Abstract
In humans, a person’s chronotype depends on environmental cues and on individual characteristics, with late chronotypes prevailing in youth. Social jetlag (SJL), the misalignment between an individual׳s biological clock and social time, is higher in late chronotypes. Strong SJL is expected in Uruguayan university students with morning class schedules and very late entertainment activities. Sleep disorders have been reported in Antarctic inhabitants, that might be a response to the extreme environment or to the strictness of Antarctic life. We evaluated, for the first time in Uruguay, the chronotypes and SJL of 17 undergraduate students of the First Uruguayan Summer School on Antarctic Research, using Munich Chronotype Questionnaire (MCTQ) and sleep logs (SL) recorded during 3 phases: pre-Antarctic, Antarctic, and post-Antarctic. The midsleep point of free days corrected for sleep debt on work days (MSFsc,) was used as proxy of individuals’ chronotype, whose values (around 6 a.m.) are the latest ever reported. We found a SJL of around 2 h in average, which correlated positively with MSFsc, confirming that late chronotypes generate a higher sleep debt during weekdays. Midsleep point and sleep duration significantly decreased between pre-Antarctic and Antarctic phases, and sleep duration rebounded to significant higher values in the post-Antarctic phase. Waking time, but not sleep onset time, significantly varied among phases. This evidence suggests that sleep schedules more likely depended on the social agenda than on the environmental light–dark shifts. High motivation of students towards Antarctic activities likely induced a subjective perception of welfare non-dependent on sleep duration.
Keywords: Circadian rhythms, Sleep habits, Munich Chronotype Questionnaire, Antarctica, Uruguayan Antarctic Scientific Base Artigas
1. Introduction
The sleep–wake cycle, whose neuroendocrine mechanisms have been well studied, is the most conspicuous cyclic behavior in humans. As any other activity–rest circadian rhythm in the animal kingdom, sleeping habits in humans depend on an endogenous biological clock daily entrained by environmental changes of the light–dark cycle [1], [2]. However, humans are subjected to complex social and environmental pressures that compromise and restrict their otherwise natural sleep schedule [3]. Modern chronobiological studies attempt to study how human biological clocks are entrained by real life conditions, rather than studying their performance in isolation or in artificial conditions of illumination and social cues as pioneering studies did [3].
Individual chronotypes, i.e., an individual’s propensity to sleep at a particular time during a 24-h period, can be easily assessed using the Munich Chronotype Questionnaire (MCTQ, online since 2000, www.theWeP.org/documentations/mctq) by computing the midsleep of free days (MSF) [4], [5]. Along with genetic factors, chronotype depends on different environmental cues, such as light exposure [6], and on individual characteristics, such as age or gender [7], [8], [9], [10]. Late chronotypes predominate in adolescents and young adults [9], [11], [12]. The distribution of chronotypes in different European populations is quasi normal, with an average midsleep point of around 04.50 h [5], [13], [14], [15]. On the other hand, Uruguay has no systematic database of the distribution of chronotypes or of sleep habits within its population.
There is no need to fly across time zones to experience a discrepancy between the endogenous clock and external time (jetlag). When this misalignment arises between an individual׳s biological clock and social time, it is called social jetlag [16]. Social jetlag, calculated as the absolute difference between midsleep on workdays (MSW) and midsleep on free days (MSF), tends to be higher in late chronotypes; and therefore more dramatically observed in young people characterized not only by their lateness but also by their strict and fixed school schedules [4]. Though there are no previous reports of chronobiological evaluations of this population, it is predictable that Uruguayan teenagers and young adults are subject to a strong social jetlag. With morning class schedules and late entertainment habits, Uruguayan youth seem to be a very advantageous population for the study of the impact of extreme swings in their sleep schedule.
During summers and winters in Antarctica, the most extreme and isolated continent on earth, the human circadian clock cannot rely on changes of photoperiod and temperature to entrain the clock day after day, and circadian rhythms might tend to free run [17]. Sleep disorders reported in Antarctic crewmembers are probably also related to this dysregulation of the external time cues [18], [19], [20], [21], [22], [23]. However, it has been hard to establish to what extent these disorders respond to the external environment rather than to the strictness of social cues and the conditions of isolation inherent to living in an Antarctic base station [24], [25].
In 2014, the School of Sciences (Facultad de Ciencias, Universidad de la República, Uruguay) organized the First Uruguayan Summer School on Introduction to Antarctic Research with the participation of 17 undergraduate students. In this study, which constitutes the first attempt to perform a chronobiological characterization in Uruguay, we evaluated the chronotypes and social jetlag of this sample of university students, and confirmed the extreme lateness of the study population as well as a strong social jetlag. Further, we analyzed the impact of the trip to Antarctica on their sleep habits and sleep quality.
2. Method
2.1. Participants
Seventeen healthy students (6 males, 11 females) from the Facultad de Ciencias, Universidad de la República, Uruguay, were selected to participate in the First Uruguayan Summer School on Introduction to Antarctic Research held from February 4 to 14, 2014 in the Uruguayan Antarctic Scientific Base Artigas, King Georges Island (62° 11’ S; 58° 52’ W). The five week long study was performed from January 21 to February 24, 2014, and was divided into three phases: pre-Antarctic (15 days before departure); Antarctic (9 days); and post-Antarctic (11 days after return). All participants were clinically assessed in order to ensure they met the required health conditions. The mean age of the participants was 23.12 years (ranging from 21 to 26 years); 16 out of 17 were normal weight adults (average BMI=22.56, ranging from 18.58 to 27.37); none showed sleep disturbances or signs of depression (Beck Depression Inventory score <10, [26]).
All procedures were approved by the ethics committee at the Hospital de Clínicas de Porto Alegre, Universidad Federal de Rio Grande do Sul, Brazil and at the Instituto de Investigaciones Biológicas Clemente Estable, Ministerio de Educación y Cultura, Uruguay (CEP/HCPA 14-0057). All participants signed an informed consent form stating that they have been told about: the aims and procedures of the study, their right to end participation without any explanatory statement at any time, their data being coded so that data evaluation could occur on an anonymous basis, and their data being communicated for scientific purposes only.
2.2. Instruments
2.2.1. Munich Chronotype Questionnaire (MCTQ)
Chronobiological parameters were assessed using the Spanish version of the Munich Chronotype Questionnaire – MCTQ [4], available on line at www.bioinfo.mpg.de/mctq/core_work_life/core/introduction.jsp?language=esp. The questionnaires were answered individually by all participants during the first day of the study (January 21, 2014). The MCTQ was used to assess the midsleep phase and sleep duration for both work (MSW, SDW, respectively) and free days (MSF, SDF, respectively) as shown in Table 1 [4], [27]. To avoid the effect of sleep debt accumulated over the workweek on midsleep phase estimates, we calculated the midsleep point sleep-corrected (MSFsc, MSF corrected for sleep debt on work days), which was used as a reliable proxy for chronotype [11], [27]. The social jetlag was calculated as the value of the difference between MSF and MSW [16].
Table 1.
Chronobiological characterization of students of the First Summer School on Introduction to Antarctic Research (n=17) obtained by Munich Chronotype Questionnaire (MCTQ) and sleep logs (SL).
| MCTQ |
SL (Sleep logs) |
|||||
|---|---|---|---|---|---|---|
| range | mean±SD | p* | range | mean±SD | p* | |
| MSFsc | 4.19–9.50 | 6.05±1.55 | 4.50–8.50 | 6.11±1.27 | ||
| MSF | 4.54–9.50 | 6.36±1.41 | 0.0004 | 4.47–9.35 | 6.64±1.40 | 0.0003 |
| MSW | 3.13–7.92 | 4.38±1.27 | 3.29–5.86 | 4.73±0.75 | ||
| SDF | 6.92–10.75 | 8.47±1.09 | 0.05 | 4.96–8.76 | 7.55±1.01 | 0.0003 |
| SDW | 5.75–8.92 | 7.69±0.86 | 3.83–7.08 | 5.44±0.89 | ||
Wilcoxon Matched-Pairs test.
2.2.2. Sleep logs (SL)
Participants were instructed to fill in sleep logs (SL) every morning after getting up for the whole study period in each one of the following phases: pre-Antarctic (15 days before departure, January 21–February 4); Antarctic (9 days, February 5-14); and post-Antarctic (10 days after return, February 15–24). Though students were in summer break before departure, we were able to identify working days (as the days they were attending the pre-Antarctic course from 9 a.m. to 4 p.m., January 21–24) and free days (weekends, January 25–26, February 1–2). As described above for the data analysis of the MCTQ, the following items (shown in Table 1) were extracted from the SL as the average values of the selected days during the pre-Antarctic phase: midsleep point and sleep duration for both work (MSW, SDW, respectively) and free days (MSF, SDF, respectively). We also calculated the social jetlag from the SL data as the value of the difference between MSF and MSW.
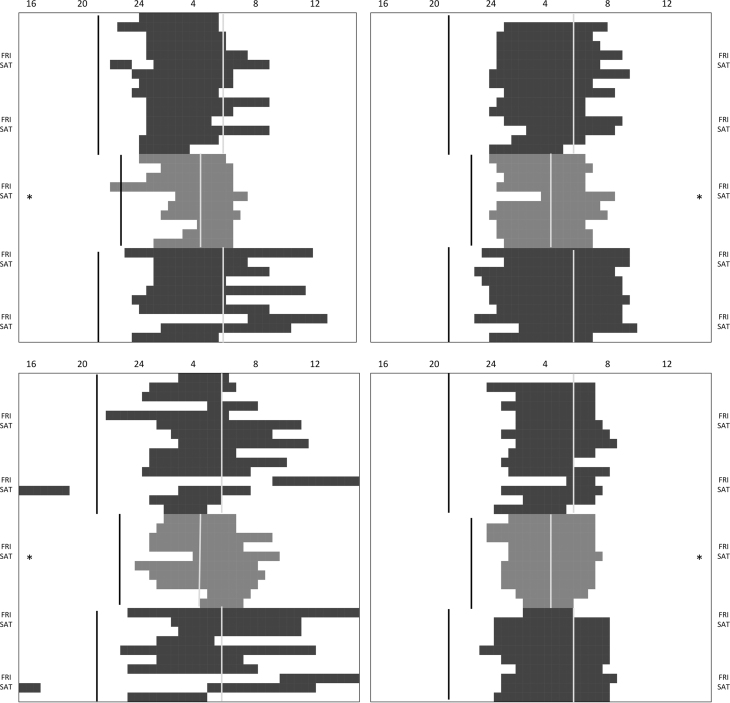
Sleep log data were used to assess the impact of the trip to Antarctica. Raw data were used to build individual 35-day-long sleep charts as shown for 4 participants in Fig. 2. To evaluate the effect of the trip on sleep duration, midsleep point, waking time, sleep onset and phase angle between sunset and bedtime, we used the average of the records of each participant in each phase (pre-Antarctic, Antarctic, and post-Antarctic), excluding the transition days (trip to and from Antarctica, change of color pattern in Fig. 2, February 4–5 and February 14) and the day distorted by the celebration of a nocturnal social event (Antarctic party, February 9) to avoid the effect of imposed exogenous predicted distortions affecting all participants (* in Fig. 2). We also sleep logs were also used to assess subjective sleep quality (0=slept very badly; 10=slept very well).
Fig. 2.
Sleep (dark bars) of 4 representative subjects of this study across the 3 phases (5 weeks): pre-Antarctic (black bars), Antarctic (gray bars) and post-Antarctic (black bars). Weekend nights are indicated at the sides of the graphs (FRI/SAT). * indicates the night of the Antarctic party. Vertical lines indicates darkness onset (black line) and its end (light gray line). Note photoperiod differences between phases (pre and post-Antarctic vs Antarctic).
2.3. Statistical analysis
Data are expressed as mean values±standard deviation throughout the text, and represented in figures using box plots to fully display the data. Values of p≤0.05 were considered statistically significant. Although some of the variables showed normal distribution, none of them was homoscedastic. For this reason, we used non-parametric tests: Wilcoxon Matched-Pairs test for free days vs work days comparisons of chronobiological variables (Table 1), and to compare sleep quality between the pre- and Antarctic phases; Mann–Whitney U test for comparisons between males and females; and Kruskal–Wallis one-way analysis of variance by ranks (Fig. 3). To test the data variability between individuals and among phases, the variance of all parameters was evaluated by one-way analysis of variance (ANOVA). Prior to the test, and to avoid the violation of homocedasticity, all variables were normalized with a Natural log transform.
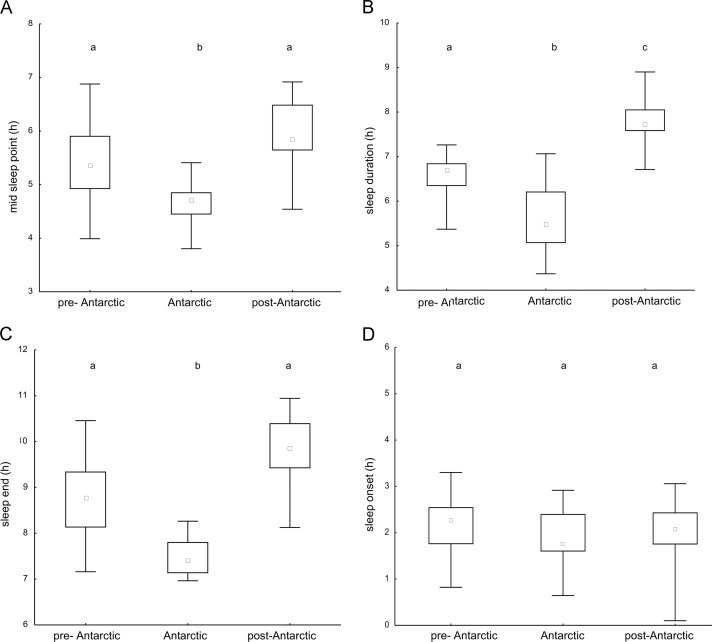
Fig. 3.
Antarctic impact on chronobiological parameters: midsleep point (A), sleep duration (B), waking time (C), and sleep onset (D). Box plot representation of each parameter compared among the three phases (pre-Antarctic, Antarctic, and post-Antarctic) through Kruskal–Wallis one-way analysis of variance by ranks and multiple comparison of mean ranks for all groups (middle point: median; box value: percentiles 25–75%; whisker value: minimum–maximum). The post-hoc test is presented above the box values using the letters “a”, “b” or “c” to represent the significant differences for each phase.
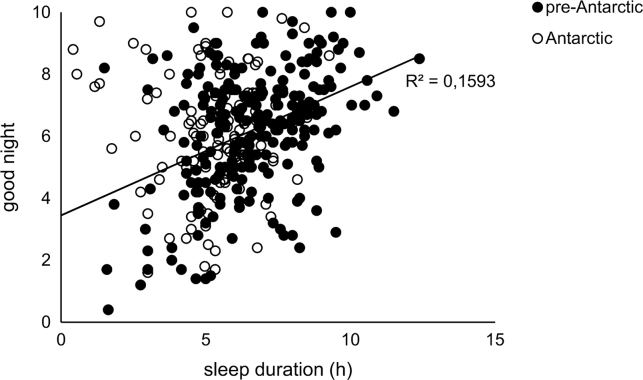
To correlate subjective perceptions of sleep quality and drowsiness with respect to sleep duration, we used all the values reported by the 17 participants for the pre-Antarctic and Antarctic phases (Fig. 4).
Fig. 4.
Linear regression between subjective perceptions of sleep quality (good night) and sleep durations. Data obtained from visual analog scales of SL. Black circles represent pre-Antarctic phase and white circles represent Antarctic phase. The predictive power of sleep duration on sleep quality can only be observed during the pre-Antarctic phase (black circles, full regression line, n=17 participants, p<0.05) but not in the Antarctic phase (white circles, n=17 participants, p>0.05).
3. Results
3.1. Characterization of the population
The chronobiological characterization of the study population is shown in Table 1. The MSFsc values obtained for this population were extremely late (mean 06.05 h±1.55) indicating that this population mainly includes individuals with extremely late chronotypes. Both the midsleep point and the sleep duration were significantly different between working days and free days, with data taken either from MCTQ or SL (* in Table 1). No significant correlations were found between the data obtained from the two instruments for any of the parameters (simple regression analysis MCTQ vs SL, p>0.05). However, similar data were obtained for midsleep parameters among instruments (MSFsc_MCTQ vs MSFsc_SL, p=0.59; MSF_MCTQ vs MSF_SL, p=0.36; MSW_MCTQ vs MSW_SL, p=0.16; Wilcoxon Matched-Pairs test). With respect to sleep duration values, SDF was significantly different from SDW when compared by both MCTQ and SL (* in Table 1). Furthermore, as we found no statistically significant difference in MSFsc between genders (Mann–Whitney U test, Z=0.201, p=0.840, nmen=6, nwomen=11), we pooled and averaged male and female data throughout.
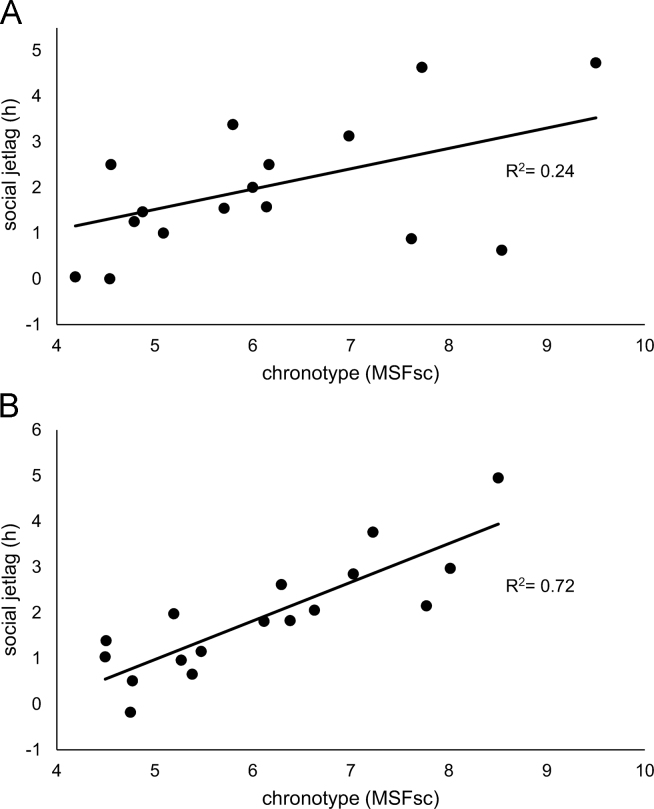
Social jetlag, the discrepancy between work and free days, was on average 1.98±1.4 h when measured by MCTQ and 1.91±1.27 h if measured by SL. Interestingly, social jetlag exhibited a wide range from 0 to 4.73 and −0.18 to 4.95, when measured by MCTQ and SL, respectively. As shown in Fig. 1, we found a positive correlation between MSFsc and social jetlag (R2=0.243, p=0.04, for MCTQ, Fig. 1A; R2=0.721, p=0.00002, for SL, Fig. 1B), confirming that late chronotypes generate a higher sleep debt during weekdays.
Fig. 1.
Linear regressions between social jet lag (SJL) and midsleep point on free days corrected for sleep debt on work days (MSFsc) obtained from Munich Chronotype Questionnaire (MCTQ) (A) and sleep logs (B) data. Both instruments show the predictive power of chronotype proxy on SJL (n=17 participants, p<0.05).
3.2. Antarctic impact
3.2.1. Longitudinal analysis of sleep
Daily profiles of the sleep–wake cycle of 4 representative individuals are illustrated in Fig. 2 during the 3 phases of the study: pre-Antarctic (dark gray), Antarctic (light gray), and post-Antarctic (dark gray). It is interesting to note the lateness of this population in the pre-Antarctic phase, as these four individuals rarely slept before midnight. Though the regularity of sleep habits was extremely variable among individuals, it is clear that the Antarctic phase represented a more synchronized period in which sleep duration clearly decreased.
The impact of the trip to Antarctica on the sleep habits of the whole study sample is shown in Fig. 3. Both, the midsleep point and sleep duration significantly decreased from the pre-Antarctic phase to the Antarctic one, and rebounded to significantly higher values in the post-Antarctic phase (Kruskal–Wallis test, midsleep point: H=20.33 p<0.001, Fig. 3A; sleep duration: H=36.77 p<0.001, Fig. 3B). As part of the base station activities and academic requirements, all of the students had a strict fixed schedule during the Antarctic phase that imposed a waking time at around 7AM. Consequently, the end of sleep varied significantly among phases from 08.76±0.89 h in the pre-Antarctic phase to 07.48±0.40 h in the Antarctic phase, and 09.80±0.78 h in the post-Antarctic phase (Kruskal–Wallis test, H=32.6 p<0.001, Fig. 3C). Darkness occurred at different times in Uruguay (approximately at 21:15) and in King Georges Island (approximately at 23:00) during the austral summer. Interestingly, no significant differences were found in the time of sleeping onset among phases (02.10±0.70 h, 1.89±0.64 h, 02.03±0.70 h in the pre-Antarctic, Antarctic, and post-Antarctic phases, respectively; Kruskal–Wallis test, H=1.20, p=0.54, Fig. 3D). The variance of all the variables decreased significantly during the Antarctic phase, indicating that the school acted as a potent synchronizer of sleep habits among its participants (ANOVA, midsleep point F=16.32 p<0.001; sleep duration F=14.38 p<0.0001; sleep onset F=5.24 p=0.008; sleep end F=59.94 p<0.0001). Further, the phase angle between sunset and sleep onset in Antarctic phase (2.89±0.64) exhibited significant differences between pre- and post-Antarctic phases (4.94±0.70 and 4.78±0.70, respectively) (Kruskal–Wallis test, H=30.7 p<0.001).
3.2.2. Subjective perception
The subjective perception of the quality of sleep that was recorded by each participant did not vary between the pre- and the Antarctic phases (good night pre- vs good night Antarctic p=0.55; Wilcoxon Matched-Pairs test). During the pre-Antarctic period, the subjective perception of sleep quality correlated positively with sleep duration (R2=0.159 p<0.001); however, this correlation disappeared during the stay in Antarctica (good night vs sleep duration, p=0.95; Fig.4).
4. Discussion
This study presents the first characterization of chronobiological parameters from a sample of Uruguayan people. We focused on a very homogeneous population: healthy young adults, attending the same semester at the same University, very restricted in age, within normal BMI, and without signs of depression or other pathologies. We corrected individual MSF for sleep debt, and used MSFsc as the best chronotype proxy, following Roenneberg et al. [11]. Age and gender differences in MSFsc have been previously reported in epidemiological studies in Europe [11], [12], [27]. We found no statistically significant difference in MSFsc between males and females, probably due to the small size of our sample. Similarly, we were unable to discriminate an effect of age due to the small age window (21–26 y/o). We found no correlation between the MCTQ and SL for any of the sleep parameters analyzed for the pre-Antarctic phase in this study, which was unexpected given the high education level and motivation of the participants to be engaged in the First Uruguayan Summer School on Introduction to Antarctic Research. Discrepancy among instruments has been previously reported [14], [28] and was always interpreted as due to inadequate completion of forms, particularly of SL. However, the participants in this study were highly-committed university students and their accuracy and responsibility in filling SL forms was supervised by the researcher team during the study period. Though MCTQ has been validated worldwide, time of-year-dependencies in subjective assessment of sleep wake times have been identified recently [29]. We can thus interpret that pre-Antarctica SL data might have been biased by the fact that students have more relaxed sleep habits during the summertime. In the future, we plan to expand this study to a larger population of students, and to repeat this characterization during the school year.
4.1. Chronobiological characterization
Diverse populations of university students have been chronobiologically characterized across the world, mainly correlating sleep habits with academic performance [7], [9], [14], [30], [31], [32], [33], [34], [35], [36]. In comparison to previous descriptions, our study population is extremely late, with the MSFsc values presented in this study being the highest ever reported. Our population exhibited its midsleep point around 1.5 h later than different European university students from Switzerland [37], Italy and Spain [38], Turkey [13], Hungary [15], Germany [14] and the Netherlands [39]. As sleep habits are profoundly influenced by the cultural environment [35], [40], [41], [42], [43], and the Uruguayan population is mostly of European descent, European chronobiological characterizations of undergraduate students serve as good reference. We were unable to compare the chronobiological traits of our study population with more closely related cultures such as Latin American university students, because they have been rarely studied [44], [45]. When compared to adolescent students from Mexico [46] and Brazil [47], expected to be more oriented toward eveningness than our study population, Uruguayan university students still show a later preference. Though this study cannot attempt to be a representative characterization of sleep habits of Uruguayan university students (as it involves less than 20 students), its extreme results point out the interest of expanding this preliminary characterization in the future.
It is well-known that chronotypes depend on age and gender [10], [41], [48]. Latest chronotypes are observed in twenty years old persons (midsleep around 05.5 h in males and 04.5 h in females) who change their sleep timing preference towards morningness later in life [9], [11], [12]. This shift in the midsleep point is not observed in our study participants, who exhibit later chronotypes than expected for their age. Moreover, these university students, who are on average 3 years older than the end of chronobiological adolescence, are even more oriented to eveningness than typical 20 years old youths.
As previously reported in several studies [30], [33], [37], [39], we confirmed that the sleep duration of weekdays was shorter than on weekends. Interestingly, students evaluated in this study sleep less than their own estimation (MCTQ vs SL), and report insufficient sleep duration during work days (<6 h, Table 1), indicating the generation of an eventually troublesome sleep debt, whose consequences have not been evaluated so far.
The trade-off between working and entertainment schedules interferes with individual sleep preferences, generating a discrepancy between biological and social time (social jetlag [16]). This study population shows a strong social jetlag of around 2 h with a wide range from 0 to almost 5 h. This is the largest average social jetlag reported in comparable university student populations [15], [32], though even higher social jetlag has been found in European adolescents [49]. Noteworthy, values above 5 h indicate a strong misalignment that has only been previously reported in nocturnal shift-workers whom are known to have their sleep habits completely distorted [50]. The constraints of early work schedules during weekdays are more likely to affect late chronotypes than early ones, leading evening-types to an increasing sleep debt over the week that is compensated for on weekends [16]. Therefore, as predicted, in this study we confirmed a positive correlation of social jetlag with chronotype, especially strong from data of SL, in which the predictive power of MSFsc on social jetlag exceeded 70%.
4.2. Antarctic impact on sleep habits
The impact of the Antarctic extreme conditions of photoperiod and temperature on sleep habits has been previously reported in several long-term studies mainly focused on crewmembers of different bases [18], [19], [20], [21], [22], [23], [25]. With the lack of cycling environmental cues during Antarctic summers and winters, circadian rhythms tend to free-run, and this disturbance, together with the social isolation and strictness of life conditions in Antarctic bases, are key factors in the high incidence of sleep disturbances in this particular population. There are scarce data on the impact of Antarctic conditions on short-trip field expeditions. For example, Weymouth and Steel [24] found no indication of sleep disturbances at the group level in 14 volunteers that traveled to the Antarctic summer and spent several days both in base and field camps, although individual differences varied markedly. To our knowledge, this is the first longitudinal study to evaluate acute Antarctic impact on a very homogeneous population followed across 3 phases: pre-Antarctic, Antarctic, and post-Antarctic. Despite the individual variability and the small sample size, our data suggest that social and academic schedules prevailed as the most important factors involved in the sleep habit changes caused by the trip to Antarctica. Moreover, preliminary data from actimetry records (unpublished data) showed that actual light exposure of participants was not different between pre-Antarctic and Antarctic phases.
The very diverse sleep records of this young population of university students on summer break became highly synchronized during the Antarctic trip (Fig. 2) implying a tight agenda of both social and academic schedules. Because of the adjustment of the study population to this fixed timetable, we observed a significantly earlier midsleep point in the Antarctic phase with respect to both pre- and post-Antarctic phases (Fig. 3A). The synchronization in sleep time among students during the Antarctic phase can also be inferred from the decrease of the dispersion of midsleep point values observed in this period (Fig. 3A). More strikingly, sleep duration significantly decreased from the pre-Antarctic to the Antarctic phase, and returned to even higher levels in the post-Antarctic phase, indicating the strong sleep debt that the Antarctic trip created for the students (Fig. 3B). Interestingly, these changes in sleep duration depended more heavily on an earlier waking time rather than on a delay of sleep onset (Fig. 3C and D). If sleep onset was controlled by sunset (darkness), we would expect a swing in sleep onset to later times during the Antarctic phase and a constant phase angle between sunset and bedtime across phases. In contrast, sleep onset did not change among phases and phase angle decreased during the Antarctic phase. We can therefore conclude that sunset was not predictive of sleeping time in any of the phases. Sleep schedules more likely depended on social agenda than on the environmental shifts of the light–dark cycle. Despite this dramatic decrease in sleep duration during the Antarctic trip, the First Uruguayan Summer School on Introduction to Antarctic Research represented a highly motivating activity for its students. This observation stands on two pieces of evidence: first, the subjective perception of sleep quality did not vary between the pre-Antarctic and Antarctic phases; and further, the perception of a good night that positively correlated with sleep duration during the pre-Antarctic phase, no longer correlated during the Antarctic trip, even though sleep duration was less during this period. In short, the subjective perception of students’ welfare in Antarctica emerged as not dependent on sleep duration.
Acknowledgments
We wish to thank the 17 students of the First Summer School on Introduction to Antarctic Research, who kindly volunteered as participants of this study, in particular Natalia Martino who helped in data acquisition and preliminary data processing. We are especially thankful to Juan Cristina, Director of the First Summer School on Introduction to Antarctic Research, its organizers and the Unidad de Estudios Antárticos, Facultad de Ciencias, Universidad de la República, its sponsors, and the Instituto Antártico Uruguayo and Laboratório de Cronobiologia, Hospital de Clinicas de Porto Alegre, Universidad Federal de Rio Grande do Sul for providing the logistics and support to carry out this study. We also need to thank Adriana Migliaro for her generous revision and suggestions to our manuscript, Daniel Naya for his help in statistics, and Gene McGinnis for the extensive English editing of our manuscript.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Golombek D., Rosenstein R. Physiol Circadian Entrain. 2010:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 2.Roenneberg T., Kantermann T., Juda M., Vetter C., Allebrandt K.V. Light and the human circadian clock. Handb Exp Pharmacol. 2013;217:311–331. doi: 10.1007/978-3-642-25950-0_13. [DOI] [PubMed] [Google Scholar]
- 3.Kronfeld-Schor N., Bloch G., Schwartz W.J. Animal clocks: when science meets nature. Proc. Biol. Sci. 2013;280(2013):1354. doi: 10.1098/rspb.2013.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythm. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 5.Roenneberg T., Kuehnle T., Juda M., Kantermann T., Allebrandt K., Gordijn M. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Wright K.P., McHill A.W., Birks B.R., Griffin B.R., Rusterholz T., Chinoy E.D. Entrainment of the human circadian clock to the natural light–dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adan A., Natale V. Gender differences in morningness–eveningness preference. Chronobiol Int. 2002;19:709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- 8.Roenneberg T., Keller L.K., Fischer D., Matera J.L., Vetter C., Winnebeck E.C. Human activity and rest in situ. Methods Enzymol. 2015;552:257–283. doi: 10.1016/bs.mie.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Randler C., Vollmer C., Beşoluk Ş., Önder İ., Horzum M.B. Age and gender differences in morningness–eveningness in Turkish adolescents and young adults. Biol Rhythm Res. 2013:1–8. [Google Scholar]
- 10.Randler C. Gender differences in morningness-eveningness assessed by self-report questionnaires: a meta-analysis. Personal Individ Differ. 2007;43:1667–1675. [Google Scholar]
- 11.Roenneberg T., Kuehnle T., Pramstaller P.P., Ricken J., Havel M., Guth A. A marker for the end of adolescence. Curr. Biol. 2004;14:1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Tonetti L., Fabbri M., Natale V. Sex difference in sleep-time preference and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol Int. 2008;25:745–759. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- 13.Beşoluk S., Onder I., Deveci I. Morningness–eveningness preferences and academic achievement of university students. Chronobiol Int. 2011;28:118–125. doi: 10.3109/07420528.2010.540729. [DOI] [PubMed] [Google Scholar]
- 14.Genzel L., Ahrberg K., Roselli C., Niedermaier S., Steiger a, Dresler M. Sleep timing is more important than sleep length or quality for medical school performance. Chronobiol Int. 2013;30:766–771. doi: 10.3109/07420528.2012.763132. [DOI] [PubMed] [Google Scholar]
- 15.Haraszti R.Á., Ella K., Gyöngyösi N., Roenneberg T., Káldi K. Social jetlag negatively correlates with academic performance in undergraduates. Chronobiol Int. 2014:1–10. doi: 10.3109/07420528.2013.879164. [DOI] [PubMed] [Google Scholar]
- 16.Wittmann M., Dinich J., Merrow M., Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 17.Kennaway D.J., Van Dorp C.F. Free-running rhythms of melatonin, cortisol, electrolytes, and sleep in humans in Antarctica. Am J Physiol. 1991;260:R1137–R1144. doi: 10.1152/ajpregu.1991.260.6.R1137. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya M., Pal M.S., Sharma Y.K., Majumdar D. Changes in sleep patterns during prolonged stays in Antarctica. Int J Biometeorol. 2008;52:869–879. doi: 10.1007/s00484-008-0183-2. [DOI] [PubMed] [Google Scholar]
- 19.Buguet A., Rivolier J., Jouvet M. Human sleep patterns in Antarctica. Sleep. 1987;10:374–382. doi: 10.1093/sleep/10.4.374. [DOI] [PubMed] [Google Scholar]
- 20.Francis G., Bishop L., Luke C., Middleton B., Williams P., Arendt J. Sleep during the Antarctic winter: preliminary observations on changing the spectral composition of artificial light. J Sleep Res. 2008;17:354–360. doi: 10.1111/j.1365-2869.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 21.Gander P.H., Macdonald J.A., Montgomery J.C., Paulin M.G. Adaptation of sleep and circadian rhythms to the Antarctic summer: a question of zeitgeber strength. Aviat Space Environ Med. 1991;62:1019–1025. [PubMed] [Google Scholar]
- 22.Premkumar M., Sable T., Dhanwal D., Dewan R. Circadian levels of serum melatonin and cortisol in relation to changes in mood, sleep, and neurocognitive performance, spanning a year of residence in Antarctica. Neurosci. J. 2013;2013:1–10. doi: 10.1155/2013/254090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usui A., Obinata I., Ishizuka Y. Seasonal changes in human sleep – wake rhythm in Antarctica and Japan. Psychiatry Clin. Neurosci. 2000:361–362. doi: 10.1046/j.1440-1819.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- 24.Weymouth W., Steel G.D. Sleep patterns during an antarctic field expedition. Mil Med. 2013;178:438–444. doi: 10.7205/MILMED-D-12-00447. [DOI] [PubMed] [Google Scholar]
- 25.Yoneyama S., Hashimoto S., Honma K. Seasonal changes of human circadian rhythms in Antarctica. Am J Physiol. 1999;277:R1091–R1097. doi: 10.1152/ajpregu.1999.277.4.R1091. [DOI] [PubMed] [Google Scholar]
- 26.Beck A.T., Steer R.A., Carbin M.G. Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 27.Roenneberg T., Merrow M. Merrow Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:293–299. doi: 10.1101/sqb.2007.72.043. [DOI] [PubMed] [Google Scholar]
- 28.Bohm S. Sleep and chronotype in adolescents – a chronobiological field-study; 2012.
- 29.Allebrandt K.V., Teder-Laving M., Kantermann T., Peters A., Campbell H., Rudan I. Chronotype and sleep duration: the influence of season of assessment. Chronobiol Int. 2014;31:731–740. doi: 10.3109/07420528.2014.901347. [DOI] [PubMed] [Google Scholar]
- 30.Kabrita C.S., Hajjar-Muça T. a, Duffy J.F. Predictors of poor sleep quality among Lebanese university students: association between evening typology, lifestyle behaviors, and sleep habits. Nat Sci Sleep. 2014;6:11–18. doi: 10.2147/NSS.S55538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund H.G., Reider B.D., Whiting A.B., Prichard J.R. Sleep Patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Heal. 2010;46:124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Önder İ., Beşoluk Ş., İskender M., Masal E., Demirhan E. Circadian preferences, sleep quality and sleep patterns, personality, academic motivation and academic achievement of university students. Learn Individ Differ. 2014;32:184–192. [Google Scholar]
- 33.Onyper S.V., Thacher P.V., Gilbert J.W., Times Gradess S.G. Class Start. Sleep, and Academic performance in college: a path analysis. Chronobiol Int. 2012;29:318–335. doi: 10.3109/07420528.2012.655868. [DOI] [PubMed] [Google Scholar]
- 34.Smith C.S., Folkard S., Schmieder R. a, Parra L.F., Spelten E., Almiral H. Investigation of morning-evening orientation in six countries using the preferences scale. Personal Individ Differ. 2002;32:949–968. [Google Scholar]
- 35.Tonetti L., Sahu S., Natale V. Circadian preference in Italy and India: a comparative study in young adults. Personal Individ Differ. 2012;53:355–358. [Google Scholar]
- 36.Tsai L.L., Li S.P. Sleep patterns in college students: gender and grade differences. J Psychosom Res. 2004;56:231–237. doi: 10.1016/S0022-3999(03)00507-5. [DOI] [PubMed] [Google Scholar]
- 37.Urner M., Tornic J., Bloch K.E. Sleep patterns in high school and university students: a longitudinal study. Chronobiol Int. 2009;26:1222–1234. doi: 10.3109/07420520903244600. [DOI] [PubMed] [Google Scholar]
- 38.Natale V., Adan A., Fabbri M. Season of birth, gender, and social-cultural effects on sleep timing preferences in humans. Sleep. 2009;32:423–426. doi: 10.1093/sleep/32.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zavada A., Gordijn M.C.M., Beersma D.G.M., Daan S., Roenneberg T. Comparison of the Munich chronotype questionnaire with the Horne–Ostberg’s morningness–eveningness score. Chronobiol Int. 2005;22:267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]
- 40.Caci H., Adan A., Bohle P., Natale V., Pornpitakpan C., Tilley A. Transcultural properties of the composite scale of morningness: the relevance of the “morning affect” factor. Chronobiol Int. 2005;22:523–540. doi: 10.1081/CBI-200062401. [DOI] [PubMed] [Google Scholar]
- 41.Paine S.-J., Gander P.H., Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years) J Biol Rhythm. 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- 42.Randler C., Díaz-Morales J.F. Morningness in German and Spanish students: a comparative study. Eur J Personal. 2007;21:419–427. [Google Scholar]
- 43.Randler C. Morningness-eveningness comparison in adolescents from different countries around the world. Chronobiol Int. 2008;25:1017–1028. doi: 10.1080/07420520802551519. [DOI] [PubMed] [Google Scholar]
- 44.Alam M.F., Tomasi E., De Lima M.S., Areas R., Menna-Barreto L. Caracterização e distribuição de cronotipos no sul do Brasil: Diferenças de gênero e estação de nascimento. J Bras Psiquiatr. 2008;57:83–90. [Google Scholar]
- 45.Rique G.L.N., Fernandes Filho G.M.C., Ferreira A.D.C., de Sousa-Muñoz R.L. Relationship between chronotype and quality of sleep in medical students at the Federal University of Paraiba, Brazil. Sleep Sci. 2014;7:96–102. doi: 10.1016/j.slsci.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrona-Palacios A., García A., Valdez P. Sleep–wake habits and circadian preference in Mexican secondary school. Sleep Med. 2015 doi: 10.1016/j.sleep.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 47.de Souza C.M., Hidalgo M.P.L. Midpoint of sleep on school days is associated with depression among adolescents. Chronobiol Int. 2014;31:199–205. doi: 10.3109/07420528.2013.838575. [DOI] [PubMed] [Google Scholar]
- 48.Roenneberg T., Kuehnle T., Juda M., Kantermann T., Allebrandt K., Gordijn M. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 49.van der Vinne V., Zerbini G., Siersema A., Pieper A., Merrow M., Hut R.A. Timing of examinations affects school performance differently in early and late chronotypes. J Biol Rhythm. 2015;30:53–60. doi: 10.1177/0748730414564786. [DOI] [PubMed] [Google Scholar]
- 50.Juda M., Vetter C., Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythm. 2013;28:141–151. doi: 10.1177/0748730412475042. [DOI] [PubMed] [Google Scholar]