Highlight
Glycolate oxidase knockouts in Cyanidioschyzon reveal that red algae harbour a plant-like photorespiratory pathway. This suggests that a photorespiratory pathway employing peroxisomal glycolate oxidase is ancient and not recently evolved.
Key words: Evolution, glycolate oxidase, knockout mutant, metabolites, photorespiration, red alga.
Abstract
Photorespiration is essential for all organisms performing oxygenic photosynthesis. The evolution of photorespiratory metabolism began among cyanobacteria and led to a highly compartmented pathway in plants. A molecular understanding of photorespiration in eukaryotic algae, such as glaucophytes, rhodophytes, and chlorophytes, is essential to unravel the evolution of this pathway. However, mechanistic detail of the photorespiratory pathway in red algae is scarce. The unicellular red alga Cyanidioschyzon merolae represents a model for the red lineage. Its genome is fully sequenced, and tools for targeted gene engineering are available. To study the function and importance of photorespiration in red algae, we chose glycolate oxidase (GOX) as the target. GOX catalyses the conversion of glycolate into glyoxylate, while hydrogen peroxide is generated as a side-product. The function of the candidate GOX from C. merolae was verified by the fact that recombinant GOX preferred glycolate over L-lactate as a substrate. Yellow fluorescent protein-GOX fusion proteins showed that GOX is targeted to peroxisomes in C. merolae. The GOX knockout mutant lines showed a high-carbon-requiring phenotype with decreased growth and reduced photosynthetic activity compared to the wild type under ambient air conditions. Metabolite analyses revealed glycolate and glycine accumulation in the mutant cells after a shift from high CO2 conditions to ambient air. In summary, or results demonstrate that photorespiratory metabolism is essential for red algae. The use of a peroxisomal GOX points to a high photorespiratory flux as an ancestral feature of all photosynthetic eukaryotes.
Introduction
The enzyme Rubisco catalyses the first step in photosynthetic carbon fixation by adding one molecule of carbon dioxide (CO2) to the acceptor molecule ribulose 1,5–bisphosphate (RuBP). The resulting two molecules of 3-phosphogylcerate (3-PGA) are fed into the Calvin–Benson cycle for the production of sugar molecules. Beside the carboxylation reaction, Rubisco also catalyses the oxygenation of RuBP in the presence of oxygen (O2). In this case, one molecule of 3-PGA and one of 2-phosphoglycolate (2-PG) are generated (Ogren and Bowes, 1971). 2-PG is detrimental to cellular metabolism, inhibiting enzymatic reactions such as those of triose-phosphate isomerase (Husic et al., 1987; Norman and Colman, 1991). Thus, 2-PG is rapidly converted to 3-PGA by the photorespiratory pathway, which is distributed between the chloroplast, peroxisome, mitochondrion, and cytosol in plants (Somerville, 2001; Bauwe et al., 2010). This pathway has nine enzymatic steps that convert two molecules of 2-PG into one molecule of 3-PGA at the expense of ATP and NADPH.
In addition to its metabolic repair function, photorespiratory metabolism is suggested to protect against acceptor limitation (Heber and Krause, 1980; Kozaki and Takeba, 1996; Takahashi et al., 2007). The essential role of photorespiration under ambient CO2 conditions is demonstrated by the lethality of mutants with a completely impaired photorespiratory metabolism. These mutants display a photorespiratory, high-carbon-requiring (HCR) phenotype. That is, the mutants become chlorotic and fade under ambient, low CO2 concentrations. High CO2 concentrations typically suppress this phenotype (Somerville, 2001). So far, mutants displaying an HCR phenotype have been identified in plants, including Zea mays (Zelitch et al., 2009) and Arabidopsis thaliana (Voll et al., 2006; Engel et al., 2007; Schwarte and Bauwe, 2007; Timm et al., 2011), the green alga Chlamydomonas reinhardtii (Suzuki et al., 1999; Nakamura et al., 2005), and the cyanobacterium Synechocystis sp. strain PCC 6803 (Eisenhut et al., 2008).
Photorespiration had already evolved in cyanobacteria, the first organisms performing oxygenic photosynthesis, and exists in all primary endosymbiotic lineages of algae as well as in plants today (Eisenhut et al., 2008; reviewed in Bauwe et al., 2010; Kern et al., 2013). Owing to increasing O2 concentrations in the atmosphere, the photorespiratory pathway needed to be optimized to deal with the enhanced oxygenation activity of Rubisco and resulting enhanced flux through the pathway. This was achieved in the plant-type photorespiratory pathway by recruiting a glycolate oxidase (GOX) instead of a glycolate dehydrogenase (GlcD) for the photorespiratory glycolate-to-glyoxylate conversion (Kehlenbeck et al., 1995). GOX has a much higher maximal rate (Vmax) than GlcD and is most efficient in an environment with a high O2 partial pressure, which enables the quick degradation of the GOX by-product hydrogen peroxide (H2O2) by catalase in the peroxisome (reviewed in Hagemann et al., 2013). Thus, the use of GOX in peroxisomes can be considered an indicator of high photorespiratory flux in the optimized plant-type photorespiratory pathway (Kehlenbeck et al., 1995; Hagemann et al., 2013).
It has been postulated that photorespiratory metabolism is essential for all organisms that perform oxygenic photosynthesis and that it evolved very early among cyanobacteria (Eisenhut et al., 2008; Bauwe et al., 2010; Hagemann et al., 2010; Hagemann et al., 2016). However, to date no detailed studies on photorespiration in the other two branches of the Archaeplastida besides Chloroplastida, namely the Glaucophyta and Rhodophyta, have been performed. The red alga Cyanidioschyzon merolae serves as a model organism for the Rhodophyta. This unicellular alga is characterized by a simple eukaryotic cell structure with each cell having a single nucleus, mitochondrion, chloroplast, and peroxisome. Its small and minimally redundant 16 Mbp genome is completely known (Matsuzaki et al., 2004) and methods for targeted gene knockout and protein localization are available (Ohnuma et al., 2008; Imamura et al., 2010). C. merolae is an extremophile and can tolerate temperatures up to 57°C and pH values <2 (Seckbach, 1995). At this acidic pH, the vast majority of inorganic carbon is present as CO2 in the aquatic environment. Given that the acidophilic and acidotolerant algae manage to maintain a neutral pH inside the cells (Zenvirth et al., 1985; Gimmler et al., 1988; Colman and Balkos, 2005), the natural pH gradient must allow the diffusive uptake of CO2 into the cytoplasm, where CO2 is captured/accumulated in the form of HCO3 −. A carbonic anhydrase recovers the CO2 and provides it to Rubisco for fixation. Importantly, red algal Rubisco has the highest specificity for CO2 over O2 (Uemura et al., 1997) measured so far.
In this study we tested the hypothesis that photorespiratory metabolism is essential in the red alga C. merolae. We focused on red algal GOX, which also allowed us to investigate the hypothesis that plant-type photorespiratory metabolism evolved early in photosynthetic eukaryotes. To this end, we generated and physiologically characterized a mutant with the candidate GOX knocked out. The mutant displayed an HCR phenotype and accumulated glycolate upon a shift from high to low (ambient) CO2 conditions. Together with the findings that the enzyme localized to the peroxisomal matrix and that recombinant GOX displayed plant-like enzymatic features, we conclude that in the evolutionary basal lineage of red algae, the photorespiratory pathway is already functioning in the plant-type mode. This indicates a high photorespiratory flux before the colonization of terrestrial environments by photosynthetic eukaryotes.
Material and methods
Strains and culture conditions
C. merolae 10D was used as the wild-type (WT) strain in this study. For the generation of the Δgox mutant, the M4 mutant, deficient in uracil synthesis, was used (Minoda et al., 2004). Cells were cultivated in 2× modified Allen’s (2×MA) medium (pH 2; Minoda et al., 2004) in glass vessels at 40°C, and aerated with high CO2 concentrations (5% CO2 in air, HC) or low CO2 concentrations (0.04% CO2 in air, LC) under continuous white light (90 µmol photons m−2 s−1). The growth medium for the M4 mutant was supplemented with 500 µg ml−1 uracil.
For the CO2 shift experiment, C. merolae WT and the Δgox mutant strains were cultivated in a multicultivator system (Photon System Instruments, Drasov, Czech Republic) at 90 µmol photons m−2 s−1 light and 40°C. For growth rate calculation, OD720 measurements were performed every hour over the experimental time by the multicultivator system.
Quantitative real-time PCR
Samples for RNA extraction were taken 0h, 3h, and 24h after the shift from HC to LC conditions, as well as 24h after the shift back to HC. RNA extraction was performed using the EURx GeneMatrix Universal RNA Purification Kit (Roboklon, Berlin, Germany) following manufacturer’s instruction for RNA cell extraction. DNase treatment was carried out using RQ1 RNA-Free DNase and cDNA synthesis was performed using M-MLV Reverse Transcriptase, RNase (H−), Point Mutant (Promega, Fitchburg, WI, USA). A MESA BLUE MasterMix for SYBR® Assay (Eurogentec, Seraing, Liège, Belgium) was employed for the quantitative (q)RT assay. The primers used for qRT-PCR were qRT-CmGOX-fw and qRT-CmGOX-rev (efficiency 2.0) for amplification of the CmGOX (CMQ436C) transcript and qRT-CmrbcL-fw and qRT-CmrbcL-rev (efficiency 2.1). The red algal homologue of the constitutively expressed gene TIP-41-like (At4g34270) in A. thaliana (Czechowski et al., 2005) was used as a reference gene. We designated this gene, CMM193C, as CmBLACK and applied it as a reference for ΔΔCt analysis using the primers qRT-CmBlack-fw and qRT-CmBlack-rev (efficiency 2.2). Primer sequences are listed in Supplementary Table S1. qRT-PCR was performed with the StepOne Plus Real-Time system (Applied Biosystems, Waltham, MA, USA). Mean normalized expression was calculated from three biological replicates, including three technical replicates, following the instructions of Simon (2003).
Subcellular localization studies
The CmGOX coding sequence was amplified using Phusion High Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA), P1 forward primer (MfeI restriction site), P2 reverse primer (NcoI restriction site), and C. merolae genomic DNA as the template. The PCR product was ligated into the pJET2.1 vector system (ThermoFisher Scientific, Waltham, MA, USA) for amplification and sequencing. For localization studies in Nicotiana benthamiana, pUBN-YFP-Dest (Grefen et al., 2010) was used as the final vector for fusion of CmGOX with an N-terminal yellow fluorescent protein (YFP) tag. Expression of the fusion protein was under the control of the constitutive UBIQUITIN 10 promoter. Transformation of N. benthamiana was carried out using Agrobacterium tumefaciens strain GV3101. For peroxisomal co-localization studies, A. tumefaciens cells containing a vector for expression of the cyan fluorescent protein (CFP) fused with the C-terminal peroxisomal target signal 1 (PTS1) were co-infiltrated into the leaf. The CFP::PTS1 construct was used as a peroxisomal-targeted fluorescent marker (Linka et al., 2008). Protoplast isolation and microscopic analysis were performed 2 d after infiltration using a Zeiss LSM 510 Meta laser microscope as described in Breuers et al. (2012).
For localization studies in C. merolae, the UBIQUITIN 10 promoter of the pUBN-YFP-Dest vector was exchanged for the apcC promoter. To this end, the apcC promoter region was synthesized by Phusion PCR using the pCG1 vector (Watanabe et al., 2011) as the template and the primer P3 (PmeI restriction site) and P4 (SpeI restriction site). The vector pJET2.1 (ThermoFisher Scientific) was used for subcloning. Transformation of C. merolae was performed as described by Ohnuma et al. (2008). Microscopic analysis was carried out 1 d after transformation using the Zeiss LSM 780 laser microscope. Primer sequences are listed in Supplementary Table S1.
Δgox mutant generation
To inactivate the CmGOX gene, the CmGOX (CMQ436C) locus was replaced by the C. merolae URA gene by homologous recombination. The CmGOX genomic region was amplified with the primers GOX_KO_F1 and GOX_KO_R1. The amplified DNA was cloned into the pQE80 vector (Qiagen, Hilden, Germany) using the In-Fusion HD Cloning Kit (Clontech, Mountain View, CA, USA). The 3′ portion, vector, and 5′ portion of CmGOX was amplified with the primers GOX_KO_F2 and GOX_KO_R2 and then the URA gene amplified with URA_F and URA_R was inserted by In-Fusion Cloning. The CmGOX genomic region with the URA insert was amplified from the vector by PCR with primers GOX_KO_F3 and GOX_KO_R3 and was transformed into C. merolae M4, a derivative of C. merolae 10D, which has a mutation in the URA gene (Minoda et al., 2004). Transformation and selection of the gene knockouts were performed as described (Imamura et al., 2010; Fujiwara et al., 2015). The occurrence of the recombination events in the CmGOX-knockout strains was confirmed by PCR with the primer sets GOX_KO_F4/GOX_KO_R4 and GOX_KO_F5/GOX_KO_R5. Absence of CmGOX transcripts in the knockout lines was verified by RT-PCR analysis using the primers GOX_KO_F2 and GOX_KO_R2. Transcripts from the CMQ432C locus adjacent to CmGOX were amplified using the primers CMQ432C-F and CMQ432C-R as a control. Primer sequences are given in Supplementary Table S1.
Metabolite extraction and analysis
Centrifugation was used to harvest 10ml cells (4°C, 5min, 3000 RCF) and the resulting pellets were frozen in liquid nitrogen. For metabolite extraction, the pellets were resuspended in ethanol (70% v/v, including 50 µM ribitol as internal standard), using acid-washed glass beads for cell disruption by vortexing (3×1min, 4°C, maximum speed). The extraction mix was centrifuged (4°C, 2min, 16000 RCF) and the supernatant was analysed by gas chromatography coupled to a time-of-flight mass spectrometer (7200 GC-QTOF; Agilent Technologies, Santa Clara, CA, USA) according to Fiehn and Kind (2007). Peak areas for all compounds were analysed using the Mass Hunter Software (Agilent) and curated manually if necessary.
For the analysis of glycine and serine, the same extract was derivatized by AccQ-Tag Ultra Reagent Powder (Waters Corporation, Milford, MA, USA) according to the manufacturer’s instructions and separated at 60°C and a flow of 0.7ml min−1 on an AccQ-Tag™ Ultra Column 2.1×100mm and particle size 1.7 μm (Waters Corporation) using the 10% AccQ-Tag™ Ultra Eluent A (Waters Corporation) and acetonitrile as eluent B (0–0.54min, 0.1% B; 0.54–5.74min, 0.1–9.1% B; 5.74–7.74min, 9.1–21.2% B; 7.74–8.04min, 21.2–59.6% B and maintained for 0.6min; 8.64–8.73min, 59.6–95% B and maintained for 0.27min; 9.0–9.1min, 95–100% B and maintained for 0.1min). Derivatized compounds were detected at 260nm. For this purpose a 1290 UHPLC system coupled to diode array detector from Agilent was used.
Photosynthetic rate measurement
Cultivation of WT and the Δgox #46 mutant was performed under continuous light (80 µmol photons m−2 s−1) at 28°C. Cells were pre-cultivated at 5% CO2. Twenty-four hours before measurements, cultures were adjusted to a cell density of OD750 of 0.7 and shifted to either HC (bubbling with 5% CO2) or LC conditions (bubbling with ambient air containing 0.04% CO2). Cells were then collected by centrifugation (4000rpm, 5min, room temperature), washed, and resuspended in CO2-free 2×MA growth medium to a final OD750 of 1. The oxygen production of 3ml of this cell culture was used to quantify the photosynthetic rate at 28°C and a saturating light intensity of 120 µmol photons m−2 s−1, using a Clark electrode (Oxygraph System; Hansatech, Norfolk, England). To determine the CO2-dependent photosynthetic rate, sodium hydrogen bicarbonate (NaHCO3) was stepwise added to a final saturating concentration of 257 µM. Oxygraph Plus software (Hansatech) and Prism (GraphPad Software, La Jolla, CA, USA) was used for data analysis.
Chlorophyll a determination
To analyse chlorophyll a (Chl a) concentration, 1ml of cell culture was centrifuged (2min, 16 000 RCF, room temperature) and 900 µl supernatant was exchanged by methanol (100% v/v). The suspension was incubated for 10min at 65°C and measured at OD665. The extinction value (extinction coefficient of 78.74l g−1 cm−1) was multiplied by 12.7 to calculate the Chl a content in micrograms per millilitre (Meeks and Castenholz, 1971).
Heterologous expression of CmGOX and enzyme assay
To generate a CmGOX overexpressing Escherichia coli strain, the coding sequence was amplified by PCR using genomic DNA from C. merolae, gene-specific primers with added cleavage sites (CMQ436C-EcoRI-fw and CMQ436C-SalI-rv; Supplementary Table S1), Taq Polymerase Mastermix (Qiagen), and proof-reading Elongase enzyme (ThermoFisher Scientific). The resulting 1182bp fragment was cloned into the pGemT-vector (Promega). After sequence confirmation (Seqlab, Göttingen, Germany), the coding sequence was cloned into the expression vector IBA43+ in frame with the N-terminal His-tag using EcoRI/SalI and subsequently transformed into the E. coli strain BL21 DE3. To improve the folding of the recombinant protein, the CmGOX-overexpressing E. coli strain was co-transformed with the pG-KJE8 plasmid coding for five chaperones (Takara, Ohtsu, Japan).
E. coli BL21 cells containing IBA43+-CmGOX and pG-KJE8 were grown in lysogeny broth medium supplemented with plasmid-specific antibiotics, l-arabinose, and tetracycline (0.05% and 0.5ng ml−1 final concentration) to an OD750 of 0.6 at 30°C. CmGOX expression was induced by the addition of 200 µg l−1 anhydrotetracycline and the cells were incubated for 16h at 30°C. The fusion protein was purified via the N-terminal His-tag using Ni-NTA Sepharose according to the protocol of the supplier (ThermoFisher Scientific). All purification steps were performed at 4°C. The cells were harvested and resuspended in homogenization buffer (20mM Tris-HCl, pH 8.0, containing 500mM NaCl, 1mM DTT, and 0.1mM FMN). Proteins were extracted by ultrasonic treatments (4×30s, 90W) in ice. Soluble protein extracts were used for affinity chromatography on Ni-NTA Sepharose using the homogenization buffer supplemented with 40–80mM imidazole as washing buffers. The elution buffer contained 300mM imidazole. The elution fractions were combined and desalted using PD-10 columns (GE Healthcare, Little Chalfont, UK). Finally, the recombinant enzymes were dissolved in 20mM Tris-HCl, pH 8.0, containing 1mM DTT, and 0.1mM FMN. The eluted proteins were checked regarding purity using SDS-PAGE. Protein concentration was determined using Bradford’s method with bovine albumin as the standard protein (Bradford, 1976).
The purified and desalted recombinant His-tagged protein was used for enzyme assays with a Hansatech oxygen electrode as described in detail by Hackenberg et al. (2011).
Results
The genome of C. merolae contains single copy genes for all enzymes of the plant-type photorespiratory pathway
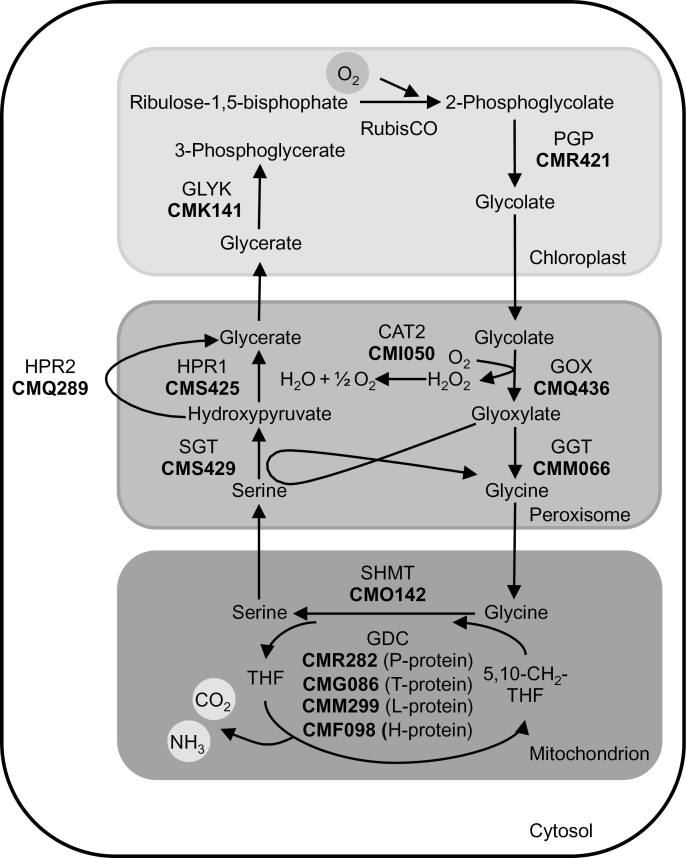
We first performed BLASTP analyses using the BLAST tools of both the C. merolae Genome Project (http://merolae.biol.s.u-tokyo.ac.jp/blast/blast.html; Matsuzaki et al., 2004) and the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi), to identify in C. merolae proteins homologous to the photorespiratory enzymes in A. thaliana. The query and match proteins from A. thaliana and C. merolae, respectively, are listed in Supplementary Table S2. As a result, we identified the full protein repertoire of a plant-type photorespiratory pathway in C. merolae (Fig. 1). In contrast to A. thaliana and other land plants, single copy genes and not gene families encode the photorespiratory enzymes. For the majority, the A. thaliana query and the identified C. merolae proteins were the best reciprocal BLAST hits. We were unable to identify a protein homologous with the glycolate dehydrogenase that acts in the photorespiratory pathway of Chlorophyta such as C. reinhardtii (Nakamura et al., 2005). This suggests that the glycolate-to-glyoxylate converting step is probably catalysed by GOX in Rhodophyta such as C. merolae. In accordance with the predicted GOX activity, we identified a catalase (Fig. 1) that is needed to decompose the H2O2, which is generated as a side-product of GOX activity.
Fig. 1.
Schematic view of the photorespiratory pathway in C. merolae. Candidate proteins in C. merolae were identified by BLAST analysis. Corresponding protein identifiers (acc. to C. merolae Genome Project, http://merolae.biol.s.u-tokyo.ac.jp/; Matsuzaki et al., 2004) are shown in bold. The enzymes are CAT2: catalase 2; GDC: glycine decarboxylase; GGT: glutamate:glyoxylate aminotransferase; GLYK, glycerate 3-kinase; GOX, glycolate oxidase; HPR1, peroxisomal hydroxypyruvate reductase 1; HPR2, cytosolic hydroxypyruvate reductase 2; PGP, 2-PG phosphatase; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; SHMT, serine hydroxymethyl transferase; SGT, serine:glyoxylate aminotransferase.
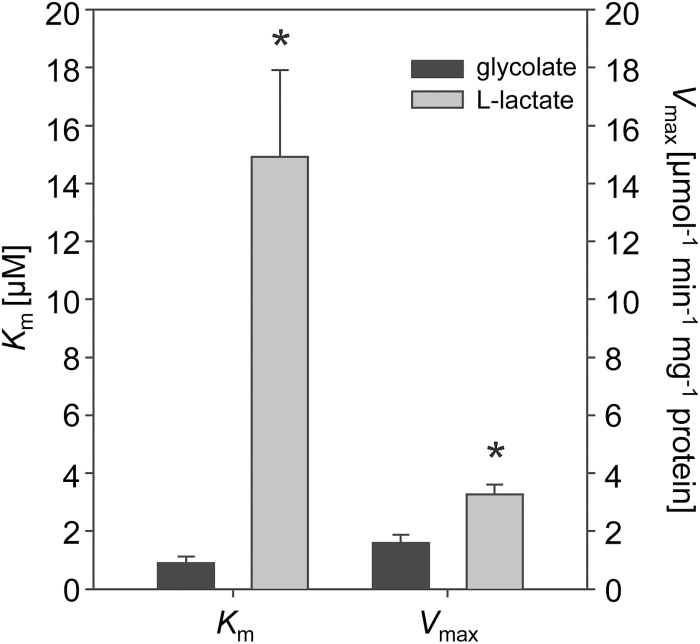
CmGOX has a higher affinity for glycolate than l-lactate
To verify the enzymatic activity of the putative GOX from C. merolae, the coding gene was heterologously expressed in E. coli. The purified His-tagged protein (see Supplementary Fig. S1) was used for enzymatic assays, in which the O2 consumption was determined depending on the substrates l-lactate or glycolate. Like the homologous protein from the plant A. thaliana, CmGOX catalysed the oxidation of both substrates, l-lactate and glycolate (Fig. 2). The low K m value for glycolate (0.9±0.2mM) and significantly higher K m value for l-lactate (14.9±3.0 µM) clearly support the hypothesis that the gene CMQ436C encodes an oxidase with higher affinity for glycolate than l-lactate. The V max for l-lactate (3.3±0.3 µmol min−1 mg−1) was twice as high as that for glycolate (1.5±0.3 µmol min−1 mg−1). The occurrence of GOX in C. merolae suggests high flux through the photorespiratory pathway, as found in vascular plants.
Fig. 2.
Biochemical characterization of recombinant CmGOX. The K m and V max values of CmGOX for the substrates glycolate and l-lactate were calculated by non-linear regression following the Michaelis–Menten kinetic implemented in SigmaPlot 11.0. The means of at least two independent enzyme preparations ± SD are given. Asterisks indicate significant differences (P < 0.05) determined with the two-tailed Student’s t-test.
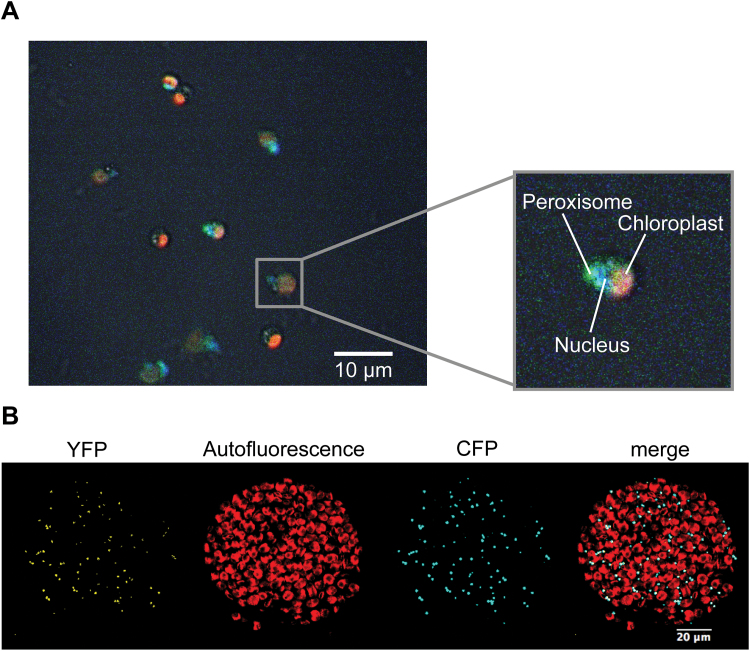
CmGOX is localized in the matrix of peroxisomes
Typically, GOX proteins reside in the peroxisomal matrix, where the critical GOX catalysis by-product H2O2 is efficiently decomposed by the activity of catalase. The CmGOX protein sequence contains a putative peroxisome targeting sequence (SKL) at the C-terminus (Matsuzaki et al., 2004). To experimentally determine the actual subcellular localization, we generated GOX protein variants that were fused with an N-terminal YFP-domain. For expression in C. merolae cells, the YFP::CmGOX construct was set under the control of the strong apcC promoter, which has been shown to be suitable for protein localization studies in C. merolae (Watanabe et al., 2011). Fluorescence microscopy demonstrated that the YFP::CmGOX resides in the peroxisome in C. merolae (Fig. 3A). To improve the resolution, we alternatively expressed the YFP::CmGOX construct under control of the UBIQUITIN 10 promoter in tobacco leaves. The overlap of the peroxisomal marker (CFP::PTS1, Linka et al., 2008) signal and the YFP::CmGOX signal in protoplasts confirmed the localization of CmGOX in the peroxisomal matrix (Fig. 3B).
Fig. 3.
Subcellular localization studies of CmGOX. (A) Localization of CmGOX in C. merolae. Red: chloroplast autofluorescence; blue: DAPI-stained nucleus; green: YFP signal from YFP::CmGOX construct. C. merolae cells were transformed 24h before microscopic analysis. (B) Localization of CmGOX in tobacco protoplasts. From left to right: YFP signal of YFP::CmGOX construct, chlorophyll autofluorescence, CFP signal as peroxisomal marker (CFP::PTS1), and merge of all three pictures. Microscopic analyses were performed with protoplasts isolated from transiently transformed N. benthamiana leaves.
The amount of CmGOX transcript increases under LC conditions
To examine if transcript amounts of CmGOX responded to changes in CO2 availability, the CmGOX steady state transcript level was analysed in C. merolae WT cells cultivated under photorespiration-suppressing HC conditions and after a shift to photorespiration-stimulating LC conditions. CmGOX transcript abundance increased 100-fold 3h after the shift to LC in comparison to constant HC conditions. After 24h under LC conditions, the CmGOX transcript level returned to the low amounts typical of HC-grown cells (Fig. 4A). We observed a comparable abundance pattern for the gene encoding the large subunit of Rubisco (CmRBCL). The transcript level significantly increased 3-fold 3h after the shift to LC, while it was similar to the HC value after 24h in LC conditions (Fig. 4B).
Fig. 4.
Effect of shift in CO2 concentrations on transcript levels of CmGOX (A) and CmRBCL (B). Samples were taken before a shift from HC (5% CO2) to LC (0.04% CO2) conditions, 3h and 24h after the shift, and 24h after a shift back to HC conditions. Shown is the mean normalized expression of three biological replicates including three technical replicates and the mean normalized standard error. CmBLACK (CMM193C) was used as the reference gene for Ct analysis. Significant differences to initial transcript levels (LC, 0h) were analysed by a two-tailed Student’s t-test (***P < 0.001).
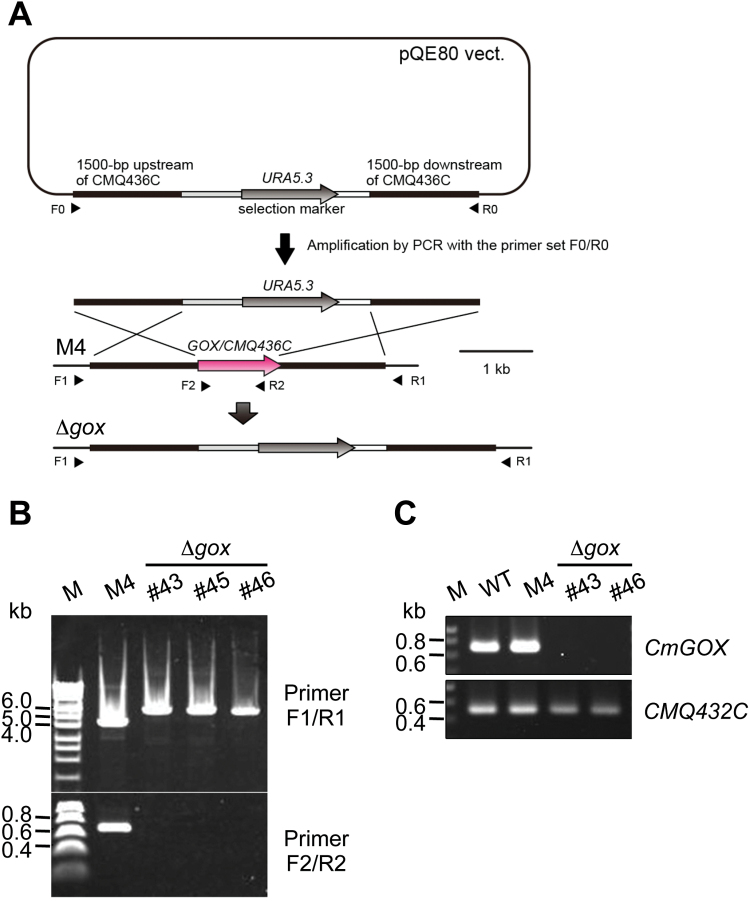
Deletion of CmGOX causes an HCR phenotype and perturbations in photorespiratory metabolism
To study the importance of photorespiration and especially the function of the GOX protein for C. merolae, we generated Δgox knockout mutant lines. Mutant generation was performed as described by Imamura et al. (2010). The CmGOX coding sequence was exchanged for the URA5.3 marker gene via homologous recombination (Fig. 5A), conferring uracil autotrophy to the otherwise uracil auxotrophic M4 mutant strain. Correct recombination events were verified for the mutant lines Δgox #43, #45, and #46 by PCR using genomic DNA as the template (Fig. 5B). Because CmGOX transcripts could not be detected for Δgox #43 and Δgox #46 (Fig. 5C) at the mRNA level, we chose these as the knockout mutant lines for further analyses.
Fig. 5.
Generation of a Δgox knockout mutant in C. merolae. (A) Schematic presentation of the strategy to generate a knockout mutant for CmGOX. For detailed information see ‘Materials and methods’. (B) Verification of Δgox mutant lines #43, #45, and #46. PCR was performed on genomic DNA of the M4 background mutant and the Δgox mutant lines #43, #45, and #46 with primers flanking the CmGOX upstream and downstream regions (F1/R1) and the CmGOX coding region (F2/R2), respectively. Expected fragment sizes were F1/R1: 4.5kb (M4), 6kb (Δgox); F2/R2: 0.66kb (M4), – (Δgox). (C) Verification of absence of CmGOX transcripts in the Δgox knockout lines #43 and #46. RT-PCR analysis was performed on cDNA isolated from WT, M4 background mutant, and the Δgox mutant lines #43 and #46 with primers flanking the CmGOX coding region (F2/R2). Transcripts from the CMQ432C locus adjacent to the CmGOX locus were used as a control. Expected fragment sizes are CmGOX: 698bp; CMQ432C: 520bp.
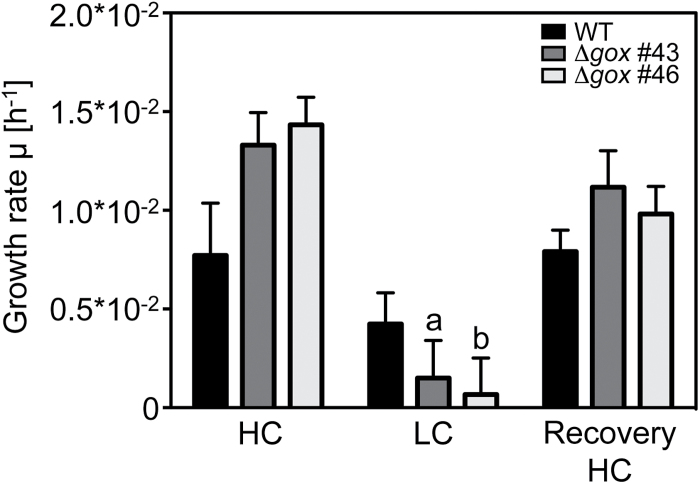
To gain insight into the impact of CmGOX deletion on the metabolism of C. merolae we performed a CO2 shift experiment, in which WT and the knockout mutant lines Δgox #43 and Δgox #46 were pre-cultivated under HC conditions, shifted to LC conditions for 24h, and then shifted back to HC conditions for another 24h. Under HC conditions, the growth performance of WT and mutants was not significantly different (Fig. 6). The shift towards LC conditions led to almost fully impaired growth in the Δgox #43 and Δgox #46 mutants, whereas WT cells continued growing. After the shift back to HC conditions, all cultures resumed growth and no difference in growth rates could be detected (Fig. 6). Chl a concentrations did not significantly differ between WT and mutant cells (Supplementary Fig. S2). Reduced growth of the Δgox #43 and Δgox #46 mutants was also observed when they were grown on solid medium under LC compared to HC conditions (Supplementary Fig. S3). Thus, the deletion of CmGOX resulted in an HCR phenotype, indicating an important role for GOX activity under ambient air conditions in C. merolae.
Fig. 6.
Growth rate of WT cells and Δgox mutant line #43 and #46 grown for 24h under HC conditions (5% CO2), 24h under LC conditions (0.04% CO2), and returned for 24h to HC conditions. OD720 measurements were performed every hour by the Multi-Cultivator system (Photons System Instruments). Shown are means of three biological replicates with standard error. Significant differences to initial growth (HC) within one cell line were determined with a two-tailed Student’s t-test and are indicated by a (P < 0.01) and b (P < 0.001). Significant differences between the mutant and corresponding WT values could not be detected by t-test.
During the shift experiment we took samples and performed a targeted metabolite analysis. Samples were taken before (0h), 3h, and 24h after the shift to LC, and 24h after the re-shift to HC conditions. When grown continuously under HC conditions, glycolate levels were below the detection limit. Importantly, 3h after the shift to LC, glycolate had accumulated to a level at which it could be measured. Its concentration was two to four times higher in Δgox mutant lines than in WT. After 24h at LC, glycolate levels declined slightly in all cultures but were still significantly elevated in the mutants compared to WT. When the cultures were allowed to recover for 24h under HC conditions, glycolate was only detectable in the Δgox mutant lines but not in WT cells (Fig. 7). Glycine-to-serine conversion in mitochondria is a central step in the photorespiratory pathway (see Fig. 1). In the WT we observed significant increases in both the glycine and the serine levels 3h after the shift to LC conditions (Supplementary Fig. S4A, B). The Δgox mutants showed a different metabolic response. Glycine concentration was already higher under HC conditions in the mutants cells compared to WT, and increased by 2-fold 3h after the shift to LC, and by 3-fold 24h after the shift. After 24h recovery under HC conditions, glycine levels were still elevated in the mutants (Supplementary Fig. S4A). Serine levels had the opposite response and were lower in the Δgox #43 and Δgox #46 mutants than in the WT. A statistically significant difference was observed 3h after the LC shift, with serine levels of only 40% compared to the WT levels (Supplementary Fig. S4B). For the photorespiratory intermediate glycerate we did not detect significant differences between WT and mutant lines (Supplementary Fig. S4C). With respect to sugars we detected a significant decline in glucose levels 24h after LC treatment in all strains. After 24h HC treatment, the values fully recovered (Supplementary Fig. S4D). Fructose concentrations were also reduced in the WT 24h after the shift to LC conditions, but were not affected in the mutant lines by changes in CO2 availability (Supplementary Fig. S4E).
Fig. 7.
Glycolate levels of WT and Δgox mutants #43 and #46 during the CO2 shift experiment. Glycolate levels of the different strains were determined by GC-MS before the shift from HC (5% CO2) to LC (0.04% CO2) conditions, 3h and 24h after the shift to LC, and after a 24h recovery phase under HC conditions. Shown are means of four biological replicates and standard errors. n.d., not detectable. Significant differences were analysed by the non-parametric Mann–Whitney test. Significant differences to initial glycolate levels (HC) within one cell line are indicated by a (P < 0.05). Significant differences between the mutant and corresponding WT values are indicated by an asterisk (P < 0.05).
The Δgox mutant has inhibited photosynthetic activity
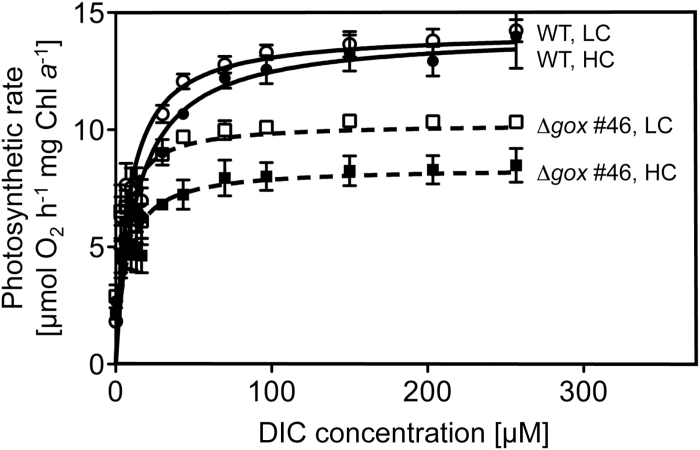
We investigated the impact of CmGOX deletion on photosynthetic activity by determining O2 production in increasing HCO3 − concentrations (Fig. 8). WT cells grown under either HC or LC conditions had a V max of 14 µmol O2 h−1 per mg Chl a (Table 1). However, WT cells grown under HC conditions had a higher apparent K m (K m = 13.9 µM) compared to WT cells grown under LC conditions (K m = 9.1 µM), which means that LC-grown cells were quicker to reach the maximal photosynthetic rate (Table 1). However, this difference was not significant. In comparison to WT, the Δgox #46 mutant line showed a significantly lower maximal photosynthetic rate, and reduced but not significantly different K m values (Table 1, Fig. 8). Mutant cells grown under LC conditions had a higher V max (V max = 10.3 µmol O2 h−1 per mg Chl a) than mutant cells grown under HC conditions (V max = 8.6 µmol O2 h−1 per mg Chl a). The different growth conditions resulted in different K m values (HC K m = 8.4 µM; LC K m = 4.9 µM; Table 1), as was the case for the WT.
Fig. 8.
Photosynthesis rates of WT and Δgox #46 mutant at increasing concentrations of externally supplied dissolved inorganic carbon (DIC). Cells were cultivated under HC conditions (5% CO2) and O2 evolution was measured using a Clark electrode. For LC measurements, cells were shifted for 24h to LC conditions (0.04% CO2). The photosynthetic rate was analysed according to increasing DIC concentration in the medium. Fitting analysis was performed using the Michaelis–Menten Kinetic (Prism 5) on the basis of three biological replicates each.
Table 1.
Effect of CO2 concentration on photosynthetic rates (V max) and Ci affinities (K m) of WT and Δgox #46 cells.
| HC | LC | |||
|---|---|---|---|---|
| WT | Δgox #46 | WT | Δgox #46 | |
|
V
max
[µmol O2 h−1 Mg−1 Chl a] |
14.12±0.49 | 8.58±1.62 | 14.20±0.49 | 10.31±0.60 |
| K m [µM] | 13.94±4.75 | 8.42±7.69 | 9.069±3.60 | 4.85±2.68 |
Results are presented as mean values ± SD from three independent determinations each. Significant differences (two-tailed Student’s t-test, P < 0.01) between WT and Δgox #46 mutant are given in bold. Within one line results for HC and LC conditions were not significantly different.
Discussion
The red alga C. merolae lives in acidic and hot aquatic habitats, which are naturally low in CO2. The solubility of CO2 and its diffusion coefficient is up to four magnitudes lower in water than in air (reviewed in Moroney et al., 2013). Consequently, the red algal Rubisco evolved a characteristically high specificity for CO2, which is indicative of low CO2 concentrations at the site of Rubisco activity (Savir et al., 2010). Despite its optimized Rubisco, red algae have been suggested to execute a carbon concentrating mechanism (CCM) that enhances the CO2 concentration near Rubisco (Zenvirth et al., 1985; Giordano et al., 2005). This raises the question of whether the survival of C. merolae under ambient conditions depends on photorespiratory metabolism. The importance of photorespiration in this organism has not been investigated to date.
The red alga C. merolae harbours a photorespiratory pathway that appears to be more similar to that of land plants than to C. reinhardtii, as revealed by BLAST analysis. All photorespiratory enzymes known from land plants, including catalase, are encoded in the genome of C. merolae and are homologous to the plant-type photorespiratory proteins (Supplementary Table S2). In contrast to land plants, all enzymes are encoded by single genes in the small and minimally redundant genome of C. merolae. Besides the enzymes catalysing the conversion of the photorespiratory intermediates, transporters shuttling the intermediates between the different organelles play a central role in photorespiratory metabolism in photosynthetic eukaryotes (reviewed in Eisenhut et al., 2013). Among the few photorespiratory transporters identified to date, only the plastidic glycolate glycerate translocator PLGG1 (Pick et al., 2013) is encoded in the genome of C. merolae. Homologues for the two plastidic carboxylate translocators DiT1 and DiT2 (Weber and Flügge, 2002; Renné et al., 2003), which are involved in photorespiratory nitrogen recycling, do not exist in C. merolae (reviewed in Eisenhut et al., 2015). We did not identify any proteins in C. merolae that were homologous with enzymes of the bacterial-type glycerate pathway, as found in cyanobacteria (Eisenhut et al., 2006). We were also unable to identify a GlcD-type glycolate dehydrogenase. Thus, we hypothesized that the candidate GOX protein identified in our study catalyses the conversion of glycolate to glyoxylate in C. merolae, which supports previous phylogenetic analyses by Kern et al. (2013).
Our analysis revealed that C. merolae and probably all Rhodophyta perform a plant-like glycolate-to-glyoxylate conversion via a specific GOX in the peroxisome. Heterologous expression of the candidate GOX from C. merolae revealed a higher affinity for glycolate than for l-lactate (Fig. 2), and thus supported the likely usage of a GOX for the conversion of glycolate to glyoxylate during photorespiration. We could furthermore demonstrate that, as in plants, CmGOX resides in the peroxisome (Fig. 3), which is a prerequisite for it to function as a photorespiratory GOX. Given that the employment of a GOX is indicative of high flux through the photorespiratory pathway (Kehlenbeck et al., 1995; Hagemann et al., 2013), we suggest that, similar to plants, photorespiratory flux is high in the red alga C. merolae. In has previously been assumed that algae such as C. reinhardtii, which use a CCM, are characterized by low photorespiratory flux rates (Birmingham et al., 1982). Indeed, glycolate-to-glyoxylate conversion by GlcD in the mitochondria of C. reinhardtii meets these low-flux requirements (Kehlenbeck et al., 1995; Nakamura et al., 2005). Moreover, in Chlorophyceae such as C. reinhardtii, the reduction of hydroxypyruvate and the transaminase steps occur in the mitochondria, and not in the peroxisomes (Atteia et al., 2009). Accordingly, peroxisomes do not seem to be involved in photorespiration in Chlorophyceae, which could be the result of a different peroxisomal enzyme repertoire in this algal lineage (Stabenau, 1974; Stabenau et al., 1993).
Further support for the involvement of CmGOX in the photorespiratory pathway in C. merolae was provided by gene expression analysis. CmGOX transcript amounts strongly increased 3h after the shift to photorespiratory conditions (Fig. 4A). The 100-fold increase of the CmGOX transcript level coincided with accumulation of glycolate in WT cells 3h after the shift to LC conditions (Fig. 7). It must be mentioned that an increase in transcript abundance does not necessarily lead to a proportional increase in protein abundance, as, for example, demonstrated for C. reinhardtii (Mettler et al., 2014). However, the transcriptional response indicates that C. merolae quickly senses a change in the CO2 environment. Comparably, genes for CCM components and photorespiratory enzymes are upregulated in the chlorophyte C. reinhardtii (Fang et al., 2012). However, it is not known how oxygenic phototrophs sense alterations in CO2 concentrations (Raven, 2006). The accumulation of photorespiratory intermediates such as 2-PG (Haimovich-Dayan et al., 2015) and glycolate (Hackenberg et al., 2012) could serve as signals that trigger the enhanced expression of the CCM genes among cyanobacteria under LC conditions.
The plant-like photorespiratory GOX is essential for survival of C. merolae under ambient conditions, as demonstrated by the occurrence of the HCR phenotype in the Δgox knockout mutants. Mutant growth almost completely stopped after a shift from 5% CO2 to ambient air, whereas it quickly recovered when mutant cells were shifted back to HC conditions (Fig. 6, Supplementary Fig. S3). This behaviour is typical for the HCR phenotype (Somerville, 2001). However, in contrast to a plant HCR phenotype, we did not observe a chlorotic phenotype in the Δgox mutants after the shift to LC conditions (Supplementary Fig. S2). A further argument for the function of photorespiratory GOX is the accumulation of glycolate in the Δgox knockout mutant lines under LC conditions. A 24h recovery phase in HC was not sufficient to reduce the amount of glycolate below detectable levels in the Δgox mutants, in contrast to what was observed for the WT (Fig. 7). Similarly, the GlcD mutant in C. reinhardtii showed a 4-fold higher accumulation of glycolate compared to the WT (Nakamura et al., 2005). The same was true for a GOX mutant in Zea mays, which showed an 11-fold higher glycolate level after 25h in ambient air compared to the stable glycolate level in WT plants (Zelitch et al., 2009).
Furthermore, the photosynthetic performance of Δgox knockout mutants was affected (Fig. 8, Table 1). WT cells showed similar maximal photosynthetic rates when grown under HC or LC conditions, but enhanced CO2 affinity was found under LC conditions, as has been previously reported (Zenvirth et al., 1985). Although not significant, cells of the mutant Δgox #46 showed an enhanced affinity towards CO2 compared to the WT cells. However, maximal photosynthetic activity was significantly reduced in the mutant, which is likely the result of toxic effects due to the impaired photorespiratory pathway. For example, accumulation of the photorespiratory intermediates 2-PG (Norman and Colman, 1991) and glycine (Eisenhut et al., 2007) is known to inhibit growth and photosynthesis.
In conclusion, all obtained results support the hypothesis that C. merolae has a plant-type photorespiratory pathway, which is an indication for high photorespiratory flux in red algae. A plant-like photorespiratory metabolism with recruitment of peroxisomal GOX to improve the bottleneck reaction glycolate-to-glyoxylate conversion is an early evolutionary strategy to adapt to increasing O2 concentrations in the atmosphere. These findings are contrary to earlier assumptions that the plant-like photorespiration pathway appeared late and only among streptophytic green algae, which was discussed to be a crucial step for the later colonization of the continents by land plants (e.g. Becker, 2013).
Supplementary data
Supplementary material is available at JXB online.
Table S1. Oligonucleotides used in this study.
Table S2. List of A. thaliana photorespiratory enzymes and identified homologous proteins in C. merolae.
Figure S1. Purification of recombinant CmGOX.
Figure S2. Chl a concentrations of WT and Δgox mutants #43 and #46 during the CO2 shift experiment.
Figure S3. CO2-dependent growth of WT and Δgox mutants #43 and #46.
Figure S4. Metabolite levels of WT and Δgox mutants #43 and #46 during the CO2 shift experiment.
Acknowledgments
The technical assistance of Samantha Kurz, Maria Graf, Elisabeth Klemp, Klaudia Michl, and Katrin Weber is greatly appreciated. Maria Graf is also acknowledged for her technical expertise in method development. Our work on photorespiration was supported by grants from the DFG (Deutsche Forschungsgemeinschaft) in the frame of the Forschergruppe FOR 1186 – Promics (WE 2231/8-2).
References
- Atteia A, Adrait A, Brugiere S, et al. 2009. A proteomic survey of Chlamydomonas reinhardtii mitochondria sheds new light on the metabolic plasticity of the organelle and on the nature of the alpha-proteobacterial mitochondrial ancestor. Molecular Biology and Evolution 26, 1533–1548. [DOI] [PubMed] [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR. 2010. Photorespiration: players, partners and origin. Trends in Plant Science 15, 330–336. [DOI] [PubMed] [Google Scholar]
- Becker B. 2013. Snow ball earth and the split of Streptophyta and Chlorophyta. Trends in Plant Science 18, 180–183. [DOI] [PubMed] [Google Scholar]
- Birmingham BC, Coleman JR, Colman B. 1982. Measurement of photorespiration in algae. Plant Physiology 69, 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Breuers FKH, Bräutigam A, Geimer S, Welzel U, Stefano G, Renna L, Brandizzi F, Weber APM. 2012. Dynamic remodeling of the plastid envelope membranes – a tool for chloroplast envelope in vivo localizations. Frontiers in Plant Science 3, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman B, Balkos KD. 2005. Mechanism of inorganic carbon acquisition in two Euglena species. Canadian Journal of Botany 83, 865–871. [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization. Plant Physiology 139, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M, Bauwe H, Hagemann M. 2007. Glycine accumulation is toxic for the cyanobacterium Synechocystis sp. strain PCC 6803, but can be compensated by supplementation with magnesium ions. FEMS Microbiology Letters 277, 232–237. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Hocken N, Weber APM. 2015. Plastidial metabolite transporters integrate photorespiration with carbon, nitrogen, and sulfur metabolism. Cell Calcium 58, 98–104. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Kahlon S, Hasse D, Ewald R, Lieman-Hurwitz J, Ogawa T, Ruth W, Bauwe H, Kaplan A, Hagemann M. 2006. The plant-like c2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria. Plant Physiology 142, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M, Pick TR, Bordych C, Weber AP. 2013. Towards closing the remaining gaps in photorespiration – the essential but unexplored role of transport proteins. Plant Biology 15, 676–685. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M. 2008. The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. PNAS 105, 17199–17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel N, van den Daele K, Kolukisaoglu U, Morgenthal K, Weckwerth W, Parnik T, Keerberg O, Bauwe H. 2007. Deletion of glycine decarboxylase in Arabidopsis is lethal under nonphotorespiratory conditions. Plant Physiology 144, 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Si Y, Douglass S, Casero D, Merchant SS, Pellegrini M, Ladunga I, Liu P, Spalding MH. 2012. Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. The Plant Cell 24, 1876–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O, Kind T. 2007. Metabolite profiling in blood plasma. Metabolomics - Methods in Molecular Biology 358, 3–17. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Kanesaki Y, Hirooka S, Era A, Sumiya N, Yoshikawa H, Tanaka K, Miyagishima S-Y. 2015. A nitrogen source-dependent inducible and repressible gene expression system in the red alga Cyanidioschyzon merolae . Frontiers in Plant Science 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmler H, Kugel H, Liebfritz D, Mayer A. 1988. Cytoplasmic pH of Dunaliella parva and Dunaliella acidophila as monitored by in vivo 31P-NMR spectroscopy and the DMO method. Physiologia Plantarum 74, 521–530. [Google Scholar]
- Giordano M, Beardall J, Raven JA. 2005. CO2 concentrating mechanism in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56, 99–131. [DOI] [PubMed] [Google Scholar]
- Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR. 2010. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant Journal 64, 355–365. [DOI] [PubMed] [Google Scholar]
- Hackenberg C, Huege J, Engelhardt A, Wittink F, Laue M, Matthijs HC, Kopka J, Bauwe H, Hagemann M. 2012. Low carbon acclimation in carboxysome-less and photorespiratory mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Microbiology 158, 398–413. [DOI] [PubMed] [Google Scholar]
- Hackenberg C, Kern R, Hüge J, Stal LJ, Tsuji Y, Kopka J, Shiraiwa Y, Bauwe H, Hagemann M. 2011. Cyanobacterial lactate oxidases serve as essential partners in N2 fixation and evolved into photorespiratory glycolate oxidases in plants. The Plant Cell 23, 2978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann M, Eisenhut M, Hackenberg C, Bauwe H. 2010. Pathway and importance of photorespiratory 2-phosphoglycolate metabolism in cyanobacteria. Advances in Experimental Medicine and Biology 675, 91–108. [DOI] [PubMed] [Google Scholar]
- Hagemann M, Fernie AR, Espie GS, Kern R, Eisenhut M, Reumann S, Bauwe H, Weber APM. 2013. Evolution of the biochemistry of the photorespiratory C2 cycle. Plant Biology 15, 639–647. [DOI] [PubMed] [Google Scholar]
- Hagemann M, Kern R, Maurino VG, Hanson DT, Weber APM, Sage RF, Bauwe H. 2016. Evolution of photorespiration from cyanobacteria to land plants considering protein phylogenies and acquisition of carbon concentrating mechanisms. Journal of Experimental Botany 67, 2963–2976. [DOI] [PubMed] [Google Scholar]
- Haimovich-Dayan M, Lieman-Hurwitz J, Orf I, Hagemann M, Kaplan A. 2015. Does 2-phosphoglycolate serve as an internal signal molecule of inorganic carbon deprivation in the cyanobacterium Synechocystis sp. PCC 6803? Environmental Microbiology 17, 1794–1804. [DOI] [PubMed] [Google Scholar]
- Heber U, Krause GH. 1980. Open question - what is the physiological role of photorespiration. Trends in Biochemical Sciences 5, 32–34. [Google Scholar]
- Husic DW, Husic HD, Tolbert NE, Clanton CBJ. 1987. The oxidative photosynthetic carbon cycle or C2 cycle. Critical Reviews in Plant Sciences 5, 45–100. [Google Scholar]
- Imamura S, Terashita M, Ohnuma M, et al. 2010. Nitrate assimilatory genes and their transcriptional regulation in a unicellular red alga Cyanidioschyzon merolae: Genetic evidence for nitrite reduction by a sulfite reductase-like enzyme. Plant and Cell Physiology 51, 707–717. [DOI] [PubMed] [Google Scholar]
- Kehlenbeck P, Coyal A, Tolbert NE. 1995. Factors affecting development of peroxisomes and glycolate metabolism among algae of different evolutionary lines of Prasinophyceae. Plant Physiology 109, 1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern R, Eisenhut M, Bauwe H, Weber APM, Hagemann M. 2013. Does the Cyanophora paradoxa genome revise our view on the evolution of photorespiratory enzymes? Plant Biology 15, 759–768. [DOI] [PubMed] [Google Scholar]
- Kozaki A, Takeba G. 1996. Photorespiration protects C3 plants from photooxidation. Nature 384, 557–560. [Google Scholar]
- Linka N, Theodoulou FL, Haslam RP, Linka M, Napier JA, Neuhaus HE, Weber AP. 2008. Peroxisomal ATP import is essential for seedling development in Arabidopsis thaliana . Plant Cell 20, 3241–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Misumi O, Shin-I T, et al. 2004. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428, 653–657. [DOI] [PubMed] [Google Scholar]
- Meeks JC, Castenholz RW. 1971. Growth and photosynthesis in an extreme thermophile, Synechococcus lividius (Cyanophya). Archives of Microbiology 78, 25–41. [DOI] [PubMed] [Google Scholar]
- Mettler T, Mühlhaus T, Hemme D, et al. 2014. Systems analysis of the response of photosynthesis, metabolism, and growth to an increase in irradiance in the photosynthetic model organism Chlamydomonas reinhardtii . The Plant Cell 26, 2310–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoda A, Sakagami R, Yagisawa F, Kuroiwa T, Tanaka K. 2004. Improvement of culture conditions and evidence for nuclear transformation by homologous recombination in a red alga, Cyanidioschyzon merolae 10D. Plant and Cell Physiology 45, 667–671. [DOI] [PubMed] [Google Scholar]
- Moroney JV, Jungnick N, DiMario RJ, Longstreth DJ. 2013. Photorespiration and carbon concentrating mechanisms: two adaptations to high O2, low CO2 conditions. Photosynthesis Research 117, 121–131. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kanakagiri S, Van K, He W, Spalding MH. 2005. Disruption of the glycolate dehydrogenase gene in the high-CO2-requiring mutant HCR89 of Chlamydomonas reinhardtii . Canadian Journal of Botany 83, 820–833. [Google Scholar]
- Norman EG, Colman B. 1991. Purification and characterization of phosphoglycolate phosphatase from the cyanobacterium Coccochloris peniocystis . Plant Physiology 95, 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren WL, Bowes G. 1971. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nature New Biology 230, 159–160. [DOI] [PubMed] [Google Scholar]
- Ohnuma M, Yokoyama T, Inouye T, Sekine Y, Tanaka K. 2008. Polyethylene glycol (PEG)-mediated transient gene expression in a red alga, Cyanidioschyzon merolae 10D. Plant and Cell Physiology 49, 117–120. [DOI] [PubMed] [Google Scholar]
- Pick TR, Brautigam A, Schulz MA, Obata T, Fernie AR, Weber APM. 2013. PLGG1, a plastidic glycolate glycerate transporter, is required for photorespiration and defines a unique class of metabolite transporters. Proceedings of the National Academy of Sciences U S A 110, 3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. 2006. Sensing inorganic carbon: CO2 and HCO3 − . Biochemical Journal 395, e5–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renné P, Dreßen U, Hebbeker U, Hille D, Flügge UI, Westhoff P, Weber APM. 2003. The Arabidopsis mutant dct is deficient in the plastidic glutamate/malate translocator DiT2. Plant Journal 35, 316–331. [DOI] [PubMed] [Google Scholar]
- Savir Y, Noor E, Milo R, Tlusty T. 2010. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proceedings of the National Academy of Sciences U S A 107, 3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarte S, Bauwe H. 2007. Identification of the photorespiratory 2-phosphoglycolate phosphatase, PGLP1, in Arabidopsis. Plant Physiology 144, 1580–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckbach J. 1995. The first eukaryotic cells - acid hot-spring algae. Journal of Biological Physics 20, 335–345. [Google Scholar]
- Simon P. 2003. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19, 1439–1440. [DOI] [PubMed] [Google Scholar]
- Somerville CR. 2001. An early Arabidopsis demonstration. Resolving a few issues concerning photorespiration. Plant Physiology 125, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H. 1974. Verteilung von Microbody-Enzymen aus Chlamydomonas in Dichtegradienten [Distribution of microbody enzymes in density gradients]. Planta 118, 35–42. [DOI] [PubMed] [Google Scholar]
- Stabenau H, Winkler U, Säftel W. 1993. Localization of glycolate dehydrogenase in two species of Dunaliella . Planta 191, 362–364. [Google Scholar]
- Suzuki K, Mamedov TG, Ikawa T. 1999. A mutant of Chlamydomonas reinhardtii with reduced rate of photorespiration. Plant and Cell Physiology 40, 792–799. [Google Scholar]
- Takahashi S, Bauwe H, Badger M. 2007. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiology 144, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm S, Florian A, Jahnke K, Nunes-Nesi A, Fernie AR, Bauwe H. 2011. The hydroxypyruvate-reducing system in Arabidopsis: multiple enzymes for the same end. Plant Physiology 155, 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Anwaruzzaman, Miyachi S, Yokota A. 1997. Ribulose-1,5-bisphosphate carboxylase/oxygenase from thermophilic red algae with a strong specificity for CO2 fixation. Biochemical and Biophysical Research Communications 233, 568–571. [DOI] [PubMed] [Google Scholar]
- Voll LM, Jamai A, Renné P, Voll H, McClung CR, Weber APM. 2006. The photorespiratory Arabidopsis shm1 mutant is deficient in SHM1. Plant Physiology 140, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Ohnuma M, Sato J, Yoshikawa H, Tanaka K. 2011. Utility of a GFP reporter system in the red alga Cyanidioschyzon merolae . The Journal of General and Applied Microbiology 57, 69–72. [DOI] [PubMed] [Google Scholar]
- Weber A, Flügge UI. 2002. Interaction of cytosolic and plastidic nitrogen metabolism in plants. Journal of Experimental Botany 53, 865–874. [DOI] [PubMed] [Google Scholar]
- Zelitch I, Schultes NP, Peterson RB, Brown P, Brutnell TP. 2009. High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiology 149, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenvirth D, Volokita M, Kaplan A. 1985. Photosynthesis and inorganic carbon accumulation in the acidophilic alga Cyanidioschyzon merolae . Plant Physiology 77, 237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.