Abstract
Context:
Tissue plasminogen activator (tPA) is underutilized for treatment of acute ischemic stroke.
Objective:
To determine whether the probability of tPA administration for patients with ischemic stroke in an integrated health care system improved from 2009 to 2013, and to identify predictors of tPA administration.
Design:
Retrospective analysis of all ischemic stroke presentations to 14 Emergency Departments between 2009 and 2013. A generalized linear mixed-effects model identified patient and hospital predictors of tPA.
Main Outcome Measures:
Primary outcome was tPA administration; secondary outcomes were door-to-imaging and door-to-needle times and tPA-related bleeding complications.
Results:
Of the 11,630 patients, 3.9% received tPA. The likelihood of tPA administration increased with presentation in 2012 and 2013 (odds ratio [OR] = 1.75; 95% confidence interval [CI] = 1.26–2.43; and OR = 2.58; 95% CI = 1.90–3.51), female sex (OR = 1.27; 95% CI = 1.04–1.54), and ambulance arrival (OR = 2.17; 95% CI = 1.76–2.67), and decreased with prior stroke (OR = 0.47; 95% CI = 0.25–0.89) and increased age (OR = 0.98; 95% CI = 0.97–0.99). Likelihood varied by Medical Center (pseudo-intraclass correlation coefficient 13.5%). Among tPA-treated patients, median door-to-imaging time was 15 minutes (interquartile range, 9–23 minutes), and door-to-needle time was 73 minutes (interquartile range, 55–103 minutes). The rate of intracranial hemorrhage was 4.2% and 0.9% among tPA- and non-tPA treated patients (p < 0.001).
Conclusion:
Acute ischemic stroke care improved over time in this integrated health system. Better understanding of differences in hospital performance will have important quality-improvement and policy implications.
INTRODUCTION
As US health care reform leads to growth of the accountable care organization model, it is important to understand how these systems perform in the care of patients with ischemic stroke. Integrated health systems, with aligned incentives and efficiencies, function similarly to accountable care organizations. However, aside from the Veterans Health Administration, patterns of acute ischemic stroke care delivery in an integrated health system have not been well described,1 and other settings have reported underuse of recommended acute ischemic stroke treatment.2–5
This study describes patterns of tissue plasminogen activator (tPA) delivery in an integrated health system (Kaiser Permanente Southern California [KPSC]), which comprises multiple Emergency Departments and hospital systems, and serves approximately 3.8 million members. Our primary objective was to determine whether the probability of tPA administration for patients with acute ischemic stroke in the KPSC system has improved from 2009 to 2013, and to identify predictors of tPA administration. Secondarily, we aimed to describe trends in door-to-imaging time and door-to-needle time metrics during the same period, and to describe complication rates among patients treated with tPA. In 2014, the KPSC health care system implemented a telemedicine stroke (“telestroke”) initiative to improve the delivery of ischemic stroke care. This report will provide important baseline performance data to inform future analyses of the telestroke implementation.
METHODS
Data Source and Populations
Structured data from electronic health and administrative records identified all patients presenting to a KPSC Emergency Department between 2009 and 2013 with a primary or secondary diagnosis of ischemic stroke (International Classification of Diseases, Ninth Revision [ICD-9] codes 433.xx, 434.xx, 436). Patients were seen at 1 of 14 KPSC Medical Center Emergency Departments (EDs) of varying size and urbanicity (degree to which a geographic region is urban), 2 of which are academic with inhospital neurology house staff. We excluded patients younger than age 18 years, those with a stroke within 90 days, and those with missing or implausible outcome variables. Human subjects approval was obtained through the KPSC institutional review board.
Outcome Measures
The primary outcome was tPA administration, identified by pharmacy code. Secondary outcome variables were door-to-imaging time and door-to-needle time for tPA delivery. Complications examined were intracranial and gastrointestinal bleeding, defined as ICD-9 codes of 432.xx, 430, 431 and 578.xx. One of the authors (AS) reviewed all patients’ charts in which there was a question about the presence of the outcome variable.
Statistical Analysis
Patient and hospital characteristics were summarized as percentages, means (standard deviation [SD]), or median (interquartile range, [IQR]) as appropriate. Descriptive statistics were used to determine the proportion of patients receiving tPA as well as the mean and median door-to-imaging and door-to-needle times. The annual trend in tPA administration was assessed by ED by plotting the annual proportions by year, separately for each ED.
We used a generalized linear mixed-effects model, with a logit link, to identify patient and hospital predictors of tPA administration. Hospital-level random intercepts were included to account for between-hospital variation in baseline rates of tPA use and to allow for any variation in tPA use caused by unmeasured hospital-level factors. Patient-level covariates included in the model were age, race, sex, prior-year stroke, and Elixhauser comorbidity score. Additionally, the following variables describing the presentation of the patient encounter were included in the analysis: arrival by ambulance, arrival from a skilled nursing facility, arrival during “off-hours” (defined as between 5 pm and 8 am Monday through Friday, or anytime Saturday or Sunday), and year of diagnosis. Variation in rates of tPA administration caused by hospital-level variables was assumed to be captured in the hospital-level random intercept. A modified version of the multivariable model treated hospital as a fixed effect to assess whether the annual trend in tPA use varied by ED, after adjusting for the relevant patient and presentation characteristics. When necessary, the likelihood ratio test was used to compare the fit of competing models.
Because change in tPA administration varied between hospitals (Figures 1 and 2), we also tested a multivariable model that included a random slope for year of diagnosis, to allow the change in stroke volume over time to vary between EDs, but we found no significant improvement in model fit compared with the final model.
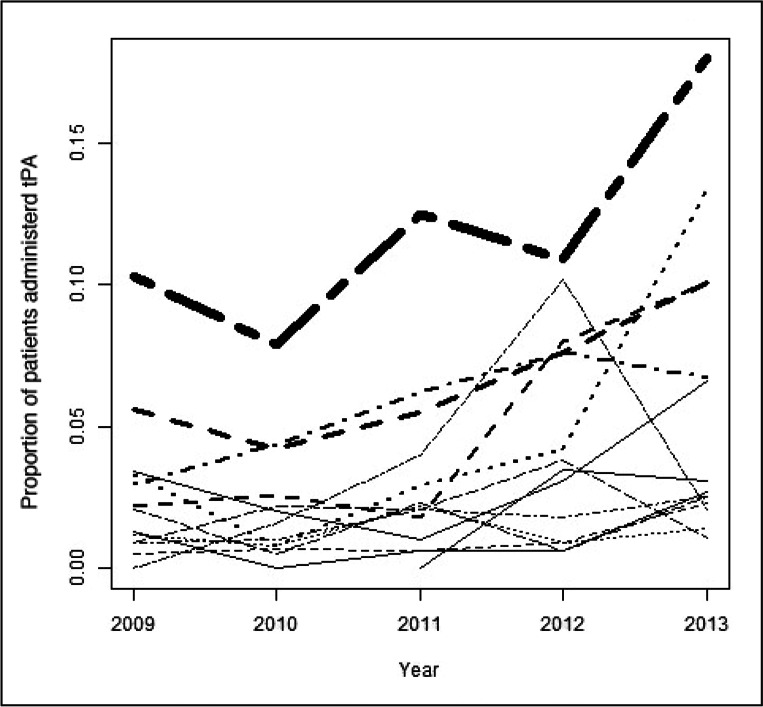
Figure 1.
Unadjusted proportions of tissue plasminogen activator received, by hospital and year.
Thickness of line represents stroke volume for different Emergency Departments.
tPA = tissue plasminogen activator.
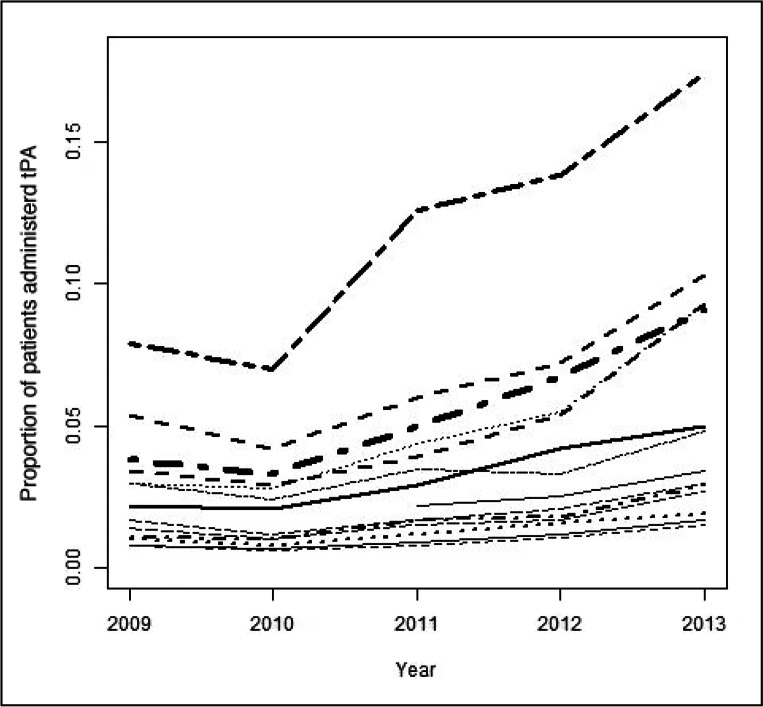
Figure 2.
Multivariable-adjusteda proportions of tissue plasminogen activator received, by hospital and year.
a On the basis of model estimates using hospital as a fixed effect.
tPA = tissue plasminogen activator.
All descriptive analyses were conducted using statistical software (SAS Version 9.3, SAS Institute Inc, Cary, NC). Figures 1 and 2 and multivariable model results were obtained via the R Project for Statistical Computing software package (Free Software Foundation, Boston, MA).
RESULTS
A total of 11,630 patients with ischemic stroke were seen at the 14 KPSC EDs during the 2009 to 2013 study period. Approximately half were women (49.6%); 47.8% of patients were white, 25.4% were Hispanic, 17.5% were black, 8.4% were of Asian or Pacific Island origin, and 0.9% were other races or ethnicities. Comorbidities included diabetes (31.9%), hypertension (63.2%), heart failure (12%), atrial fibrillation (13.9%), and valvular heart disease (8.6%). A minority of patients arrived by ambulance (37.2%).
Of the overall sample, 3.9% of patients were treated with tPA; the proportion of tPA-treated patients increased during the study period from 2.6% in 2009 to 6.4% in 2013 (Table 1). In the multivariate analysis, the likelihood of tPA administration was increased for patients presenting in 2012 and 2013 (OR = 1.75; 95% CI = 1.26–2.43; and OR = 2.58; 95% CI = 1.90–3.51, respectively), female sex (OR = 1.27; 95% CI = 1.04–1.54), and arrival by ambulance (OR = 2.17; 95% CI = 1.76–2.67). Conversely, prior stroke and increasing age were associated with a decreased likelihood (OR = 0.47; 95% CI = 0.25–0.89; and OR = 0.98; 95% CI = 0.97–0.99, respectively). Both unadjusted and multivariable-adjusted rates of tPA treatment varied by ED (pseudo-intraclass correlation coefficient, 13.5%; Figures 1 and 2).
Table 1.
Brain imaging and thrombolytic metrics, 2009 to 2013
| Metric | 2009 | 2010 | 2011 | 2012 | 2013 | All years |
|---|---|---|---|---|---|---|
| Total no. of patients | 2475 | 2343 | 2334 | 2163 | 2315 | 11,630 |
| tPA-treated patients, no. (%) | 64 (2.6) | 53 (2.3) | 87 (3.7) | 100 (4.6) | 149 (6.4) | 453 (3.9) |
| Door-to-imaging time | ||||||
| All patients, median minutes (IQR) | 58 (35–87) | 55 (34–84) | 48 (25–79) | 47 (23–76) | 46 (23–78) | 51 (29–81) |
| tPA-treated patients, median minutes (IQR) | 23 (14.5–28) | 16 (8–29) | 13 (9–20) | 13 (8–22) | 15 (9–22) | 15 (9–23) |
| Door-to-needle time | ||||||
| Minutes, median (IQR) | 94 (67–128) | 79 (62–112) | 74 (58–96) | 73.5 (54.5–103.5) | 67 (50–89) | 73 (55–103) |
| Percentage of patients < 60 minutes | 20.3 | 22.6 | 27.6 | 32 | 40.3 | 31.1 |
IQR = interquartile range; tPA = tissue plasminogen activator.
The median door-to-imaging time among all patients was 51 minutes (IQR = 29-81 minutes), and among tPA-treated patients was 15 minutes (IQR = 9–23 minutes). The door-to-imaging time improved during the study period from 58 minutes in 2009 to 46 minutes in 2013. The median door-to-needle time was 73 minutes overall (IQR = 55–103 minutes), and 31.1% of patients had a door-to-needle time within the 60-minute guideline-recommended window.6 The door-to-needle time also improved during the study period (Table 1).
Of the overall sample, 124 patients (1.1%) experienced intracranial bleeding after a primary ischemic stroke diagnosis. Patients who did not receive tPA had a lower rate of intracranial bleeding than those receiving tPA (0.9% vs 4.2%, p < 0.001) but did not have a higher rate of gastrointestinal bleeding (0.4% vs 0.2%, p = 0.55).
DISCUSSION
In this analysis of all patients with ischemic stroke in an integrated health system, we found that the probability of tPA administration has improved over time, with concordant improvement in door-to-imaging and door-to-needle times during the same period. Emergency care of acute patients with ischemic stroke is improving in this integrated health system.
This improvement in emergent care of acute ischemic stroke is similar to that observed in larger reports of national trends.7–9 Although our overall rate of tPA administration is low, we were limited by our inability to determine patient eligibility for tPA, and it is likely that a substantial portion of the patients in our sample were ineligible for treatment because of a prolonged onset-to-arrival time or other reasons for ineligibility. Furthermore, the rates we report are similar to rates from other hospitals and health systems when the proportion of tPA delivered among all patients with ischemic stroke is reported.10–13 We found that tPA was less likely to be used in patients with a history of stroke and in those with increased age. We suspect that this reduced likelihood is because of exclusion criteria of tPA in randomized controlled trials, which have excluded patients with recent stroke (within the past 3 months)14 and patients older than age 80 years.15
We found a longer median door-to-imaging time than has been recently reported in other settings.8,9 However, one of these reports was isolated to only tPA-treated patients.8 When we examined door-to-imaging time among tPA-treated patients, our results were similar, with most patients receiving imaging within the guideline-recommended 25-minute window. We also noted that fewer patients in our sample arrived by ambulance than in other reports.7,8 Given that ambulance arrival often correlates with stroke severity, this may be an indication that overall stroke severity in our sample was lower than in other reports. Unfortunately, we did not have a measure of stroke severity available in our data to assess this.
It is also important to note that in this integrated health system composed of 14 hospitals of varying size, urbanicity, and academic status, the rate of intracerebral hemorrhage among tPA-treated patients was similar to rates in the randomized controlled trials and larger effectiveness reports.7,14
Our findings confirm that in our integrated health system, delivery of emergency care for patients with acute ischemic stroke is similar to national trends. Yet as Figure 1 illustrates, even in a single integrated system, the delivery of tPA varies widely between hospitals, both with respect to baseline performance and improvement during the study period. Some hospitals showed marked improvement, whereas others remained more static. Thus, it is important to better understand factors that drive differences in stroke care delivery between hospitals and to explore varying approaches to quality-improvement processes to improve care at lower-performing centers and ensure high-quality care across the board.
Our study has several limitations. Because we were unable to determine the time of symptom onset because of inherent limitations of our data, we were unable to define the population of patients eligible for tPA. However, other reports have described rates of tPA administration among all patients with ischemic stroke, and our results can be interpreted in this context. Our use of ICD-9 codes for sample specification and to identify treatment complications may have introduced bias to the sample; however, on a limited chart review by the authors, we found that the ICD-9 codes were appropriately identifying patients with ischemic stroke, and we did not note any change in documentation over the study period to suggest that this would bias the trends we report. We were further limited by our data in our inability to capture the National Institutes of Health Stroke Scale for all patients. Although we were unable to account for stroke severity, we did include the Elixhauser comorbidity index to capture the acuity of patients’ presentation. We were also unable to determine patient eligibility for tPA; thus, it is possible that other factors led to increased tPA utilization. For example, public awareness campaigns may have increased the proportion of patients arriving within the appropriate time window for eligibility, or clinicians may have become more aware of or more comfortable with administration of the treatment. Finally, we may have introduced bias by excluding patients with missing or implausible door-to-imaging times; however, we opted to include only those patients in whom the ED time course and care delivery were consistent with presentation of acute ischemic stroke to avoid the alternative bias of a misspecified sample.
CONCLUSION
In the KPSC integrated health system, acute ischemic stroke care delivery is improving over time, as evidenced by increased rates of tPA delivery and improved door-to-imaging and door-to-needle times. Better understanding of differences in hospital performance in an integrated system will have important implications for quality improvement and policy development.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Apoplexy
This apoplexy, as I take it, is a kind of lethargy, an’t please your lordship; a kind of sleeping in the blood, a whoreson tingling ... It hath its original from much grief, from study and perturbation of the brain. I have read the cause of his effects in Galen. It is a kind of deafness.
— Henry IV, Part II, I, ii, 126, William Shakespeare, 1564–1616, English poet, playwright, and actor
References
- 1.Keyhani S, Arling G, Williams LS, et al. The use and misuse of thrombolytic therapy within the Veterans Health Administration. Med Care. 2012 Jan;50(1):66–73. doi: 10.1097/MLR.0b013e3182294092. DOI: http://dx.doi.org/10.1097/MLR.0b013e3182294092. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Suri MF, Nasar A, et al. Thrombolysis for ischemic stroke in the United States: data from National Hospital Discharge Survey 1999–2001. Neurosurgery. 2005 Oct;57(4):647–54. DOI: http://dx.doi.org/10.1227/01.NEU.0000175546.62088.D6. [PubMed] [Google Scholar]
- 3.Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000 Mar 1;283(9):1151–8. doi: 10.1001/jama.283.9.1151. DOI: http://dx.doi.org/10.1001/JAMA.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 4.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM, Cleveland Clinic Health System Stroke Quality Improvement Team Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004 Mar;61(3):346–50. doi: 10.1001/archneur.61.3.346. DOI: http://dx.doi.org/10.1001/archneur.61.3.346. [DOI] [PubMed] [Google Scholar]
- 5.Katzan IL, Hammer MD, Furlan AJ, Hixson ED, Nadzam DM, Cleveland Clinic Health System Stroke Quality Improvement Team Quality improvement and tissue-type plasminogen activator for acute ischemic stroke: a Cleveland update. Stroke. 2003 Mar;34(3):799–800. doi: 10.1161/01.STR.0000056944.42686.1E. DOI: http://dx.doi.org/10.1161/01.STR.0000056944.42686.1E. [DOI] [PubMed] [Google Scholar]
- 6.Jauch EC, Saver JL, Adams HP, et al. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease, Council on Clinical Cardiology. Stroke: AHA/ASA guideline [Internet] Dallas, TX: American Heart Association; 2015 [cited 2015 Nov 30]. Available from: http://stroke.ahajournals.org/content/early/2013/01/31/STR.0b013e318284056a.full. [Google Scholar]
- 7.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013 Jun 19;309(23):2480–8. doi: 10.1001/jama.2013.6959. DOI: http://dx.doi.org/10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 8.Sauser K, Burke JF, Levine DA, Scott PA, Meurer WJ. Time to brain imaging in acute stroke is improving: secondary analysis of the INSTINCT trial. Stroke. 2014 Jan;45(1):287–9. doi: 10.1161/STROKEAHA.113.003678. DOI: http://dx.doi.org/10.1161/STROKEAHA.113.003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly AG, Hellkamp AS, Olson D, Smith EE, Schwamm LH. Predictors of rapid brain imaging in acute stroke: analysis of the Get With the Guidelines-Stroke program. Stroke. 2012 May;43(5):1279–84. doi: 10.1161/STROKEAHA.111.626374. DOI: http://dx.doi.org/10.1161/STROKEAHA.111.626374. [DOI] [PubMed] [Google Scholar]
- 10.Kleindorfer D, de los Rios La Rosa F, Khatri P, Kissela B, Mackey J, Adeoye O. Temporal trends in acute stroke management. Stroke. 2013 Jun;44(6 Suppl 1):S129–31. doi: 10.1161/STROKEAHA.113.001457. DOI: http://dx.doi.org/10.1161/STROKEAHA.113.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakaran S, McNulty M, O’Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke. 2012 Mar;43(3):875–7. doi: 10.1161/STROKEAHA.111.640060. DOI: http://dx.doi.org/10.1161/STROKEAHA.111.640060. [DOI] [PubMed] [Google Scholar]
- 12.Mullen MT, Kasner SE, Kallan MJ, Kleindorfer DO, Albright KC, Carr BG. Joint Commission primary stroke centers utilize more rt-PA in the nationwide inpatient sample. J Am Heart Assoc. 2013 Mar 26;2(2):e000071. doi: 10.1161/JAHA.112.000071. DOI: http://dx.doi.org/10.1161/JAHA.112.000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasr DM, Brinjikji W, Cloft HJ, Rabenstein AA. Utilization of intravenous thrombolysis is increasing in the United States. Int J Stroke. 2013 Dec;8(8):681–8. doi: 10.1111/j.1747-4949.2012.00844.x. DOI: http://dx.doi.org/10.1111/j.1747-4949.2012.00844.x. [DOI] [PubMed] [Google Scholar]
- 14.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995 Dec 14;333(24):1581–7. doi: 10.1056/NEJM199512143332401. DOI: http://dx.doi.org/10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317–29. doi: 10.1056/NEJMoa0804656. DOI: http://dx.doi.org/10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]