Abstract
Cohesins function in almost all aspects of chromosome biology. Two new studies confirm that a subset of cohesin subunits form a flexible but compressed ring that can be opened through degradation. X-ray crystallography supports potentially differing regulation of subunit associations.
Prior to dividing, the cell copies its genetic material to produce two identical sets of chromosomes that are termed sister chromatids. The time interval between replicating the genome (S phase of the cell cycle) and segregating the sister chromatids into newly forming daughter cells (M phase of the cell cycle) can be hours, months or even years. Given that our genomes are chock full of repeated DNA sequences, gene families and oft-used motifs, identifying over time which chromatids are sisters can be a sticky business. The solution appears simple: glue sisters together from the time of synthesis until segregation. Elucidating both the structure of that glue, a protein complex termed cohesin, and mechanisms through which cohesins are regulated fostered a diversity of models [1]. Resolving these models is of significant interest given that cohesins are also critical for chromosome condensation, DNA replication and repair, ribosome maturation and proper deployment of transcription programs (Figure 1A) [2]. Notably, mutations in cohesin can result in aneuploidy (a characteristic of cancer cells), severe developmental maladies, or both [3]. Two articles published in Science by Gligoris and colleagues and Huis in 't Veld and colleagues solidify an expansive body of evidence that three cohesin subunits, Mcd1(Scc1/RAD21), Smc1 and Smc3, form a closed ring [4,5].
Figure 1.
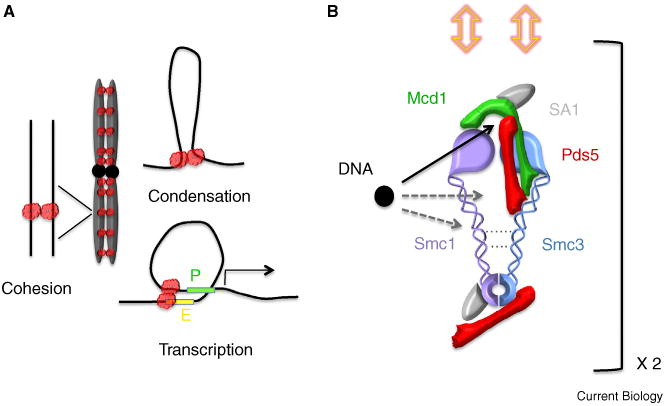
Cohesin functions, subunit interactions, and potential modes of DNA binding.
(A) Cohesin functions in chromosome biology include sister chromatid cohesion, chromosome condensation, and transcription regulation (P, promoter; E, enhancer). Regardless of cohesin structure each DNA molecule associates with its own cohesin (amorphous red cloud shown to reflect unknown details regarding cohesin dimerization in vivo). (B) Approximate map of cohesin subunit interactions includes a compressed ring with flexible coiled coils (cross-links between coiled coils indicated by dashed lines). Smc1,3 head-to-hinge binding through folding of flexible coiled coils may allow cohesin to clamp onto DNA (not shown). Possible binding sites between DNA (black circle) and cohesin are indicated (arrows). Only DNA retention between Smc1,3 heads and the Mcd1 cap is directly supported (note solid black arrow). Cohesion requires two cohesins, with dimerization most likely occuring through Mcd1 and requiring both Pds5 and Scc3/SA1 (yellow double-headed arrows). Drawing not to scale.
X-ray crystallographic analysis of a subset of cohesin interactions further suggest that, while SMC proteins are highly conserved, Mcd1 binds to distinct domains within Smc1 and Smc3, suggesting that each association may be differentially regulated during cohesin–DNA interactions. Here, I discuss the broader implications of the cohesin ring and why the study of cohesin remains in its infancy.
What Does Structure Have To Do with It?
At least five proteins are required to maintain sister chromatid cohesion: Smc1, Smc3, Mcd1(Scc1/RAD21), Scc3(Irr1/SA1,2) and Pds5 (all capitals denote vertebrate proteins). Vertebrate cells contain a sixth cohesin-binding factor, Sororin, which is also essential for cohesion. Early findings in yeast revealed that cohesins are recruited to DNA during S phase and subsequently converted to a cohesion-competent state by the S phase factor Ctf7/Eco1. Interactions between Ctf7/Eco1 and PCNA (DNA replication processivity factor) and other studies thus led to the model that cohesion is established through the tethering together of cohesins bound on each sister [6]. Structural analyses of cohesins, however, significantly altered the cohesion landscape [7–10]. SMC proteins are elongated proteins (∼100 nm) that fold in half at a centrally located hinge. Anti-parallel coiled coils extend from the hinge, bringing globular amino and carboxyl termini in registration to form an ATPase head domain. Smc1,3 proteins dimerize through hinge–hinge interactions on one end with additional evidence that Smc1,3 heads transiently associate at the other end. Smc1,3 head associations are capped (or bridged) by Mcd1 to form a contiguous ring. In turn, Mcd1 recruits Scc3 and Pds5 (Figure 1B). Similar to other cohesin subunits, Scc3 and Pds5 are essential for cohesion even though they do not participate in the contiguous ring structure [11]. The notion that cohesins form a ring spawned an ‘entrapment’ model of cohesion. If cohesin rings could be deposited on DNA before S phase, then subsequent passage of the DNA replisome would entrap both sister chromatids [8,9]. In pursuing this model, Huis in 't Veld and colleagues examined transmission electron microscopy (TEM) micrographs of recombinant dimeric (SMC1,3) and tetrameric (SMC1,3, SA1 and Mcd1/RAD21) human cohesins, focusing on complexes in which elongated coiled-coil structures were easily discernible. SMC1,3 dimers (tethered together by hinge–hinge association) form flexible and often open (SMC1,3 heads apart) structures, although a significant population of dimers retained SMC1,3 head interactions. In contrast, tetrameric cohesins formed a closed ring-like structure with SMC1,3 heads capped by Mcd1 that were uniformly positioned ∼25 nm apart [5]. In the adjoining article, Gligoris and colleagues analyzed cohesins assembled in vivo. Here, the authors modified each of the three subunit interfaces (Mcd1–Smc3, Smc3–Smc1 and Smc1–Mcd1) to allow for inducible covalent cross-links that resist detergent denaturation. Indeed, cross-links produced structures that migrated during gel electrophoresis as trimeric complexes, indicative of a closed ring [4]. Thus, both studies confirm that SMC1,3 heads are bridged by Mcd1/RAD21 to produce a closed ring [4–10].
If DNA (or two DNA molecules) is trapped within a ring, how might it escape? Huis in 't Veld and colleagues modified Mcd1 to contain a TEV-dependent cleavage site and reassembled tetrameric cohesins that contained SMC1,3 and SA1(Scc3). TEM micrographs revealed that SMC1,3 head domains return to a random (i.e., separated) disposition upon Mcd1 cleavage, similar to SMC1,3 dimers [5]. These findings provide structural evidence that Mcd1/RAD21 cleavage can result in cohesin ring dissolution. Mcd1 point mutations that target the Mcd1–Smc3 interface resulted in dissociation of both SA1 and the cleaved amino-terminal portion of Mcd1 from SMC1,3 in vitro, although the SA1–Mcd1 fragment complex remained chromatin associated in vivo. Mcd1 mutations that targeted the Mcd1–Smc3 interface, however, decreased the residence time of cohesins onto DNA as measured by fluorescence recovery after photobleaching (FRAP) [5], adding credence to the closed ring structure.
Think Outside the Single Ring: Is Cohesion Mediated through Cohesin–Cohesin Interactions?
By any account, “cohesin complexes that mediate sister chromatid cohesion must dissociate from DNA to allow chromosome segregation” [5]. When tested directly, however, the single ring entrapment model proved incorrect [12,13]. Pds5 is an essential cohesion factor that is required for cohesion maintenance during mitosis. Cells synchronized in mitosis contain tightly paired sister chromatids, but separate upon Pds5 inactivation. The single ring entrapment model requires that one or both of the sister chromatids lose cohesin for cohesion loss. Instead, chromosomes fully retained cohesin binding — sister chromatids did not dissociate by escaping from a single ring [12,13]. The inescapable conclusion is that deposition factors decorate each sister with cohesins and that cohesion arises through subsequent modification (Eco1/Ctf7) to produce cohesin–cohesin interactions that tether together the two sisters. If true, surely there must be evidence of such interactions. To date, a limited number of studies report co-immunoprecipitation of alternatively tagged Mcd1 and the ability of Mcd1 to link alternatively tagged Smc1,3 complexes [9,14]. SMC capping protein associations may be conserved given evidence of Mre11 dimerization atop SMC-like Rad50 ATPase head domains [15]. Gligoris and colleagues exploited their covalently cross-linked cohesin complex (Mcd1–Smc3, Smc3–Smc1 and Smc1–Mcd1) to test for cohesin–cohesin interactions by immunoprecipitation [4]. Cross-links did not extend to either Pds5 or Scc3/SA1,2, however, both of which are essential for cohesion. Thus, the inability to co-immunoprecipitate alternatively tagged cohesin complexes provides an important starting point from which additional cross-links can be incorporated to test for intermolecular cohesin associations.
Really — Think Outside the Ring: Is DNA Entrapped within the Ring Lumen?
While the formation of a cohesin ring is now certain, numerous issues remain regarding cohesin structures that mediate cohesion. Front and center is whether the ring represents the final cohesin conformation. Keep in mind that the cohesins analyzed were assemblies of recombinant proteins required to survive mechanical disruption, detergents and TEM staining procedures [5]. Moreover, Huis in 't Veld analyzed only those structures in which elongated coiled-coil domains were readily identifiable — excluding analyses of a significant percentage of folded or potentially oligomerized structures. The question is worth considering given evidence from atomic force microscopy that cohesins adopt conformations that are half the length of those selected for analyses by Huis in 't Veld and colleagues [5,16]. It is at least worth entertaining models that cohesin complexes fold over — possibly to clamp onto DNA [11]. If true, SMC1,3 head and hinge domains may come into close proximity. Indeed, fluorescence resonance energy transfer (FRET) studies suggest that Pds5 associates with both SMC1,3 hinges and heads [17]. Folding may be a conserved feature given that Nse5,6 appear to bind Smc5,6 hinges in addition to other SMC domains [18]. Findings by Huis in 't Veld and colleagues are especially intriguing. These authors induced extensive cross-links along Smc1,3 to map cohesin proximity domains. Mass spectroscopy provided some surprises in that intermolecular cross-links between Smc1,3 extend significantly up from the hinge to link coiled-coil regions [5], reducing the effective lumen size (Figure 1B). EM micrographs similarly document that Smc2,4 coiled coils in condensin complexes remain closely apposed along their length [7,10], despite arguments to the contrary [19].
The most significant obstacle in understanding cohesin function in vivo is the lack of a map regarding the path of DNA in/around/through cohesin. To date, the only findings relevant to this question emanate from the SMC-like Mre11, Nbs1, Rad50 complex in which DNA does not pass through the lumen but instead threads between ATPase head domains of Rad50 dimers that are capped by Mre11 dimers [15]. This is a satisfying possibility in which DNA becomes positioned nearest the ATPase domains most likely to exert force (DNA looping for condensin, DNA tethering by cohesin, registration of distal cis DNA elements for transcription) (Figure 1). Regardless, popular models focus on DNA entrapment with Smc1,3 coiled coils. X-ray crystallography mapped Mcd1 binding to a Smc3 head-proximal region of the coiled coil, an ‘exit gate’ interface through which DNA might escape from cohesin ring entrapment [4,20]. While this remains a viable and important model, expressing amino-to-carboxy terminal fusions of Smc3–Mcd1 (distal from the site mapped above) support cell viability, suggesting that any linkage that closes the ring will suffice [20]. The field looks forward to not only future testing of this model, but considered discussions of any model that appears supported by persistent yet inconvenient truths.
References
- 1.Huang CE, Milutinovich M, Koshland D. Rings, bracelet or snaps: fashionable alternatives for Smc complexes. Philos Trans R Soc Lond B Biol Sci. 2005;360:537–542. doi: 10.1098/rstb.2004.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remeseiro S, Cuadrado A, Losada A. Cohesin in development and disease. Development. 2013;140:3715–3718. doi: 10.1242/dev.090605. [DOI] [PubMed] [Google Scholar]
- 3.Skibbens RV, Colquhoun JM, Green MJ, Molnar CA, Sin DN, Sullivan BJ, Tanzosh EE. Cohesinopathies of a feather flock together. PLoS Genet. 2013;9:e1004036. doi: 10.1371/journal.pgen.1004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gligoris TG, Scheinost JC, Bürmann F, Petela N, Chan KL, Uluocak P, Beckouët F, Gruber S, Nasmyth K, Löwe J. Closing the cohesin ring: structure and function of its Smc3-kleisin interface. Science. 2014;346:963–967. doi: 10.1126/science.1256917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huis in 't Veld PJ, Herzog F, Ladurner R, Davidson IF, Piric S, Kreidl E, Bhaskara V, Aebersold R, Peters JM. Characterization of a DNA exit gate in the human cohesin ring. Science. 2014;346:968–972. doi: 10.1126/science.1256904. [DOI] [PubMed] [Google Scholar]
- 6.Skibbens RV. Holding your own: establishing sister chromatid cohesion. Genome Res. 2000;10:1664–1671. doi: 10.1101/gr.153600. [DOI] [PubMed] [Google Scholar]
- 7.Melby TE, Ciampaglio CN, Briscoe G, Erickson HP. The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J Cell Biol. 1998;142:1595–1604. doi: 10.1083/jcb.142.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruber S, Haering CH, Nasmyth K. Chromosomal cohesin forms a ring. Cell. 2003;112:765–777. doi: 10.1016/s0092-8674(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 9.Haering C, Lowe J, Hochwagen A, Nasmyth K. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol Cell. 2002;9:773–788. doi: 10.1016/s1097-2765(02)00515-4. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DE, Losada A, Erickson HP, Hirano T. Condensin and cohesin display different arm conformations with characteristic hinge angles. J Cell Biol. 2002;156:419–424. doi: 10.1083/jcb.200111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skibbens RV. Sticking a fork in cohesin – it's not done yet! Trends Genet. 2011;27:499–506. doi: 10.1016/j.tig.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Tong K, Skibbens RV. Cohesin without cohesion: a novel role for Pds5 in Saccharomyces cerevisiae. PLoS One. 2014;9:e100470. doi: 10.1371/journal.pone.0100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulemzina I, Schumacher MR, Verma V, Reiter J, Metzler J, Failla AV, Lanz C, Sreedharan VT, Rätsch G, Ivanov D. Cohesin rings devoid of Scc3 and Pds5 maintain their stable association with the DNA. PLoS Genet. 2012;8:e1002856. doi: 10.1371/journal.pgen.1002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang N, Jiang Y, Mao Q, Demeler B, Tao YJ, Pati D. Characterization of the interaction between the cohesin subunits Rad21 and SA1/2. PLoS One. 2013;8:e69458. doi: 10.1371/journal.pone.0069458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mockel C, Lammens K, Schele A, Hopfner KP. ATP driven structural changes of the bacterial Mre11:Rad50 catalytic head complex. Nucleic Acids Res. 2012;40:914–927. doi: 10.1093/nar/gkr749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai A, Hizume K, Sutani T, Takeyasu K, Yanagida M. Condensin but not cohesin SMC heterodimer induces DNA reannealing through protein-protein assembly. EMBO J. 2003;22:2764–2775. doi: 10.1093/emboj/cdg247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntyre J, Muller EG, Weitzer S, Snydsman BE, Davis TN, Uhlmann F. In vivo analysis of cohesin architecture using FRET in the budding yeast Saccharomyces cerevisiae. EMBO J. 2007;26:3783–3793. doi: 10.1038/sj.emboj.7601793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan X, Yang Y, Chen YH, Arenz J, Rangi GK, Zhao X, Ye H. Architecture of the Smc5/6 complex of Saccharomyces cerevisiae reveals a unique interaction between the Nse5-6 subcomplex and the hinge regions of Smc5 and Smc6. J Biol Chem. 2009;284:8507–8515. doi: 10.1074/jbc.M809139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuylen S, Metz J, Haering CH. Condensin structures chromosomal DNA through topological links. Nat Struct Mol Biol. 2011;18:894–901. doi: 10.1038/nsmb.2087. [DOI] [PubMed] [Google Scholar]
- 20.Chan KL, Roig MB, Hu B, Beckouet F, Metson J, Nasmyth K. Cohesin's DNA exit gate is distinct from its entrance gate and is regulated by acetylation. Cell. 2012;150:961–974. doi: 10.1016/j.cell.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


