Abstract
OBJECTIVE
To describe the case of a patient with castration-resistant, metastatic prostate cancer who achieved a complete and durable biochemical response after treatment with sipuleucel-T while continuing with enzalutamide and to explore the immunologic basis for such a response.
MATERIALS AND METHODS
We obtained serial prostate-specific antigen (PSA) measurements and bone scans to assess the patient’s response to enzalutamide followed by the addition of sipuleucel-T. Using preclinical and clinical data, we describe his response through known immunobiologic mechanisms.
RESULTS
This patient’s PSA level became undetectable during treatment with enzalutamide and began to increase again after 14 months. He opted for treatment with sipuleucel-T, while continuing with the enzalutamide. This resulted in another complete PSA response 6 months after exposure to sipuleucel-T.
CONCLUSION
Sipuleucel-T typically does not produce significant PSA reductions, and, to the best of our knowledge, only 1 previous report of a durable complete PSA response in a patient with metastatic disease has been published. The timing of this response supports an immune mechanism. The biologic rationale for the combination, coupled with the clinical result observed in our patient, provides a basis for studies of the combination of sipuleucel-T and enzalutamide.
Until 2010, docetaxel chemotherapy was the principal treatment option for men with meta-static, castration-resistant prostate cancer (CRPC), demonstrating a survival benefit in randomized controlled studies.1,2 Until recently, patients with meta-static CRPC had few other treatment options. Several additional agents, including androgen-signaling inhibitors, cytotoxic chemotherapy, radiopharmaceuticals, and immunotherapy, have subsequently been shown to also extend survival. Because all these agents have been studied as monotherapies, little is known about the potential for combining these agents with 1 another. With 6 different agents now demonstrating a survival benefit, both clinical observations and an understanding of the biologic interactions are important in considering how to best begin combining (or sequencing) these agents with each other.
Sipuleucel-T was approved by the Food and Drug Administration for men with asymptomatic or minimally symptomatic metastatic CRPC in 2010 after the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) study demonstrated a median survival benefit of 4.1 months compared with placebo.3 Sipuleucel-T is an adoptive cellular immunotherapy designed to activate an immune response directed against prostatic acid phosphatase. Enzalutamide (MDV3100) is a novel androgen receptor antagonist recently reported to extend survival in patients with docetaxel-treated CRPC. Enzalutamide lacks the partial agonist activity of first-generation androgen receptor antagonists and has been shown to block androgen receptor translocation to the nucleus.4
In this report, we present a patient who achieved a durable complete PSA response when sipuleucel-T was added to enzalutamide. To the best of our knowledge, this is the first report of these 2 agents administered concurrently. One other report has been published of a complete and durable response in a patient with metastatic disease after sipuleucel-T.5
CASE REPORT
A 69-year-old man was diagnosed with adenocarcinoma of the prostate in 1997 at 54 years old. He underwent radical prostatectomy, with the final pathologic examination showing a pT3aN0Mx, Gleason score 3+5 cancer. In September 1999, he developed a PSA relapse. He first participated in a clinical trial of high-dose calcitriol and then in another trial of granulocyte-macrophage colony-stimulating factor (GM-CSF). He experienced PSA declines with GM-CSF given 150 μg/m2 every 14 of 28 days. As reported in the published data,6 treatment resulted in a saw-tooth–like pattern of PSA declines during treatment followed by a rebound during the off-treatment period. The most significant on-treatment decline was from 22.6 to 9.3 ng/mL. He received GM-CSF immunotherapy for approximately 9 months.
In 2002 (while receiving GM-CSF), the patient developed evidence of osseous metastases. He declined treatment with a luteinizing hormone-releasing hormone (LHRH) agonist at that time and, instead, initiated treatment with bicalutamide 150 mg/d plus breast irradiation to prevent gynecomastia. His disease remained well-controlled with this regimen until 2005; at that time, finasteride was added to his bicalutamide regimen. In 2007, in response to additional disease progression, he was treated with a LHRH agonist and subsequently received combined androgen ablation (LHRH agonist plus bicalutamide).
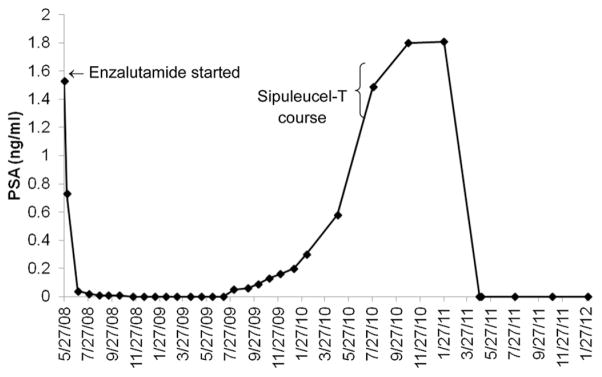
When his cancer again progressed in 2008, the patient enrolled in the phase I study of enzalutamide (NCT00510718) while continuing his LHRH agonist therapy, which is the standard. Enzalutamide was initially administered at a dose of 360 mg/d by mouth in June 2008. This therapy resulted in bone scan improvement (resolution of 1 lesion and stability of 2 remaining lesions) and a nadir PSA of <0.1 ng/mL. In January 2009, the dose was reduced to 240 mg/d by the sponsor. After another year of treatment, his dose was decreased again to 160 mg/d, as directed by the study sponsor, because of toxicities at the greater dose observed in the other study participants. The patient remained in PSA remission with stable bone scintigraphy findings for 14 months, until his serum PSA level again became detectable and began to consistently increase (Fig. 1). When his PSA level reached 1.49 ng/mL, he received on-label sipuleucel-T while continuing enzalutamide and LHRH agonist therapy. After a delay of approximately 6 months, his serum PSA level unexpectedly declined, eventually again reaching an undetectable level. His PSA level remained undetectable 1 year later. His computed tomography scan showed no evidence of metastatic disease, and his nuclear medicine bone scans have not shown disease progression.
Figure 1.
Patient’s prostate-specific antigen (PSA) levels graphed by time. He started enzalutamide on May 27, 2008 and received a course of sipuleucel-T from August 30, 2010 to September 27, 2010. His PSA level remained undetectable since April 28, 2011.
COMMENT
PSA reductions after sipuleucel-T are rare, and the present case raises several interesting points. First, a strong need exists to identify the predictors of benefit from immunotherapy in prostate cancer. It was intriguing that our patient experienced PSA declines with GM-CSF therapy several years before his sipuleucel-T treatment. Sipuleucel-T is an autologous cellular product consisting of mono-nuclear cells incubated with a prostatic acid phosphatase/GM-CSF fusion protein. It would be interesting to further explore the possibility that the PSA changes in response to GM-CSF are a marker of sensitivity to subsequent sipuleucel-T therapy.
Second, the present case also raises the question of whether enzalutamide boosts the effect of sipuleucel-T or vice versa. The immunologic effects of androgen ablation are fairly well-accepted.7 In animal models, androgen ablation reverses the involution of the thymus that occurs with age, resulting in increased output of naive T cells.8 In prostate cancer, several groups have demonstrated that castration results in an influx of immune cells into the prostate gland.9,10 The combination of androgen ablation and vaccination has been studied in animal models. In general, these studies have shown that castration reverses tumor-specific T-cell tolerance, suggesting the possibility of an additive or synergistic effect in patients.11,12 In a small clinical study, the viral-vector based PSA-targeted vaccine ProstVac appeared to augment the effects of subsequently administered niluta-mide.13 In the present case, the delay of approximately 6 months between sipuleucel-T treatment and PSA reduction is consistent with an immune mechanism, because T-cell responses to vaccination typically take weeks to months to develop. An association was found between antigen presenting cell upregulation, as measured by CD54, and overall survival in subjects who participated in the phase III trials, Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) and D9901/D9902A.14 Our patient had a robust increase in antigen presenting cell activation at the third dose relative to the other patients. The increase in antigen presenting cell activation was 23.6-fold. In contrast, the reported median increase was 10.6-fold (25th to 75th percentile 7.8–13.7; Table 1). The increase in activation with doses 1 and 2 was near the median seen in the phase III studies. Taken together, these data support an immunologic basis for the PSA response seen in our patient. However, controlled clinical studies are required to formally test that hypothesis. Additionally, it is possible that other clinical features of our patient were associated with the success of sipuleucel-T, but we were not able to confirm that in a case report.
Table 1.
Patient’s immune parameters
| Dose
|
|||
|---|---|---|---|
| 1 | 2 | 3 | |
| Total nucleated cells (×106 cells) | 3883 | 6421 | 4179 |
| CD54 upregulation (x-fold increase) | 6.6 | 13.9 | 23.6 |
| Number of CD54-positive cells (×106 cells) | 1454 | 2800 | 750 |
Third, it is important to note that, unlike several other prostate cancer treatments, enzalutamide is administered without immunosuppressive doses of corticosteroids and, therefore, might be an ideal agent to evaluate in combination with sipuleucel-T immunotherapy. An ongoing trial testing the relative sequencing of sipuleucel-T with an LHRH agonist (Eligard) in early-stage disease (biochemical relapse; NCT01431391) has completed accrual. The results in the present case argue that the approach could also be helpful in men with later disease stages.
CONCLUSION
We have described an unusual case of a patient with CRPC with PSA progression after an initial response to enzalutamide, who experienced a complete PSA response to the immunotherapy sipuleucel-T while continuing with enzalutamide. The present case suggests that the combination of enzalutamide and sipuleucel-T is worthy of investigation in advanced disease, as well as the interesting possibility that the PSA response to GM-CSF might be predictive of sipuleucel-T efficacy.
Footnotes
Financial Disclosure: T. M. Beer is a consultant to and receives funding from Dendreon, the maker of sipuleucel-T; C. G. Drake is a consultant to Dendreon.
References
- 1.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration–resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Dumas L, Payne H, Chowdhury S. The evolution of antiandrogens: MDV3100 comes of age. Expert Rev Anticancer Ther. 2012;12:131–133. doi: 10.1586/era.11.210. [DOI] [PubMed] [Google Scholar]
- 5.Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 6.Small EJ, Reese DM, Um B, et al. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 7.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 9.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon PO, Poisson AO, Delvoye N, et al. Characterization of the intraprostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh YT, Gray A, Higgins SA, et al. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. doi: 10.1007/s00262-012-1317-2. Epub 2012 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]