Abstract
Objective
The levels of leptin, a major regulator of lipid metabolism, may increase in obesity, and contribute to the development of metabolic syndrome. Leptin is produced by adipose tissue and is a peptide hormone, which has strong association with obesity, elevated cardiovascular risk, and morbidity. The present study was designed to evaluate the relationships between leptin levels, obesity, and cardiovascular risk factors in men with acute myocardial infarction.
Methods and results
Twenty-four obese and twenty-three nonobese male patients, who had experienced their first myocardial infarction, were included in the study. Their leptin levels, biochemical parameters, and anthropometric measures were obtained. Mean leptin levels were significantly higher in the obese group compared to the nonobese group (2.53 ng/mL versus 1.23 ng/mL; p < 0.01). Leptin levels correlated positively with anthropometric measurements, triglyceride, fasting glucose, C-reactive protein, and uric acid levels, and negatively with high-density lipoprotein cholesterol levels.
Conclusion
Findings indicate high leptin levels to be positively correlated with obesity and diastolic blood pressure in male patients with myocardial infarction.
Keywords: Leptin, Obesity, Anthropometric measurements, Myocardial infarction
1. Introduction
Obesity is associated with a high risk of developing cardiovascular (CV) and metabolic diseases, such as hypertension, coronary atherosclerosis, myocardial hypertrophy, diabetes, dyslipidemia, and increased CV morbidity and mortality.1 Leptin is a 16-kDa polypeptide (167 amino acids) hormone synthesized and secreted into the circulation primarily by white adipocytes.2, 3 There is a strong relationship between body fat mass and the amount of synthesized and secreted leptin.4, 5 Leptin is considered to be an antiobesity hormone. The first major action of leptin to be described is the control of body weight and fat deposition through its effects on hypothalamic receptors, which leads to appetite inhibition, as well as its effects on metabolic rate stimulation and thermogenesis.2, 3 Leptin levels decrease during fasting and increase after several days of overfeeding, in a mechanism that helps to regulate energy balance in humans. Thus, increased leptin concentrations would be expected to correlate with decreased weight. However, serum leptin levels are in fact strongly correlated with body fat mass in obese individuals. Recent studies suggest the existence of an endogenous leptin-resistance mechanism in obesity6 that may explain this unexpected correlation.
In obesity, elevated leptin levels are not sufficient to prevent disturbances in energy balance, suggesting that obese people are leptin-resistant.7 Alternatively, another possibility is that nonleptin-mediated mechanisms play a more powerful role than leptin under the altered physiological conditions present in obesity. Obesity is associated with increased sympathetic nerve activity, and leptin, partly by increasing renal sympathetic nerve activity, has been proven to participate in autonomic nervous system control. Therefore, the contribution of leptin to sympathetic activation in a leptin-resistant state, like obesity, is contradictory. This has led to the novel concept of selective leptin resistance, in which resistance appears to be primarily limited to the metabolic (satiety and weight-reducing) functions of leptin, sparing the other functions in obese individuals.8
It has been suggested that leptin could be an important link between obesity and the development of CV diseases.9 This might be mediated through various effects of leptin, including effects on blood pressure,10 platelet aggregation,11 formation of arterial thrombosis,12 and inflammatory vascular response.13 High levels of leptin are believed to be associated with reduced arterial distensibility, an index of circulatory function, and leptin is involved in the pathogenesis of the atherosclerotic process through mechanisms other than vascular relaxation.14 Leptin has also been shown to promote angiogenesis, regulate osteoblastic differentiation, enhance the calcification of vascular cells, and potentiate prothrombotic platelet aggregation through a novel leptin receptor mechanism.11
Several studies have shown leptin to be a predictor of myocardial infarction, coronary events, and stroke, independent of body mass index (BMI).15, 16 Plasma leptin was observed to be higher in individuals with a paternal history of premature myocardial infarction than in those without family history of CV events.17 Plasma leptin was also higher in male patients, who subsequently developed first-ever myocardial infarction than in control subjects.16
The aim of the present study was to assess the relationships between blood leptin concentrations and several anthropometric parameters and CV risk factors in obese and nonobese men with acute myocardial infarction (AMI) treated with primary coronary intervention (PCI).
2. Methods
2.1. Study population
The study subjects included 24 obese male patients and 23 age-matched nonobese men as the control group, who experienced their first acute myocardial infarction. Patients who had previous ischemic heart disease, atrial fibrillation, bundle branch block, and significant valvular heart disease, or who were >65 years of age, were excluded from the study.
Ethical approval was taken from local ethics committee.
Anthropometric measurements, clinical definitions, and treatment.
AMI was diagnosed on the basis of symptoms, electrocardiographic signs, and elevation of myocardial injury markers.
A BMI of 30, calculated as body weight divided by the square of height (kg/m2), was used as the cutoff value for obesity. Patients with a BMI <25 were designated as nonobese. Weight and height were measured on the third or fourth day after admission, while the subjects were fasting and wearing only their undergarments. Waist circumference (WC), a measure of abdominal subcutaneous and visceral fat, was measured at the widest diameter between the xiphoid process of the sternum and the iliac crest. Hip circumference (HC), representing subcutaneous fat alone, was measured at the widest diameter over the greater trochanters. From these measurements, the waist-to-hip ratio (WHR) was calculated. Systolic and diastolic blood pressures (SBP, DBP) were measured before blood sampling.
2.2. Laboratory measurements
C-reactive protein (CRP) and uric acid levels were assessed as part of a complex analysis of the blood samples taken upon admission to the hospital. Fasting blood glucose levels, lipid profile and leptin levels were determined from the blood drawn the following day. Plasma samples taken for leptin concentration measurement were frozen at −70 °C, until analysis with a sandwiched enzyme-linked immunosorbent assay (ELISA) (ELx 800 Absorbance Microplate Reader [BioTek, Winooski, USA] and Assay Max Human Leptin ELISA Kit [Assaypro, St. Charles, MO, USA]).
2.3. Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) for Windows (version 15.0; SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as mean ± standard deviation. Variables were log-transformed before statistical analysis, if necessary. Comparison between the two groups was performed using the two-tailed, nonpaired student's t-test or the Mann–Whitney test, as appropriate. Categorical variables were presented as numbers or percentages, and were analyzed using the chi-square test. Association between the parameters was assessed using the Spearman correlation coefficient. A p value <0.05 was considered statistically significant.
3. Results
The proportion of patients with hypertension and smoking were similar in the two groups, as were SBP levels. The proportion of patients with high-density-lipoprotein cholesterol (HDL-CH) <40 mg/dl, total CH >200 mg/dl, and triglycerides (TG) >150 mg/dl were significantly higher in the obese group than in the control group, as were the anthropometric measures (BMI, WC, HC, WHR) (p < 0.001). The clinical characteristics and the anthropometric measurements for the obese and nonobese patients are shown in Table 1.
Table 1.
Clinical characteristics and anthropometric measurements for the study participants.
| Obese [n = 24] | Nonobese [n = 23] | p value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age | 48.5 (11.4) | 55.8 (8.8) | 0.027 |
| n(%) | n(%) | ||
| Smoking | 19 (79.2) | 19 (82.6) | 1.000 |
| Hypertension | 15 (62.5) | 9 (39.1) | 0.109 |
| Mean (SD) | Mean (SD) | ||
| Systolic blood pressure (SBP) | 145.7 (26.4) | 134.7 (28.3) | 0.176 |
| Diastolic blood pressure (DBP) | 87.9 (17.6) | 71.4 (27.5) | 0.018 |
| n(%) | n(%) | ||
| Total cholesterol > 200 mg/dl | 14 (58.3) | 4 (17.4) | 0.004 |
| LDL cholesterol > 100 mg/dl | 17 (70.8) | 12 (52.2) | 0.188 |
| HDL cholesterol < 40 mg/dl | 15 (62.5) | 7 (30.4) | 0.028 |
| Triglycerides (TG) > 150 mg/dl | 17 (70.8) | 4 (17.4) | <0.001 |
| Mean (SD) | Mean (SD) | ||
| Body mass index (kg/m−2) | 31.5 (2.1) | 24.0 (1.3) | <0.001 |
| Waist circumference (cm) | 111.3 (13.7) | 91.7 (11.9) | <0.001 |
| Hip circumference (cm) | 106.9 (10.1) | 92.1 (15.7) | <0.001 |
| Waist-to-hip ratio | 1.04 (0.06) | 0.97 (0.05) | <0.001 |
In obese patients, all biochemical parameters were higher except fasting glucose and CRP levels. The mean values of the biochemical parameters assessed are presented in Table 2.
Table 2.
Biochemical parameters in the study groups.
| Obese [n = 24] | Nonobese [n = 23] | p | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Fasting glucose (mg/dl) | 167.5 (85.2) | 138.8 (58.7) | 0.142 |
| Total cholesterol (mg/dl) | 206.8 (48.3) | 160.4 (45.8) | 0.002 |
| LDL-cholesterol (mg/dl) | 135.9 (42.5) | 102.7 (38.0) | 0.007 |
| HDL-cholesterol (mg/dl) | 37.7 (5.7) | 44.9 (12.7) | 0.018 |
| Triglycerides (mg/dl) | 185.8 (68.2) | 98.7 (43.1) | <0.001 |
| C-reactive protein (mg/dl) | 26.6 (40.3) | 20.6 (22.1) | 0.941 |
| Uric acid (mg/dl) | 5.8 (1.4) | 4.5 (1.6) | 0.009 |
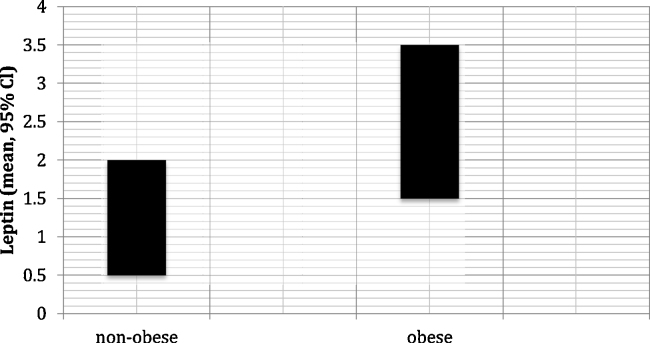
The mean leptin level in obese patients (2.53 ng/mL) was significantly higher than the mean leptin level in nonobese patients (1.23 ng/mL; p < 0.001) (Fig. 1). There was a positive correlation between leptin and the anthropometric measurements (Table 3).
Fig. 1.
Mean leptin values in obese and nonobese groups.
Table 3.
Correlation between leptin and anthropometric measurements.
| Anthropometric measurements | Leptin |
|
|---|---|---|
| rho | p | |
| BMI (kg/m2) | 0.527 | <0.001 |
| WC (cm) | 0.579 | <0.001 |
| HC (cm) | 0.430 | 0.003 |
| WHR | 0.633 | <0.001 |
The relationship of leptin and anthropometric measurements to obesity-related risk factors is shown in Table 4. Leptin was only positively correlated with DBP (p = 0.040, r = 0.301). There were also positive correlations between BMI and DBP, total CH, low-density-lipoprotein CH (LDL-CH), TG, and uric acid, whereas there was an inverse correlation between BMI and HDL-CH. In addition, WC and HC exhibited positive correlations with fasting blood glucose, total-CH, LDL-CH, and TG. Finally, WHR had a positive correlation with fasting blood glucose and TG.
Table 4.
The relationship of leptin and anthropometric measurements to obesity-related risk factors.
| Leptin | BMI (kg/m2) | WC (cm) | HC (cm) | WHR | |
|---|---|---|---|---|---|
| SBP (mmHg) | |||||
| rho | 0.166 | 0.137 | 0.041 | −0.002 | 0.008 |
| p | 0.264 | 0.357 | 0.785 | 0.988 | 0.959 |
| DBP (mmHg) | |||||
| rho | 0.301 | 0.307 | 0.151 | 0.111 | 0.143 |
| p | 0.040 | 0.036 | 0.310 | 0.457 | 0.337 |
| Fasting glucose (mg/dl) | |||||
| rho | 0.141 | 0.148 | 0.381 | 0.296 | 0.309 |
| p | 0.345 | 0.322 | 0.008 | 0.044 | 0.034 |
| Total CH (mg/dl) | |||||
| rho | 0.240 | 0.448 | 0.337 | 0.318 | 0.275 |
| p | 0.104 | 0.002 | 0.020 | 0.029 | 0.061 |
| LDL-CH (mg/dl) | |||||
| rho | 0.153 | 0.447 | 0.318 | 0.325 | 0.240 |
| p | 0.304 | 0.002 | 0.029 | 0.026 | 0.104 |
| HDL-CH (mg/dl) | |||||
| rho | −0.218 | −0.291 | −0.120 | −0.117 | −0.120 |
| p | 0.140 | 0.048 | 0.423 | 0.433 | 0.422 |
| TG (mg/dl) | |||||
| rho | 0.248 | 0.612 | 0.480 | 0.432 | 0.405 |
| p | 0.093 | 0.000 | 0.001 | 0.002 | 0.005 |
| CRP (mg/dl) | |||||
| rho | 0.092 | 0.078 | 0.169 | 0.225 | 0.054 |
| p | 0.538 | 0.600 | 0.255 | 0.128 | 0.720 |
| Uric acid (mg/dl) | |||||
| rho | 0.269 | 0.357 | 0.207 | 0.134 | 0.215 |
| p | 0.068 | 0.014 | 0.164 | 0.367 | 0.146 |
4. Discussion
The question of whether leptin, which has an important role in obesity etiology, has a direct or indirect effect on coronary artery disease has been previously studied.18 In the present study, we analyzed the relationship between leptin, CV risk factors, and anthropometric measures in 47 obese and nonobese males with AMI. The leptin levels of the obese group were found to be significantly higher than that of the nonobese group. In previous studies19 conducted on patients with coronary heart disease (CHD), leptin levels in obese patients were found to be higher than that of nonobese patients, as we also found in the present study. Since leptin levels may be affected by age, we included only patients <65 years of age, and the average age of the obese group was significantly lower than that of the nonobese group. This situation may indicate earlier development of CHD in the obese. In other studies, obesity has not been found to correlate with leptin levels in people over the age of 65.19, 20 As individuals age, leptin levels fall. Ostlund et al.5 found a negative correlation between age and leptin levels, independent of body fat, in patients between the ages of 18 and 80 (mean (SD) 52.8 (15.8) years). This indicates that plasma leptin levels in people over 65 are expected to be significantly lower than that of the people under the age of 65. A decrease in leptin levels produced by adipose tissue and/or an increase in plasma leptin clearance may be responsible for this difference. On the other hand, certain studies have found a positive correlation between plasma leptin levels and age.21
In this study, the smoking rate of the obese group was similar with the nonobese group. Previous studies have shown that body fat ratio and leptin levels are lower in smokers.22 This may be explained by the fact that total fat mass is lower in smokers. In addition, leptin resistance has been found to increase in smokers compared with nonsmokers.22
In our study, a positive correlation was found between leptin levels and BMI, WC, HC, and WHR. Previous studies reported contradictory results; positive20, 23 and negative4, 19, 24 correlations were obtained between leptin levels and WHR. The positive correlation between leptin levels and WHR is predominantly seen in males and the negative correlation is predominantly detected in females. This gender-related difference may be explained by the greater abundance of subcutaneous fat tissue in females and of visceral fat tissue in males. In addition, previous studies have found higher leptin secretion in subcutaneous fat tissue than visceral fat tissue.25 In one study, WC was found to be a better measure of visceral adipose tissue than WHR.26 This study supports these previous studies.
Hyperleptinemia may co-occur with other atherosclerosis risk factors associated with obesity. This study evaluated the total CH, HDL-CH, LDL-CH, uric acid, and TG levels. Along with the significant difference in leptin levels between obese and nonobese groups, significant differences in LDL-CH, total CH, HDL-CH, uric acid, and TG levels were also found between obese and nonobese groups. In a study conducted by Piestrzeniewicz et al.,27 TG, uric acid, and fasting blood glucose levels showed significant differences between obese and nonobese groups.
In this study, the relationship of leptin levels with CV risk factors (SBP, DBP, fasting blood glucose, total CH, HDL-CH, LDL-CH, and TG levels) between obese and nonobese groups was evaluated. DBP was the only one of these factors that was found to show a significant correlation with leptin. Results from other studies support this finding. Dunbar et al.28 observed that when rats were infused with intracerebroventricular leptin, blood pressure increased, along with the sympathetic activity. A study investigating the correlation between leptin and high blood pressure in humans found that hyperleptinemia leading to insulin intolerance might be responsible for the pathogenesis of primary hypertension.29
Several studies have shown positive correlations between plasma leptin and TG levels.24, 30 On the other hand, no correlation has been detected between plasma leptin and LDL-CH levels.19, 20 The relationship between plasma leptin and HDL-CH is controversial. While a negative correlation was found between plasma leptin and HDL-CH levels in some studies,30 the others failed to show such a correlation.4, 19 As a result, the correlation between the known lipid risk factors for CHD and leptin remains in question. In our study, no correlation was found between leptin and lipid parameters. In addition, the positive correlation found between leptin levels and uric acid31 and fasting blood glucose levels, which are known to be CHD risk factors, has not been observed in this study. This may be due to the relatively low number of patients in this study, compared to other studies.
Inflammation plays an important role in all stages of atherosclerosis,32 and CRP, interleukin 6 (IL-6), tumor necrosis factor (TNF)-alpha, adiponectin and resistin, mediators of inflammation, are secreted from adipose tissue in response to leptin.33 Although CRP's role in chronic inflammatory processes is not its only function, it is known to lead to the development of atherosclerosis.34 It has also been shown that leptin plays a part in reacting to certain acute stress situations.35 Increases in serum leptin levels have been observed within 24 h of acute myocardial infarct.35, 36 Pro-inflammatory cytokines may show an increase along with leptin; in other words, leptin may have a role in the development of inflammation under acute stress.36 This relationship may be explained by the similarities between the IL-6 receptor and the leptin receptor.37 In our study, leptin and CRP levels in obese and nonobese male patients who had AMI were checked and the correlation between them was investigated. No correlation was found between leptin and CRP levels. This was in contrast to several previous studies that found a positive correlation between leptin and CRP levels in obese and nonobese patients, both in healthy individuals and in patients with coronary artery disease.21, 38 However, the results supported those of Fujimaki,39 who also failed to find such a positive correlation. These different results may be related to the differences in patients’ reactions to stress and inflammation.
5. Conclusion
This study indicated leptin levels of obese male patients <65 years of age with AMI to be higher than that of the nonobese subjects. Leptin was positively correlated with WC, HC, WHR, and BMI, in both obese and nonobese patients.
Our search for correlation between leptin and several CV risk factors yielded a positive correlation only between DBP and leptin. Opinions from different research groups vary on the correlation between CV risk factors and leptin. These differences may stem from the ethnicity, age, the existence and stage of obesity, co-morbid diseases, insulin intolerance, leptin intolerance, and other such factors of the individuals.
This study shows that obese males with AMI have higher levels of leptin compared with nonobese males. This may lead us to conclude that leptin level may represent a useful tool for evaluating CV risks in obese males. However, the results also indicate that leptin level measurement, which is relatively expensive, has no significant advantage over evaluating CV risks through assessment of obesity using anthropometric measurements.
Conflicts of interest
The authors have none to declare.
References
- 1.Hall J.E., Crook E.D., Jones D.W., Wofford M.R., Dubbert P.M. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Sci. 2002;324:127–137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Misra A., Garg A. Leptin, its receptor and obesity. J Investig Med. 1996;44:540–548. [PubMed] [Google Scholar]
- 3.Lönnqvist F. The obese (ob) gene and its product leptin: a new route towards obesity treatment in man. Q J Med. 1996;89:327–332. [Google Scholar]
- 4.Liuzzi A., Savia G., Tagliaferri M. Serum leptin concentration in moderate and severe obesity: relationship with clinical, anthropometric, and metabolic factors. Int J Obes Relat Metab Disord. 1999;23:1066–1073. doi: 10.1038/sj.ijo.0801036. [DOI] [PubMed] [Google Scholar]
- 5.Ostlund R.E., Jr., Yang J.W., Klein S., Gingericj R. Relation between plasma leptin concentration and body fat, gender, diet, age and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- 6.Lundasen T., Liao W., Angelin B., Rudling M. Leptin induces the hepatic high density lipoprotein receptor scavenger receptor b type I (SR-BI) but not cholesterol 7alpha-hydroxylase (Cyp7a1) in leptin-deficient (ob/ob) mice. J Biol Chem. 2003;278:43224–43228. doi: 10.1074/jbc.M302645200. [DOI] [PubMed] [Google Scholar]
- 7.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53:152–158. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 8.Montani J.P., Antic V., Yang Z., Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord. 2002;26:28–38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan D.E., Vaile J.C., Petley G.W. Gender differences in the relationship between leptin, insulin resistance and the autonomic nervous system. Regul Pept. 2007;140:37–42. doi: 10.1016/j.regpep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Cooke J.P., Oka R. Does leptin cause vascular disease. Circulation. 2001;106:1904–1905. doi: 10.1161/01.cir.0000036864.14101.1b. [DOI] [PubMed] [Google Scholar]
- 11.Chaldakov G.N., Fiore M., Stankulov I.S. BDNF, leptin, and mast cells in human coronary atherosclerosis and metabolic syndrome. Arch Physiol Biochem. 2001;109:357–360. doi: 10.1076/apab.109.4.357.4249. [DOI] [PubMed] [Google Scholar]
- 12.Bełtowski J., Wojcicka G., Górny D., Marciniak A. Human leptin administered intraperitoneally stimulates natriuresis and decreases renal medullary Na+, K+-ATPase activity in the rat – impaired effect in dietary-induced obesity. Med Sci Monit. 2002;8:BR221–BR229. [PubMed] [Google Scholar]
- 13.Bodary P.F., Westrick R.J., Wickenheiser K.J., Shen Y., Eitzman D.T. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 14.Chu N.F., Spiegelman D., Rifai N., Hotamisligil G.S., Rimm E.B. Glycemic status and soluble tumor necrosis factor receptor levels in relation to plasma leptin concentrations among normal weight and overweight US men. Int J Obes Relat Metab Disord. 2000;24:1085–1092. doi: 10.1038/sj.ijo.0801361. [DOI] [PubMed] [Google Scholar]
- 15.Ren J. Leptin and hyperleptinemia – from friend to foe for cardiovascular function. J Endocrinol. 2004;181:1–10. doi: 10.1677/joe.0.1810001. [DOI] [PubMed] [Google Scholar]
- 16.Schulze P.C., Kratzsch J., Linke A. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail. 2003;5:33–40. doi: 10.1016/s1388-9842(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 17.Makris T.K., Hatzizacharias A.N., Krespi P.G. Markers of risk in young offspring with paternal history of myocardial infarction. Int J Cardiol. 2003;89:287–293. doi: 10.1016/s0167-5273(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 18.Friedman L.M., Leibel R.L. Tackling a weighty problem. Cell. 1992;69:217–220. doi: 10.1016/0092-8674(92)90402-x. [DOI] [PubMed] [Google Scholar]
- 19.Lönnqvist F., Wennlund A., Arner P. Relationship between circulating leptin and peripheral fat distribution in obese subjects. Int J Obes Relat Metab Disord. 1997;21:255–260. doi: 10.1038/sj.ijo.0800394. [DOI] [PubMed] [Google Scholar]
- 20.Smith J.D., Al-Amri M., Sniderman A.D., Cianflone K. Leptin and adiponectin in relation to body fat percentage, waist to hip ratio and the apoB/apoAl ratio in Asian Indian and Caucasian men and women. Nutr Metab. 2006;3:18–26. doi: 10.1186/1743-7075-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamsuzzaman A.S., Winnicki M., Wolk R. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–2185. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 22.Walley D. Serum leptin level in cigarette smoking. Comment on: Ann Epidemiol. 1969;7:79–80. [Google Scholar]
- 23.Staiger H., Tschritter O., Machann J. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes Res. 2003;11:368–372. doi: 10.1038/oby.2003.48. [DOI] [PubMed] [Google Scholar]
- 24.Lyoussi B., Ragala M.A., Mguil M., Chraibi A., Israili Z.H. Gender-specific leptinemia and its relationship with some components of the metabolic syndrome in Moroccans. Clin Exp Hypertens. 2005;4:377–394. [PubMed] [Google Scholar]
- 25.Minocci A., Savia G., Lucantoni R. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Telat Metab Disord. 2000;24:1139–1144. doi: 10.1038/sj.ijo.0801385. [DOI] [PubMed] [Google Scholar]
- 26.Lemieux S., Prud’homme D., Bouchard C., Tremblay A., Després J.P. A single threshold value of waist girth subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 27.Piestrzeniewicz K., Luczak K., Komorowski J., Maciejewski M., Goch J.H. The relationship between leptin and obesity and cardiovascular risk factors in men with acute myocardial infarction. Cardiol J. 2007;14:252–259. [PubMed] [Google Scholar]
- 28.Dunbar J.C., Hu Y., Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 29.Suter P.M., Locher R., Hasler E., Vetter W. Is there a role for the ob gene product leptin in essential hypertension. Am J Hypertens. 1998;11:1305–1311. doi: 10.1016/s0895-7061(98)00162-9. [DOI] [PubMed] [Google Scholar]
- 30.Wannamethee S.G., Tchernova J., Whincup P. Plasma leptin: associations with metabolic, inflammatory and haemostatic risk factors for cardiovascular disease. Atherosclerosis. 2007;191:418–426. doi: 10.1016/j.atherosclerosis.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Fruehwald-Schultes B., Peters A., Kern W., Beyer J., Pfützner A. Serum leptin is associated with serum uric acid concentrations in humans. Metabolism. 1999;48:677–680. doi: 10.1016/s0026-0495(99)90163-4. [DOI] [PubMed] [Google Scholar]
- 32.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 33.Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 34.Torzewski M., Rist C., Mortensen R.F. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in angiogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–2099. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 35.Meisel S.R., Ellis M., Pariente C. Serum leptin levels increase following acute myocardial infarction. Cardiology. 2001;95:206–211. doi: 10.1159/000047373. [DOI] [PubMed] [Google Scholar]
- 36.Heymsfield S., Greenberg A.S., Fujioka K. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 37.Baumann H., Morella K.K., White D.W. The full length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Nat Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazumi T., Kawaguchi A., Hirano T., Yoshino G. C-reactive protein in young, apparently healthy men: associations with serum leptin, QTc interval, and high-density lipoprotein-cholesterol. Metabolism. 2003;52:1113–1116. doi: 10.1016/s0026-0495(03)00184-7. [DOI] [PubMed] [Google Scholar]
- 39.Fujimaki S., Kanda T., Fujita K., Tamura J., Kobayashi I. The significance of measuring plasma leptin in acute myocardial infarction. J Int Med Res. 2001;29:108–113. doi: 10.1177/147323000102900207. [DOI] [PubMed] [Google Scholar]