benn M, Mogelvang R, Jensen JS, Schnohr P, Rehfeld JF, et al. Impact of hemoglobin on plasma pro-B-type natriuretic peptide concentrations in the general population. Clin Chem. 2007;53:1921–7. doi: 10.1373/clinchem.2007.089391. [DOI] [PubMed] [Google Scholar]
- 34.Gustafsson F, Steensgaard-Hansen F, Badskjaer J, Poulsen AH, Corell P, Hildebrandt P. Diagnostic and prognostic performance of N-terminal ProBNP in primary care patients with suspected heart failure. J Card Fail. 2005;11(Suppl):S15–20. doi: 10.1016/j.cardfail.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Zaphiriou A, Robb S, Murray-Thomas T, Mendez G, Fox K, McDonagh T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: Results of the UK natriuretic peptide study. Eur J Heart Fail. 2005;7:537–41. doi: 10.1016/j.ejheart.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Berger R, Moertl D, Peter S, Ahmadi R, Huelsmann M, Yamuti S, et al. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55:645–53. doi: 10.1016/j.jacc.2009.08.078. [DOI] [PubMed] [Google Scholar]
- 37.Giannoni A, Tani C, Clerico A, Passino C, Tavoni A, d’Ascanio A, et al. When the heart is burning: Amino-terminal pro-brain natriuretic peptide as an early marker of cardiac involvement in active autoimmune rheumatic disease. Int J Cardiol. 2011;148:161–7. doi: 10.1016/j.ijcard.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 38.Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas G. B-type natriuretic peptide in rheumatic diseases: A cardiac biomarker or a sophisticated acute phase reactant? Autoimmun Rev. 2012;11:837–43. doi: 10.1016/j.autrev.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Gölbaşý Z, Uçar O, Yüksel AG, Gülel O, Aydoğdu S, Ulusoy V. Plasma brain natriuretic peptide levels in patients with rheumatic heart disease. Eur J Heart Fail. 2004;6:757–60. doi: 10.1016/j.ejheart.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Pande S, Agarwal SK, Dhir U, Chaudhary A, Kumar S, Agarwal V. Pulmonary arterial hypertension in rheumatic mitral stenosis: Does it affect right ventricular function and outcome after mitral valve replacement? Interact Cardiovasc Thorac Surg. 2009;9:421–5. doi: 10.1510/icvts.2009.206607. [DOI] [PubMed] [Google Scholar]


