Highlight
C2 photosynthesis in grasses is facilitated by organelle enrichment in tandem with enhanced levels of GDC in the carbon-concentrating cells consistent with changes in expression of a single GLDP gene.
Key words: Arthropogoninae, bundle sheath, C2 Kranz anatomy, C2 photosynthesis, glycine decarboxylase, grasses, mitochondria, Homolepis.
Abstract
Photorespiratory glycine shuttling and decarboxylation in bundle sheath (BS) cells exhibited by C2 species is proposed to be the evolutionary bridge to C4 photosynthesis in eudicots. To evaluate this in grasses, we compare anatomy, cellular localization of glycine decarboxylase (GDC), and photosynthetic physiology of a suspected C2 grass, Homolepis aturensis, with these traits in known C2 grasses, Neurachne minor and Steinchisma hians, and C3 S. laxum that is sister to S. hians. We also use publicly available genome and RNA-sequencing data to examine the evolution of GDC subunits and enhance our understanding of the evolution of BS-specific GDC expression in C2 and C4 grasses. Our results confirm the identity of H. aturensis as a C2 species; GDC is confined predominantly to the organelle-enriched BS cells in H. aturensis and S. hians and to mestome sheath cells of N. minor. Phylogenetic analyses and data obtained from immunodetection of the P-subunit of GDC are consistent with the hypothesis that the BS dominant levels of GDC in C2 and C4 species are due to changes in expression of a single GLDP gene in M and BS cells. All BS mitochondria and peroxisomes and most chloroplasts in H. aturensis and S. hians are situated centripetally in a pattern identical to C2 eudicots. In S. laxum, which has C3-like gas exchange patterns, mitochondria and peroxisomes are positioned centripetally as they are in S. hians. This subcellular phenotype, also present in eudicots, is posited to initiate a facilitation cascade leading to C2 and C4 photosynthesis.
Introduction
C4 photosynthesis has independently evolved >60 times in angiosperms (R.F. Sage et al., 2011; Grass Phylogeny Working Group II, 2012). Within the angiosperms, the Poaceae represents the most prolific family of C4 origins, with approximately twice as many origins as any other family (Sage, 2016). C4 grasses also make up the greatest number of C4 species, comprising ~60% of the 8000 estimated number of C4 species (Still et al., 2003; Sage, 2016). Roughly a quarter of global net primary productivity on land is due to C4 photosynthesis (Still et al., 2003), of which the vast majority is contributed by grasses (Sage et al., 1999). C4 grasses also have great significance for humanity as they dominate the fraction of biomass entering the human food chain as grain (maize, sorghum, and millets), sugar (sugarcane), and fodder for animals, and efforts are underway to engineer the C4 pathway into C3 grass crops such as rice and wheat to exploit the superior productivity of C4 photosynthesis (Peterhansel, 2011; von Caemmerer et al., 2012). Given these considerations, there is now a great interest in understanding how C4 photosynthesis evolved in grasses, to understand both how this complex trait repeatedly arose, and how we might learn from the evolutionary examples to direct C4 engineering in major crops (Hibberd et al., 2008).
Studies of C4 evolution are informed by the presence of species exhibiting intermediate stages between fully expressed C3 and C4 life forms within a single evolutionary clade (Sage et al., 2014). Ideally, there should be C3–C4 relatives from multiple independent lineages of C4 photosynthesis to facilitate evaluation of evolutionary hypotheses using comparative approaches. Multiple independent clades provide the possibility to assess whether evolutionary trends are replicated, as they should be if C4 photosynthesis evolved along common trajectories (Heckmann et al., 2013). To date, the majority (85%) of C3–C4 species occur in eudicots, with the genus Flaveria standing as the major group used in studies of C4 evolution (R.F. Sage et al., 2011, 2014). Flaveria has over twice the number of intermediates (10) as any other evolutionary clade, and these form two distinct clades that each evolved C3- and C4-like species (McKown et al., 2005; Lyu et al., 2015). In addition to Flaveria, at least 11 other C4 evolutionary lineages have been identified with C3–C4 intermediates branching in sister positions to the C4 line (Sage et al., 2014). Most of these have only one or two species, although in recent years there is evidence of three clades (Heliotropium, Anticharis, and Blepharis) potentially having more than five intermediates (Muhaidat et al., 2011; Khoshravesh et al., 2012; Fisher et al., 2015). All but three of the C4 lineages with C3–C4 intermediates are eudicots. Among monocots, the genus Neurachne contains a C3–C4 intermediate that branches in a sister position to a C4 (Christin et al., 2012). A recent study has reported C3, C3–C4, and C4 photosynthetic genotypes in Alloteropsis semialata (Lundgren et al., 2015). The genus Steinchisma also contains C3–C4 intermediates (Brown et al., 1983; Hylton et al., 1988), but they lack close C4 relatives in their subtribe, Otachyrinae (Grass Phylogeny Working Group II, 2012). As a consequence of the discrepancy between C3–C4 numbers in eudicots and monocots, our understanding of C4 evolution is dominated by information from eudicot clades. If there is important variation in eudicot versus monocot patterns of C4 evolution, as suggested by recent theoretical treatments (Williams et al., 2013), it could be missed because of low monocot representation in the C3–C4 intermediate population.
The South American subtribe Arthropogoninae is a hot-spot for C4 evolution within the grasses, with four putative distinct C4 origins, once in Mesosetum/Arthropogon, a second time in Oncorachis, and twice in Coleataenia (Grass Phylogeny Working Group II, 2012). As such, the Arthropogoninae is a strong candidate to contain numerous C3–C4 species. This possibility is bolstered by an image from Homolepis aturensis in Supplementary fig. S1 of Christin et al. (2013) that illustrates enlarged bundle sheath (BS) cells with chloroplasts arranged around the periphery in addition to chloroplast clusters adjacent to the vascular tissue. Although this centripetal chloroplast arrangement led to tentative identification of H. aturensis as C4 (Christin et al., 2013), the presence of centrifugal chloroplasts in the BS cells is a common feature in C3–C4 intermediate species (Monson et al., 1984; Sage et al., 2014). Significantly, because the genus Homolepis is sister to a clade that contains only C4 species, it has been identified as a genus that might exhibit precursor traits that could have enabled the evolution of the C4 phenotype (Grass Phylogeny Working Group II, 2012). To evaluate this possibility, we have collected H. aturensis in Costa Rica for study.
A prominent physiological feature of C3–C4 intermediates is the transport of photorespiratory glycine from mesophyll (M) to BS cells for decarboxylation by glycine decarboxylase (GDC), with the released CO2 then being refixed by BS Rubisco (Monson and Rawsthorne, 2000). Photorespiratory glycine shuttling exhibited by C3–C4 intermediates has also been termed C2 photosynthesis in reference to the number of carbons shuttled from M to BS cells (Vogan et al., 2007; Bauwe, 2011). The BS mitochondria of most C2 species examined to date contain the majority of the GDC within the leaf, with small amounts in M cells potentially for C1 metabolism (Hylton et al., 1988; Morgan et al., 1993; Rawsthorne et al., 1998; Voznesenskaya et al., 2001; Ueno et al., 2003; Marshall et al., 2007; Voznesenskaya et al., 2010; Muhaidat et al., 2011; T.L. Sage et al., 2011). Decarboxylation of glycine in the BS cells establishes a glycine gradient between M and BS cells, and rapid movement to BS cells is facilitated by enhanced vein density in C2 relative to C3 species (Monson and Rawsthorne, 2000). Subsequent glycine decarboxylation within BS cells increases CO2 around BS Rubisco ~3-fold (Keerberg et al., 2014), and the resulting increase in Rubisco efficiency reduces the CO2 compensation point in C2 species relative to C3 by 10–40 μmol mol−1 (Holaday et al., 1984; Monson et al., 1984; Vogan et al., 2007; T.L. Sage et al., 2011, 2013).
The functional GDC holoenzyme consists of four subunits encoded by individual genes, GLDH, GLDL, GLDP, and GLDT (Bauwe 2011). Decarboxylase activity of the complex is located in the P-subunit encoded by the GDLP gene. In Flaveria, the C4 photosynthetic mechanism was established through gradual pseudogenization of a ubiquitously expressed GLDP gene, and full activation of a second GLDP gene that shows BS-specific expression in C3 Flaveria species (Schulze et al., 2013). The ancestral duplication of the GLDP gene in Flaveria is considered a genetic enabler of C4 evolution in the genus (Schulze et al., 2013). BS cell-dominant expression of GDC has been reported in the C2 grass Steinchisma hians (=Panicum milioides; Hylton et al., 1988). To date, the molecular evolution of this trait in S. hians or other C2 and C4 grasses has not been assessed. Schulze et al. (2013), highlighting the presence of two GLDP genes in rice [a member of the C3 BEP (Bambusoideae, Ehrhartoideae, Pooideae) clade] and a single copy in maize, sorghum, and Setaria italica [of the C4 PACMAD (Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristoideae, Danthonioideae) clade], posited that the ubiquitously expressed GLDP gene(s) in C4 grasses were pseudogenized as in Flaveria and subsequently lost from the genomes.
The purpose of this study was to determine whether H. aturensis exhibits C2 photosynthesis or is a C4 species as previously suggested (Christin et al., 2013). We compared anatomy, localization of GLDP, and photosynthetic physiology of H. aturensis with patterns previously identified in the C2 grasses Steinchisma hians and Neurachne minor (Morgan and Brown, 1979; Hylton et al., 1988). Current phylogenies place the subtribe Otachyrinae, containing Steinchisma, as sister to the Arthropogoninae (Grass Phylogeny Working Group II, 2012). In addition, we examined S. laxum which has also been identified as a species that might provide information on the early stages of C2 and C4 evolution (Grass Phylogeny Working Group II, 2012; Sage et al., 2013). Finally, we use genome sequence data from publicly available databases (the Phytozome and NCBI), as well as assembled RNA-sequencing (RNA-seq) from 16 additional grass species, to examine evolution of GLDP and genes encoding the other GDC subunits and provide a broader understanding of the evolution of BS-specific GDC expression in C2 and C4 grasses.
Materials and methods
Plant material
Plants of Homolepis aturensis Chase., Steinchisma hians Raf., and S. laxum (Sw.) Zuloaga obtained from sources described in Supplementary Table S1 at JXB online were grown at the University of Toronto in a greenhouse in 10–20 liter pots of a sandy-loam soil and were watered daily to avoid water stress. Fertilizer was supplied weekly as a 50:50 mixture of Miracle-Grow 24-10-10 All Purpose Plant Food and Miracle Grow Evergreen Food (30-10-20) at the recommended dosage (22ml of fertilizer salt per 6 liters; Scotts Miracle-Gro; www.scotts.ca). Plants of Neurachne minor from localities previously described (Christin et al., 2012) were grown in a naturally illuminated glasshouse with mean temperatures of 25 °C/13 °C (day/night) at the Plant Growth Facility (PGF) of the University of Western Australia, Perth, Western Australia (latitude 33°89'S). To provide C3 and C4 grass species for comparison, we also examined leaves of PACMAD species Dichanthelium oligosanthes (Schult.) Gould (C3), Panicum bisulcatum Thunb. (C3), and two C4 species (Panicum virgatum L., NAD-ME subtype and Setaria viridis P. Beauv., NADP-ME subtype). Seed of these plants, obtained from sources described in Supplementary Table S1, were also grown at the University of Toronto.
Leaf anatomy, ultrastructure, and immunolocalizations
The internal anatomy of leaves was assessed on sections sampled from the middle of the most recent, fully expanded leaves (one leaf per plant; three plants per species). Plants were sampled from 09:00h to 11:00h between April and August when day length was >11.5h and light intensity in the greenhouse regularly exceeded 1400 µmol photons m−2 s−1. The youngest cohort of fully expanded leaves was sampled in full sun for all procedures. Samples were prepared for light and transmission electron microscopy (TEM) to assess anatomy as previously described (T.L. Sage et al., 2011, 2013; Stata et al., 2014). For immunolocalization, tissue from the same region of the leaf was fixed overnight in 1% (v/v) paraformaldehyde and 1% (v/v) glutaraldehyde in 0.05M sodium cacodylate buffer. Tissue was then dehydrated and embedded in LR White (Voznesenskaya et al., 2013). Immunolocalization of GLDP was conducted as outlined by Khosravesh et al. (2012). Primary and secondary antibody (18nm anti-rabbit IgG gold conjugate; Jackson Immunoresearch) dilutions were 1:50 and 1:20, respectively. Immunodetection of the Rubisco large subunit was modified from Ueno (1992). Sections were blocked in 0.5% BSA prior to incubation in primary antibody (1:100) for 3h. Incubation in secondary antibody (1:40; 18nm anti-rabbit IgG gold conjugate; Jackson Immunoresearch) was for 1h. To quantify all BS and M cellular features, TEM images from BS and M cells of the same grids used for immunogold labeling were analyzed using Image J software (Schneider et al., 2012) as previously described (Sage et al., 2013; Stata et al., 2014). The anti-GLDP antiserum was commercially produced (GL Biochem) against a 17 amino acid peptide showing high conservation in both monocots and dicots. Antisera recognizing the Rubisco large subunit (RBCL) were obtained from AgriSera. Three replicate immunolocalizations were conducted on different days with each replicate including sectioned tissue from all species.
Leaf gas exchange analysis
Gas exchange of intact, attached leaves was determined using a LiCor 6400 gas exchange system as previously described (Sage et al., 2013). Measurement conditions were 31±1 °C and a vapor pressure difference between leaf and air of 2±0.2 kPa. For measurement of the response of net CO2 assimilation rate (A) to intercellular CO2 concentration (C i) at light saturation, leaves were first equilibrated to 1200–1500 µmol photons m−1 s−1 at an ambient CO2 concentration of 400 µmol m−2 s−1. Ambient CO2 levels were then reduced in steps to 30–50 µmol mol−1 (lower end of this range for C2 and C4 species, upper end of this range for C3 species), with measurements at each step after rate equilibration. The ambient CO2 was then returned to 400 µmol photons m−1 s−1, and A re-measured. If A was within 10% of the original rate, the CO2 concentration around the leaf was increased in steps to near 1600 µmol mol−1, with measurements made at each step. The linear initial slope of the A/C i response was used as an estimate of carboxylation efficiency (CE).
For estimation of the apparent CO2 compensation point in the absence of day respiration (C*), the Laisk method was used as modified by Sage et al. (2013). Values of C* were not estimated in C4 plants. Leaves were first equilibrated at 400 µmol mol−1 and a light intensity near saturation (900–1500 µmol photons m−2 s−1). The CO2 was then reduced to provide a C i near 100 µmol mol−1, and, after stabilization, the rate was reduced in a series of steps to near the CO2 compensation point (Γ), with measurements at each step after signal stabilization. The C i was then returned to near 100 µmol mol−1, and the procedure was repeated at a lower light intensity. This cycle was repeated such that when measurements were completed, there were 4–5 A versus C i response curves with over five measurements at C i values <100 µmol mol−1. Each A versus C i response at a given light intensity was then fitted with a linear regression using the lowest 4–6 measurement points that fell on the regression. Points that fell below the regression line above ~80 µmol mol−1 were not included in the regression, as A/C i responses below light saturation may be prone to non-linearity above 60–100 µmol CO2 mol−1 air. The estimate of C* was taken as the C i value where the 4–5 curves at different light intensities converged. Rarely, however, did all curves converge at the exact same C i; instead, they intersected with each other over a 5–10ppm range. In such cases, the middle of the intersects was taken as the C* estimate. However, if there was evidence of a shift to lower photosynthetic photon flux density (PPFD) of the high light response, which often occurs in C2 species, the high light response was not included in the analysis (Sage et al., 2013).
Phylogenetic analysis of GDC subunit genes
Genome sequences for Zea mays, Sorghum bicolor, Panicum hallii, Se. viridis, S. italica, Oryza sativa, Brachypodium distachyon, and B. stacei were downloaded from the Phytozome v10.3 (https://phytozome.jgi.doe.gov/pz/portal.html). Polyploid species Triticum aestivum and P. virgatum were omitted. Amborella trichopoda was used as the outgroup, and we included the Brassicaceae species Arabidopsis thaliana, Capsella grandiflora, and Boechera stricta to show which duplications in the model plant A. thaliana are conserved across the land plants and which are lineage specific. RNA-seq data for 14 BEP clade species as well as the C3 PACMAD Dicanthelium clandestinum and its close C4 relative Megathyrsus maximus were downloaded from the NCBI short read archive (Supplementary Table S2). Reads mapping to each GDC subunit gene were identified using BLASTN with orthologs in Z. mays and O. sativa as queries (S. bicolor was used in lieu of Z. mays for one of the two GLDL paralogs as Z. mays appears to have lost this copy). For each gene, alignments were generated based on the Phytozome genomes using Muscle (Edgar, 2004), a highly conserved region was selected, and a consensus sequence was generated. These consensus sequences were used as reference for assembling sequences based on the retrieved reads using Geneious 8 (http://www.geneious.com).
The assemblies and sequences from Phytozome genomes were aligned using Muscle, trimmed using Trimal (Capella-Gutierrez et al., 2009) with the ‘strict’ heuristic option, and used to generate Bayesian phylogenies using MrBayes (Ronquist and Huelsenbeck, 2003) as follows: four runs, four chains, GTR substitution model, 2 million generations for all trees except GLDT, which was run for 10 million generations to allow better convergence; ≥10 000 trees were sampled from portions of the end of each run where the average SD of split frequencies remained below 1%. Tree figures were generated using Fig Tree (http://tree.bio.ed.ac.uk/software/figtree).
Data analysis
Results were analyzed with Sigmaplot version 12.5 (Systat Software., San Jose, CA, USA) using one-way ANOVA followed by a Tukey’s means comparison test. For characterization of leaf anatomy and ultrastructure, leaf samples were collected from three plants. For all traits measured, the values per plant were averaged to give one value for a plant. These individual plant values were the unit of replication for statistical analysis. For characterization of anatomical features, data from 3–5 sections per plant were averaged. For quantitative assessment of organelles in M and BS cells and GLDP immunodetection, the data from 10 imaged cells per cell type per plant were averaged. For leaf gas exchange, 4–15 measurements were conducted on 4–7 plants per species. In Neurachne species, vascular tissue is surrounded by two layers of cells, an outermost BS and innermost mestome sheath that functions in the C4 species as the site of CO2 refixation (Hattersley et al., 1986). Comparing data collected from the mestome sheath of N. minor with data from the BS of the other grasses makes statistical comparisons invalid except for comparisons between either the presence or absence of GDLP in M versus the BS or mestome sheath cells. Hence, N. minor was not included in the statistical tests involving these other grasses.
Results
Homolepis aturensis possesses structural features common to C2 species
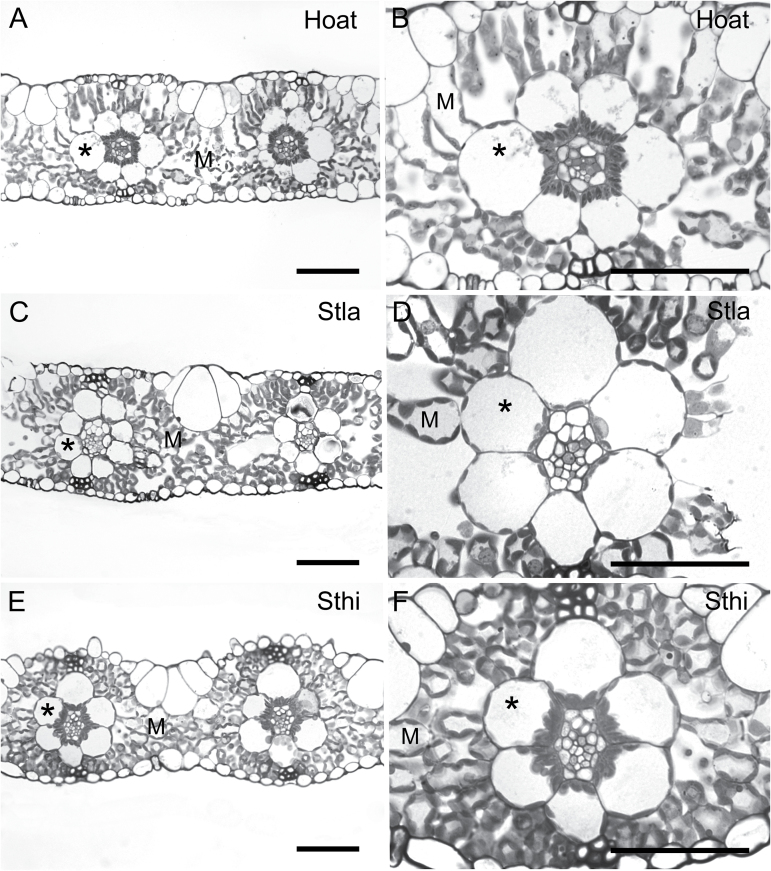
Leaves of H. aturensis are anatomically similar to those of the C2 species S. hians and S. laxum with respect to M cell structure (Fig. 1). One layer of M cells extends from BS cells to the adaxial and abaxial epidermis (Fig. 1). Approximately six M cells separate the BS of adjacent veins in S. laxum whereas four cells separate the BS of adjacent veins in H. aturensis and S. hians (Fig. 1; Supplementary Table S3). The M:BS tissue ratio for S. laxum, H. aturensis, and S. hians is ~2 (Fig. 1; Supplementary Table S3), which is similar to that of the C4 species P. virgatum but >2.5 times less than that of the C3 species D. oligosanthes and P. bisulcatum. A large M and small BS volume contributes to an M:BS of almost 5 in Se. viridis even though there are only two cells between each vein (Supplementary Fig. S1C; Supplementary Table S3). M cells in D. oligosanthes, P. bisulcatum, and Se. viridis are more loosely packed and elongate than those of P. virgatum (Supplementary Fig. S1). The cellular features of N. minor are similar to those previously reported (Hattersley et al., 1986), with 2–3M cells between veins (Supplementary Table S3).
Fig. 1.
Leaf cross-sections from (A, B) Homolepis aturensis (Hoat), (C, D) Steinchisma laxum (Stla), and (E, F) S. hians (Sthi). M, mesophyll; asterisk, bundle sheath. Scale bars=50 µm.
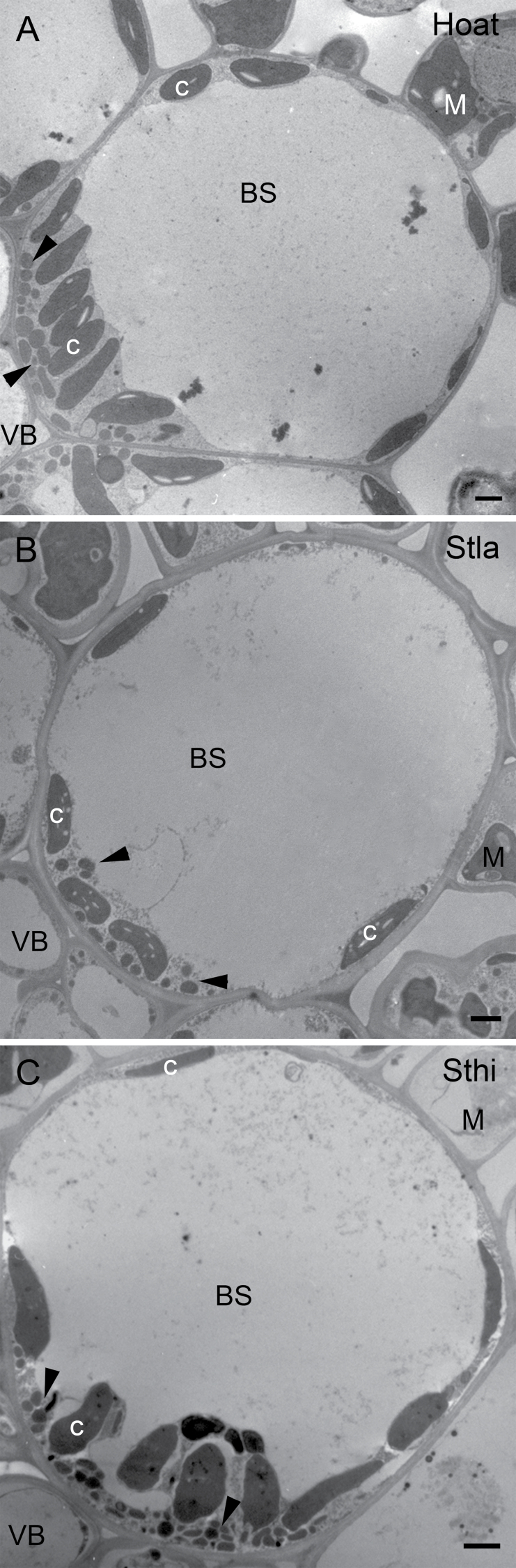
The BS cells of H. aturensis contain chloroplasts arranged around the periphery, with a significantly greater number clustered centripetally (Figs 1A, B, 2A; Supplementary Table S4). Mitochondria and peroxisomes localize almost exclusively to the centripetal BS pole (Fig. 2A; Supplementary Table S4). These spatial arrays of chloroplasts, mitochondria, and peroxisomes are similar in BS cells of S. hians (Figs 1E, F, 2C; Supplementary Table S4). As observed for H. aturensis and S. hians, a significantly greater number of mitochondria and peroxisomes are positioned at the centripetal BS pole in S. laxum, although chloroplasts are arranged equally around BS cells (Fig 1C, D; Supplementary Table S4). BS mitochondria are commonly surrounded by chloroplasts in S. hians (Fig 3E), H. aturensis, and S. laxum in a pattern similar to previous reports for Steinchisma species (Brown et al., 1983). In contrast to centripetal chloroplasts displayed with their long axis parallel to the BS wall in S. laxum, the long axis of these chloroplasts in H. aturensis and S. hians are perpendicular to the BS wall (Figs 1, 2). BS chloroplasts are situated primarily in a centrifugal position in C3 grasses D. oligosanthes and P. bisulcatum (Supplementary Table S4; Supplementary Figs S1, S2), as noted in C3 eudicots (Muhaidat et al., 2011; Sage et al., 2013). Approximately 65–69% of mitochondria and 19% (D. oligosanthes) and 41% (P. bisulctum) of peroxisomes, respectively, are located in the centripetal position of the C3 grasses (Supplementary Table S4).
Fig. 2.
Bundle sheath ultrastructure of (A) Homolepis aturensis (Hato), (B) Steinchisma laxum (Stla), and (C) S. hians (Sthi). BS, bundle sheath; C, chloroplast; M, mesophyll; VB, vascular tissue; arrowheads, mitochondria. Scale bars=2 µm.
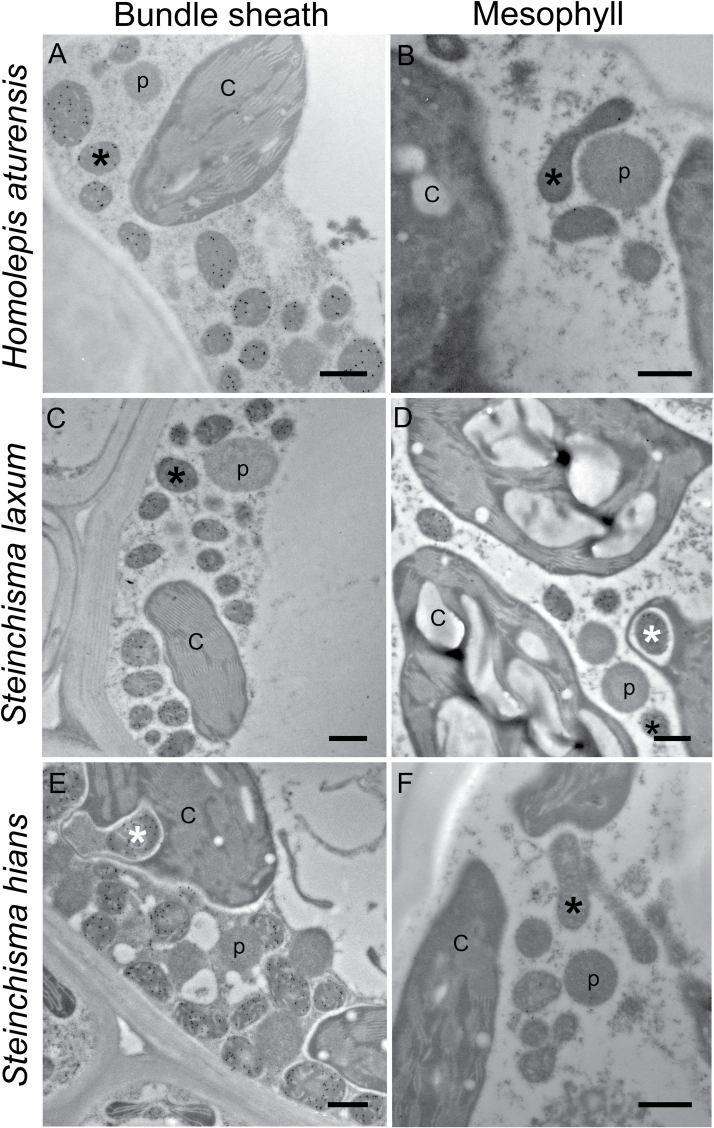
Fig. 3.
Immunolocalization of GLDP in bundle sheath and mesophyll cells of (A, B) Homolepis aturensis, (C, D) Steinchisma laxum, and (E, F) S. hians. C, chloroplasts; p, peroxisomes; black asterisk, mitochondria; white asterisk, mitochondria surrounded by chloroplast. Scale bars=500nm.
Quantitative parameters of organelle traits of BS and M cells are summarized in Supplementary Table S5. BS organelle parameters of H. aturensis and S. hians are not statistically different from each other except for a significantly greater number of mitochondria per planar cell area in S. hians. Mitochondria planar area per planar BS cell area of H. aturensis and S. hians is significantly greater than for S. laxum. In addition, mitochondria number per planar BS cell area, chloroplast and peroxisome number, and planar area per planar BS cell area are significantly greater in S. hians than in S. laxum. Results from a one-way ANOVA on ranks comparing peroxisome planar area per planar BS cell area between S. hians, S. laxum, and H. aturensis only indicated that this trait was significantly higher in S. hians and H. aturensis than in S. laxum (P≤0.001). The C3 grasses have the lowest mitochondria planar area per planar BS cell area; however, BS mitochondria parameters of C4 grasses are not different from those of the C2 grasses H. aturensis and S. hians. Mitochondria, peroxisomes, and chloroplasts are abundant in mestome sheath cells of N. minor (Hattersley et al., 1986; Sage et al., 2014). Unlike H. aturensis, S. hians, and S. laxum, organelles are not polarized in their distribution (Supplementary Fig. S3), but are instead positioned around the mestome sheath cell periphery (Hattersley et al., 1986; Sage et al., 2014).
When considering M cell organelle features, significantly fewer mitochondria and peroxisomes are observed in C4 species relative to the C3 species and the C2 species H. aturensis, S. hians, and S. laxum (Supplementary Table S5). The C4 species as well as H. aturensis have significantly lower chloroplast planar area per M planar cell area relative to S. hians, S. laxum, and the C3 grass species, and these changes result from either smaller (H. aturensis, P. virgatum) or fewer (Se. viridis) M cell chloroplasts (Supplementary Table S5). M cells of C4-like and C4 Flaveria and other C4 species have recently been reported to have significant reductions in chloroplast volume (Stata et al., 2014, 2016).
Homolepis aturensis exhibits C2 levels of GLDP in bundle sheath and mesophyll cells
Results from quantification of gold particles conjugated to secondary antibodies that bind to anti-GLDP are summarized in Supplementary Table S5. GLDP is almost exclusively located in BS cells of H. aturensis and S. hians, and mestome sheath cells of N. minor (Fig. 3A, B, E, F; Supplementary Fig. S3). Both BS and M cells contained high levels of GLDP labeling in S. laxum (Fig. 3C, D). In comparison with all other C3 species examined, S. laxum had the highest GLDP labeling in BS mitochondria (Supplementary Table S5). These patterns in GLDP distribution in M and BS mitochondria of S. laxum and S. hians are similar to earlier reports on these species (Hylton et al., 1988). The gold density in C3 species D. oligosanthes and P. bisulcatum is higher in mitochondria of M than BS cells and is significantly greater on a planar M cell area basis than in those of H. aturensis, S. hians, and the C4 species (Supplementary Table S5; Supplementary Fig. S4).
Homolepis aturensis exhibits C2 levels of Rubisco in bundle sheath and mesophyll cells
Rubisco occurs in both M and BS cell chloroplasts of H. aturensis, and this pattern was similar to that observed in S. hians, S. laxum, and M and mestome sheath cells of N. minor (Supplementary Fig. S5). As expected, Rubisco labeling is present only in the BS chloroplasts of the C4 species (Supplementary Fig. S6). Although Rubisco is also present in the M and BS cells of the C3 species D. oligosanthes and P. bisulcatum, there is qualitatively less labeling in the BS cells (Supplementary Fig. S6).
Homolepis aturensis exhibits photosynthetic characteristics of a C2 species
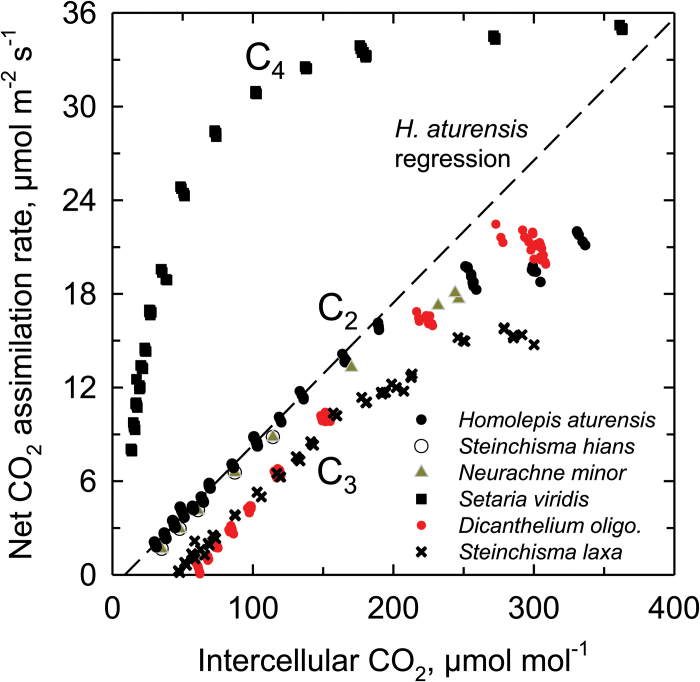
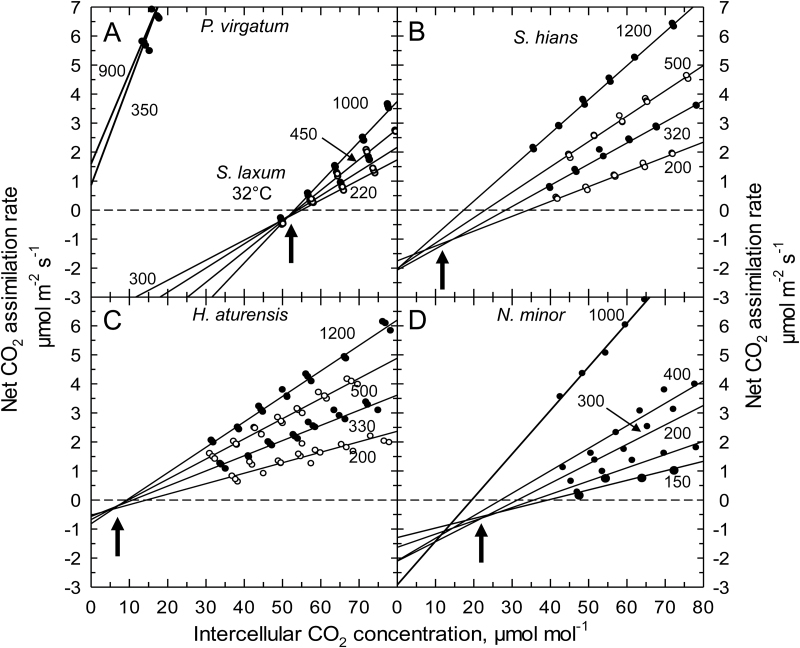
Values of A at 400 µmol CO2 mol−1 air are statistically similar, being 21±3 (mean±range) µmol m−2 s−1 for all species in the study except for the C4 plant Se. viridis, which has higher A (Table 1). Thus, any differences in carboxylation efficiency (CE), Γ, or intrinsic water use efficiency (estimated as A/g s at 400 µmol CO2 mol−1 air) should reflect photosynthetic pathway effects and not variation in photosynthetic capacity. Comparison of the A versus C i responses at saturating light intensities show three equivalent sets of curves that corresponded to the photosynthetic pathway (Fig. 4). C3 and C2 species have similar responses, with the exception that Γ was reduced ≥30 µmol mol−1 in the C2 species; consequently, their A/C i responses were shifted to lower C i values. Homolepis aturensis has A/C i responses identical to those of S. hians and N. minor, leading us to classify H. aturensis as a C2 species. Carboxylation efficiencies and A/g s of all the C2 and C3 species are statistically identical and less than those of the C4 species (Table 1).
Table 1.
Summaries of gas exchange values for species included in this study. Values
are means ±SE.
| Species | n |
C
*
µmol mol−1 |
CE
mol m−2 s−1 |
A
at 400
µmol m−2 s−1 |
A/g
s at 400
µmol mol−1 |
|
|---|---|---|---|---|---|---|
| C 3 species | ||||||
| Dicanthelium oligosanthes | 3, 6 | 48±1 a | 0.13±0.01 b | 24.5±1.5 b | 56±2 | |
| Panicum bisulcatum | 6, 6 | 50±2 a | 0.11±0.01 b | 18.7±1.7 b | 62±8 | |
| C 3 -Protokranz | ||||||
| Steinchisma laxum | 5, 7 | 53±1 a | 0.12±0.0 b | 21.7±2.4 b | 62±11 | |
| C 2 species | ||||||
| Homolepis aturensis | 4, 7 | 10.8±1.4 c | 0.09±0.01 b | 19.8±1.4 b | 57±10 | |
| Neurachne minor | 3, 3 | 20.0±2.5 b | 0.11±0.02 b | 17.7±1.9 b | 43±4 | |
| Steinchisma hians | 5, 8 | 11.8±1.4 c | 0.10±0.01 b | 21.6±1.1 b | 58±7 | |
| C 4 species | ||||||
| Panicum virgatum | 0, 2 | NA | 0.36±0.01 a | 24.5±2.5 b | 152±5 | |
| Setaria viridis | 0, 3 | NA | 0.54±0.05 a | 31.9±0.8 a | 76±12 |
Measurement temperature was 31±1 °C.
Sample sizes given are n = 3-5 for C* estimates, and n = 3–8 for all other data, with the exception of P. virgatum where n= 2).
Letters indicate statistical groupings at P<0.5 via one-way ANOVA followed by a Student’s–Neumann–Kuehls test.
NA, not applicable.
400 refers to ambient CO2 concentration in µmol mol−1.
Fig. 4.
Response of net CO2 assimilation rate to intercellular CO2 concentration in Homolepis aturensis, two C2 species (Neurachne minor and Steinchisma hians), a C4 species (Setaria viridis), a C3 species (Dicanthelium oligosanthes), and a C3 species with proto-Kranz anatomy (Steinchisma laxum). Measurement conditions were 31±1 °C and saturating light intensities (1200–1500 µmol m−2 s−1). The curves shown are representative of 2–3 individual A/C i responses per species. The linear regression for points below 100 µmol mol−1 is shown for H. aturensis (dashed line).
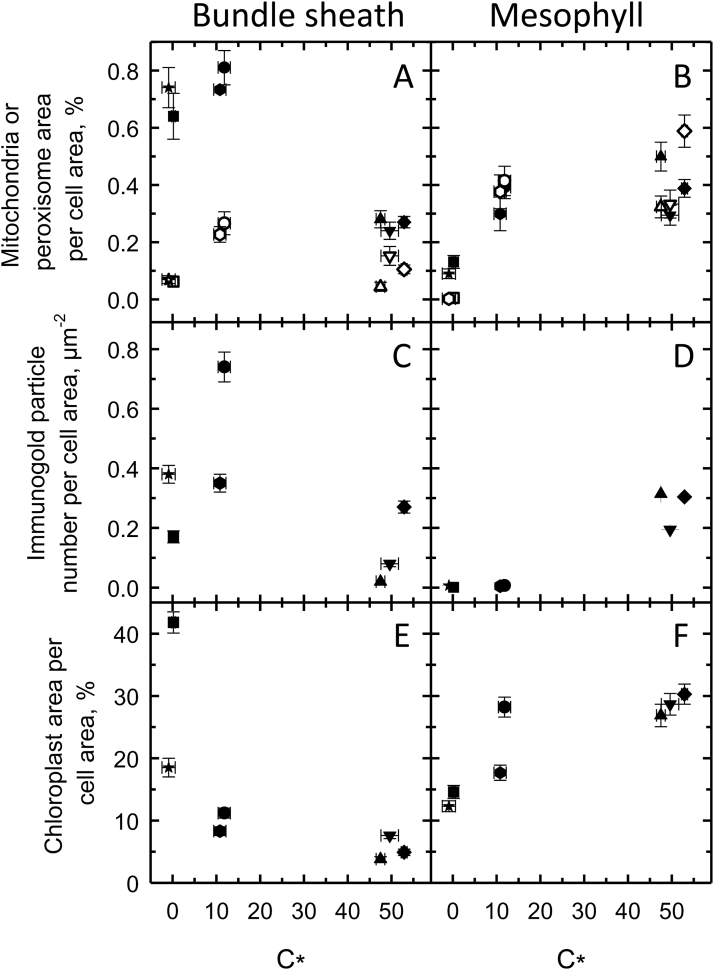
In the C3 species D. oligosanthes and P. bisulcatum, we observed C* values near 50 µmol mol−1 (Table 1) which is typical for C3 species at 31 °C (Busch et al., 2013). Steinchisma laxum had a similar C* value (53 µmol mol−1; Table 1; Fig. 5 A) indicating it is functionally C3 despite the potential function of the BS organelles. The C* values of the C2 species were 10.8−20 µmol mol−1 (Table 1; Fig. 5B–D); the 10.8 value is on the lower end of C* for species with this physiology (Edwards and Ku, 1987). In the grasses studied here, lower C* values correspond to higher values of BS mitochondria per planar cell area (Fig. 6A) and higher BS GLDP density per planar cell area, except for Se. viridis (Fig. 6C). C* was lower in species where BS chloroplast area per planar cell area was greater and M chloroplast area per planar cell area was lower (Fig. 6E, F).
Fig. 5.
Representative A/C i responses below 80 µmol mol−1 determined on single leaves at four distinct light intensities of (A) Panicum virgatum (C4) and Steinchisma laxum (C3 proto-Kranz), (B) the C2 species Steinchisma hians, (C) the C2 species Homolepis aturensis, and (D) the C2 species Neurachne minor. Measurement light intensities are indicated beside each curve in µmol photons m−1 s−1. The C* estimate is indicated by arrows. Curves shown are representative of 3–6 measurement sets per species, except for P. virgatum where two sets of measurements were obtained. Measurement temperature was 31±1 °C.
Fig. 6.
C* (Γ, for C4 species), organelle traits, and GLDP labeling in bundle sheath (A, C, E) and mesophyll (B, D, F) cells of Dichanthelium oligosanthes (triangle, C3), Panicum bisulcatum (inverted triangle, C3), Steinchisma laxum (diamond, C3), S. hians (circle, C2), Homolepis aturensis (hexagon, C2), Se. viridis (square, C4), and P. virgatum (star, C4). Filled and open symbols represent mitochondria and peroxisomes, respectively. Mean ±SE.
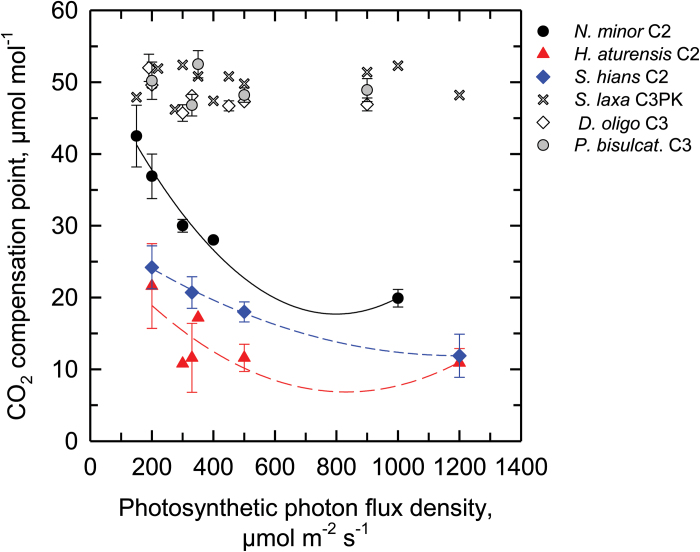
There is little observed shift to lower C i in the high light response of A versus C i in H. aturensis, such that all A versus C i curves converge near a common intersection point (Fig. 5). In S. hians and N. minor there is a slight reduction by a C i of ~5-7 µmol mol−1 in the high light response. There is no change in Γ in the C3 species with variation in light intensity (Fig. 7), while in each of the C2 species, Γ increases at the lower light intensity (Fig. 7). Neurachne minor exhibits the greatest increase in Γ as light declines, while the light response of Γ is negligible in H. aturensis above 300 µmol m−2 s−1. Notably, Γ of N. minor is twice that of H. aturensis across the range of light intensities.
Fig. 7.
The response of the CO2 compensation point of A (Γ) as a function of measurement light intensity in the C2 species Homolepis aturensis, Neurachne minor, Steinchisma hians, the proto-Kranz species Steinchisma laxum, and the C3 species Dicanthelium oligosanthes and Panicum bisulcatum. Values of Γ were determined from the A/C i curves used in the sequence of measurements to determine C*. Symbols are means ±SE (n=2–6), except where no error bar is shown, in which case n=1.
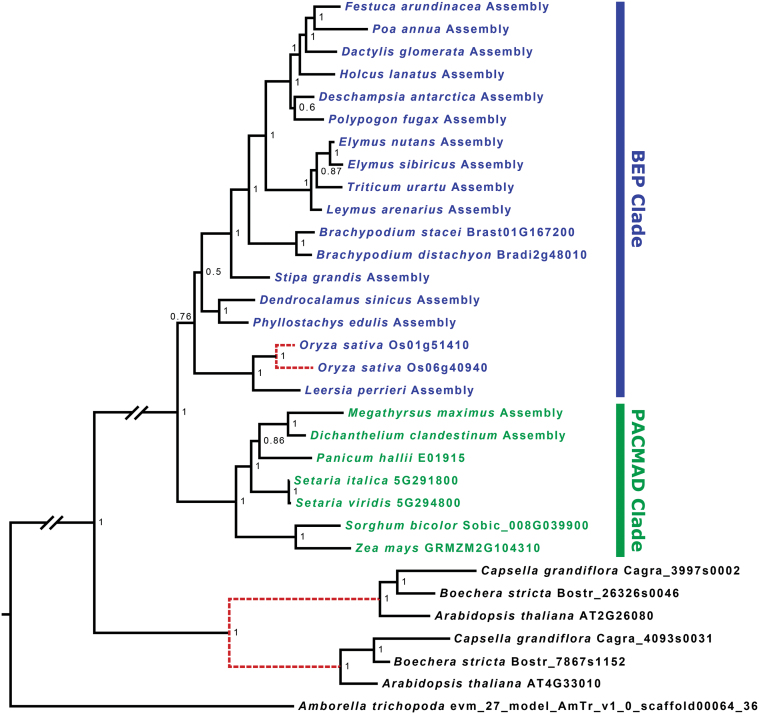
GDC BS specificity in C2 and C4 grasses probably results from changes in expression of a single GLDP gene
Phylogenetic analyses reveal that within the Poaceae, O. sativa is the only species examined with two gene copies encoding GLDP, and these sequences are most closely related to each other, even with inclusion of 14 additional BEP clade species (Fig. 8). We find no evidence of broader GLDP gene duplication or loss of gene copies in C4 grass species. Two paralogs encoding GLDH are present in all angiosperms, owing to an ancient duplication event (Supplementary Figs S7, S8). The two paralogs were treated independently as GLDH1 and GLDH2. Preliminary analysis indicates that there is a Poaceae-wide duplication only of GLDL, and targeted assemblies were made independently for each paralog, which we label GLDL1 and GLDL2 (Supplementary Fig. S9). During each assembly, mapped reads were scrutinized manually for evidence of additional paralogs, and none was detected. No reads were found for GLDL2 in the BEP grass Dendrocalamus sinicus, suggesting that it may have been lost in this species. Most other grass species possess both GLDL gene copies, with the exception of Z. mays, which also lacks GLDL2; S. bicolor, which shares a common C4 origin with Z. mays, has both paralogs. For all grass species, full GLDL1 and GLDL2 sequences from genomes and all assemblies which were completed to the start codon were strongly predicted to be mitochondrial-localized by TargetP (Emanuelsson et al., 2000; data not shown). This is consistent with targeting of the two copies in Arabidopsis (AT3G17240, AT1G48030; Fig. S9), which are both mitochondrial (Lutziger and Oliver, 2001; Rajinikanth et al., 2007). A plastidial dihydrolipoyl dehydrogenase exists in land plants as well, but is more distantly related and likely dates to a much earlier duplication (Lutziger and Oliver, 2000; Rajinikanth et al., 2007). Finally, GLDT is present as a single-copy gene in all species examined (Supplementary Fig. S10). While we find evidence of local duplication (GLDP, GLDH2) and conserved paralogs (GLDL), we find no evidence of C4 lineage loss of any GDC subunit genes.
Fig. 8.
A Bayesian phylogenetic tree of GLDP nucleotide sequences from 24 grasses, three Brassicaceae species, and Amborella. Long branches between distantly related groups are condensed for visibility, denoted with a gap. Dashed red lines denote inferred gene duplication events. Numbers at nodes indicate posterior probability.
Discussion
Photorespiratory glycine shuttling exhibited by C2 species is considered to be the evolutionary bridge from C3 photosynthesis to C4 photosynthesis, based largely on studies from eudicot species, particularly Flaveria (Bauwe, 2011; Sage et al., 2013; Schulze et al., 2013; Mallmann et al., 2014). It has been posited that C2 photosynthesis may serve a bridging role to C4 photosynthesis in grasses as well (Schulze et al., 2013), although the timing of trait acquisition, to include GDC BS cell specificity and abundance, has been proposed to differ between eudicots and monocots (Williams et al., 2013). Here, we evaluated photosynthetic pathway characteristics and cellular features of BS and M cells in H. aturensis, a candidate C2 species which branches in a position sister to a C4 clade in the subtribe Arthropogoninae. We used comparative studies with confirmed C2 grasses, S. hians and N. minor, and the C3 grass S. laxum to facilitate our classification of the photosynthetic type of H. aturensis. In addition, we conducted a phylogenetic study of genes for the GDC subunits to test the Schulze et al. (2013) inference that the evolution of BS-specific GDC expression in C4 grasses was similar to that in Flaveria. From our physiological and structural results, we conclude that H. aturensis is indeed a C2 species, supporting a hypothesis that the photorespiratory glycine shuttle is a bridge to C4 photosynthesis in grasses in the subtribe Arthropogoninae. The characterization of S. laxum and S. hians also allows us to conclude that activation of the BS cells during transition from C3 to C2 in grasses in the subtribe sister to the Arthropogoninae is similar to what has been reported for eudicots with respect to BS organelle positioning and organelle and GDC enrichment (Muhaidat et al., 2011; Sage et al., 2013). Finally, our phylogenetic and immunohistochemical data are consistent with the notion that BS GDC in C2 and C4 grasses results from changes in expression levels of a single GLDP gene in M and BS cells.
Homolepis aturensis exhibits characteristic features common to species that concentrate photorespired CO2 in BS cells using the C2 metabolic cycle. One of the key proteins essential for GDC activity, GLDP, localizes almost exclusively in BS mitochondria in H. aturensis and S. hians. This pattern is ubiquitous in C2 eudicots (Hylton et al., 1988; Voznesenskaya et al., 2001; Ueno and Sentoku, 2006; Voznesenskaya et al., 2010; Muhaidat et al., 2011; T.L. Sage et al., 2011). A second characteristic of C2 species is an abundance of Rubisco in M and BS cells (Monson et al., 1984). Rubisco is abundant in M and BS cells in H. aturensis and S. hians, in contrast to the typical C3 pattern (high M Rubisco; low BS Rubisco) and C4 pattern (Rubisco only in BS cells). Lastly, the number of M cells between BS cells in H. aturensis and S. hians results in a reduced M:BS ratio and increased vein density common to C2 species (reviewed in Sage et al., 2012). These features promote rapid flux of photorespiratory metabolites between M and BS compartments (Monson and Rawsthorne, 2000), improve water relations under high photorespiratory conditions (Osborne and Sack, 2012), and facilitate an increase in the volume of leaf tissue where photorespired CO2 is concentrated around Rubisco in C2 species.
As the refixed fraction of photorespiratory CO2 increases, C* (and Γ) declines (von Caemmerer, 2000). The effectiveness of the C2 process in H. aturensis as well as S. hians is reflected in the Γ and C* values that are at the lower range of values reported for C2 species (Holaday et al., 1984; Monson et al., 1984; Ueno et al., 2003; Vogan et al., 2007; Voznesenskaya et al., 2010; T.L. Sage et al., 2011, 2013). Values of Γ and C* below 15ppm indicate either that a C4 cycle is active to complement the C2 cycle, or that the CO2 trap in the inner BS is particularly effective at recapturing the photorespired CO2 (von Caemmerer, 2000). Steinchisma hians has weak to negligible C4 cycle activity (Edwards et al., 1982), and cellular characteristics of organelle orientation in BS cells may explain the low C* in H. aturensis and S. hians. The organelle-enriched BS cells of these two species exhibit polarity in organelle positioning such that almost all peroxisomes and GDC-containing mitochondria, and over half of the chloroplasts are situated adjacent to the vascular tissue. This arrangement of BS organelles is posited to be particularly effective in enhancing recapture efficiency of photorespired CO2 before it can escape the BS cell in C2 species (Rawsthorne, 1992). One notable feature we observed is that the parallel orientation of the centripetal BS chloroplasts to the inner wall in S. laxum shifts to a perpendicular orientation in S. hians. A similar perpendicular chloroplast orientation is present in H. aturensis. This pattern allows for packaging of the more numerous, larger chloroplasts in the centripetal position, which could be important for increasing the surface area for refixing photorespired CO2 from adjacent mitochondria. Moreover, BS mitochondria are physically surrounded by chloroplasts in S. hians and H. aturensis, and these close physical associations have been proposed to enhance refixation of photorespired CO2 (Brown et al., 1983).
The fine structure of C2 BS cells is defined as C2 Kranz, reflecting a view that this photosynthetic carbon-concentrating mechanism is associated with its own enabling Kranz-like structure (Sage et al., 2014). Multiple convergence of C2 Kranz in eudicots and grasses is strong evidence that this particular BS anatomy is specifically adapted for the C2 pathway. The earliest recognizable subcellular events that ‘increase the accessibility’ (Grass Phylogeny Working Group II, 2012) of C2 Kranz from C3 in eudicots are an enhancement in numbers and size of mitochondria per BS cell, and positioning of these organelles from the centrifugal C3 position to the centripetal BS pole. The BS chloroplast numbers also increase in tandem with alterations in mitochondria placement, along with a rearrangement of many, but not all, BS chloroplasts from the centrifugal to centripetal pole (Muhaidat et al., 2011; Sage et al., 2013; Voznesenskaya et al., 2013). The anatomy associated with these earliest subcellular events has been termed proto-Kranz (Muhaidat et al., 2011; Sage et al., 2013). The transition to full C2 BS patterns from proto-Kranz in eudicots results from further amplification in centripetal mitochondria volume (size and numbers) and relocation of a greater fraction of enlarged chloroplasts to the centripetal pole (Muhaidat et al., 2011; Sage et al., 2013). Proto-Kranz and the shift to C2 Kranz occurs with increasing vein density in Flaveria and Heliotropium (Muhaidat et al., 2011; Sage et al., 2013).
The subcellular framework of C3 S. laxum BS cells and subsequent changes to that configuration from S. laxum to C2 S. hians are similar to those observed in eudicots, supporting a hypothesis that proto-Kranz facilitates the C3 to C2 transition (Sage et al., 2014). In comparison with the C3 species D. oligosanthes and P. bisulcatum, mitochondria and peroxisomes are situated along the centripetal poles of BS cells in S. laxum, classifying this species as proto-Kranz. Previous characterizations of proto-Kranz species have not presented data on peroxisomes, and this additional focus in the present study provides critical information on the positioning of the other organelle involved in C2 photosynthesis in the BS. A 3-fold increase in mitochondrial volume and corresponding increase in GDC, as well as a 2.5-fold increase in peroxisome volume, and 2-fold increase in chloroplast volume accompany the transition to the C2 BS pattern in S. hians. Also, as observed in eudicots, the increase in BS chloroplast volume in S. hians from proto-Kranz is associated with more of these organelles in the centripetal location. Notably, although the number and size of mitochondria per BS cell area are lower in S. laxum than in S. hians, the GLDP label intensity is similar per mitochondrion and significantly higher than that observed in the C3 grasses. These results indicate that increased BS GDC per mitochondrion is also a functionally important development early in C2 evolution in grasses. The enhanced BS GDC density per mitochondrion in S. laxum is present in tandem with C3-like values of vein density. The C2 levels of BS GDC in S. hians are present in high vein density leaves. The high levels of BS GDC in S. laxum and S hians contrasts with the theoretical predictions of Williams et al. (2013) who modeled C4 evolution in eudicots and monocots based on observed patterns of trait acquisition in C3–C4 intermediates. For monocots, they predicted that an increase in vein density preceded enhanced GDC specificity and abundance in BS cells. However, their model relied on a relatively small data set with significant gaps. The results here indicate that C2 evolution in grasses follows a pattern more typical of eudicots, which the model of Williams et al. (2013) may support when reparameterized with a richer data set.
In Neurachne, as in many other grasses, the mestome sheath cell is the site of the Calvin–Benson cycle in C4 species (Hattersley and Browning, 1981; Hattersley et al., 1986; Dengler and Nelson, 1999; Edwards and Voznesenskaya, 2011). We demonstrate that the mestome sheath cells in the C2 species N. minor are functionally similar to the C2 BS cells of H. aturensis and S. hians because GDC is almost exclusively located in organelle-enriched mestome sheath cells in the high vein density leaves. However, unlike H. aturensis and S. hians, there is no polarized orientation of organelles in the GDC-enriched mestome sheath cells of N. minor (Hattersley et al., 1986; this study), indicating that N. minor utilizes a different strategy from H. aturensis and S. hians to trap photorespired CO2. In Neurachne, as in many other grasses, the thick mestome sheath cell wall with a suberized lamella becomes the trap (Hattersley and Browning, 1981; Hattersley et al., 1986; Dengler and Nelson, 1999; Edwards and Voznesenskaya, 2011). The C2 species Alloteropsis semialata ssp semialata has a similar C2 Kranz anatomy to N. minor; GDC levels are highest in mestome sheath cells with a suberized lamella and the abundant organelles are equally partitioned within those cells (Hattersley and Browning, 1981; Ueno and Sentoku, 2006). Intriguingly, although organelle orientation in mestome sheath cells is not important in the evolution of C2 photosynthesis in N. minor, chloroplasts do have a centrifugal orientation in C4 Neurachne species (Hattersley et al., 1986). Qualitative observations on C3 Neurachne species indicate that some of the C3 species have enhanced numbers of chloroplasts and mitochondria in mestome sheath cells (Hattersley et al., 1986), leading us to posit that organelle and GDC enrichment may have been important during the early stages of C2 evolution in the genus.
The evolutionary transition from C3 to C2 has been proposed first to involve a change in cell type-specific expression of GDC from M to BS in tandem with a loss of M GDC (Bauwe, 2011). A comparison of the cellular site of GDC expression in proto-Kranz S. laxum with that of the sister species S. hians indicates that the severe reduction in M GDC in the C2 species is preceded by increased expression in BS cells. This is consistent with patterns observed in the eudicots Flaveria and Heliotropium, and supports a model of gradual GDC loss in M cells following a physiological activation of the BS (Muhaidat et al., 2011; Sage et al., 2013; Schulze et al., 2013). C3 species of Flaveria contain two copies of the gene encoding GLDP resulting from gene duplication (Schulze et al., 2013). One of these is BS dominant in expression and the second is expressed ubiquitously throughout the leaf in C3 Flaveria (Schulze et al., 2013). During the evolution of C4 photosynthesis, the loss of M GDC function in Flaveria resulted from pseudogenization of the gene coding for the ubiquitously expressed GLDP (Schulze et al., 2013). Schulze et al. (2013) speculated that BS-dominant GLDP expression arose in a similar manner in C4 grasses, because O. sativa (C3 BEP clade) has two GLDP genes, but Z. mays and other C4 species in the C4 PACMAD clade have only one. To provide an understanding of the evolution of BS-specific GDC expression in C2 and C4 grasses, we conducted phylogenetic analyses of genes encoding GLDP using 17 BEP and seven PACMAD grass species, three Brassicaceae species, and Amborella as outgroup. Our analyses demonstrate that, with the exception of O. sativa, all grasses have one copy of GLDP. The two copies of the genes encoding GLDP in rice are more closely related to each other than to any other GLDP gene included in the analysis and therefore represent a local duplication. Combined, the phylogenetic and immunohistochemical observations on C3 and C4 PACMAD species are consistent with a hypothesis that BS-dominant quantities of GDC in C2 and C4 grasses resulted from modifications in regulatory mechanisms controlling the levels of expression of a single GLDP gene present in M and BS cells; C3 species have high levels of M GDC and low levels of BS GDC, and the opposite pattern is present in the C2 and C4 species. Mesophyll tissue specificity of phosphoenolpyruvate carboxylase has evolved through modification of cis-regulatory elements in C4 Flaveria (Gowik et al., 2004). Studies examining promoter regions of the GLDP subunits in closely related C3, C2, and C4 species should provide insights into the evolution of the regulatory mechanisms that confer the requisite expression patterns for C2 and subsequently C4 photosynthesis in grasses.
Since it is conceivable that BS specificity of the GDC complex arose via duplication and pseudogenization of one of the other subunits, we also included analyses for GLDL, GLDH1 and GLDH2, and GLDT. In land plants, GLDH1 functions in photorespiration and GLDH2 is associated with C1 metabolism (Rajinikanth et al., 2007). We found no evidence of broad duplication for either of the ancient GLDH copies or GLDT in the grasses; however, GLDL is encoded by two conserved paralogs in most grass species, and may have arisen from the whole-genome duplication in the ancestor of the grass family. GLDL is the only GDC subunit gene for which we find evidence of subfunctionalized and conserved paralogs analogous to the two GLDP copies in Flaveria. There is, however, no evidence that either of these copies has been lost or pseudogenized (e.g. via nonsense or frameshift mutation) in any C4 species except Z. mays, which has a local duplication of GLDL1 and lacks a gene encoding GLDL2. Yet it is unlikely that this loss was involved in the evolution of C4 photosynthesis, as GLDL2 is present in S. bicolor, which shares a common C4 origin with maize (Grass Phylogeny Working Group II, 2012). The two GLDL paralogs in grasses may be partially reduntant with one another (Rajinikanth et al., 2007). The nature of the subfunctionalization of GLDL which resulted in the evolutionary retention of two paralogs in Poaceae is not known, but our results indicate that these paralogs did not play a role in the evolution of C4 photosynthesis analogous to the GLDP paralogs in Flaveria. It is similarly unlikely that the two ancestral copies of GLDH played a role in C4 evolution analogous to that of GLDP in Flaveria as both are present in all grass species examined here, and only GLDH1 plays a role in photorespiration (Rajinikanth et al., 2007).
Conclusion
Evolution of C2 photosynthesis in the grasses and eudicots is conferred by organelle enrichment, BS- or mestome sheath-dominant GDC accumulation, and centripetal positioning of organelles when the BS is the carbon-concentrating tissue. In Steinchisma, the earliest recognized subcellular event that facilitates C2 photosynthesis is the placement of BS mitochondria and peroxisomes exclusively to the centripetal pole. This feature, also present in eudicots, is posited to set in motion a feed-forward facilitation cascade that leads to C2 and subsequently C4 photosynthesis (Sage et al., 2013). How and why changes in BS or mestome sheath GDC levels and organelle volume, and BS organelle positioning were initiated during the early stages of C2 evolution in grasses remains a mystery. Identification of mechanisms controlling these processes should be a primary focus of research. The Arthropogoninae, the subtribe containing Steinchisma (Otachyrinae), and Neurachninae will be key to these future studies.
Supplementary data
Supplementary data are available at JXB online.
Table S1. List of species studied and source of species.
Table S2. Species for which RNA-seq data were downloaded and assembled for phylogenetic analysis of GDC subunits.
Table S3. Anatomical parameters of C3, C2, and C4 leaves.
Table S4. Organelle distribution in bundle sheath cells of C3 and C2 species.
Table S5. Quantification of organelle numbers, size, and density of gold labeling (GLDP) in mesophyll and bundle sheath cells of C3, C2, and C4 leaves.
Figure S1. Light micrographs of C3 and C4 species.
Figure S2. Bundle sheath cell ultrastructure of C3 and C4 species.
Figure S3. Leaf structure and anatomy and immunolocalization of GLDP in N. minor.
Figure S4. Immunolocalization of GLDP in M and BS cells of C3 and C4 species.
Figure S5. Immunolocalization of Rubisco large subunit in M and BS cells of C3 and C2 species.
Figure S6. Immunolocalization of Rubisco large subunit in M and BS cells of C3 and C4 species.
Figure S7. A Bayesian phylogenetic tree of GLDH1.
Figure S8. A Bayesian phylogenetic tree of GLDH2.
Figure S9. A Bayesian phylogenetic tree of GLDL.
Figure S10. A Bayesian phylogenetic tree of GLDT.
Acknowledgements
We thank E. Kellog, T. Brutnell, and M. Tanaiguchi for providing seed, and N. Dakin for assistance with preparation of N. minor leaves. This research was supported by funding from the Natural Science and Engineering Research Council of Canada (grant nos 2015-04878 to TLS and 154273-2012 to RFS) and an Australian Research Council Discovery Project Grant to ML, TLS, and RFS (DP130102243).
References
- Bauwe H. 2011. Photorespiration: the bridge to C4 photosynthesis. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Advances in Photosynthesis and Respriration, Vol. 32 Dordrecht, The Netherlands: Springer, 81–108. [Google Scholar]
- Brown H, Bouton JH, Rigsby L, Rigler M. 1983. Photosynthesis of grass species differing in carbon dioxide fixation pathways. VIII. Ultrastuctural characteristics of Panicum species in the LAXA group. Plant Physiology 71, 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch FA, Sage TL, Cousins AB, Sage RF. 2013. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2 . Plant, Cell and Environment 36, 200–212. [DOI] [PubMed] [Google Scholar]
- Capella-Gutierrez S, Silla-Martinez J, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proceedings of the National Academy of Sciences, USA 110, 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Wallace MJ, Clayton H, Edwards EJ, Furbank RT, Hattersley PW, Sage RF, Macfarlane TD, Ludwig M. 2012. Multiple photosynthetic transitions, polyploidy, and lateral gene transfer in the grass subtribe Neurachninae. Journal of Experimental Botany 63, 6297–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler NG, Nelson T. 1999. Leaf structure and development in C4 plants In: Sage RF, Monson RK, eds. C4 plant biology . New York: Academic Press, 133–172. [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Ku MSB. 1987. Biochemistry of C3–C4 intermediates. In: Hatch MD, Boardman HK, eds. The biochemistry of plants, Vol. 10. London: Academic Press, 275–325. [Google Scholar]
- Edwards GE, Ku MSB, Hatch MD. 1982. Photosynthesis in Panicum milioides, a species with reduced photorespiration. Plant and Cell Physiology 23, 1185–1195. [Google Scholar]
- Edwards GE, Voznesenskaya EV. 2011. C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Advances in Photosynthesis and Respriration, Vol. 32 Dordrecht, The Netherlands: Springer, 29–61. [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology 300, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Fisher AE, McDade LA, Kiel CA, Khoshravesh R, Johnson MA, Stata M, Sage TL, Sage RF. 2015. Evolutionary history of Blepharis (Acanthaceae) and the origin of C4 photosynthesis in section Acanthodium. International Journal of Plant Sciences 176, 770–790. [Google Scholar]
- Gowik U, Burscheidt J, Akyildiz M, Schlue U, Koczor M, Streubel M, Wessthoff P. 2004. cis-Regulatory elements for mesophyll-specific gene expression in the C4 plant Flaveria trinervia, the promoter of the C4 phosphoenolpyruvate carboxylase gene. The Plant Cell 16, 1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group II 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytologist 193, 304–312. [DOI] [PubMed] [Google Scholar]
- Hattersley PW, Browning AJ. 1981. Occurrence of the suberized lamella in leaves of grasses of different photosynthetic types. I. In parenchymatous bundle sheaths and PCR (‘Kranz’) sheaths. Protoplasma 109, 371–401. [Google Scholar]
- Hattersley PW, Wong S-C, Perry S, Roksandic Z. 1986. Comparative ultrastructure and gas exchange characteristics of the C3–C4 intermediate Neurachne minor S. T. Blake (Poaceae). Plant, Cell and Environment 9, 217–233. [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber APM, Lercher MJ. 2013. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA. 2008. Using C4 photosynthesis to increase the yield of rice—rationale and feasibility. Current Opinion in Plant Biology 11, 228–231. [DOI] [PubMed] [Google Scholar]
- Holaday AS, Lee KW, Chollet R. 1984. C3−C4 intermediate species in the genus Flaveria: leaf anatomy, ultrastructure, and the effect of O2 on the CO2 compensation concentration. Planta 160, 25–32. [DOI] [PubMed] [Google Scholar]
- Hylton CM, Rawsthorne S, Smith AM, Jones DA, Woolhouse HW. 1988. Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3–C4 intermediate species. Planta 175, 452–459. [DOI] [PubMed] [Google Scholar]
- Keerberg O, Pärnik T, Ivanova H, Bassüner B, Bauwe H. 2014. C2 photosynthesis generates about 3-fold elevated leaf CO2 levels in the C3–C4 intermediate species Flaveria pubescens . Journal of Experimental Botany 65, 3649–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshravesh R, Akhani H, Sage TL, Nordenstam B, Sage RF. 2012. Phylogeny and photosynthetic pathway distribution in Anticharis Endl. (Scrophulariaceae). Journal of Experimental Botany 63, 5645–5658. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Christin PA, Gonzalez Escobar E, Ripley BS, Besnard G, Long CM, Hattersley PW, Ellis RP, Leegood RC, Osborne CP. 2015. Evolutionary implications of C3–C4 intermediates in the grass Alloteropsis semialata . Plant, Cell and Environment (in press). [DOI] [PubMed] [Google Scholar]
- Lutziger I, Oliver DJ. 2000. Molecular evidence of a unique lipoamide dehydrogenase in plastids: analysis of plastidic lipoamide dehydrogenase from Arabidopsis thaliana FEBS Letters 484, 12–16. [DOI] [PubMed] [Google Scholar]
- Lutziger I, Oliver DJ. 2001. Characterization of two cDNAs encoding mitochondrial lipoamide dehydrogenase from Arabidopsis. Plant Physiology 127, 615–623. [PMC free article] [PubMed] [Google Scholar]
- Lyu MJ, Gowik U, Kelly S, et al. 2015. RNA-Seq based phylogeny recapitulates previous phylogeny of the genus Flaveria (Asteraceae) with some modifications. BMC Evolutionary Biology 15, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Brautigam A, Lercher MJ, Weber AP, Westhoff P, Gowik U. 2014. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria . Elife 3, e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DM, Muhaidat R, Brown NJ, Liu Z, Stanley S, Griffiths H, Sage RF, Hibberd JM. 2007. Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. The Plant Journal 51, 886–896. [DOI] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG. 2005. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. American Journal of Botany 92, 1911–1928. [DOI] [PubMed] [Google Scholar]
- Monson R, Rawsthorne S. 2000. CO2 assimilation in C3–C4 intermediate plants. In: Leegood R, Sharkey T, von Caemmerer S, eds. Photosynthesis, Vol. 9 Dordrecht, The Netherlands: Springer, 533–550. [Google Scholar]
- Monson RK, Edwards GE, Ku MSB. 1984. C3–C4 intermediate photosynthesis in plants. BioScience 34, 563–574. [Google Scholar]
- Morgan JA, Brown RH. 1979. Photosynthesis in grass species differing in carbon dioxide fixation pathways: II. A search for species with intermediate gas exchange and anatomical characteristics. Plant Physiology 64, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CL, Turner SR, Rawsthorne S. 1993. Coordination of the cell-specific distribution of the 4 subunits of glycine decarboxylase and of serine hydroxymethyltransferase in leaves of C3–C4 intermediate species from different genera. Planta 190, 468–473. [Google Scholar]
- Muhaidat R, Sage TL, Frohlich MW, Dengler NG, Sage RF. 2011. Characterization of C3–C4 intermediate species in the genus Heliotropium L. (Boraginaceae): anatomy, ultrastructure and enzyme activity. Plant, Cell and Environment 34, 1723–1736. [DOI] [PubMed] [Google Scholar]
- Osborne CP, Sack L. 2012. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 583–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhansel C. 2011. Best practice procedures for the establishment of a C4 cycle in transgenic C3 plants. Journal of Experimental Botany 62, 3011–3019. [DOI] [PubMed] [Google Scholar]
- Rawsthorne S. 1992. C3–C4 intermediate photosynthesis—linking physiology to gene-expression. The Plant Journal 2, 267–274. [Google Scholar]
- Rawsthorne S, Morgan CL, O’Neill CM, Hylton CM, Jones DA, Frean ML. 1998. Cellular expression pattern of the glycine decarboxylase P protein in leaves of an intergeneric hybrid between the C3 –C4 intermediate species Moricandia nitens and the C3 species Brassica napus . Theoretical and Applied Genetics 96, 922–927 [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rajinikanth M, Harding SA, Tsai CJ. 2007. The glycine decarboxylase complex multienzyme family in Populus . Journal of Experimental Botany 58, 1761–1770. [DOI] [PubMed] [Google Scholar]
- Sage R. 2016. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and hall of fame. Journal of Experimental Botany 67, 2919–2922. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage RF, Khoshravesh R, Sage TL. 2014. From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis. Journal of Experimental Botany 65, 3341–3356. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Sage RF, Wedin DA, Li M. 1999. The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage RF, Monson RK, eds. C4 plant biology . San Diego: Academic Press: 313–374. [Google Scholar]
- Sage TL, Busch FA, Johnson DC, Friesen PC, Stinson CR, Stata M, Sultmanis S, Rahman BA, Rawsthorne S, Sage RF. 2013. Initial events during the evolution of C4 photosynthesis in C3 species of Flaveria . Plant Physiology 163, 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Sage RF, Vogan PJ, Rahman B, Johnson DC, Oakley JC, Heckel MA. 2011. The occurrence of C2 photosynthesis in Euphorbia subgenus Chamaesyce (Euphorbiaceae). Journal of Experimental Botany 62, 3183–3195. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze S, Mallmann J, Burscheidt J, Koczor M, Streubel M, Bauwe H, Gowik U, Westhoff P. 2013. Evolution of C4 photosynthesis in the genus Flaveria: establishment of a photorespiratory CO2 pump. The Plant Cell 25, 2522–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata M, Sage TL, Hoffman N, Covshoff S, Ka-Shu Wong G, Sage RF. 2016. Mesophyll chloroplast investment in C3, C4 and C2 species of the genus Flaveria . Plant and Cell Physiology 57 (in press). [DOI] [PubMed] [Google Scholar]
- Stata M, Sage TL, Rennie TD, Khoshravesh R, Sultmanis S, Khaikin Y, Ludwig M, Sage RF. 2014. Mesophyll cells of C4 plants have fewer chloroplasts than those of closely related C3 plants. Plant, Cell and Environment 37, 2587–2600. [DOI] [PubMed] [Google Scholar]
- Still CJ, Berry JA, Collatz GJ, DeFries RS. 2003. Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochemical Cycles 17. [Google Scholar]
- Ueno O. 1992. Immunogold localization of photosynthetic enzymes in leaves of Aristida latifolia, a unique C4 grass with a double chlorenchymatous bundle sheath. Physiologia Plantarum 85, 189–196. [Google Scholar]
- Ueno O, Bang SW, Wada Y, Kondo A, Ishihara K, Kaneko Y, Matsuzawa Y. 2003. Structural and biochemical dissection of photorespiration in hybrids differing in genome constitution between Diplotaxis tenuifolia (C3–C4) and radish (C3). Plant Physiology 132, 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno O, Sentoku N. 2006. Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies. Plant, Cell and Environment 29, 257–268. [DOI] [PubMed] [Google Scholar]
- Vogan PJ, Frohlich MW, Sage RF. 2007. The functional significance of C3–C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant, Cell and Environment 30, 1337–1345. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis . Australia: CSIRO publishing. [Google Scholar]
- von Caemmerer S, Quick WP, Furbank RT. 2012. The development of C4 rice: current progress and future challenges. Science 336, 1671–1672. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Artyusheva EG, Franceschi VR, Pyankov VI, Kiirats O, Ku MSB, Edwards GE. 2001. Salsola arbusculiformis, a C3–C4 intermediate in Salsoleae (Chenopodiaceae). Annals of Botany 88, 337–348. [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Akhani H, Roalson EH, Edwards GE. 2013. Structural and physiological analyses in Salsoleae (Chenopodiaceae) indicate multiple transitions among C3, intermediate, and C4 photosynthesis. Journal of Experimental Botany 64, 3583–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G. 2010. Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3–C4 intermediate in genus Portulaca L. (Portulacaceae). Journal of Experimental Botany 61, 3647–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Johnston IG, Covshoff S, Hibberd JM. 2013. Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. Elife 2, e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.