Abstract
Needle fear typically begins in childhood and represents an important health-related issue across the lifespan. Individuals who are highly fearful of needles frequently avoid health care. Although guidance exists for managing needle pain and fear during procedures, the most highly fearful may refuse or abstain from such procedures. The purpose of a clinical practice guideline (CPG) is to provide actionable instruction on the management of a particular health concern; this guidance emerges from a systematic process. Using evidence from a rigorous systematic review interpreted by an expert panel, this CPG provides recommendations on exposure-based interventions for high levels of needle fear in children and adults. The AGREE-II, GRADE, and Cochrane methodologies were used. Exposure-based interventions were included. The included evidence was very low quality on average. Strong recommendations include the following. In vivo (live/in person) exposure-based therapy is recommended (vs. no treatment) for children seven years and older and adults with high levels of needle fear. Non-in vivo (imaginal, computer-based) exposure (vs. no treatment) is recommended for individuals (over seven years of age) who are unwilling to undergo in vivo exposure. Although there were no included trials which examined children < 7 years, exposure-based interventions are discussed as good clinical practice. Implementation considerations are discussed and clinical tools are provided. Utilization of these recommended practices may lead to improved health outcomes due to better health care compliance. Research on the understanding and treatment of high levels of needle fear is urgently needed; specific recommendations are provided.
Keywords: Fear, phobia, needle, blood–injection–injury, exposure, clinical practice guideline
Introduction
Needle fear: epidemiology and impact
Needle procedures (e.g. vaccinations and venipunctures) are common in health care. They are used to diagnose, treat, prevent, and monitor different conditions across the lifespan. As an example, vaccinations are one of the most important advances in medicine and have been responsible for the reduction and eradication of a host of infectious diseases (Ulmer, Valley, & Rappuoli, 2006; Worboys, 2007); at approximately 12 billion injections per year, vaccinations are the most commonly occurring painful medical procedure worldwide (Miller & Pisani, 1999).
Fear of needles is a prevalent yet under recognized and under prioritized health issue. Fear of needles is common in both children (~33–63%) and adults (~14–38%) and can contribute to negative experiences with needle procedures and health care for patients, caregivers, and health professionals (Deacon & Abramowitz, 2006; Nir, Paz, Sabo, & Potasman, 2003; Taddio et al., 2012). While many children and adults are able to manage their apprehension and successfully undergo needle procedures, some individuals have sufficiently elevated levels of anxiety and fear that they respond in ways that interfere with clinicians’ abilities to carry out procedures (e.g. due to flailing and attempts to escape). Significant fear can also undermine the efficacy of pain interventions at the time of the needle (McMurtry, Pillai Riddell et al., 2015). Needle fear can also result in a host of deleterious consequences beyond a given procedure, including vaccination noncompliance and more general health care avoidance (Hamilton, 1995; McMurtry, Pillai Riddell et al., 2015; Taddio et al., 2012). Individuals with chronic and life-threatening health conditions (e.g. diabetes and multiple sclerosis) and high levels of needle fear who require repeated injections are a particularly vulnerable group, as they may make important treatment decisions based on fear rather than medical recommendations (Ellinwood & Hamilton, 1991; Hamilton, 1995; Mohr, Boudewyn, Likosky, Levine, & Goodkin, 2001; Wright, Yelland, Heathcote, Ng, & Wright, 2009).
A high level of needle fear typically begins in childhood and can follow a chronic course without treatment (American Psychiatric Association, 2013; Goisman et al., 1998; Öst, 1992). Needle fears can be conceptualized along a spectrum from mild to high to clinically significant phobia depending on the level of distress and impairment/interference [e.g. avoidance of medical care; for a detailed consideration of pain, fear, anxiety, phobia, and fainting in the context of needle procedures see (McMurtry, Pillai Riddell et al., 2015)]. For the purposes of this guideline, we are distinguishing between the formal diagnosis of a needle-related phobia and focusing on the broader clinical category of high needle fear.
The formal diagnosis of a needle-related phobia falls under the blood–injection–injury type of specific phobia in the Diagnostic and Statistical Manual of Mental Disorders - Fifth Edition [DSM-5 (American Psychiatric Association, 2013)] and the Neurotic, Stress-related and Somatoform Disorders F40.2 Specific (Isolated) Disorders within the World Health Organization’s (2015) International Classification of Diseases. To meet diagnostic threshold, an individual must display persistent, excessive, and severe anxiety in anticipation of, and fear when confronted with, a particular stimulus (i.e. needle procedures). A unique feature of blood–injection–injury phobia is the higher preponderance of a vasovagal (fainting) response compared to the general population; this is not seen in other phobias (Oar, Farrell, Waters, & Ollendick, in press; Öst, 1992).
For this guideline, we included individuals with a high degree of needle fear who may or may not have a diagnosis of a specific phobia of needles because: (1) sub-threshold but high needle fears are important, given the risk they pose for avoidance of medical procedures; (2) this enables a practical approach to screening individuals who would benefit from specific treatment to manage their fear before they undergo routine needle procedures (Taddio, McMurtry, Shah, Pillai Riddell et al., 2015).
Guideline team, scope, and purpose
In 2010, a multidisciplinary team from across Canada, Help ELiminate Pain in KIDS (HELPinKIDS), published the first clinical practice guideline (CPG) on pediatric vaccination pain mitigation (Taddio et al., 2010). Funded by a federal grant in 2013 (Canadian Institutes of Health Research, co-principal investigators: Taddio & McMurtry), the team reconvened and expanded its membership to include individuals with expertise in working with adults and specialists on fear and anxiety to allow consideration of the full lifespan and the management of high levels of needle fear. It was recognized that: (1) needle fear is prevalent across the lifespan and poses a significant deterrent to vaccination and other health-promoting behaviors; (2) efforts should be made to better manage pain in order to help prevent needle fears from developing; and (3) guidance was needed regarding the management of individuals currently suffering from high levels of needle fear. Given that there was no prior CPG for needle fears, the team undertook an expanded knowledge synthesis and revision of the original pain management guideline in 2015 to examine pain management and interventions for the management of high levels of needle fear across the lifespan. To reflect the expanded scope, the name of the team was changed to HELPinKids&Adults.
The following is a brief summary of the HELPinKids&Adults’ CPG recommendations for the management of high levels of needle fear [the guideline on vaccination pain management appears elsewhere (Taddio, McMurtry, Shah, Pillai Riddell et al., 2015)]. The purpose of a CPG is to provide instruction to clinicians and patients on the management of a particular health concern; this guidance emerges from a systematic process with a multidisciplinary team that considers risks, benefits, and patient preferences while adhering to particular standards in order to be rigorous, transparent, reduce bias, and ultimately increase the usefulness of the CPG (Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, 2011; Woolf, Grol, Hutchinson, Eccles, & Grimshaw, 1999). Critically, in spite of limitations of the evidence base, a neutral stance (i.e. no recommendation) and/or simple calls for more research are discouraged in a CPG as a clinician requires actionable recommendations to assist a particular patient/client. A CPG may also change over new time as new evidence emerges (Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, 2011). The current work is the first CPG for needle fear across the lifespan. As noted, a CPG is designed to provide recommendations for how to proceed with a given patient; thus, for individuals who are skilled in a particular area, it may seem less “novel” if they are already using the intervention(s) in question. However, a CPG has value in that it: (1) can help optimize patient care through consistent evidence-based practice; (2) act as a refresher or resource for experienced professionals (for example, in this case: social workers, psychologists, and/or psychiatrists); (3) provide an introduction for trainees; (4) provide basic information for non-specialists who may be called on to make treatment referrals (e.g. here nurses and other health care professionals responsible for delivering needle procedures); and (5) highlight issues for future research. The included exposure-based interventions in this CPG are wholly distinct from interventions designed to manage low to moderate levels of fear and pain implemented at the time of needle procedures [e.g. distraction; topical anesthetics; (Birnie et al., 2014; Pillai Riddell et al., 2015; Taddio, McMurtry, Shah, Pillai Riddell et al., 2015; Uman et al., 2013)]. For example, distraction is recommended for procedural pain but is actually thought to interfere with the extinction of fear which is desirable in exposure (Rodriguez & Craske, 1993) so could be counter-productive in treating high levels of needle fear [further discussion of safety behaviors appears later]. The interventions contained in the current CPG are to be implemented outside the vaccination context (i.e. before the individual undergoes needle procedures).
Method
Guideline development
The Grading of Assessments, Recommendations, Development and Evaluation (GRADE) methodology was used to formulate the recommendations (with additional consideration of the extant literature when needed), while the overall guideline process followed the Appraisal of Guidelines for Research and Evaluation - II (AGREE-II; www.agreetrust.org) framework. Full details regarding the guideline team composition are available separately (Taddio, McMurtry, Shah, Yoon et al., 2015). In brief, the individuals were from diverse disciplines including psychology, nursing, medicine, pharmacy, and library sciences with expertise in fear, pain, pediatrics, anxiety, phobias, public health, health policy, infectious diseases, epidemiology, and family advocacy. Team members were involved in all aspects of the project including determination of scope, clinical questions, and recommendation development.
Clinical questions and outcomes
The included clinical questions were formatted using the PICOS (participants, intervention, comparison, outcomes, and study design) framework (below). Following the GRADE process, clinical questions were selected via voting by team members and associated outcomes were categorized as “critically important” or as “important” through this process (Taddio, McMurtry, Shah, Yoon et al., 2015). Altogether, six clinical questions were included that pertained to exposure-based interventions for the management of high levels of needle fear. While other treatments (e.g. hypnosis, relaxation, and coping skills training) have been evaluated in the context of needle fears, exposure-based therapies were focused on given that they are the most widely studied and considered an evidence-based treatment for specific phobias as a group (Choy, Fyer, & Lipsitz, 2007; Davis, May, & Whiting, 2011; Wolitzky-Taylor, Horowitz, Powers, & Telch, 2008). No restrictions were made in terms of included ages and developmental stage was considered within the constraints of available literature.
PICOS
Participants
Children of any age and adults with high levels of needle fear. This included individuals: (1) with a DSM-based diagnosis of blood–injection–injury phobia; (2) who were diagnosed with a related phobia (e.g. injection phobia) in a manner consistent with the DSM series; or (3) with high levels of needle fear and impairment in functioning, such as being unable to self-inject medication needed for a chronic illness (but without a formal diagnosis). In the absence of the aforementioned, individuals with other specific phobias diagnosed according to the DSM series were included; N.B. this occurred only for children and is clearly identified below by clinical question.
Interventions
Exposure-based interventions including in vivo, non-in vivo, single session, multi-session, and applied tension (muscle tension + exposure).
Comparisons
No treatment (i.e. waitlist control) or a specified comparator (e.g. education, support group).
Outcomes (designated critically important or important)
Specific fear (i.e. fear of needles; in the case of an alternate fear/phobia, fear directed toward that particular stimulus/event) was typically the critically important outcome. Important outcomes included general fear (a change in general fear was not necessarily expected as these interventions are tailored to address the specific fear), compliance (typically measured using a behavioral avoidance test 1 in which an individual is presented with a series of steps/tasks of increasing difficulty focusing on the feared situation), parent report of child distress, fainting, and satisfaction.
Study design
Randomized and quasi-randomized controlled trials.
Search strategy, data extraction, data synthesis, and evidence quality
As part of the larger project (Taddio, McMurtry, Shah, Yoon et al., 2015), a single broad search strategy was developed for the following databases: MEDLINE, EMBASE, PsycINFO, CINAHL, and ProQuest Dissertations & Theses Global (inception to 26 February 2015). No language restrictions were applied. The Cochrane Collaboration methodology (www.handbook.cochrane.org) was used for meta-analysis and the Risk of Bias tool for individual study quality assessment (Hartling et al., 2012). Across studies, the GRADE framework was used to assess methodologic quality (McMurtry, Noel et al., 2015).
Evidence interpretation, recommendation formulation, and external review
The intervention was said to have benefit if meta-analyses showed a statistically significant response in favor of the intervention on the critical outcome(s); if the evidence was inconsistent, it was described as showing mixed results. As per the GRADE approach, a neutral stance was avoided (Andrews et al., 2013) and each clinical question was “answered.” Thus, a recommendation was made for or against an intervention in accordance with the following: (1) the strength of the recommendation is indicated by the words “recommend” (strong) or “suggest” (weak); (2) the quality of evidence on which the recommendation is based is categorized into high, moderate, low, or very low and leads to a rating of “confidence” in the effects. Recommendations were formulated based on the magnitude of and confidence in the effect, risk benefit analysis, consideration of values and preferences, resource use, and extant literature (where available and if needed). The perspective of the individual with a high level of needle fear was emphasized over other perspectives (e.g. clinicians and society). The recommendations underwent external review using the AGREE-II methodology as a framework (Brouwers et al., 2010). Feedback was incorporated by the panel and the guideline was finalized.
Practice recommendations
Exposure-based interventions
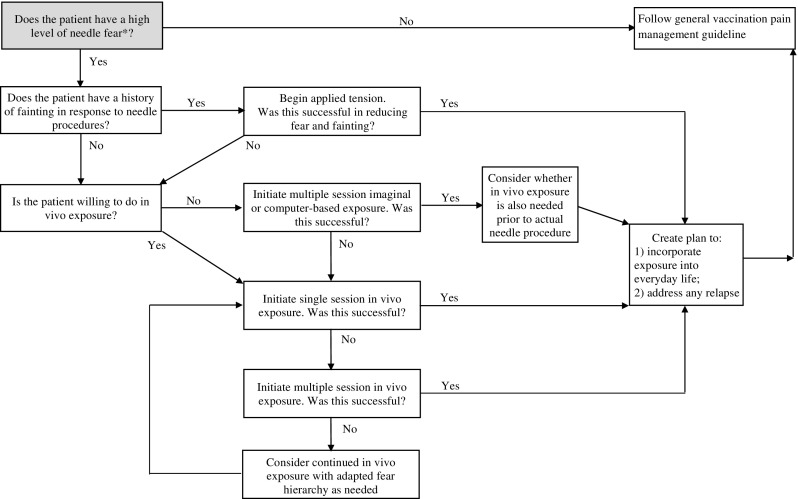
A summary of the recommendations (Table 1) and a treatment algorithm to guide implementation (Figure 1) are provided. Only strong recommendations are described below. Accordingly, the addition of non-exposure-based techniques (i.e. muscle tension 2 ) to exposure and the clinical question relating to dosage (i.e. multiple vs. single sessions of exposure) are not described in the body of the CPG and readers are referred to the online supplemental material for these weak recommendations. Across all questions and outcomes, the quality of evidence was very low. As no trials were identified with children under seven years of age, recommendations have been specified for individuals seven years and older. However, as exposure-based interventions are expected to have a similar impact on children under seven (Freeman et al., 2014; Lewin et al., 2014), we have included guidance for this patient population in our implementation considerations.
Table 1. Interventions for reducing fear and/or fainting in individuals with high levels of needle fear.
| Treatment | Recommendation | Strength | Children (7–12 yr) | Adolescents (>12–17 yr) | Adults (≥ 18 yr) | Confidence |
|---|---|---|---|---|---|---|
| Exposure-based therapy | We recommend in vivo exposure-based therapy (vs. no treatment) | Strong | 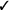 |
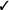 |
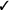 |
Very low |
| If in vivo exposure-based therapy is not used, we recommend non-in vivo exposure-based therapy (vs. no treatment) | Strong | 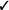 |
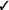 |
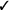 |
Very low | |
| If in vivo exposure-based therapy is used, we suggest a single session rather than multiple sessions* | Weak | 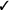 |
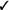 |
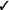 |
Very low | |
| Applied tension (muscle tension and exposure) | We suggest applied tension in individuals with fainting* (vs. exposure alone) | Weak | 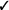 |
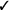 |
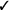 |
Very low |
Described in online supplementary material only.
Figure 1.
Treatment algorithm (for further details and justification see Implementation Considerations sections of the Discussion and the online supplementary information).
*Individuals with a high level of needle fear will show high distress (e.g. shaking, crying, behavioral resistance, flailing, and attempts to escape) prior to a needle or potentially even the mention of a needle and may repetitively avoid/cancel appointments in which a needle might occur. Note that all exposure in this algorithm is hierarchical in nature. For individuals who have a high level of needle fear and a history of fainting, if the initial implementation of applied tension does not successfully reduce both fear and fainting, then targeted exposure (without muscle tension) may also be necessary to address the remaining fear. A plan for incorporating exposure into everyday life and for potential relapse should be included at the end of the treatment. Everyday exposure could take many forms: watching medical TV shows or movies containing needle content, posting pictures of syringes where they will be seen (or as the desktop or screen saver on a computer, phone, or tablet), accompanying family members undergoing needle procedures, becoming a blood donor, or volunteering in a health care setting. Planning for particularly challenging times (e.g. flu shot season) and what to do in times of stress are important for relapse prevention. For a further discussion of relapse planning see Antony and Watling (2006). Once the needle fear has resolved, individuals can then follow the general guideline for vaccination pain management (Taddio, McMurtry, Shah, Pillai Riddell et al., 2015). For simplicity, pain management is pictured as occurring once needle fear is resolved; however, pain management strategies should be put in place for all needle procedures.
Exposure-based therapy is a psychologically based, behavioral intervention that involves individuals being exposed to their fear in a controlled manner that allows them to learn that fear-relevant stimuli are unlikely to cause serious harm and that related distress is manageable. For needle fear, exposure-based therapy typically includes a gradual presentation of aspects of needle procedures in a hierarchical manner of ascending fear. The exposure needs to be of sufficient duration to allow individuals to experience a reduction in fear, learn that catastrophic beliefs of harm are unlikely to occur, or, that if they do occur, they can be tolerated (Öst, 1989). The extent to which safety behaviors (e.g. avoidance, controlled breathing, and distraction) interfere with fear reduction during exposure has been the subject of much debate (Parrish, Radomsky, & Dugas, 2008), but it is typically recommended that the use of these behaviors be reduced during exposure. In addition to in-session hierarchical exposure, these therapies may also include psychoeducation, modeling, “homework” (exposure or other tasks completed outside of the session), and a cognitive component (e.g. exposure targeting catastrophic belief(s) or other cognitive restructuring). Exposure-based therapy can be conducted either in vivo (i.e. live/in person) or through a non-in vivo method (i.e. using computer-based techniques or one’s own imagination) (Choy et al., 2007; Wolitzky-Taylor et al., 2008).
Should in vivo exposure-based therapy be used rather than no treatment/control for children with high levels of needle fear? We recommend in vivo exposure-based therapy (vs. no treatment/control) for children seven years and older with high levels of needle fear (strong recommendation, very low confidence in estimates of effect).
No randomized or quasi-randomized studies were identified for children with high levels of needle fear alone requiring reliance on indirect evidence. Five studies including 263 7–17-year-old children were included in the analysis (Flatt & King, 2010; Leutgeb, Schäfer, Köchel, & Schienle, 2012; Muris, Merckelbach, Holdrinet, & Sijsenaar, 1998; Ollendick et al., 2009; Öst, Svensson, Hellström, & Lindwall, 2001). The children had various phobias including blood–injection–injury phobia (n = 20), phobias of spiders, other animals, and enclosed spaces, among others; of note, the Ollendick et al. (2009) sample excluded individuals with blood–injection–injury phobia. Benefit was observed on both the critical outcome of specific fear measured posttreatment and the important outcome of compliance. There was no evidence of a benefit on the important outcome of general fear. Of note, all included studies delivered the treatment in a single session (see online supplemental material for a clinical question on single vs. multiple sessions).
Should in vivo exposure-based therapy be used rather than no treatment/control for adults with high levels of needle fear? We recommend in vivo exposure-based therapy (vs. no treatment/control) for adults with high levels of needle fear (strong recommendation, very low confidence in estimates of effect).
In one randomized study including 20 adults with blood–injection–injury phobia (Öst, Fellenius, & Sterner, 1991), in vivo exposure-based therapy showed additional benefit over muscle tension on specific fear (critical outcome) immediately posttreatment but no benefit at one-year follow-up. There was no benefit on the important outcomes of general fear and compliance either posttreatment or at one-year follow-up. Finally, there was no evidence of a benefit on the important outcome of fainting at posttreatment or at one-year follow-up.
Should non-in vivo (imaginal) exposure-based therapy rather than no treatment/control be used for children with high levels of needle fear? We recommend non-in vivo (imaginal) exposure-based therapy (vs. no treatment/control) for children seven years and older with high levels of needle fear (strong recommendation, very low confidence in estimates of effect).
No randomized or quasi-randomized studies were identified that examined participants with high levels of needle fear. Two studies including 41 children aged 7–17 years old with phobias of spiders or darkness (Cornwall, Spence, & Schotte, 1996; Muris et al., 1998) showed benefit for the critical outcome of specific fear both immediately after treatment and at three-month follow-up. There were mixed results for the important outcome of general fear, with no benefit seen immediately after treatment but a benefit at three-month follow-up. Parents’ ratings of their children’s distress in their feared situation (important outcome) showed benefit both immediately posttreatment and at three-month follow-up. When both studies were included for the immediate posttreatment compliance indicator, there was no benefit observed; however, when the study which used a potentially problematic “placebo” comparator [computer-delivered stimuli; (Muris et al., 1998)] was removed, a benefit of the intervention was seen. Increased compliance was seen in the imaginal exposure group at three-month follow-up.
Should non-in vivo (imaginal, computer-based) exposure-based therapy rather than no treatment/control be used for adults with high levels of needle fear? We recommend non-in vivo (imaginal, computer-based) exposure-based therapy (vs. no treatment/control) for adults with high levels of needle fear (strong recommendation, very low confidence in estimates of effect).
Two randomized studies including 114 adults with a high degree of needle fear undergoing dental injections or self-injection of medication were included (Heaton, Leroux, Ruff, & Coldwell, 2013; Mohr, Cox, & Merluzzi, 2005). The results were mixed for different indicators of specific fear (critical outcome); there was a benefit for fear assessed via questionnaire but not for a rating of acute fear during a voluntary needle procedure (Heaton et al., 2013). For the important outcome of compliance, there was evidence of a benefit.
Discussion
To our knowledge, this is the first time that recommendations for the management of high levels of needle fear, across the lifespan, have been assembled in a Clinical Practice Guideline for mental health practitioners. The strength of this tool for clinical practice also lies with its synergy with the companion guideline for the management of pain during vaccine injections (Taddio, McMurtry, Shah, Pillai Riddell et al., 2015). While the latter guideline also includes psychological interventions, they are distinct from the psychological interventions recommended in the present guideline as those pain mitigation strategies are to be employed in individuals without significant levels of needle fear during actual vaccine injection procedures and also include distraction-based approaches (Birnie et al., 2014, 2015; Boerner et al., 2015; Pillai Riddell et al., 2015; Uman et al., 2013). The use of distraction and other neutralization or safety behaviors within exposure-based treatments for fear and anxiety is typically discouraged as they are thought to interfere with extinction of the fear response (Rodriguez & Craske, 1993); none of the included trials reported measuring such safety behavior. This guideline serves as an important foundation to move the field forward; future research directions are explored below. High needle fear is ubiquitous is medical settings from community practices to outpatient clinics to inpatient hospital rooms. Our CPG has clearly defined the parameters of the literature and the dire need for more high-quality work in this crucial area. However, despite the state of the field, high needle fear and phobia must be treated. The following are our recommendations for practice based on the current literature available.
Implementation considerations
General principles
We are confident that children, their parents, as well as adults suffering from high levels of needle fear value a reduction in their fear (Taddio, Ilersich, Ilersich, & Wells, 2014; Taddio et al., 2012). In fact, this recognition was a major factor in determining the strength of our strong recommendations. Although resources and specific mental health expertise are required to provide these interventions, these costs are considered justified as needle fear/phobia does not usually resolve without intervention and, downstream, increases risk of illness and morbidity/mortality via decreased engagement in positive health behaviors (e.g. adherence to medical care) across the lifespan (Hamilton, 1995; McMurtry, Pillai Riddell et al., 2015; Mohr et al., 2001; Sokolowski, Giovannitti, & Boynes, 2010; Taddio et al., 2012; Wright et al., 2009).
In accordance with patient-/client-centered care, health care providers trained in exposure therapy need to: (1) conduct a thorough functional assessment prior to creation of fear hierarchies and exposure; (2) gauge the fit between an individual (readiness and tolerance) and the mode of delivery to provide the “dose” and type of exposure required; the use of single- vs. multiple-session treatment is explored in the online supplementary materials. An individual’s response to the interventions should be assessed and can be achieved in part through the use of in-session exposure (fear ratings, notation of successfully completed steps on the fear hierarchy). Response to intervention would clearly impact the frequency, duration, and focus of the treatment. In-depth assessment at the end of formal treatment would typically be consistent with pretreatment measures, with the addition of measures of treatment satisfaction (see online supplementary material). The end of treatment should also include explicit planning for the integration of ongoing exposure into everyday life as well as how to address any relapse (see Figure 1).
Delivery: type of exposure
Both in vivo and non-in vivo delivery of exposure-based therapy are recommended for individuals with high levels of needle fear (vs. no treatment/control/placebo). It is important to note that while these approaches have been dichotomized in the current CPG, intermediary forms such as virtual reality also exist. In formulating our algorithm, we prioritized in vivo (vs. non-in vivo via computer or imagination). This was determined by considering the evidence base for specific phobias as a whole which contains more accumulated evidence with in vivo delivery and some evidence of its higher effectiveness (Choy et al., 2007; Wolitzky-Taylor et al., 2008). Thus, we suggest as good practice that in vivo exposure be offered as the primary intervention with non-in vivo exposure-based therapy reserved for individuals who would prefer to pursue that form. Individuals can be asked about their preferences regarding in vivo vs. non-in vivo treatment. Preferences might play an important role in treatment adherence and efficacy, although this was not assessed. Included trials varied in terms of the number of non-in vivo sessions delivered (1–6 sessions). For individuals who select non-in vivo exposure-based therapy, the number of sessions required will likely vary with the individual and the particular mode of delivery (e.g. imaginal vs. computer). More sessions will likely be required for exposures that are further removed from reality in order to sufficiently reduce the needle fear and adequately prepare an individual for the actual needle procedure. Applied tension should be considered for individuals with high levels of needle fear and a history of fainting; our recommendation supporting this treatment as well as implementation considerations appear in the guideline’s online supplemental materials.
Good clinical practice with younger children
The interventions included in this guideline must be tailored to the individual patient. With respect to children, the age of seven years and older was used as a cut-off for the recommendations because no trials meeting the inclusion criteria were identified with younger children. However, the peak age of onset of needle fears is between 5 and 10 years of age (Bienvenu & Eaton, 1998; LeBeau et al., 2010). Thus, guidance regarding treatment in young children is needed. Based on the broader exposure-based treatment literature in pediatric anxiety disorders (Freeman et al., 2014; Lewin et al., 2014) and existing case studies for needle phobia (Dash, 1981), we are confident that exposure-based treatments can be adapted to younger children. For instance, the fear hierarchy could involve children’s books about needles, having the child give a teddy bear a needle, and working up to a real needle procedure.
The role of the parent in exposure-based therapy is unclear. A recent trial comparing parent augmented exposure treatment with child-focused exposure treatment for specific phobias found no benefit of parent involvement (Ollendick et al., 2015). Only one of the included trials systematically manipulated parental involvement (present or not); however, when parents were present, the degree of their participation was described as highly variable and generally no difference was found between conditions (Öst et al., 2001). Prior anxiety research also demonstrates that the level of parental involvement is not uniformly related to child outcome (Barrett, Dadds, & Rapee, 1996; Öst et al., 2001; Reynolds, Wilson, Austin, & Hooper, 2012). The younger a child is, the more likely his/her parent(s) will be involved, particularly for activities outside treatment sessions (e.g. homework, ongoing exposure, and relapse planning) (Barrett et al., 1996; Freeman et al., 2008, 2014). Also, as fears can be acquired and maintained through familial vicarious and instrumental learning processes, clinicians should consider parental needle fear and behaviors which may exacerbate children’s fear (Davis, Ollendick, Reuther, & Munson, 2012).
One adult behavior that may exacerbate child fear and should therefore be avoided is simplistic or uninformative reassurance that fails to address the worry (e.g. “don’t worry” and “it’s okay”). Ineffective use of reassurance is problematic in procedural pain (McMurtry, Chambers, McGrath, & Asp, 2010; McMurtry, McGrath, & Chambers, 2006), longer lasting pain (Pincus et al., 2013; Traeger et al., 2015), and other health contexts (Coia & Morley, 1998; Lucock, Morley, White, & Peake, 1997; McDonald, Daly, Jelinek, Panetta, & Gutman, 1996; Short, Kitchiner, & Curran, 2004), while excessive reassurance seeking by individuals with anxiety disorders is often a treatment target (Asmundson, Abramowitz, Richter, & Whedon, 2010; Salkovskis & Warwick, 2001; Warwick & Salkovskis, 1990). Future research should consider the roles of reassurance and reassurance seeking in the maintenance of needle fears.
Limitations and future research
There are important limitations in the evidence base that warrant consideration and illuminate critical areas for future research. These limitations point to the need to develop a stronger evidence base and re-examine these recommendations in light of emerging data in the future. Although the GRADE process encourages formulation of a recommendation (i.e. avoidance of a neutral stance) to guide clinical practice based on existing evidence, this is particularly challenging when the evidence base is sparse, as it was in the present work. There were also highly limited randomized controlled trials on adults with high levels of needle fear and no pediatric trials. As described in the “Evidence Interpretation, Recommendation Formulation, and External Review” section, the strength of the recommendations (strong and weak) was determined based not only on methodological rigor (among included trials there was a notable lack of methodological rigor), but also a risk benefit analysis, consideration of values and preferences (with an emphasis on the perspective of the individual with needle fear and his/her caregivers), resource use, and extant literature (where available and if needed). The lack of data from randomized controlled trials on exposure-based interventions for needle fear (particularly for children) may be surprising to clinicians, particularly when clearly presented within each clinical question. The companion review (McMurtry, Noel et al., 2015) which informed the current guideline was the first to systematically review the literature on exposure-based treatments for high levels of needle fear across the lifespan, perform complete meta-analyses, and consider quality of the original studies. Much of the existing literature consists of case studies (i.e. 37 specific phobia case studies were excluded from the systematic review), rather than randomized trials; critically, the current recommendations are consistent with the results of these pediatric and adult case studies, a recent narrative review which suggested modified one-session exposure-based treatment for blood–injection–injury phobia (Oar, Farrell, & Ollendick, 2015), as well as the conclusions of reviews for phobias in general (Choy et al., 2007; Davis et al., 2011; Wolitzky-Taylor et al., 2008). The current guideline focused on exposure-based treatments; however, other treatments (e.g. cognitive therapy, hypnosis, and pharmacotherapy) exist. As these treatments are not discussed in the current guideline, the reader is directed to other resources [as listed in the online supplemental information; also (Hood & Antony, 2012; Ollendick & Davis, 2012)].
Table 2 highlights important areas for future research. Additional trials of exposure-based interventions specifically for children and adults with high needle fears and blood–injection–injury phobia are recommended, including the examination of a variety of delivery formats (e.g. computer programs, virtual reality, and remote delivery) and manualized approaches (Chapman & DeLapp, 2014). Future trials should also be designed to provide guidance regarding: (1) typical number of sessions required, (2) transitioning between exposure types (e.g. non-in vivo to in vivo), (3) the use or avoidance of safety behaviors, (4) the role of parents in treatment of children, (5) optimal exposure maintenance plans to prevent relapse, and (6) the use and utility of cognitive-based interventions both alone and in combination with exposure. Future investigation of cost and third-party reimbursement of treatment is also warranted. As noted above, as further research is conducted on this topic, the findings may change the current recommendations. In order to increase the quality and interpretability of the evidence base, we strongly urge researchers to use the established methods put forth by CONSORT and the Cochrane Collaboration when designing and reporting their trials.
Table 2. Selected directions for future research (focusing on high levels of needle fear unless otherwise noted).
| Domain | Knowledge gap |
|---|---|
| Nature of needle fear |
|
|
|
|
|
|
|
|
|
| Assessment |
|
| Treatment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The extent to which explicit and overt cognitive restructuring by a therapist (vs. a process assumed to naturally occur within individuals as a result of exposure) was utilized in the included trials was frequently unclear. The focus of individuals’ fear invariably influences their response to the feared stimulus and intervention and was presumably addressed within the fear hierarchies themselves. Even among individuals with high levels of needle fears, there may be considerable heterogeneity in this regard [e.g. individuals may fear blood, needles, fainting, etc. (Dalley, Creary, & McMurtry, 2014; Oar et al., in press)]. Together, these factors emphasize the need for more targeted assessment of the focus of fear and its relation to outcomes.
The current guideline is based on a state-of-the-art knowledge synthesis utilizing the GRADE and Cochrane approaches. A strength of this evidence base was the inclusion of multiple assessment time points following intervention. Exposure appears to have efficacy for immediate posttreatment outcomes for adults with needle fear and children with other phobias; however, reduced benefit was seen for longer term outcomes (e.g. one-year follow-up). Given the nature of fears, it is likely that if exposure to the feared stimulus does not continue, its efficacy on future fear fades over time. Hence, our algorithm suggests planning for incorporation of exposure into everyday life. More formally, booster sessions have been successfully used as part of cognitive behavioral treatments (Gearing, Schwalbe, Lee, & Hoagwood, 2013) and would likely be of benefit in the context of treating needle fears. Researchers should examine the impact of long-term exposure planning and providing booster sessions in future trials.
Updates and conclusion
Updates to this guideline will be made depending on the emergence of new evidence, stakeholder interest, and availability of funding. The recommendations may change if more research evidence becomes available. This is the first evidence-based CPG for the management of high levels of needle fear across the lifespan. The recommendations were formulated based on a rigorous knowledge synthesis interpreted by an expert panel and support the use of exposure-based approaches (vs. no treatment/control/placebo) to reduce high levels of needle fear. In addition to clinical practice recommendations and recommended resources, this guideline also outlines numerous avenues for future research in this clinically important area.
Funding
Funding for HELPinKids&Adults activities was procured through the Canadian Institutes of Health Research [Knowledge Synthesis Grant KRS 132031; co-PIs: Taddio & McMurtry]; open access funding for publications was provided by The Mayday Fund (United States). The funding bodies had no input into the choice of clinical questions and outcomes, synthesis methods, or development of the clinical practice guidelines [for further details including the handling of conflict of interest, please see (Taddio, McMurtry, Shah, Pillai Riddell et al., 2015)].
Endorsing and supporting organizations
This guideline is endorsed by the following organizations: Canadian Association of Paediatric Health Centres, Canadian Child & Youth Health Coalition, Canadian Family Advisory Network, Canadian Nursing Coalition for Immunization, Canadian Paediatric Society, Canadian Pharmacists Association, Canadian Psychological Association, Canadian Public Health Association, The College of Family Physicians of Canada, Immunize Canada, AnxietyBC, and Nurse Practitioners’ Association of Ontario. This guideline is supported by the following organizations: Canadian Center for Vaccinology and British Columbia Centre for Disease Control.
Disclosure statement
A Taddio declares a grant from Pfizer, and study supplies from Natus and Ferndale. CT Chambers declares consultation fees from Abbvie. E. Lang is a member of the GRADE working group and declares consultation fees from the International Liaison Committee on Resuscitation (ILCOR). L. Bucci declares a relationship with government agencies and grants from Merck, GSK, Novartis, Sanofi, and Pfizer. SA Halperin declares grants from GSK, Sanofi, Novartis, Pfizer, Merck, PREVENT, ImmunoVaccine, NovaVax, Janssen, and Folia.
Supplementary Material
Acknowledgments
Dianne Alexander, Manager, Immunization Policy and Programs, Public Health Division, Ontario Ministry of Health and Long-Term Care, participated as an observer. Thank you to the reviewers of the guideline. Firstly, in combination with the general guideline, this guideline was reviewed by stakeholder organizations with liaison members on the HELPinKids&Adults team, including: British Columbia Centre for Disease Control, Canadian Center for Vaccinology, Canadian Family Advisory Network, Canadian Paediatric Society, Canadian Psychological Association, College of Family Physicians of Canada, and Immunize Canada. Secondly, external review was conducted by individuals with the relevant content expertise, individuals representing stakeholder organizations, or individuals that were members of stakeholder organizations but did not represent them, including: Theresa Agnew (Nurse Practitioners’ Association of Ontario), Oliver Baclic (Public Health Agency of Canada), Katie Birnie, Eliana Castillo (Society of Obstetricians and Gynaecologists of Canada), Shelley Deeks (Public Health Ontario), Philippe De Wals (Comité sur l’Immunisation du Québec), Blaine Ditto, Eve Dube (Institut national de santé publique du Quebec), Martine Dubuc (Public Health Agency of Canada), Philip Emberley (Canadian Pharmacists Association), Lucy Gagliese, Frank Gavin (Canadian Child and Youth Health Coalition), Arielle Goldman Smith (Canadian Nursing Coalition for Immunization), Mary Jerrett (Canadian Institute of Child Health), Judith Law (AnxietyBC), Janet McElhaney (Canadian Geriatric Society), Margaret McIntyre (Public Health Ontario), Kathy Reid, Joan Robinson (Canadian Paediatric Society), Anne Smith (Baby Friendly Initiative), Carl von Baeyer, Gary Walco, Kelly Warmington (The Hospital for Sick Children), Arlene Young, and William Zempsky. The guideline also underwent formal review by the World Health Organization Secretariat with an in-person meeting held with representatives of the HELPinKids&Adults team. Individuals involved in this review included: K.O. Antwi-Agyei, Philippe Duclos, Liesbet Goubert, Darunee Jongudomkarn, Kevin Pottie, Nikki Turner, and Winnie Siu.
Notes
Please note that Öst typically refers to the behavioral measure of fear as a behavioral avoidance test. As his work comprises much of the foundational evidence for this CPG, we have chosen to use the term “behavioral avoidance test.” Completion of steps on a BAT could also be thought of as engagement in the task and behavioral approach.
Muscle tension is a technique designed to raise blood pressure for individuals with a history of fainting; as noted previously, vasovagal syncope is more common in individuals with blood–injection–injury phobia than the typical population or populations with other phobias. The addition of muscle tension to exposure-based therapy is referred to as applied tension.
Supplemental data
Supplemental data for this article can be accessed here. [http://dx.doi.org/10.1080/16506073.2016.1157204]
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Andrews J., Guyatt G., Oxman A. D., Alderson P., Dahm P., Falck-Ytter Y., Schünemann H. J.2013GRADE guidelines: 14. Going from evidence to recommendations: The significance and presentation of recommendations Journal of Clinical Epidemiology ,719–725. 10.1016/j.jclinepi.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Asmundson G. J. G., Abramowitz J. S., Richter A. A., Whedon M.2010Health anxiety: Current perspectives and future directions Current Psychiatry Reports ,306–312. 10.1007/s11920-010-0123-9 [DOI] [PubMed] [Google Scholar]
- Barrett P. M., Dadds M. R., Rapee R. M.1996Family treatment of childhood anxiety: A controlled trial Journal of Consulting & Clinical Psychology ,333–342. [DOI] [PubMed] [Google Scholar]
- Bienvenu O. J., Eaton W. W.1998The epidemiology of blood-injection-injury phobia Psychological Medicine ,1129–1136. 10.1017/S0033291798007144 [DOI] [PubMed] [Google Scholar]
- Birnie K. A., Chambers C. T., Taddio A., McMurtry C. M., Noel M., Pillai Riddell R., HELPinKids&Adults Team 2015Psychological interventions for vaccine injections in children and adolescents: Systematic review of randomized and quasi-randomized controlled trials The Clinical Journal of Pain ,S72–S89. 10.1097/AJP.0000000000000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie K. A., Noel M., Parker J. A., Chambers C. T., Uman L. S., Kisely S. R., McGrath P. J.2014Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents Journal of Pediatric Psychology ,783–808. 10.1093/jpepsy/jsu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner K. E., Birnie K. A., Chambers C. T., Taddio A., McMurtry C. M., Noel M., HELPinKids&Adults Team 2015Simple psychological interventions for reducing pain from common needle procedures in adults: Systematic review of randomized and quasi-randomized controlled trials The Clinical Journal of Pain ,S90–S98. 10.1097/AJP.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers M. C., Kho M. E., Browman G. P., Burgers J. S., Cluzeau F., Feder G., AGREE Next Steps Consortium 2010AGREE-II: Advancing guideline development, reporting and evaluation in health care Canadian Medical Association Journal ,E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman L. K., DeLapp R. C. T.2014Nine session treatment of a blood-injection-injury phobia with manualized cognitive behavioral therapy: An adult case example Clinical Case Studies ,299–312. 10.1177/1534650113509304 [DOI] [Google Scholar]
- Choy Y., Fyer A. J., Lipsitz J. D.2007Treatment of specific phobia in adults Clinical Psychology Review ,266–286. 10.1016/j.cpr.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Coia P., Morley S.1998Medical reassurance and patients’ responses Journal of Psychosomatic Research ,377–386. [DOI] [PubMed] [Google Scholar]
- Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Institute of Medicine of the National Academies Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E., editors. Clinical practice guidelines we can trust. 2011 http://www.iom.nationalacademies.org/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx [PubMed] [Google Scholar]
- Cornwall E., Spence S. H., Schotte D.1996The effectiveness of emotive imagery in the treatment of darkness phobia in children Behaviour Change ,223–229. [Google Scholar]
- Dalley J. S., Creary P., McMurtry C. M. Poster presented at the International Association for the Study of Pain’s 15th World Congress on Pain. Buenos Aires, Argentina: 2014. What are you afraid of? Exploring the specific fears of children and adults regarding painful and other aspects of needle procedures. [Google Scholar]
- Dash J.1981Rapid hypno-behavioral treatment of a needle phobia in a five-year-old cardiac patient Journal of Pediatric Psychology ,37–42. 10.1093/jpepsy/6.1.37 [DOI] [PubMed] [Google Scholar]
- Davis T. E. I., May A., Whiting S. E.2011Evidence-based treatment of anxiety and phobia in children and adolescents: Current status and effects on the emotional response Clinical Psychology Review ,592–602. 10.1016/j.cpr.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Davis T. E. I., Ollendick T. H., Reuther E. T., Munson M. S. One-session treatment: Principles and procedures with children and adolescents. In: Davis III T. E. I., editor. Intensive one-session treatment of specific phobias. New York, NY: Springer; 2012. pp. 97–125. [DOI] [Google Scholar]
- Deacon B., Abramowitz J.2006Fear of needles and vasovagal reactions among phlebotomy patients Journal of Anxiety Disorders ,946–960. 10.1016/j.janxdis.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Ellinwood E. H., Hamilton J. G.1991Case report of a needle phobia The Journal of Family Practice ,420–422. [PubMed] [Google Scholar]
- Flatt N., King N.2010Brief psycho-social interventions in the treatment of specific childhood phobias: a controlled trial and a 1-year follow-up Behaviour Change ,130–153. 10.1375/bech.27.3.130 [DOI] [Google Scholar]
- Freeman J. B., Garcia A. M., Coyne L., Ale C., Przeworski A., Himle M., Leonard H. L.2008Early childhood OCD: Preliminary findings from a family-based cognitive-behavioral approach Journal of the American Academy of Child and Adolescent Psychiatry ,593–602. 10.1097/CHI.0b013e31816765f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J. B., Sapyta J., Garcia A., Compton S., Khanna M., Flessner C., Franklin M.2014Family-based treatment of early childhood obsessive-compulsive disorder: The pediatric obsessive-compulsive disorder treatment study for young children (POTS Jr) - a randomized clinical trial JAMA Psychiatry ,689–698. 10.1001/jamapsychiatry.2014.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing R. E., Schwalbe C. S., Lee R., Hoagwood K. E.2013The effectiveness of booster sessions in CBT treatment for child and adolescent mood and anxiety disorders Depression & Anxiety ,800–808. [DOI] [PubMed] [Google Scholar]
- Goisman R. M., Allsworth J., Rogers M. P., Warshaw M. G., Goldenberg I., Vasile R. G., Keller M. B.1998Simple phobia as a comorbid anxiety disorder Depression & Anxiety ,105–112. [PubMed] [Google Scholar]
- Hamilton J. G.1995Needle phobia: A neglected diagnosis The Journal of Family Practice ,169–175. [PubMed] [Google Scholar]
- Hartling L., Hamm M., Klassen T., Chan A.-W., Meremikwu M., Moyer V., StaR Child Health Group 2012Standard 2: Containing risk of bias Pediatrics ,Suppl.3S124–S131. [DOI] [PubMed] [Google Scholar]
- Heaton L. J., Leroux B. G., Ruff P. A., Coldwell S. E. Computerized dental injection fear treatment: A randomized clinical trial. Journal of Dental Research. 2013;92(7):S37–S42. doi: 10.1177/0022034513484330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood H. K., Antony M. M. Evidence-based assessment and treatment of specific phobias in adults. In: Davis T. E. I., Ollendick T. H., Öst L.-G., editors. Intensive one-session treatment of specific phobias. New York, NY: Springer; 2012. pp. 19–42. [DOI] [Google Scholar]
- LeBeau R. T., Glenn D., Liao B., Wittchen H. U., Beesdo-Baum K., Ollendick T., Craske M. G.2010Specific phobia: A review of DSM-IV specific phobia and preliminary recommendations for DSM-V Depression & Anxiety ,148–167. [DOI] [PubMed] [Google Scholar]
- Leutgeb V., Schäfer A., Köchel A., Schienle A.2012Exposure therapy leads to enhanced late frontal positivity in 8- to 13-year-old spider phobic girls Biological Psychology ,97–104. 10.1016/j.biopsycho.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A. B., Park J. M., Jones A. M., Crawford E. A., De Nadai A. S., Menzel J., Storch E. A.2014Family-based exposure and response prevention therapy for preschool-aged children with obsessive-compulsive disorder: A pilot randomized controlled trial Behaviour Research and Therapy ,30–38. 10.1016/j.brat.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Lucock M. P., Morley S., White C., Peake M. D.1997Responses of consecutive patients to reassurance after gastroscopy: Results of self administered questionnaire survey British Medical Journal ,572–575. 10.1136/bmj.315.7108.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald I. G., Daly J., Jelinek V. M., Panetta F., Gutman J. M.1996Opening Pandora’s box: The unpredictability of reassurance by a normal test result British Medical Journal ,329–332. 10.1136/bmj.313.7053.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry C. M., Chambers C. T., McGrath P. J., Asp E.2010When “don’t worry” communicates fear: Children’s perceptions of parental reassurance and distraction during a painful medical procedure Pain ,52–58. 10.1016/j.pain.2010.02.021 [DOI] [PubMed] [Google Scholar]
- McMurtry C. M., McGrath P. J., Chambers C. T.2006Reassurance can hurt: Parental behavior and painful medical procedures The Journal of Pediatrics ,560–561. 10.1016/j.jpeds.2005.10.040 [DOI] [PubMed] [Google Scholar]
- McMurtry C. M., Noel M., Taddio A., Antony M. M., Asmundson G. J. G., Pillai Riddell R., HELPinKids&Adults Team 2015Interventions for the management of high levels of needle fear: Systematic review of randomized controlled trials and quasi-randomized controlled trials The Clinical Journal of Pain ,S109–S123. 10.1097/AJP.0000000000000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry C. M., Pillai Riddell R., Taddio A., Racine N., Asmundson G. J. G., Noel M., HELPinKids&Adults Team 2015Far from “just a poke”: Common painful needle procedures and the development of needle fear The Clinical Journal of Pain ,S3–S11. 10.1097/AJP.0000000000000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Pisani E.1999The cost of unsafe injections Bulletin of the World Health Organization ,808–811. [PMC free article] [PubMed] [Google Scholar]
- Mohr D. C., Boudewyn A. C., Likosky W., Levine E., Goodkin D. E.2001Injectable medication for the treatment of multiple sclerosis: The influence of self-efficacy expectations and infection anxiety on adherence and ability to self-inject Annals of Behavioral Medicine ,125–132. 10.1207/S15324796ABM2302_7 [DOI] [PubMed] [Google Scholar]
- Mohr D. C., Cox D., Merluzzi N.2005Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications Multiple Sclerosis ,182–185. 10.1191/1352458505ms1146oa [DOI] [PubMed] [Google Scholar]
- Muris P., Merckelbach H., Holdrinet I., Sijsenaar M.1998Treating phobic children: Effects of EMDR versus exposure Journal of Consulting & Clinical Psychology ,193–198. [DOI] [PubMed] [Google Scholar]
- Nir Y., Paz A., Sabo E., Potasman I.2003Fear of injections in young adults: Prevalence and associations American Journal of Tropical Medicine & Hygiene ,341–344. [PubMed] [Google Scholar]
- Oar E. L., Farrell L. J., Ollendick T. H.2015One session treatment for specific phobias: An adaptation for paediatric blood–injection–injury phobia in youth Clinical Child and Family Psychology Review ,370–394. 10.1007/s10567-015-0189-3 [DOI] [PubMed] [Google Scholar]
- Oar E. L., Farrell L. J., Waters A. M., Ollendick T. H. Blood-injection-injury phobia and dog phobia in youth: Psychological characteristics and associated features in a clinical sample. Behavior Therapy. in press doi: 10.1016/j.beth.2016.01.004. http://www.sciencedirect.com/science/article/pii/S0005789416000058 [DOI] [PubMed] [Google Scholar]
- Ollendick T. H., Davis T. E. I. Evidence-based assessment and treatment of specific phobias in children and adolescents. In: Davis T. E. I., Ollendick T. H., Öst L.-G., editors. Intensive one-session treatment of specific phobias. New York, NY: Springer; 2012. pp. 43–56. [DOI] [Google Scholar]
- Ollendick T. H., Halldorsdottir T., Fraire M. G., Austin K. E., Noguchi R. J. P., Lewis K. M., Cunningham N. R.2015Specific phobias in youth: A randomized controlled trial comparing one-session treatment to a parent-augmented one-session treatment Behavior Therapy ,141–155. 10.1016/j.beth.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollendick T. H., Öst L.-G., Reuterskiöld L., Costa N., Cederlund R., Sirbu C., Jarrett M. A.2009One-session treatment of specific phobias in youth: A randomized clinical trial in the United States and Sweden Journal of Consulting & Clinical Psychology ,504–516. [DOI] [PubMed] [Google Scholar]
- Öst L.-G.1989One-session treatment for specific phobias Behaviour Research and Therapy ,1–7. [DOI] [PubMed] [Google Scholar]
- Öst L.-G.1992Blood and injection phobia: Background and cognitive, physiological, and behavioral variables The Journal of Abnormal Psychology ,68–74. [DOI] [PubMed] [Google Scholar]
- Öst L.-G., Fellenius J., Sterner U.1991Applied tension, exposure in vivo, and tension-only in the treatment of blood phobia Behaviour Research & Therapy ,561–574. [DOI] [PubMed] [Google Scholar]
- Öst L.-G., Svensson L., Hellström K., Lindwall R.2001One-session treatment of specific phobias in youths: a randomized clinical trial Journal of Consulting & Clinical Psychology ,814–824. [PubMed] [Google Scholar]
- Parrish C. L., Radomsky A. S., Dugas M. J.2008Anxiety-control strategies: Is there room for neutralizing behavior in successful exposure treatment? Clinical Psychology Review ,1400–1412. 10.1016/j.cpr.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Pillai Riddell, R.,, Taddio, A, McMurtry, C. M., Chambers, C. T.,, Shah, V, Noel, M, HELPinKids&Adults Team Psychological interventions for vaccine injections in young children 0 to 3 years: Systematic review of randomized controlled trials and quasi-randomized controlled trials. The Clinical Journal of Pain. 2015:S64S71. doi: 10.1097/AJP.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Holt N., Vogel S., Underwood M., Savage R., Walsh D. A., Taylor S. J.2013Cognitive and affective reassurance and patient outcomes in primary care: A systematic review Pain ,2407–2416. 10.1016/j.pain.2013.07.019 [DOI] [PubMed] [Google Scholar]
- Reynolds S., Wilson C., Austin J., Hooper L.2012Effects of psychotherapy for anxiety in children and adolescents: A meta-analytic review Clinical Psychology Review ,251–262. 10.1016/j.cpr.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Rodriguez B. I., Craske M. G.1993The effects of distraction during exposure to phobic stimuli Behaviour Research and Therapy ,549–558. 10.1016/0005-7967(93)90106-5 [DOI] [PubMed] [Google Scholar]
- Salkovskis P. M., Warwick H. M. Making sense of hypochondriasis: A cognitive model of health anxiety. In: Asmundson G. J. G., Taylor S., Cox B. J., editors. Health anxiety: Clinical and research perspectives on hypochondriasis and related conditions. New York, NY: Wiley; 2001. pp. 46–64. [Google Scholar]
- Short N. P., Kitchiner N. J., Curran J.2004Unreliable evidence Journal of Psychiatric and Mental Health Nursing ,106–111. 10.1111/j.1365-2850.2004.00695.x [DOI] [PubMed] [Google Scholar]
- Sokolowski C. J., Giovannitti J. A., Boynes S. G.2010Needle phobia: Etiology, adverse consequences, and patient management Dental Clinics of North America ,731–744. 10.1016/j.cden.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Taddio A., Appleton M., Bortolussi R., Chambers C., Dubey V., Halperin S., Shah V.2010Reducing the pain of childhood vaccination: An evidence-based clinical practice guideline (summary) Canadian Medical Association Journal ,1989–1995. 10.1503/cmaj.092048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A., Ilersich A. F., Ilersich A. N., Wells J.2014From the mouth of babes: Getting vaccinated doesn’t have to hurt The Canadian Journal of Infectious Diseases & Medical Microbiology ,196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A., Ipp M., Thivakaran S., Jamal A., Parikh C., Smart S.2012Survey of the prevalence of immunization non-compliance due to needle fears in children and adults Vaccine ,4807–4812. 10.1016/j.vaccine.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Taddio A., McMurtry C. M., Shah V., Pillai Riddell R., Chambers C. T., Noel M., HELPinKids&Adults Team 2015Reducing pain during vaccine injections: Clinical practice guideline Canadian Medical Association Journal ,975–982. 10.1503/cmaj.150391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A., McMurtry C. M., Shah V., Yoon E., Uleryk E., Pillai Riddell R., HELPinKids&Adults Team 2015Methodology for Knowledge Synthesis of the Management of Vaccination Pain and Needle Fear The Clinical Journal of Pain ,S12–S19. 10.1097/AJP.0000000000000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traeger A. C., Hübscher M., Henschke N., Moseley G. L., Lee H., McAuley J. H. Effect of primary care-based education on reassurance in patients with acute low back pain: Systematic review and meta-analysis. JAMA Internal Medicine. 2015:733–743. doi: 10.1001/jamainternmed.2015.0217. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Valley U., Rappuoli R.2006Vaccine manufacturing: Challenges and solutions Nature Biotechnology ,1377–1383. 10.1038/nbt1261 [DOI] [PubMed] [Google Scholar]
- Uman L. S., Birnie K. A., Noel M., Parker J. A., Chambers C. T., McGrath P. J., Kisely S. R. The Cochrane database of systematic reviews. 2013. Psychological interventions for needle-related procedural pain and distress in children and adolescents. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick H. M., Salkovskis P. M.1990Hypochondriasis Behaviour Research & Therapy ,105–117. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor K. B., Horowitz J. D., Powers M. B., Telch M. J.2008Psychological approaches in the treatment of specific phobias: A meta-analysis Clinical Psychology Review ,1021–1037. 10.1016/j.cpr.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Woolf S. H., Grol R., Hutchinson A., Eccles M., Grimshaw J.1999Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines British Medical Journal ,527–530. 10.1136/bmj.318.7182.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worboys M.2007Vaccines: Conquering untreatable diseases British Medical Journal ,S19. 10.1136/bmj.39045.558889.94 [DOI] [PubMed] [Google Scholar]
- World Health Organization International statistical classification of diseases and related health problems -10th Revision. 2015 http://apps.who.int/classifications/icd10/browse/2015/en [Google Scholar]
- Wright S., Yelland M., Heathcote K., Ng S. K., Wright G.2009Fear of needles: Nature and prevalence in general practice Australian Family Physician ,172–176. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.