Abstract
Upregulation of the C4 metabolic cycle is a major step in the evolution of C4 photosynthesis. Why this happened remains unclear, in part because of difficulties measuring the C4 cycle in situ in C3-C4 intermediate species. Now, Alonso-Cantabrana and von Caemmerer (2016) have described a new approach for quantifying C4 cycle activity, thereby providing the means to analyze its upregulation in an evolutionary context.
Key words: Carbon isotope discrimination, C3-C4 intermediate species, C4 evolution, Flaveria, F. brownii, F. floridana, intermediate photosynthesis.
C4 photosynthesis is a complex trait arising from evolutionary modifications to dozens of traits in C3 ancestral species (Box 1). Despite this complexity, it is also one of the most convergent of evolutionary phenomena, with over 60 independent origins (Sage et al., 2011). The leading hypothesis for C4 evolution proposes that glycine decarboxylase, a critical enzyme in photorespiration, is localized to the bundle sheath (BS) cells, thereby forcing all photorespiratory glycine to migrate from the mesophyll to BS tissues (Box 2; Rawsthorne, 1992). The resulting release of photorespired CO2 within the BS elevates its concentration by 200% or more, thus increasing the activity of BS Rubisco (Keerberg et al., 2014). Photorespiratory glycine shuttling, or C2 photosynthesis as it is now termed, is thus considered to be the evolutionary bridge between C3 and C4 photosynthesis (Sage et al., 2012). Because of the glycine shuttle, many features associated with C4 photoynthesis evolved, including Kranz-like anatomy and increased mesophyll to BS transport capacity (Sage et al., 2012). In short, C2 photosynthesis is the foundation upon which the C4 metabolic cycle became established, a possibility supported by phylogenetic studies which show that C2 species are closely related to many C4 lineages (Christin et al., 2011; Sage et al., 2014; Fisher et al., 2015).
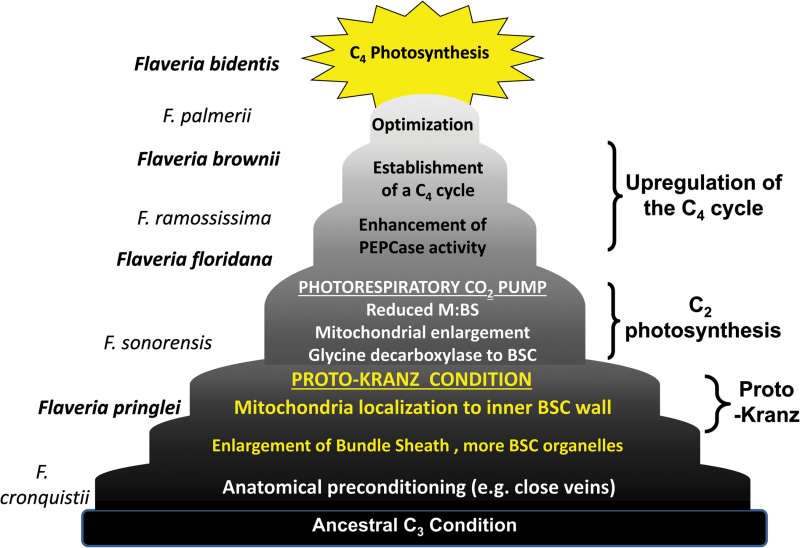
Box 1. C4 photosynthesis in Flaveria
The diagram shows a conceptual model of how the C4 photosynthetic pathway is assembled in the genus Flaveria (after Sage et al., 2012; 2014). A successive series of traits are layered onto previously existing traits to assemble a C4 phenotype from a C3 ancestor. Key stages in the process are initial enlargement and organelle enhancement in BS cells (BSCs) to create a proto-Kranz condition. Next, restrictions of glycine decarboxylase activity to the BS creates a photorespiratory glycine shuttle that concentrates CO2 into the BS, enhancing Rubisco efficiency (in what is termed C2 photosynthesis). The C4 metabolic cycle is then upregulated, beginning with enhancement of PEP carboxylase (PEPCase) activity, and following a series of optimizing adaptations, an efficient, fully functioning C4 pathway is created. To the left of the diagram, the Flaveria species that correspond to specific evolutionary stages are shown, with those studied by Alonso-Cantabrana and von Caemmerer (2016) highlighted in bold. M, mesophyll.
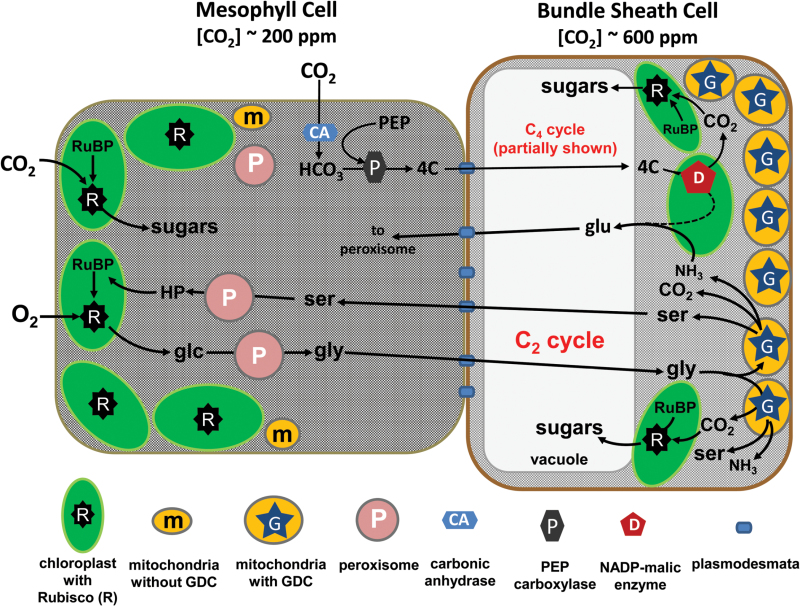
Box 2. The photorespiratory glycine shuttle (C2 photosynthesis)
The diagram summarizes the photorespiratory glycine (gly) shuttle, showing how glycolate (glc) produced after Rubisco (R) oxygenation of RuBP in the mesophyll cells diffuses into the BS cells where the glycine decarboxylase (GDC or G)-containing mitochondria can metabolize it to CO2, serine (ser) and ammonia (NH3). The CO2 in the BS can then accumulate to levels two to three times that in the mesophyll cells and stimulate Rubisco activity in nearby BS chloroplasts, while the ser returns to the mesophyll cells to be metabolized back to RuBP in a series of steps. A C4 metabolic cycle can also function in species conducting C2 photosynthesis, to provide additional CO2 to the BS, but also possibly to provide carbon skeletons to facilitate NH3 reassimilation in the BS, as indicated by the dashed line in the BS cell (Rawthorne, 1992; Mallmann et al., 2014). glu, glutamate; HP, hydroxy pyruvate.
While the evidence supports a C2 photosynthetic bridge to C4 photosynthesis, it is only the first half of the bridge. The second half involves the upregulation of the C4 metabolic cycle. Why C4 metabolism became upregulated is unknown, although a recent hypothesis suggests it happened to provide carbon skeletons for re-assimilation of ammonia released by BS glycine decarboxylase (Mallmann et al., 2014). A way to test this and other evolutionary hypotheses is to use a comparative approach, where the appearance of a given trait is evaluated in multiple yet distinct evolutionary lineages (Ackerly, 1999). Exploitation of the comparative approach has been facilitated by phylogenetic characterization of numerous C4 lineages in recent years (Sage et al., 2011; Kadereit et al. 2012; Christin et al. 2013); however, given the potential numbers of lines to analyze, it is necessary to have a quick method to quantify the C4 cycle in an evolutionary context. This has been lacking, particularly since 14C-tracer methods have fallen out of favor for safety and feasibility reasons.
Using a method that involves real-time observations of steady-state 13C and 12C discrimination in plants, Alonso-Cantabrana and von Caemmerer (2016) present a rapid means to assay the contribution of C3 and C4 metabolism to carbon gain in intact leaves of C3, C2 and C4 species, and then test the method on four species of Flaveria (Asteraceae), the model genus for studying C4 evolution. The four species differ in degree of C4 cycle engagement: F. bidentis is a full C4 plant; F. pringlei is a C3 species with no C4 cycle activity; and two species are C3-C4 intermediates, one with a modest C4 cycle to compliment the dominant C2 cycle (F. floridana), the second with a strong C4 cycle but retaining slight Rubisco expression in the mesophyll cells (F. brownii, which is described as being a C4-like intermediate).
Assessing C4 cycle activity – challenges and solutions
The challenge for assessing C4 cycle activity in C3-C4 intermediates is that the C4 cycle operates in parallel with a C2 cycle (which transports CO2 into the BS via the glycine shuttle, Box 2) and the C3 cycle (which is responsible for all net CO2 fixation in the leaf), such that the biochemical signatures of their respective activities are difficult to segregate. Historically, the relative contribution of the C4 cycle to carbon gain was assessed using pulse-chase experiments to determine 14C incorporation into the initial metabolites fixed by PEP carboxylase and Rubisco, a time consuming procedure that required sample destruction and chromatographic separation of radioactive compounds (Monson et al., 1986; Moore et al., 1987).
Analytical gas exchange has been widely used to identify C3-C4 intermediacy by measuring reductions in the CO2 compensation point of photosynthesis (Γ); however, it cannot delineate C4 cycle contributions because Γ is affected by glycine shuttling and the C4 cycle (Alonso-Cantabrana and von Caemmerer, 2016). Carbon isotope ratios (δ13C) can identify C4 cycle activity, because PEP carboxylase discriminates against 13C less than Rubisco. This leads to the well-known difference in δ13C between C3 and C4 plants, where the δ13C of C3 plants is –22‰ to –32‰ while in C4 plants it is –9‰ to –16‰. This difference is easily detectable with a mass spectrometer, which has been valuable for screening C3 to C4 transitions in phylogenetic clades using plant material from herbarium collections (e.g. Christin et al., 2011; Fisher et al., 2015).
As noted by Alonso-Cantabrana and von Caemmerer (2016), however, δ13C of dried plants cannot precisely delineate C4 metabolism in C3-C4 intermediates. Multiple processes contribute to the δ13C signal, including Rubisco and PEP carboxylation, refixation of photorespired CO2, diffusion of CO2 and various biosynthetic processes. Dry matter δ13C also integrates environmental variation during a plant’s life, and the δ13C in the air around the leaf can vary with position in the canopy and proximity to fossil fuel sources (an issue in urban areas, where many labs are located). To avoid these complications, real-time, mass spectroscopy should be coupled to gas exchange analyses, producing ‘on-line’ carbon isotope assessments that reflect the immediate biochemistry of CO2 fixation. On-line measurements are facilitated by tunable-diode laser absorbance spectrometers (TDLASs), which are best known from mesophyll conductance studies (Evans and von Caemmerer, 2013).
The on-line process factors out variation in source gas δ13C, producing a direct measure of discrimination (∆) against 13C by photosynthesis. The C4 versus C3 cycle activity can then be determined by simultaneously measuring and model-fitting CO2 exchange and ∆ responses to variation in atmospheric CO2 and O2, as described by Alonso-Cantabrana and von Caemmerer (2016). A key contribution of their paper is a new equation that describes ∆ responses for C3, C3-C4 intermediate and C4 photosynthesis, and incorporates contributions from mesophyll conductance and transpiration rate. This is important, because CO2 provided to BS Rubisco by glycine decarboxylase increases ∆, while CO2 provided by PEP carboxylation and the C4 cycle decreases ∆, such that the two signals offset. Through their approach, Alonso-Cantabrana and von Caemmerer overcome this conflict to reveal the C4 cycle contribution.
New measurements with Flaveria
With their approach, Alonso and von Caemmerer (2016) estimate that C4 cycle activity at current atmospheric CO2 levels contributes about 12% of the carbon assimilated in F. floridana and 80% of the carbon assimilated by F. brownii (see Fig. 8 in Alonso-Cantabrana and von Caemmerer), which is comparable to pulse-chase estimates using 14C (Moore et al., 1987). Of note, they are able to examine the change in the C3 and C4 contributions across a range of intercellular CO2 levels in the same leaf. Thus, for example, at CO2 levels approximating pre-industrial values (280ppm), the C4 cycle contribution increases to 15% in F. floridana and 90% in F. brownii. At high CO2, the C4 contribution dropped off in F. brownii, to only 75%, reflecting a marked increase in the efficiency of the residual Rubisco left in its mesophyll tissue. This increase in the contribution of Rubisco to CO2 fixation causes a substantial rise in the biochemical ∆ from below one at low CO2 to near six at high CO2 (Fig. 5 in Alonso-Cantabrana and von Caemmerer). Moreover, the ability to predict the CO2 response of photosynthesis in the intermediates was much improved by incorporating their estimated C4 cycle contribution, as was the modelled CO2 response of ∆.
These responses highlight how small amounts of C4 cycle activity can improve carbon gain at low CO2 levels, yet become less significant at elevated CO2 if a modest amount of Rubisco is present in the mesophyll tissue. In F. floridana, the function of the C4 cycle has been questioned, since it seemed to contribute little to photosynthesis, and thus was suggested to initiate a futile cycle (Monson et al., 1986). Alonso-Cantabrana and von Caemmerer demonstrate that the C4 cycle does indeed contribute to CO2 fixation, and thus is not futile and could be adaptive in low CO2 conditions of recent geological time, when atmospheric CO2 fell below 200ppm (Gerhart and Ward, 2010).
In summary, Alonso-Cantabrana and von Caemmerer have provided researchers with a powerful approach that can be quickly applied to many C3-C4 intermediates from a range of lineages, thereby enabling comparative analyses for addressing hypotheses explaining how evolution upregulated C4 metabolism. When coupled with genomic and ecological data, C4 researchers should now be able to evaluate in detail how one of the most evolutionary complex traits on Earth repeatedly evolved in recent geological time.
References
- Ackerly DD. 1999. Comparative plant ecology and the role of phylogenetic information. In: Press MC, Scholes JD, Barker MG, eds. Physiological plant ecology. Oxford, UK: Blackwell, 391–413. [Google Scholar]
- Alonso-Cantabrana H, von Caemmerer S. 2016. Carbon isotope discrimination as a diagnostic tool for C4 photosynthesis in C3-C4 intermediate species. Journal of Experimental Botany 67, 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ. 2013. Anatomical enablers and the evolution of C4 photosynthesis. Proceedings of the National Academy of Sciences, USA 110, 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF. 2011. Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65, 643–660. [DOI] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S. 2013. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant, Cell and Environment 36, 745–756. [DOI] [PubMed] [Google Scholar]
- Fisher AE, McDade LA, Kiel CA, Khoshravesh R, Johnson MA, Stata M, Sage TL, Sage RF. 2015. History of Blepharis (Acanthaceae) and the origin of C4 photosynthesis in section Acanthodium . International Journal of Plant Sciences 176, 770–790. [Google Scholar]
- Gerhart LM, Ward JK. 2010. Plant responses to low [CO2] of the past. New Phytologist 188, 674–695. [DOI] [PubMed] [Google Scholar]
- Kadereit G, Ackerly D, Pirie MD. 2012. A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proceedings of the Royal Society, B: Biological Science 279, 3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerberg O, Parnik T, Ivanova H, Bassuner B, Bauwe H. 2014. C2 photosynthesis generates about 3-fold elevated leaf CO2 levels in C3-C4 intermediate species Flaveria pubescens . Journal of Experimental Botany 65, 3649–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshravesh R, Akhani H, Sage TL, Nordenstam B, Sage RF. 2012. Phylogeny and photosynthetic pathway distribution in Anticharis Endl. (Scrophulariaceae). Journal of Experimental Botany 63, 5645–5658. [DOI] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Brautigam A, Lercher MJ, Weber APM, Westhoff P, Gowik U. 2014. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria . eLife 3, e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Moore BD, Ku MSB, Edwards GE. 1986. Co-function of C3- and C4-photosynthetic pathways in C3, C4 and C3-C4 intermediate Flaveria species. Planta 168, 493–502. [DOI] [PubMed] [Google Scholar]
- Moore BD, Ku MSB, Edwards GE. 1987. C4 photosynthesis and light-dependent accumulation of inorganic carbon in leaves of C3-C4 and C4 Flaveria species. Australian Journal of Plant Physiology 14, 657–668. [Google Scholar]
- Rawsthorne S. 1992. C3-C4 intermediate photosynthesis: linking physiology to gene expression. The Plant Journal 2, 267–274. [Google Scholar]
- Sage RF, Christin PA, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage RF, Khoshravesh R, Sage TL. 2014. From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis. Journal of Experimental Botany 65, 3341–3356. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]