Highlight
Accurate measurement of small fluxes is critical for photosynthesis models. This review highlights improved methods of measuring CO2 diffusion into and within leaves, CO2 concentrations inside leaves, and CO2 efflux.
Key words: Diffusion, internal leaf CO2, mesophyll conductance, photosynthesis, respiration, stomatal conductance, xylem CO2.
Abstract
Since its inception, the Farquhar et al. (1980) model of photosynthesis has been a mainstay for relating biochemistry to environmental conditions from chloroplast to global levels in terrestrial plants. Many variables could be assigned from basic enzyme kinetics, but the model also required measurements of maximum rates of photosynthetic electron transport (J max), carbon assimilation (Vcmax), conductance of CO2 into (g s) and through (g m) the leaf, and the rate of respiration during the day (R d). This review focuses on improving the accuracy of these measurements, especially fluxes from photorespiratory CO2, CO2 in the transpiration stream, and through the leaf epidermis and cuticle. These fluxes, though small, affect the accuracy of all methods of estimating mesophyll conductance and several other photosynthetic parameters because they all require knowledge of CO2 concentrations in the intercellular spaces. This review highlights modified methods that may help to reduce some of the uncertainties. The approaches are increasingly important when leaves are stressed or when fluxes are inferred at scales larger than the leaf.
Introduction
The accepted standard for interpreting measurements of photosynthesis was developed over 35 years ago (Farquhar et al., 1980) and it remains the foundation for a large array of research topics, ranging from genetic modification to global climate modes (Friedlingstein et al., 2006; Price et al., 2011; Bodman et al., 2013; Peterhansel et al., 2013; Lin et al., 2014a,b; Borland et al., 2015; Carmo-Silva et al., 2015; Long et al., 2015; Schimel et al., 2015). The strength of the Farquhar et al. (1980) model that led to its universal adoption was the judicial use of simplifications to mathematically represent the complexity of linking biochemistry to the prevailing environmental conditions experienced by leaves. A major advance was the concept of single limiting factors (see Sharkey, 1989), also known as the photosynthetic teeter-totter or see-saw (Farquhar et al., 2001). This concept is based on the assumption that losses in the coupling between the light and dark (i.e. carbon) reactions are minimal, so the rate of photosynthetic CO2 assimilation (A) can be estimated from the minimum of the light and carbon reactions estimated separately (von Caemmerer, 2013).
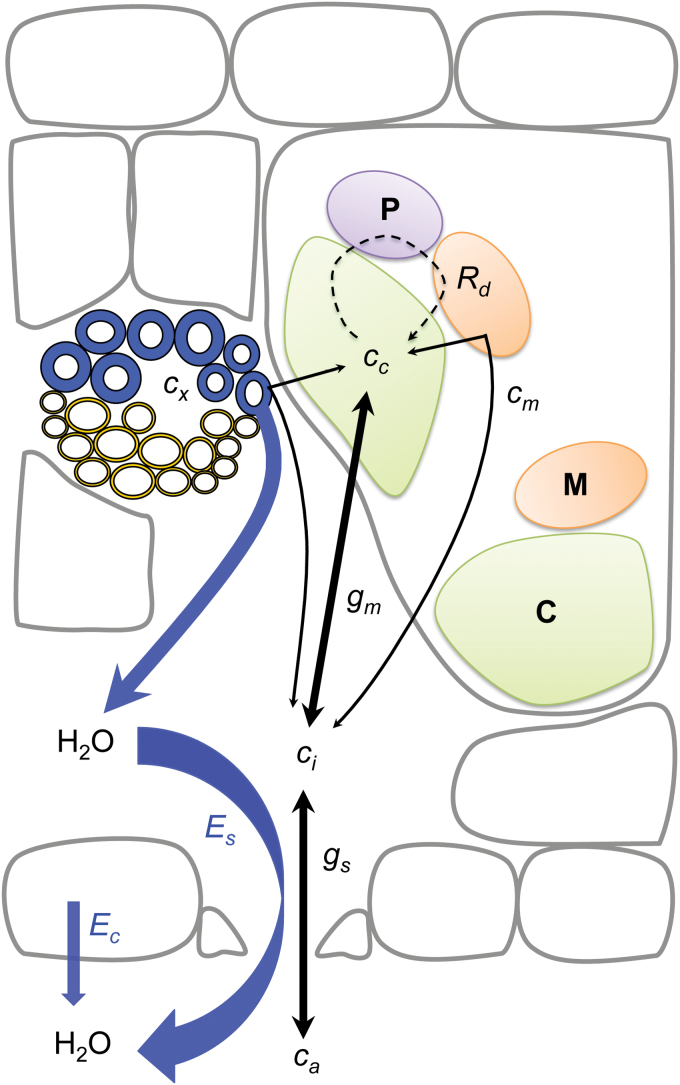
Here, we consider some of the carbon reaction assumptions from the Farquhar et al. (1980) paper that affect the CO2 partial pressure in the chloroplast stroma (c c) because it is central to the model. In particular, we examine assumptions about sources of CO2 that originate as small fluxes within the plant and either diffuse out of the leaf or are recycled by photosynthesis. We also examine assumptions about the effects of small fluxes of water and CO2 on the diffusion of CO2 from the atmosphere (c a) into the sub-stomatal air spaces inside the leaf (ci) and, finally, through the cell to the chloroplast stroma (Fig. 1). There have been several advances in knowledge and methodology in the intervening 35 years that have parameterized some of the assumptions, while others have been mostly overlooked.
Fig. 1.
Leaf cross section showing small fluxes and conductances in leaves. CO2 fluxes are labelled with solid black arrows between concentrations at each location (c a = above the leaf, c i = intercellular spaces, c c = chloroplast, c m = mesophyll cytosol, c x = xylem sap) along with stomatal (g s) and mesophyll (g m) conductances. Water fluxes are shown with blue arrows, along with transpiration through stomata (E s) and the cuticle/epidermis (E c). The dashed circular arrow represents photorespiration and R d is respiration in the absence of photorespiration. Organelles in the exaggerated palisade layer cell (upper right) are coloured and labelled with a bold letter (C = chloroplast, M = mitochondrion, P = peroxisome), blue circles represent xylem in a leaf vein.
It is becoming evident that errors in small fluxes at the subcellular to leaf levels may have large consequences when using measurements to parameterize current carbon–climate coupled Earth system models and make predictions about the global carbon cycle. Behaviour of the carbon cycle is the second greatest source of uncertainty in climate model predictions of global temperature (Bodman et al., 2013) and errors in small fluxes could be contributing significantly. Over 15% of the uncertainty can be attributed to the temperature feedbacks on respiration and net primary productivity, while another 11% arises from other biological responses, including CO2 feedbacks (Bodman et al., 2013). Even though leaf respiration is often an order of magnitude lower than photosynthesis (i.e. a small flux), current estimates indicate that gross global leaf respiration releases around 30 Pg C year−1, which is approximately four times larger than all emissions from fossil fuel burning globally (Atkin et al., 2015). Therefore, even a 1% error in predictions of leaf respiration (which is so small a flux that some instruments cannot measure it) would add up to 0.3 Pg C year−1 globally and would account for almost half of the 0.7 Pg C year−1 uncertainty in global carbon models (Canadell et al., 2007).
The rate of leaf respiration in the light is challenging to measure (see ‘Efflux of CO2 from leaves’ below), and is the smallest of the three critical fluxes for determining carbon assimilation (A) in Farquhar et al. (1980):
| (1) |
where V c is the rate of carboxylation by the photosynthetic enzyme ribulose-1,5-carboxylase/oxygenase (Rubisco), V o is the rate of oxygenation by Rubisco, and R d is the rate of respiration in the day (excluding photorespiratory CO2 release).
The flux, V o, is difficult to directly measure so it is often described by the kinetics of Rubisco and connected to the carbon reaction equations through the CO2 loss resulting from the processing of phosphoglycolate by photorespiratory metabolism. Equation 1 assumes a loss of 0.5mol CO2 for every mole of O2 assimilated. It has been proposed that this constant is actually variable, being affected by temperature and catalase activity (Hanson and Peterson, 1986; Zelitch, 1992; Brisson et al., 1998). Mutants have also been generated with much higher loss of CO2 (Cousins et al., 2008, 2011), though the mechanism for generating the extra CO2 is unclear. We will continue to use the constant of 0.5 here, but note that this assumption should be tested more widely across plant types and environmental conditions, especially in the case of plants whose metabolism has been re-engineered. Defining the term ϕ as the ratio of V o to V c, Eq. 1 can then be rearranged:
| (2) |
It is useful to expand the description of ϕ in terms of the kinetics of Rubisco (Laing et al., 1974):
| (3) |
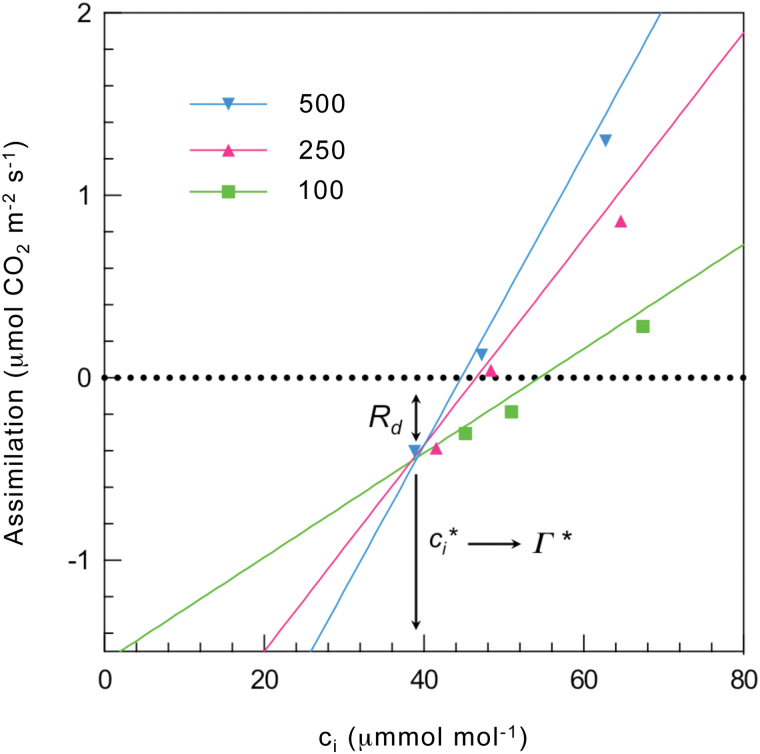
where V cmax, V omax, K c, and K o are the maximal rates and the Michaelis–Menten constants of carboxylation and oxygenation, respectively; c c and O c are the partial pressures of CO2 and O2 in the chloroplast; and S c/o is the relative specificity of Rubisco for CO2 over O2 (note that S c/o is expressed with the CO2 reactions in the numerator and O2 reactions in the denominator, the inverse of the standard for ϕ). Gas exchange methods can readily be used to measure the CO2 compensation point of photosynthesis by reducing c a until A balances R d and photorespiration (i.e. net A = 0) (Fig. 2). This point is a simple and fundamental way to represent the ability of a leaf to use CO2 (Woodrow and Berry, 1988). However, for modelling it is a bit more useful to define a related compensation point using Eq. 2 and realizing that A = 0 when ϕ = 2 and R d = 0. This point is called Γ * (Laisk, 1977; Farquhar et al., 1980) and can be written as:
Fig 2.
Common approach for determining respiration in the day (R d), the compensation point within the cell in the absence of respiration (Γ *), and the internal leaf CO2 concentration at which photosynthesis is balanced by photorespiration (c i*). The method of Laisk (1977) as modified by von Caemmerer et al. (1994) uses the intersection of the linear portion (low CO2) of multiple photosynthetic CO2 response curves, each measured at a different light intensity (low light is favoured for at least one, here Populus deltoides was measured at 500, 250, and 100 µmol m−2 s−1). The usual interpretation is that the intersection of the lines occurs at c i* and R d, and Γ * is at a higher CO2 concentration due to the effects of mesophyll conductance and respiration. This approach has several assumptions that are not always valid and may cause significant errors (von Caemmerer, 2013).
| (4) |
ϕ can then be re-written as:
| (5) |
and, finally, substituting Eq. 5 into Eq. 2 yields:
| (6) |
Unlike Eq. 2, there are commonly used gas exchange methods for determining all the variables in Eq. 6, but the measurements are challenging and not entirely independent. As discussed below, calculations of Γ * and c c each require knowing mesophyll conductance (g m), while calculations of g m include R d and Γ *. In addition, there is growing evidence that the magnitude of variation in g m, R d, and Γ * is important, but they are still generally treated as constants. Our technical ability to measure Γ *, R d, and g m has greatly improved, but they are all based on measurements of small fluxes that are very sensitive to errors in our assumptions.
The small fluxes that we are considering all interact within the intercellular space of the leaf to create c i (Fig. 1); therefore, accurate determination of c i is essential (see below). For ease of discussion, we have compiled these important parameters into two topic areas: (i) factors affecting the efflux of CO2 from leaves, which include photorespiration, day respiration, and a relatively unknown flux of CO2 dependent on transpiration and the inorganic carbon concentration in the xylem (c x); and (ii) factors affecting the gradient of CO2 between leaves and the atmosphere, including stomatal conductance (g s), transpiration through stomata (E s), transpiration through the cuticle/epidermis (E c), and mesophyll conductance (g m).
Efflux of CO2 from leaves
Photorespiratory CO2 efflux
One of the largest complications in understanding carbon reactions is the fact that CO2 and O2 compete for the active site of Rubisco (Bowes et al., 1971). This competition greatly impacts our ability to unravel these reactions both because the rate of oxygenation is substantial, often leading to the loss of ~25% of the CO2 assimilated by carboxylation via photorespiration (Sharkey, 1988), and because measuring or modelling the flux is technically challenging (Busch, 2013). Direct measurements of V o would require separating it from R d, which is a significant challenge because both occur simultaneously with photosynthesis and have the opposite effect on gas exchange (i.e. they release CO2 and consume O2). The idea that one might have to correct for this kind of flux led it to be called “a nightmare oppressing all who are concerned with the exact measurement of [gross] photosynthesis” (see p. 170 of Zelitch [2001] for the context and attribution).
Instead of directly measuring V o, Eq. 6 allows A to be modelled by measuring Γ *. This is most commonly achieved using the method of Laisk (1977) as modified by von Caemmerer et al. (1994), which uses the intersection of the linear portion (low CO2 where V c is small) of multiple photosynthetic CO2 response curves each measured at a different light intensity (low light is favoured for at least one). Figure 2 illustrates how these data are used to calculate constants for R d and the internal leaf CO2 concentration at which photosynthesis is balanced by photorespiration (c i*) (also described as the compensation point in the absence of R d). A separate measurement is needed for g m (discussed later), which is then used with R d and c i* to calculate the equivalent compensation point within the cell, Γ * (Eq. 7) (for recent reviews, see Busch, 2013; von Caemmerer, 2013).
| (7) |
Although this method has been widely used, it is problematic. There is frequently no common intersection of the multiple CO2 responses, so criteria need to be developed for excluding data or measurements have to be repeated (Weise et al., 2015). A new fitting approach has recently been proposed that improves this process (Walker and Ort, 2015), but two conceptual problems with interpreting the gas exchange for determining Γ * remain. One is that Eq. 7 requires R d, but R d may not be constant (see the ‘Non-photorespiratory CO2 efflux’ section below). The other is that Eq. 7 also requires g m, and the pathway for diffusion of CO2 back out of leaf cells may be more complicated than originally envisioned. In the Farquhar et al. (1980) model, it is assumed that all respired and photorespired CO2 passes back through the chloroplast before entering the intercellular air spaces of the leaf. However, the CO2 could also enter the cytosol and escape between the chloroplasts, which is a different pathway with a separate set of resistances. Therefore, there would be a different concentration of CO2 in the cytosol of mesophyll cells (c m) than in the chloroplast (Fig. 1). The resistance of this additional path needs to be estimated in order to determine Γ * (von Caemmerer, 2013). A modelling effort by Walker and Ort (2015) used a series of fixed ratios of the resistances through each pathway and argued that the errors caused by not accounting for the two pathways should be small. However, this also assumes that the resistances for each are constant across the measurement conditions. This may not be a safe assumption because the extent to which chloroplasts cover the surface of mesophyll cells exposed to intercellular air spaces (S c /S m) is known to affect the diffusion of CO2 out of leaves, and S c /S m changes between the high and low light intensities (Busch et al., 2013) used for the Laisk (1977) approach. Given the wide diversity in chloroplast movement among plants (Koniger, 2014), more studies are needed to explicitly examine the frequency with which chloroplast movement is a problem for determining Γ * and R d.
Aside from chloroplast positioning, diffusion across the chloroplast membrane is dependent on the expression of CO2 permeable aquaporins (Flexas et al., 2006; Uehlein et al., 2008; Heckwolf et al., 2011) and has been modelled as a major resistance between c i and c c (Tholen and Zhu, 2011). Furthermore, there is controversy over the extent to which g m changes in response to CO2 and light and how widely the responses can be generalized across species (Flexas et al., 2007; Tazoe et al., 2009, 2011; Douthe et al., 2011; Gu and Sun, 2014; Nishida et al., 2015; Xiong et al., 2015). If g m is changing when CO2 and light are changed for the Laisk (1977) method, then determining Γ * from c i* would be much more complicated and a single point of convergence for c i* may not exist.
Non-photorespiratory CO2 efflux
R d is generally an order of magnitude smaller than the maximum rates of V c and V o, so for simplicity, Farquhar et al. (1980) assumed R d was equal to the rate of leaf respiration in the dark (Eq. 1), even though they recognized it was likely to be lower. Since then, there has been a growing consensus that leaf respiration differs significantly between day and night. However, measurements of the inhibition of leaf respiration in the light range widely from 16 to 77% and are affected by light intensity (Sharp et al., 1984; Villar et al., 1994; Atkin et al., 1998, 2000; Shapiro et al., 2004; Ayub et al., 2011; Way et al., 2015). Much of this may be due to a reduction in the cyclic nature of the citric acid cycle when leaves are illuminated (Tcherkez et al., 2009). It has also been shown that rates of day and night leaf respiration can respond differently to sustained drought, with day respiration inhibited by drought more than night respiration (Ayub et al., 2011). In some cases, the ratio of light to dark respiration increases with rising leaf temperature through a reduction in the light suppression of respiration at high temperatures (Way et al., 2015). When considering the importance of environmental effects on respiration, it is important to realize that any condition reducing photosynthesis (e.g. low light, low CO2, low water potential, high and low temperature) often increases the relative importance of respiration.
Beyond respiration of leaf cells, recent work has highlighted the flux of CO2 and bicarbonate from roots to stems, leaves, and, ultimately, the atmosphere via the transpiration stream (for reviews, see Teskey et al., 2008; Bloemen et al., 2015b; Steppe et al., 2015). Respired CO2 from root and stem cells was generally thought to exit the plant near the location it was generated; however, it is now known that a large portion of the CO2 dissolves in the xylem sap (c x) and is transported to other parts of the plant, where some is used for photosynthesis (Teskey and McGuire, 2002; Wertin and Teskey, 2008; McGuire et al., 2009; Aubrey and Teskey, 2009; Bloemen et al., 2013). We are only now beginning to understand how much c x contributes to c i (Fig. 1) and how much is recycled through photosynthesis versus being lost to the atmosphere (Bloemen et al., 2013, 2015a). Given that the gas phase concentration of c x has been measured as high as 26% (i.e. the concentration in air equilibrated with the total dissolved CO2 and bicarbonate; McGuire and Teskey, 2002; Teskey et al., 2008), even a small amount entering leaves could have a large effect. As described in the introduction, a 1% error in leaf respiration scaled up globally represents a flux similar to the uncertainty of global carbon-cycle models.
Since the Laisk (1977) method measures CO2 responses at high and low light intensity, the transpiration rate (and the associated c x efflux) would likely differ between CO2 responses and confound the estimate of c i* and R d. Efflux of c x into the intercellular spaces is also complicated by the fact that c x is much less likely to have equilibrated with c c before entering the c i pool (Fig. 1). As described earlier for the diffusion of CO2 from the cytosol to the intercellular spaces between chloroplasts, the additional diffusional pathway makes calculation of Γ * from c i* exceedingly complex. Finally, this internal transpiration-dependent CO2 source is not included in calculations of c i, which could contribute to some of the errors discussed below.
Gradient of CO2 between the atmosphere and the chloroplast
CO2 in sub-stomatal cavities
CO2 diffuses as a gas from the atmosphere into the intercellular spaces inside leaves. The diffusion is mostly through stomatal pores that can vary in size and control the diffusion rate. Stomatal dimensions are microscopic, making it difficult to measure the c i. However, the wet internal surfaces of the cells provide a source of water vapour that diffuses out through the same stomata. Realizing this, Moss and Rawlins (1963) suggested that water vapour moving out could act as a tracer for CO2 moving in. The concentration of CO2 inside could then be calculated from the rate of water loss according to Eq. 8:
| (8) |
where c a is the partial pressure of CO2 in the bulk air above the leaf (we convert partial pressures here and below to concentrations, mol mol−1, because of their greater familiarity for the reader), A s is the assimilation rate for CO2 moving into the leaf through stomata (mol m−2 s−1), E s is the transpiration rate for water vapour moving out of the leaf through stomata (mol m−2 s−1; Fig. 1), and w i and w a are the water vapour partial pressures in the leaf and bulk air, respectively (converted to concentrations, mol mol−1). The ratio of diffusivities for water vapour and CO2 in air is used to convert the rate of water diffusion into a rate of CO2 diffusion (at 25°C, water vapour diffuses about 1.58 times as fast as CO2, see Massman 1998). In order for CO2 to diffuse in, the partial pressure in the intercellular spaces must be lower than that outside. Accordingly, the terms to the right of the minus sign are the amount by which c i is lower than outside.
This equation and some of its variants (von Caemmerer and Farquhar, 1981; Boyer and Kawamitsu, 2011) are now the norm for determining c i. This equation has the great advantage that gradients for diffusion are undisturbed, and the leaf acts as it normally would. Because c i is calculated inside the leaf, the equation gives the concentration actually available to the photosynthetic process after accounting for effects of stomata. The c i is also the starting concentration for CO2 diffusion through the mesophyll cells to the chloroplasts where assimilation occurs. The Moss/Rawlins relationship thus stands as a great achievement.
Several aspects of the relationship are noteworthy. First, several of the terms are easily measured, such as w a and ca in bulk air above the leaf (i.e. inside a stirred gas exchange chamber), and w i determined from the temperature of the water in the leaf (i.e. the leaf temperature). Second, the crucial measurement is the ratio of A s to E s, when E s is 100–1000 times larger than A s, it causes the ratio to be very small (0.01 to 0.001). Third, the flood of vapour diffusing out inhibits the trickle of CO2 diffusing in. The reverse is also true, but the trickle does not affect the flood very much. These ternary interactions were first shown by von Caemmerer and Farquhar (1981) and expanded upon by Boyer and Kawamitsu (2011) but will be ignored here for the sake of simplicity. Fourth, all the diffusion is assumed to be in the gas phase moving through stomata. Diffusion through the cuticle and underlying epidermis that cover the leaf between individual stomata is assumed to be small enough to ignore.
Impact of the cuticle on calculated CO2 in leaves
Diffusion through the cuticle and underlying epidermis inevitably does influence the calculations, but when are the affects large enough to cause important errors? As the stomata begin to close, the relative amount of diffusion through the cuticle and epidermis increases. The cuticle/epidermis transmits water vapour 20–40 times faster than it transmits CO2, a rate that is thus vastly different from the 1.58 in Eq. 8 (Boyer et al., 1997; Boyer, 2015a). Consequently, using water vapour as a tracer for CO2 tends to overestimate the amount of CO2 entering the leaf.
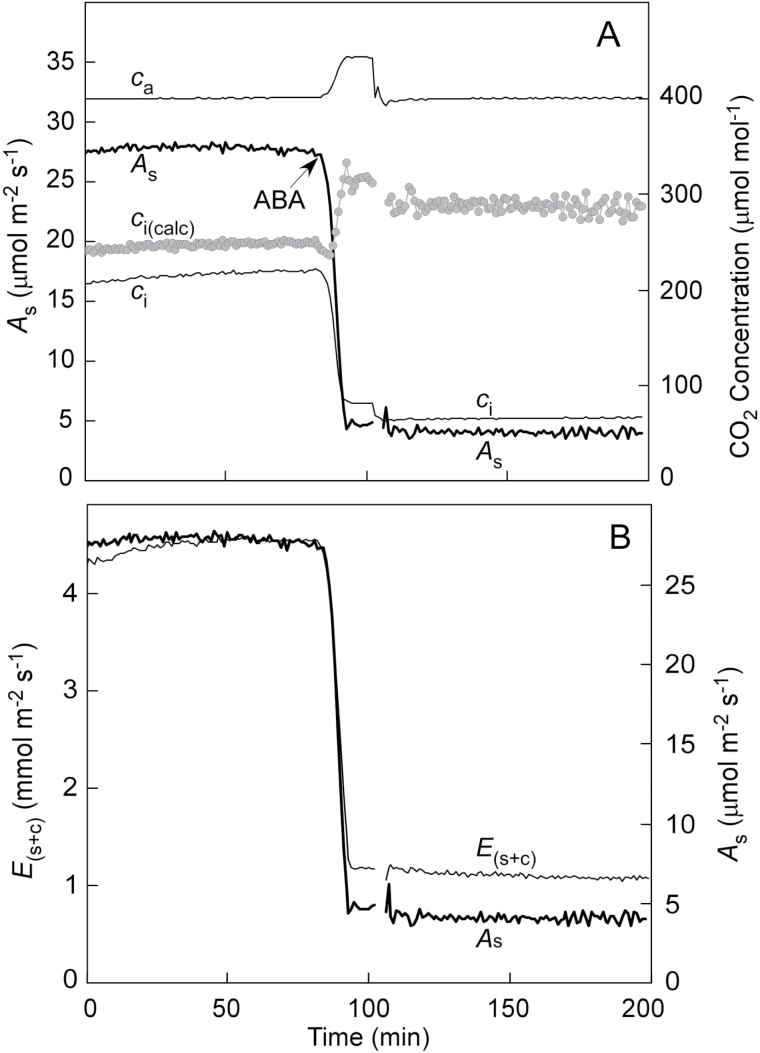
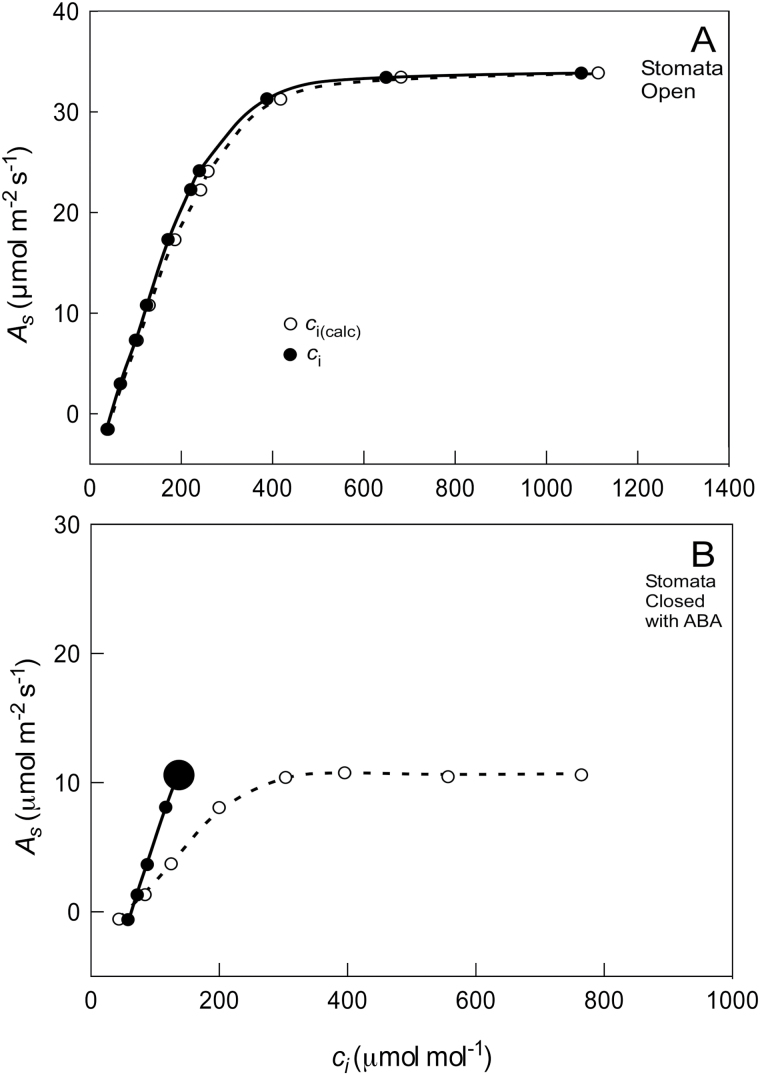
This effect can be seen when directly measuring c i by clamping a cup to the abaxial (lower) surface with an airtight seal and allowing it to equilibrate with the CO2 concentration in the leaf (Fig. 3A) (Sharkey et al., 1982). When stomata were open, the cup indicated that measured c i was below calculated c i [c i(calc)]. When abscisic acid (ABA) was fed through the petiole of a detached sunflower (Helianthus annus) leaf to close the stomata, the cup indicated that measured c i was even farther below c i(calc). In this situation, photosynthesis (A s) and measured c i decreased while c i(calc) increased. Evidently, the CO2 inside the leaf was being consumed by photosynthesis, and the cup reflected this behaviour whereas c i(calc) did not. The reason for the discrepancy is shown in Fig. 3B, where water loss as a tracer for CO2 clearly overestimated the amount of CO2 entering the leaf as stomata close. The net result in Eq. 8 is that E s is too rapid because of the additional cuticular water loss (E c) (Fig. 1). The ratio A s/E s becomes too small and c i(calc) becomes too high whether stomata are open or not (Boyer, 2015b).
Fig. 3.
(A) Gas exchange of a sunflower leaf fed ABA to close the stomata. The leaf was excised and initially fed water, to which 10–4 M ABA was added at the arrow. Shown are the assimilation rate (A s), CO2 concentration in the bulk air (c a), CO2 concentration directly measured inside the leaf by sealing a cup to the abaxial surface (c i), and the CO2 concentration calculated for the same leaf [c i(calc)), round grey points] according to Boyer (2015a). Note that A s and c i decrease when the stomata close but c i(calc) increases. (B) Transpiration [E (s+c)] and A s for the leaf in (A). Transpiration is shown as E (s+c) because water vapour moves through both stomata and the cuticle/epidermis while CO2 moves mostly through stomata. The axes in (B) have been adjusted so the diffusion of water vapour and CO2 are superimposed before feeding ABA. This demonstrates the overestimation of water vapour diffusion after feeding with ABA. Data in (A) are redrawn from Boyer (2015a).
Notice that this result was obtained because the cuticle/epidermis contributes substantial water loss. Measurements of transpiration include both stomatal and cuticle loss (E (s+c) instead of E s; Fig. 1). By contrast, the cuticle/epidermis conducts so little CO2 that it can be ignored (A s). This happens because water vapour is thought to move only through the wax layer of the cuticle whereas CO2 moves through both wax and epidermis before it enters the intercellular spaces. In effect, the cuticle/epidermis is less of a barrier to water vapour than to CO2 (Boyer et al., 1997; Boyer, 2015a, b).
This fact can be used to determine how much transpiration moves through stomata. By ignoring cuticular transport of CO2 and assuming all CO2 moves through the stomata, CO2 diffusion can be used to estimate how much water vapour should move through stomata (E s). Boyer (2015b) and Tominaga and Kawamitsu (2015b) used this method. Applying it to the experiment in Fig. 3A when stomata were open indicated that 82% of the water vapour moved through stomata and 18% through the cuticle. Consequently, 82% of the measured transpiration should have been used in the calculation instead of the whole amount. When this was done for the data with open stomata in Fig. 3A, c i calculated from Eq. 8 became 231 µmol mol−1 instead of the 269 µmol mol−1 shown in Fig. 3A. In other words, calculated c i became much closer to the c i measured with the cup (220 µmol mol−1). This was also true when ABA was fed through the petiole to close the stomata. With the stomata closed, the difference between calculated and measured c i became very large. Similarly correcting the discrepancy by using CO2 to estimate the stomatal component of transpiration brought the values much closer. Boyer (2015b) and Tominaga and Kawamitsu (2015b) found that c i(calc) then followed the depleted c i measured with the cup. Evidently, the cuticular transport of water vapour makes a difference to c i(calc) whether the stomata are open or not.
Does the cuticle always transport so much water vapour? The amounts vary with leaf and species. Replicates of Fig. 3A displayed stomatal transport of 84%, 91%, and 95% of the total transpiration in sunflower. Stomatal transport in a hypostomatous species (stomata primarily on lower leaf surface) in the genus Vitis ranged from 78 to 97.6% among replicates (Boyer et al., 1997), showing a wide variation between leaves of the same species. Between species, Holmgren et al. (1965) reported stomatal transport of 98% in Betula, 95% in Quercus, 93% in Acer, 84% in Circaea, and 65% in Lamium on average. Kerstiens (1996) reviewed stomatal transport in 200 species measured with various methods and found similar variability among species. Boyer (2015a) found that losses in turgor diminished gas transport across cuticles as the leaves shrank, inevitably shrinking the cuticle. This might account for some of the variability reviewed by Kerstiens (1996) because turgor was typically unknown in these measurements.
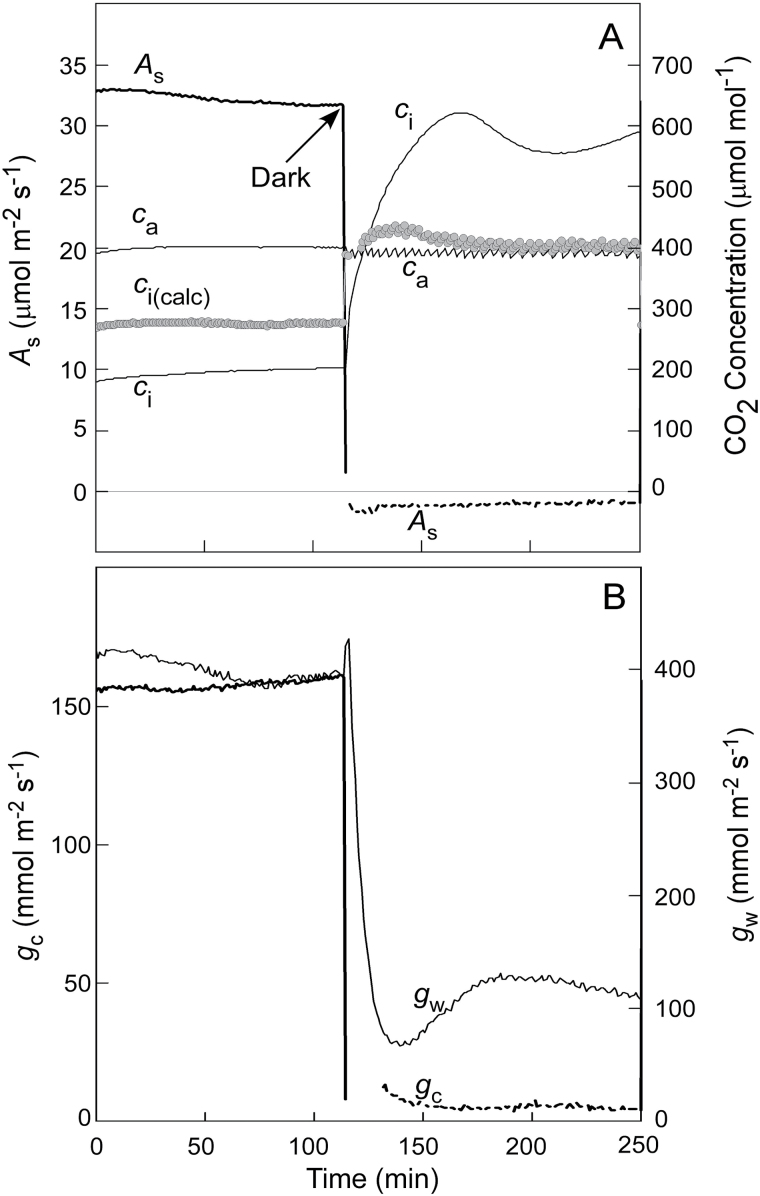
The effect of the cuticle is also seen when stomata close at night in a sunflower leaf (Fig. 4A). The c i calculated from Eq. 8 was scarcely above that in the atmosphere (i.e. suggesting low resistance to efflux of CO2) but the atmosphere inside the cup attached to the same leaf showed high resistance to CO2 efflux, such that c i became high inside the leaf (about 600 µmol mol−1). The cup results are expected because A s becomes negative (respiration) and CO2 is released inside the leaf. The cup captures this CO2 and the concentration rises until it is the same as inside the leaf. The reason c i(calc) scarcely rose appears to be the rapid diffusion of water vapour used as a tracer for CO2. The conductance for water vapour was higher than for CO2 at night (Fig. 4B).
Fig. 4.
(A) Gas exchange of a sunflower leaf darkened at the arrow. Shown are the assimilation rate (A s), CO2 concentration in the bulk air (c a), CO2 concentration directly measured inside the leaf by sealing a cup to the abaxial surface (c i), and the CO2 concentration calculated for the same leaf [c i(calc), round grey points] according to Boyer (2015a). Note that A s becomes negative in the dark and the leaf produces CO2. (B) Conductances for CO2 (g c) and water vapour (g w) for the leaf in (A). The axes in (B) have been adjusted so the conductances in the light are superimposed in order to highlight the differences after darkening.
Patchiness effects on measurements of cuticle/epidermis diffusion
Terashima et al. (1988) and Mizokami et al. (2015) considered cuticle/epidermal transport but concluded it was unlikely to be important in the calculations of c i. The only cuticle/epidermal properties available to them were from Boyer et al. (1997) showing a low conductance for grape leaves. Later, cuticle properties were compared in sunflower and grape (Boyer, 2015a) and larger cuticle/epidermal conductances were reported for sunflower than for grape. If the cuticle properties from sunflower had been available to Terashima and co-workers, it is likely they would have included cuticle effects. Holmgren et al. (1965) similarly did not measure cuticle transport of CO2 but noted that it was less than that of water vapour. They showed large variability among species, preventing cuticle properties in one species from predicting those in another. There was also the problem of changes in leaf size with changes in turgor. By working with cuticle properties while the cuticle was intact on the leaf, it was possible for Boyer (2015a) to test whether the inevitable stretching or shrinking resulting from altered turgor changed cuticle properties. As sunflower or grape leaves shrank with decreasing turgor, the cuticle conductance diminished for both gases (Boyer, 2015a).
With this turgor effect plus the water/CO2 discrimination described above, it is difficult to include cuticle properties in Eq. 8. The aforementioned cup is an alternative solution and was first suggested by Sharkey et al. (1982). Because the cuticle/epidermis transmits CO2 slowly, the cup communicates through both the cuticle and stomata. In other words, cuticle properties are taken into account by the cup measurement but not by the calculation in Eq. 8.
If stomatal closure is patchy, stomata in certain areas of the leaf could be closed while others are open. When there are patches that are completely sealed (i.e. no CO2 can be exchanged), it would be expected that the cup would only reflect those areas where stomata are open. However, this model was proved to be incorrect, with CO2 continuing to diffuse slowly through the cuticle of grape leaves despite an absence of stomata, that is, with the stomata on the other surface as closed (sealed) as possible (Boyer et al., 1997; Boyer, 2015b). Moreover, in the dark where closure should be substantial, CO2 continued to be exchanged with the cup (Fig. 4A). Instead, the data indicate that c i measured with the cup communicated with all areas of the leaf, whether stomata were open or not. The c i would probably differ between areas with slow CO2 entry and areas with rapid entry, but the measured c i would reflect averages for the leaf area sealed under the cup. For patchy closure, the smaller the area, the greater the expected variation among c i measured on the same leaf.
Many investigators recognize that Eq. 8 oversimplifies the diffusion situation in leaves. For example, stomata often display an array of sizes in light. As light diminishes, some stomata may close more than others and create patchiness in gas exchange, which could affect the calculations from Eq. 8. Numerous studies have explored these kinds of effects (Beyschlag et al., 1992; Laisk, 1983; Downton et al., 1988; Robinson et al., 1988; Terashima et al., 1988; Sharkey and Seeman, 1989; Daley et al., 1989; van Gardingen et al., 1989; van Kraalingen, 1990; Cheeseman, 1991; Gunasekara and Berkowitz, 1992; Terashima, 1992; Mott et al., 1993; Cardon et al., 1994; Mott, 1995; Buckley et al., 1997; Meyer and Genty, 1998; West et al., 2005). However, Cheeseman (1991) ran numerical models and concluded that patchiness was unlikely to be extreme enough to be important. Gunasekara and Berkowitz (1992) found evidence of patchiness in short-term experiments but not in longer ones. Moreover, patchy closure would affect c i differently from the results shown in Figs 3 and 4. If patchy closure caused the results seen in these figures, water vapour and CO2 diffusion would decrease to the same extent, or water vapour would decrease more than CO2 if CO2 was saturating for photosynthesis. In fact, the reverse occurred. As stomata closed, water vapour diffused out more rapidly than CO2 entered (Figs 3B and 4B).
Although the cup measurements shown would not have been affected, patchiness of stomatal aperture, CO2 assimilation, light harvesting, and even leaf temperature are not routinely monitored. The spatial variation in photosynthesis is an area of research that requires more attention.
Calculated versus directly measured CO2 in leaves
The simplicity of Eq. 8 for calculating c i is advantageous. However, the omission of cuticle properties is a problem and, given the high variability between species and even between replicates of the same species, it would be necessary to know the cuticle properties for each leaf. Add to this the effect of turgor, which can vary cuticle conductance without visual symptoms (i.e. without wilting the leaf), and it seems impossible for the calculations to take cuticle properties into account. If a way to include cuticle properties in Eq. 8 could be found without altering leaf turgor, calculated c i might be useful. Gradients in the leaf would be unaltered and c i could be determined from standard gas exchange measurements.
Without that possibility, however, Tominaga and Kawamitsu (2015a) recently modified a commercially available instrument to allow cup-based measurements of c i (Li-Cor LI6400XT). Instead of clamping a cuvette onto a leaf to calculate c i from both leaf surfaces, the cup measurements were made on the abaxial surface while the calculated measurements were made on the adaxial (upper) surface of the same leaf area. This allowed a rigorous comparison between direct measurements and calculated ones. In general, the direct measurements were lower than calculated ones. If stomata were open, the difference had little effect on the A s–c i relationship (Fig. 5A). When stomata were closed by feeding ABA through the petioles of the excised leaves, the A s–c i relationship became quite different (Fig. 5B). The closure caused the cuticle properties to dominate the calculation, and the initial slope of the calculated A s–c i relationship reflected cuticle properties that overestimated CO2 entry (i.e. c i was higher when calculated than when directly measured). Taken at face value, photosynthetic metabolism appeared highly altered by stomatal closure if calculated c i were used but not when measurements were made with the cup on the same area of leaf. Tominaga and Kawamitsu (2015b) attributed the difference to the cuticle/epidermis, pointing out that the calculations did not include cuticle/epidermal effects (Eq. 8) but the cup did. This altered A s–c i relationship may have large consequences for the determination and interpretation of Γ * (Fig. 2).
Fig. 5.
(A) A s–c i relationship for sunflower leaf with open stomata. (B) A s–c i relationship after closing the stomata with 10–4 M ABA. The large data point indicates multiple data on top of each other at higher c i because stomata closed more tightly as c i increased. Redrawn from Tominaga and Kawamitsu (2015a, b).
In principle, perhaps the Tominaga and Kawamitsu (2015a, b) approach could be applied more widely. Care would be needed to ensure the seal between the cup and the leaf was completely airtight, which can be tricky when thick vasculature is present. While there is no doubt that this closed cup system will result in a change in the gradient of CO2 in leaves that are amphistomatous (stomatal numbers similar on both leaf surfaces) (Parkhurst et al., 1988; Parkhurst and Mott, 1990), it is also possible to attach the cup to the top of the leaf. An optically clear cup sealed to the adaxial surface would change the CO2 gradient of an amphistomatous leaf to one resembling that of a hypostomatous leaf. This is similar to attaching the cup to the bottom of the leaf but with the gradient inverted. Even in a hypostomatous leaf, there would be slow exchange of CO2 across the adaxial astomatous cuticle, but miniaturization of the flow path might improve the rate of equilibration. Having the ability to measure the c i of amphistomatous and hypostomatous leaves would open up an array of uses for the instrument. It also might be possible to investigate the mesophyll conductance using the Tominaga–Kawamitsu unit.
In summary, c i measured with the cup was more accurate than the c i calculated from Eq. 8. This finding appears to be because the equation neglects to include the cuticle properties. Without the ability to include cuticle properties, the cup measurement is preferred.
CO2 in the chloroplast
Equation 6 illustrates one of the ways in which c c is essential for the Farquhar et al. (1980) model, and calculating c c requires knowledge of g m, as shown here:
| (9) |
Equation 9 illustrates how errors in c i, as discussed in the prior section, cause a direct offset in c c such that neglecting cuticular/epidermal conductance could cause errors over 200ppm. However, Eq. 9 glosses over a second need for c i in the determination of g m. All methods for measuring g m require c i (Pons et al., 2009), so we emphasize the need for more direct measurements of c i in order to avoid unnecessary artefacts. The additional assumptions and potential artefacts of all methods were also recently reviewed (Gu and Sun, 2014). One significant problem was the reliance on the Laisk (1977) method for determining R d and Γ *, a method that also neglects the contribution of the cuticle (some of the circularity was discussed earlier).
However, examining the simple and complete equations for isotopic discrimination (Farquhar et al., 1982; Evans et al., 1986) reveals a way to screen for and even correct artefacts caused by errors in estimates of R d and Γ *.
Biochemical and diffusional processes occurring during photosynthesis result in net discrimination against 13CO2. A simplified two-step model of diffusion followed by carboxylation that assumes (i) c c equals c i (as is the case if g m is very large) and (ii) that there are no fractionations associated with mitochondrial respiration or photorespiration can be used to generate a predicted discrimination (Δi). The equation in C3 leaves, modified to include boundary layer conductance, is:
| (10) |
where a b and a are the fractionations occurring during diffusion through the boundary layer and through still air (respectively). Fractionation values for diffusion through air are derived from theoretical estimates of the ratio of diffusivities of 12CO2/13CO2 in the slightly turbulent boundary layer of a leaf (1.0029; see Farquhar, 1983) and through still air based on their reduced masses (1.0044; Craig, 1954; O’Leary, 1981). Net fractionation from carboxylation (b) by Rubisco and phosphoenolpyruvate carboxylase (PEPC) is generally assumed to be 1.029 for Nicotiana tabacum (Evans et al., 1994). In a few species, the Rubisco portion of b has been measured independently (McNevin et al., 2006, 2007) and it is important to use the best data available for b (Gu and Sun, 2014). Ambient, leaf surface, and internal leaf partial pressures of CO2 (c a, c s, and c i, respectively) are used to scale each fractionation proportionately to the CO2 drop between each location.
A more detailed model of discrimination is necessary for investigating g m, and for effects of fractionation by mitochondrial respiration in the light (e) and photorespiratory fractionation (f). The full classical model of 13C discrimination during photosynthesis is then (Farquhar et al., 1982):
| (11) |
where a 1 (1.0007) is the fractionation associated with diffusion through leaf water, b s is fractionation as CO2 moves into solution (1.0011 at 25°C), and k is the carboxylation efficiency. Formal equations for correcting b (above) with a value (β) that accounts for non-negligible amounts of PEPC and other carboxylase activities in C3 plants are described elsewhere (Farquhar and Richards, 1984; Raven and Farquhar, 1990).
The isotopic approach for determining g m is accomplished by combining Eqs 8, 9, and 10 (as in Evans et al. 1986):
| (12) |
obtaining Δi and other variables from standard gas exchange (or assumed values for constants) and replacing Δ with observed discrimination (Δobs) via online stable isotope gas exchange as follows:
| (13) |
where δe and δo are the isotopic compositions of CO2 entering and leaving the leaf cuvette, respectively. ξ = c e/(c e – c o), with c e and c o being the partial pressures of CO2 in air entering and leaving a well-mixed leaf cuvette.
It should be noted that the above discrimination model was derived with the simplifying assumptions that respiratory substrates have an isotopic composition given by recent photosynthetic processes that include the decarboxylation of glycine, and that after Rubisco fixation there are no further fractionations (Farquhar et al., 1982). These assumptions may be unjustified (Tcherkez et al., 2004), and modifications to better accommodate additional fractionations have been developed (see Evans and von Caemmerer, 2013 and references therein) as well as corrections for ternary effects associated with stomatal conductance (Farquhar and Cernusak, 2012). We have left out these more recent modifications for the sake of clarity when discussing the following approach.
When considering Eq. 11, it is important to realize that if there is no CO2 efflux in the light (i.e. from respiration or photorespiration), then the value of δe will have no effect on Δobs in Eq. 13 because fractionations from diffusion and carboxylation will generate the same difference between δe and δo, irrespective of the absolute value of δe. However, if there is any CO2 efflux out of the leaf, it will have an isotopic composition that reflects the source material and any additional fractionations. If we assume that the isotopic composition of effluxed CO2 is around −20‰, then if δe is also −20‰ the effluxed CO2 would not change δo − δe. However, the more that δe deviates from −20‰, the larger the effect on δo − δe. The potential for this effect is included in the right-hand side of Eq. 11 and the bottom of Eq. 12, (eR d /k + fΓ *)/c a, where e and f are the isotopic compositions of respired and photorespired CO2, respectively. Unfortunately, R d and Γ * are required to scale the effect to the magnitude of the efflux through each pathway. As noted earlier, this is problematic for determining g m because g m is needed to calculate Γ * (Eq. 7). Gu and Sun (2014) highlight additional problems with methods for determining R d and Γ *, and conclude that existing data on g m, R d, and Γ * should be treated with great caution.
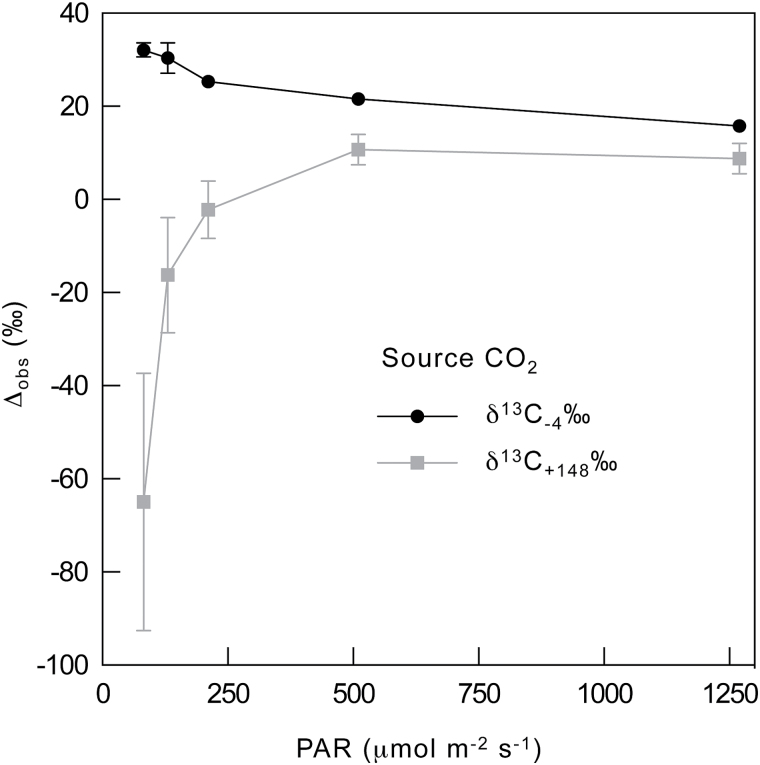
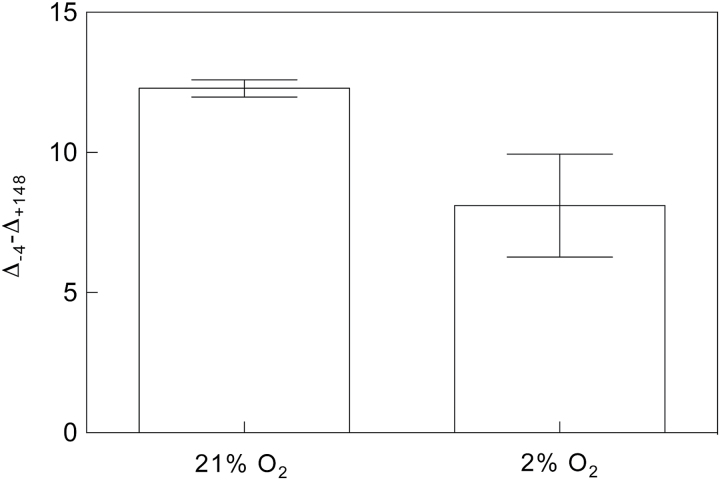
Fortunately, we can exploit the sensitivity of Eq. 11 to δe by measuring Δobs. This is done by using a δe near the isotopic composition of effluxed CO2, and then rapidly repeating the measurement of Δobs while changing δe to a value far from that of effluxed CO2. The difference in Δobs between these two measurements (dΔobs) then represents the combined effect of the rate and isotopic composition of effluxed CO2 from all sources, represented as (eR d /k + fΓ *)/c a in Eqs 10 and 11. Conveniently, this would also incorporate effects of xylem-transported CO2 and eliminate the need to determine R d, Γ *, e, and f to determine g m in a manner that is independent of R d and Γ *. All that would be needed would be to scale dΔobs to the magnitude of the difference between the two values of δe and use the resulting value as an approximation of (eR d /k + fΓ *)/c a in Eq. 12. Furthermore, if the magnitude of dΔobs is the same between treatments, then one can reasonably conclude that any changes in g m would not be artefacts of changing CO2 efflux. As a proof of concept, we tested this approach using δe values of −4‰ and +148‰ across a light-response curve (Fig. 6). If R d is constant or increasing as A declines with light intensity, then dΔobs should increase at low light, which is what we see in Fig. 6, especially at and below photosynthetically active radiation (PAR) values of 200 µmol m−2 s−1. We also compared measurements of dΔobs at 2% and 21% oxygen and found that dΔobs decreased when photorespiration was suppressed, as expected (Fig. 7). Therefore, we believe that this approach has great promise for magnifying the effects of small but critical fluxes, thereby improving our ability to detect and quantify them. Future studies describing the approach in detail are forthcoming.
Fig. 6.
Light response of photosynthetic discrimination, Δobs, in Nicotiana tabacum measured at 400ppm CO2 with δe values of −4‰ and +148‰. The same leaf area was measured for each δe by starting with −4‰, transiently switching to +148‰ for 5–10min, and then returning to −4‰ until Δobs matched the prior Δobs at −4‰ (usually less than 10min) before proceeding to the next light intensity. This demonstrates that CO2 efflux from leaves has large effects on Δobs when leaves are provided air with 13C-enriched CO2, and that the effect is not constant across light intensities. N = 2.
Fig. 7.
Oxygen sensitivity of dΔobs (Δ-4–Δ+148) in Populus deltoides measured at a PAR of 200 µmol m−2 s−1 and 400ppm CO2 with δe values of −4‰ and +148‰. The same method was used as described for Fig. 6. This demonstrates the utility of this method for detecting CO2 efflux from photorespiration. N = 3.
Why are these small flux errors so important now?
Significant efforts are underway to characterize plant functional traits such as photosynthesis and respiration in non-model species under a broad range of environmental conditions (e.g. Atkin et al., 2015). This effort is driven by the need to improve predictions of plant responses to a ‘new normal’ of warmer, drier (i.e. stressful) environmental conditions and the impact on Earth system models of the global carbon cycle and climate change (Friedlingstein et al., 2006; Bodman et al., 2013; Schimel et al., 2015). This has been given a greater sense of urgency owing to the assessment that mean precipitation will likely decrease in many mid-latitude and subtropical dry regions during this century (IPCC, 2014). Furthermore, water limitation is already a global problem, with 97% of the terrestrial surface experiencing periods of water deficit at least one month per year (Jenerette et al., 2012), several observations of widespread plant stress and mortality during intense droughts (Allen et al., 2010), and predictions of future ‘megadroughts’ in the Southwest and Central Plains of Western North America (Cook et al., 2015).
This motivation along with the growing population is also pushing efforts to re-engineer plants for ultra-high photosynthesis and reduced water requirements (Price et al., 2011; Peterhansel et al., 2013; Lin et al., 2014a, b; Borland et al., 2015; Carmo-Silva et al., 2015; Long et al., 2015) and to improve our understanding of poorly studied (non-broad leaf) photosynthetic tissues, such as photosynthetic branches that are especially important in arid environments (Ávila et al., 2014). Each of these efforts is affected by assumptions made in the methods used for measuring carbon and water fluxes and by how those data are interpreted and used in models.
Recently, serious concerns have been raised about the validity of common approaches for measuring critical parameters in photosynthetic models (Gu and Sun, 2014; Boyer, 2015a, b). Here, we have reviewed these concerns, highlighted additional problems, and offered potential solutions. Fortunately, the problems are tractable, but they will require significant research efforts to be resolved.
Acknowledgements
This work was supported by NSF grant IOS-0719118, the NSF EPSCoR Program under Award # IIA-1301346, and NIH grant NIH-NCRR P20RR18754, all at the University of New Mexico. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
References
- Allen CD, Macalady AK, Chenchouni H, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259, 660–684. [Google Scholar]
- Atkin OK, Bloomfield KJ, Reich PB, et al. 2015. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. The New Phytologist 206, 614–636. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Evans JR, Ball MC, Lambers H, Pons TL. 2000. Leaf respiration of snow gum in the light and dark. Interactions between temperature and irradiance. Plant Physiology 122, 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Evans JR, Ball MC, Siebke K, Pons TL, Lambers H, Moller IM, Gardestrom P, Gliminius K, Glaser E. 1998. Light inhibition of leaf respiration: the role of irradiance and temperature. In: Moller IM, Gardestrom P, Gliminus K, Glaser E, eds. Plant mitochondria: from gene to function . Leiden, the Netherlands: Bluckhuys Publishers, 25–32. [Google Scholar]
- Aubrey DP, Teskey RO. 2009. Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. The New Phytologist 184, 35–40. [DOI] [PubMed] [Google Scholar]
- Ávila E, Herrera A, Tezara W. 2014. Contribution of stem CO2 fixation to whole-plant carbon balance in nonsucculent species. Photosynthetica 52, 3–15. [Google Scholar]
- Ayub G, Smith RA, Tissue DT, Atkin OK. 2011. Impacts of drought on leaf respiration in darkness and light in Eucalyptus saligna exposed to industrial-age atmospheric CO2 and growth temperature. The New Phytologist 190, 1003–1018. [DOI] [PubMed] [Google Scholar]
- Beyschlag W, Pfanz H, Ryel RJ. 1992. Stomatal patchiness in Mediterranean evergreen sclerophylls. Phenomenology and consequences for the interpretation of the midday depression in photosynthesis and transpiration. Planta 187, 546–553. [DOI] [PubMed] [Google Scholar]
- Bloemen J, Bauweraerts I, De Vos F, Vanhove C, Vandenberghe S, Boeckx P, Steppe K. 2015. a. Fate of xylem-transported 11C- and 13C-labeled CO2 in leaves of poplar. Physiologia Plantarum 153, 555–564. [DOI] [PubMed] [Google Scholar]
- Bloemen J, McGuire MA, Aubrey DP, Teskey RO, Steppe K. 2013. Transport of root-respired CO2 via the transpiration stream affects aboveground carbon assimilation and CO2 efflux in trees. The New Phytologist 197, 555–565. [DOI] [PubMed] [Google Scholar]
- Bloemen J, Teskey RO, McGuire MA, Aubrey DP, Steppe K. 2015. b Root xylem CO2 flux: an important but unaccounted-for component of root respiration. Trees , 1–10. doi 10.1007/s00468-015-1185-4 [Google Scholar]
- Bodman RW, Rayner PJ, Karoly DJ. 2013. Uncertainty in temperature projections reduced using carbon cycle and climate observations. Nature Climate Change 3, 725–729. [Google Scholar]
- Borland AM, Wullschleger SD, Weston DJ, Hartwell J, Tuskan GA, Yang X, Cushman JC. 2015. Climate-resilient agroforestry: physiological responses to climate change and engineering of crassulacean acid metabolism (CAM) as a mitigation strategy. Plant, Cell & Environment 38, 1833–1849. [DOI] [PubMed] [Google Scholar]
- Bowes G, Ogren WL, Hageman RH. 1971. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochemical and Biophysical Research Communications 45, 716–722. [DOI] [PubMed] [Google Scholar]
- Boyer JS. 2015. a. Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. Journal of Experimental Botany 66, 2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. 2015. b. Impact of cuticle on calculations of the CO2 concentration inside leaves. Planta 242, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Boyer JS, Kawamitsu Y. 2011. Photosynthesis gas exchange system with internal CO2 directly measured. Environmental Control in Biology 49, 193–207. [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD. 1997. CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiology 114, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson LF, Zelitch I, Havir EA. 1998. Manipulation of catalase levels produces altered photosynthesis in transgenic tobacco plants. Plant Physiology 116, 259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Farquhar GD, Mott KA. 1997. Qualitative effects of patchy stomatal conductance distribution features on gas exchange calculations. Plant, Cell & Environment 20, 867–880. [Google Scholar]
- Busch FA. 2013. Current methods for estimating the rate of photorespiration in leaves. Plant Biology 15, 648–655. [DOI] [PubMed] [Google Scholar]
- Busch FA, Sage TL, Cousins AB, Sage RF. 2013. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2 . Plant, Cell & Environment 36, 200–212. [DOI] [PubMed] [Google Scholar]
- Canadell JG, Le Quere C, Raupach M, Field C, Buitenhuis E, Ciais P, Conway T, Gillett N, Houghton R, Marland G. 2007. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proceedings of the National Academy of Sciences USA 104, 18866–18870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon ZG, Mott KA, Berry JA. 1994. Dynamics of patchy stomatal movements, and their contribution to steady-state and oscillating stomatal conductance calculated with gas-exchange techniques. Plant, Cell & Environment 17, 995–1008. [Google Scholar]
- Carmo-Silva AE, Scales JC, Madgwick PJ, Parry MAJ. 2015. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant, Cell & Environment 38, 1817–1832. [DOI] [PubMed] [Google Scholar]
- Cheeseman JM. 1991. PATCHY: simulating and visualizing the effects of stomatal patchiness on photosynthetic CO2 exchange studies. Plant, Cell & Environment 14, 593–599. [Google Scholar]
- Cook BI, Ault TR, Smerdon JE. 2015. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Science Advances 1, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Pracharoenwattana I, Zhou W, Smith SM, Badger MR. 2008. Peroxisomal malate dehydrogenase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Plant Physiology 148, 786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Walker BJ, Pracharoenwattana I, Smith SM, Badger MR. 2011. Peroxisomal hydroxypyruvate reductase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Photosynthesis Research 108, 91–100. [DOI] [PubMed] [Google Scholar]
- Craig H. 1954. Carbon 13 in plants and the relationship between carbon 13 and carbon 14 variations in nature. Journal of Geology 62, 115–149. [Google Scholar]
- Daley PF, Raschke K, Ball JT, Berry JA. 1989. Topography of photosynthetic activity of leaves obtained from video images of chlorophyll fluorescence. Plant Physiology 90, 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthe C, Dreyer E, Epron D, Warren CR. 2011. Mesophyll conductance to CO2, assessed from online TDLAS records of 13CO2 discrimination, displays small but significant short-term responses to CO2 and irradiance in Eucalyptus seedlings. Journal of Experimental Botany 62, 5335–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downton WJS, Loveys BR, Grant WJR. 1988. Non-uniform stomatal closure induced by water stress causes putative non-stomatal inhibition of photosynthesis. The New Phytologist 110, 503–509. [DOI] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S. 2013. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant, Cell & Environment 36, 745–756. [DOI] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S, Setchell BA, Hudson GS. 1994. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Australian Journal of Plant Physiology 21, 475–495. [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. 1986. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Australian Journal of Plant Physiology 13, 281–292. [Google Scholar]
- Farquhar GD. 1983. On the nature of carbon isotope discrimination in C4 species. Australian Journal of Plant Physiology 10, 205–226. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 2001. Models of photosynthesis. Plant Physiology 125, 42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Cernusak LA. 2012. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant, Cell & Environment 35, 1221–1231. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Leary MHO, Berry JA. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology 9, 121–137. [Google Scholar]
- Farquhar GD, Richards RA. 1984. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology 11, 539. [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M. 2007. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell & Environment 30, 1284–98. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R. 2006. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant Journal 48, 427–439. [DOI] [PubMed] [Google Scholar]
- Friedlingstein P, Cox P, Betts R, et al. 2006. Climate–carbon cycle feedback analysis: Results from the C4MIP model intercomparison. Journal of Climate 19, 3337–3353. [Google Scholar]
- Gu L, Sun Y. 2014. Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods. Plant, Cell & Environment 37, 1231–1249. [DOI] [PubMed] [Google Scholar]
- Gunasekera D, Berkowitz GA. 1992. Heterogeneous stomatal closure in response to leaf water deficits is not a universal phenomenon. Plant Physiology 98, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KR, Peterson RB. 1986. Regulation of photorespiration in leaves: evidence that the fraction of ribulose bisphosphate oxygenated is conserved and stoichiometry fluctuates. Archives of Biochemistry and Biophysics 246, 332–346. [DOI] [PubMed] [Google Scholar]
- Heckwolf M, Pater D, Hanson DT, Kaldenhoff R. 2011. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant Journal 67, 795–804. [DOI] [PubMed] [Google Scholar]
- Holmgren P, Jarvis PG, Jarvis MS. 1965. Resistances to carbon dioxide and water vapour transfer in leaves of different plant species. Physiologia Plantarum 18, 557–573. [Google Scholar]
- IPCC. . 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change . [Core Writing Team, RK Pachauri and LA Meyer (eds.)]. IPCC, Geneva, Switzerland, 151 pp. [Google Scholar]
- Jenerette GD, Barron-Gafford GA, Guswa AJ, McDonnell JJ, Villegas JC. 2012. Organization of complexity in water limited ecohydrology. Ecohydrology 5, 184–199. [Google Scholar]
- Kerstiens G. 1996. Cuticular water permeability and its physiological significance. Journal of Experimental Botany 47, 1813–1832. [Google Scholar]
- Koniger M. 2014. Chloroplast movement in higher plants, ferns and bryophytes: a comparative point of view. In Hanson DT, Rice SK, eds. Photosynthesis in bryophytes and early land plants . Dordrecht: Springer Netherlands, 131–150. [Google Scholar]
- Laing WA, Ogren WL, Hageman RH. 1974. Regulation of soybean net photosynthetic CO2 fixation by the interaction of CO2, O2, and ribulose 1,5-diphosphate carboxylase. Plant Physiology 54, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk AK. 1977. Kinetics of photosynthesis and photorespiration in C3 plants . Moscow: Nauka. [Google Scholar]
- Laisk A. 1983. Calculation of leaf photosynthetic parameters considering the statistical distribution of stomatal apertures. Journal of Experimental Botany 34, 1627–1635. [Google Scholar]
- Lin MT, Occhialini A, Andralojc PJ, Devonshire J, Hines KM, Parry MAJ, Hanson MR. 2014. a β-Carboxysomal proteins assemble into highly organized structures in Nicotiana chloroplasts. Plant Journal 79, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Occhialini A, Andralojc PJ, Parry MAJ, Hanson MR. 2014. b A faster Rubisco with potential to increase photosynthesis in crops. Nature 513, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Marshall-Colon A, Zhu X-G. 2015. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66. [DOI] [PubMed] [Google Scholar]
- Massman WJ. 1998. A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO, and NO2 in air, O2 and N2 near STP. Atmospheric Environment 32, 1111–1127. [Google Scholar]
- McGuire MA, Marshall JD, Teskey RO. 2009. Assimilation of xylem-transported 13C-labelled CO2 in leaves and branches of sycamore (Platanus occidentalis L.). Journal of Experimental Botany 60, 3809–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire MA, Teskey RO. 2002. Microelectrode technique for in situ measurement of carbon dioxide concentrations in xylem sap of trees. Tree Physiology 22, 807–11. [DOI] [PubMed] [Google Scholar]
- McNevin DB, Badger MR, Kane HJ, Farquhar GD. 2006. Measurement of (carbon) kinetic isotope effect by Rayleigh fractionation using membrane inlet mass spectrometry for CO2-consuming reactions. Functional Plant Biology 33, 1115–1128. [DOI] [PubMed] [Google Scholar]
- McNevin DB, Badger MR, Whitney SM, von Caemmerer S, Tcherkez GGB, Farquhar GD. 2007. Differences in carbon isotope discrimination of three variants of D-ribulose-1,5-bisphosphate carboxylase/oxygenase reflect differences in their catalytic mechanisms. The Journal of Biological Chemistry 282, 36068–76. [DOI] [PubMed] [Google Scholar]
- Meyer S, Genty B. 1998. Mapping intercellular CO2 mole fraction (C i) in Rosa rubiginosa leaves fed with abscisic acid by using chlorophyll fluorescence imaging. Significance of C i estimated from leaf gas exchange. Plant Physiology 116, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami Y, Noguchi K, Kojima M, Sakakibara H, Terashima I. 2015. Mesophyll conductance decreases in the wild type but not in an ABA-deficient mutant (aba1) of Nicotiana plumbaginifolia under drought conditions. Plant, Cell & Environment 38, 388–398. [DOI] [PubMed] [Google Scholar]
- Moss DN, Rawlins SL. 1963. Concentration of carbon dioxide inside leaves. Nature 197, 1320–1321 [Google Scholar]
- Mott KA, Cardon ZG, Berry JA. 1993. Asymmetric patchy stomatal closure for the two surfaces of Xanthium strumarium L. leaves at low humidity. Plant, Cell & Environment 16, 25–34. [Google Scholar]
- Mott KA. 1995. Effects of patchy stomatal closure on gas exchange measurements following abscisic acid treatment. Plant, Cell & Environment 18, 1291–1300. [Google Scholar]
- Nishida K, Kodama N, Yonemura S, Hanba YT. 2015. Rapid response of leaf photosynthesis in two fern species Pteridium aquilinum and Thelypteris dentata to changes in CO2 measured by tunable diode laser absorption spectroscopy. Journal of Plant Research 128, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary MH. 1981. Carbon isotope fractionation in plants. Phytochemistry 20, 553–567. [Google Scholar]
- Parkhurst DF, Mott KA. 1990. Intercellular diffusion limits to CO2 uptake in leaves. Studies in air and helox. Plant Physiology 94, 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst DF, Wong SC, Farquhar GD, Cowan IR. 1988. Gradients of intercellular CO2 levels across the leaf mesophyll. Plant Physiology 86, 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhansel C, Krause K, Braun H-P, Espie GS, Fernie AR, Hanson DT, Keech O, Maurino VG, Mielewczik M, Sage RF. 2013. Engineering photorespiration: current state and future possibilities. Plant Biology 15, 754–758. [DOI] [PubMed] [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E. 2009. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. Journal of Experimental Botany 60, 2217–34. [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR, von Caemmerer S. 2011. The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiology 155, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Farquhar GD. 1990. The influence of N metabolism and organic acid synthesis on the natural abundance of isotopes of carbon in plants. The New Phytologist 116, 505–529. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Grant WJR, Loveys BR. 1988. Stomatal limitation of photosynthesis in abscisic acid-treated and in water-stressed leaves measured at elevated CO2 . Australian Journal of Plant Physiology 15, 495–503. [Google Scholar]
- Schimel D, Stephens BB, Fisher JB. 2015. Effect of increasing CO2 on the terrestrial carbon cycle. Proceedings of the National Academy of Sciences USA 112, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JB, Griffin KL, Lewis JD, Tissue DT. 2004. Response of Xanthium strumarium leaf respiration in the light to elevated CO2 concentration, nitrogen availability and temperature. The New Phytologist 162, 377–386. [Google Scholar]
- Sharkey TD. 1988. Estimating the rate of photorespiration. Physiologia Plantarum 73, 147–152. [Google Scholar]
- Sharkey TD. 1989. Evaluating the role of Rubisco regulation in photosynthesis of C3 plants. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 323, 435–448. [Google Scholar]
- Sharkey TD, Imai K, Farquhar GD, Cowan IR. 1982. A direct confirmation of the standard method of estimating intercellular partial pressure of CO2 . Plant Physiology 69, 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Seemann JR. 1989. Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiology 89, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Matthews MA, Boyer JS. 1984. Kok effect and the quantum yield of photosynthesis. Plant Physiology 75, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppe K, Sterck F, Deslauriers A. 2015. Diel growth dynamics in tree stems: linking anatomy and ecophysiology. Trends in Plant Science 20, 1–9. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Badger MR, Evans JR. 2009. Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. Journal of Experimental Botany 60, 2291–301. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Estavillo GM, Evans JR. 2011. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant, Cell & Environment 34, 580–591. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Farquhar G, Badeck F, Ghashghaie J. 2004. Theoretical considerations about carbon isotope distribution in glucose of C3 plants. Functional Plant Biology 31, 857. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Mahé A, Gauthier P, Mauve C, Gout E, Bligny R, Cornic G, Hodges M. 2009. In folio respiratory fluxomics revealed by 13C isotopic labeling and H/D isotope effects highlight the noncyclic nature of the tricarboxylic acid ‘cycle’ in illuminated leaves. Plant Physiology 151, 620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima I. 1992. Anatomy of non-uniform leaf photosynthesis. Photosynthesis Research 31, 195–212. [DOI] [PubMed] [Google Scholar]
- Terashima I, Wong SC, Osmond CB, Farquhar GD. 1988. Characterization of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant and Cell Physiology 29, 385–394. [Google Scholar]
- Teskey RO, McGuire MA. 2002. Carbon dioxide transport in xylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant, Cell & Environment 25, 1571–1577. [Google Scholar]
- Teskey RO, Saveyn A, Steppe K, McGuire MA. 2008. Origin, fate and significance of CO2 in tree stems. The New Phytologist 177, 17–32. [DOI] [PubMed] [Google Scholar]
- Tholen D, Zhu X-G. 2011. The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiology 156, 90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga J, Kawamitsu Y. 2015. a. Tracing photosynthetic response curves with internal CO2 measured directly. Environmental Control in Biology 53, 27–34. [Google Scholar]
- Tominaga J, Kawamitsu Y. 2015. b. Cuticle affects calculations of internal CO2 in leaves closing their stomata. Plant and Cell Physiology 56, 1900–1908. doi 10.1093/pcp/pcv109. [DOI] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. 2008. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20, 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gardingen PR, Jeffree CE, Grace J. 1989. Variation in stomatal aperture in leaves of Avena fatua L. observed by low temperature scanning electron microscopy. Plant, Cell & Environment 12, 887–898. [Google Scholar]
- van Kraalingen DWG. 1990. Implications of non-uniform stomatal closure on gas exchange calculations. Plant, Cell & Environment 13, 1001–1004. [Google Scholar]
- Villar R, Held AA, Merino J. 1994. Comparison of methods to estimate dark respiration in the light in leaves of 2 woody species. Plant Physiology 105, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S. 2013. Steady-state models of photosynthesis. Plant, Cell & Environment 36, 1617–1630. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. 1994. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195, 88–97. [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- Walker BJ, Ort DR. 2015. Improved method for measuring the apparent CO2 photocompensation point resolves the impact of multiple internal conductances to CO2 to net gas exchange. Plant, Cell & Environment 38, 2462–2474. [DOI] [PubMed] [Google Scholar]
- Way DA, Holly C, Bruhn D, Ball MC, Atkin OK. 2015. Diurnal and seasonal variation in light and dark respiration in field-grown Eucalyptus pauciflora . Tree Physiology 35, 840–849. [DOI] [PubMed] [Google Scholar]
- Weise SE, Carr DJ, Bourke AM, Hanson DT, Swarthout D, Sharkey TD. 2015. The arc mutants of Arabidopsis with fewer large chloroplasts have a lower mesophyll conductance. Photosynthesis Research 124, 117–126. [DOI] [PubMed] [Google Scholar]
- Wertin TM, Teskey RO. 2008. Close coupling of whole-plant respiration to net photosynthesis and carbohydrates. Tree Physiology 28, 1831–1840. [DOI] [PubMed] [Google Scholar]
- West JD, Peak D, Peterson JQ, Mott KA. 2005. Dynamics of stomatal patches for a single surface of Xanthium strumarium L. leaves observed with fluorescence and thermal images. Plant, Cell & Environment 28, 633–641. [Google Scholar]
- Woodrow IE, Berry JA. 1988. Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annual Review of Plant Physiology and Plant Molecular Biology 39, 533–594. [Google Scholar]
- Xiong D, Liu X, Liu L, Douthe C, Li Y, Peng S, Huang J. 2015. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant, Cell & Environment 38, 2541–2550. [DOI] [PubMed] [Google Scholar]
- Zelitch I. 1992. Control of plant productivity by regulation of photorespiration. Regulation of photorespiration can have beneficial effects on net photosynthesis. Bioscience 42, 510–516. [Google Scholar]
- Zelitch I. 2001. Travels in a world of small science. Photosynthesis Research 67, 157–176. [DOI] [PubMed] [Google Scholar]