Abstract
PURPOSE
To investigate the safety, tolerability, and bioactivity of intravenous infusions of bevacizumab in patients with choroidal neovascularization (CNV) attributable to causes other than age-related macular degeneration.
DESIGN
Nonrandomized clinical trial.
METHODS
Ten patients with CNV received infusions of 5 mg/kg of bevacizumab. The primary efficacy outcome measure was change in visual acuity (VA; Early Treatment Diabetic Retinopathy Study letters read at 4 meters) at 24 weeks and secondary measures were changes from baseline in excess foveal thickness (center subfield thickness), area of fluorescein leakage, and area of CNV.
RESULTS
Infusions were well tolerated and there were no ocular or systemic adverse events. At baseline, median VA was 25.5 letters read at 4 meters (20/80) and median foveal thickness was 346 μm. At the primary endpoint (24 weeks), median VA was 48.5 letters (20/32), representing four lines of improvement from baseline (P = .005), median foveal thickness was 248 μm representing a 72% reduction in excess foveal thickness (P = .007). Four of nine patients had complete elimination of fluorescein leakage, three had near complete elimination (reductions of 91%, 88%, and 87%), two had modest reductions, and one had no reduction. All patients except one showed a reduction in area of CNV with a median reduction of 43%.
CONCLUSIONS
Despite the small number of patients studied, the marked improvement in VA accompanied by prominent reductions in foveal thickness, fluorescein leakage, and area of CNV suggest a beneficial effect. It may be worthwhile to consider further evaluation of systemic bevacizumab in young patients with CNV.
Choroidal neovascularization (cnv) attributable to age-related macular degeneration (AMD) is the most common cause of severe vision loss in elderly people of developed countries. The pathogenesis of CNV attributable to AMD has not been completely elucidated, but it is clear that vascular endothelial growth factor (VEGF) plays a central role. Ranibizumab, a specific antagonist of VEGF, slows growth of the new vessels, reduces leakage, and causes significant improvement in visual acuity in 30% to 40% of patients.1,2 The source of increased production of VEGF is uncertain, but increased staining for VEGF has been demonstrated in retinal pigmented epithelial (RPE) cells in CNV removed by surgery, so RPE cells are a likely source.3–5 RPE cells increase their production of VEGF when they are grown on abnormal extracellular matrix6 and abnormal extracellular matrix in the form of drusen and diffuse thickening of the Bruch membrane is a defining feature of AMD.7
Young patients also develop CNV from a variety of causes including pathologic myopia, ocular histoplasmosis, angioid streaks, and certain types of ocular inflammatory disease. Drusen and thickening of the Bruch membrane are not seen in these diseases, which instead tend to show breaks in the Bruch membrane or inflammation in close proximity to the Bruch membrane. Therefore, the pathogenesis of CNV formation in these diseases may be quite different from that in AMD, and angiogenic stimuli other than VEGF, such as tumor necrosis factor-α (a proangiogenic cytokine that is secreted by inflammatory cells), might play a primary role. However, intravenous infusions of bevacizumab caused dramatic improvements in two patients with CNV attributable to pathologic myopia, suggesting that VEGF may play an important role in pathologic myopia as well as in AMD.8 These encouraging results caused us to initiate an open-label trial investigating the effects of infusions of bevacizumab in patients with CNV attributable to a variety of causes other than AMD. Since then, intraocular injections of bevacizumab have become widely used in patients with CNV attributable to AMD or other causes. Herein, we report the results of our study that was initiated prior to the use of intraocular bevacizumab.
METHODS
STUDY DESIGN
This was an open-label pilot study to investigate the effect of intravenous infusions of bevacizumab in 10 subjects with CNV secondary to diseases other than AMD (non AMD CNV). Patients were given two infusions of 5 mg/kg of bevacizumab two weeks apart followed by re-evaluation at six, eight, 10, and 12 weeks for evidence of leakage on fluorescein angiography (FA). Patients with evidence of persistence leakage on FA at these visits were given up to two more infusions two weeks apart. Patients were subsequently followed up at 16, 24 (primary endpoint), 36, and 48 weeks.
The primary outcome measure was the median change from baseline in best-corrected visual acuity (BCVA) in letters read at 4 meters on an Early Treatment Diabetic Retinopathy Study (ETDRS) chart. The following were secondary outcome measures: 1) the change from baseline in excess foveal thickness with foveal thickness defined as the central subfield with a diameter of 1 mm centered on the fovea measured by optical coherence tomography (OCT) using the fast macular thickness map; 2) the change from baseline in CNV lesion size measured 60 seconds after fluorescein injection; and 3) the change from baseline in leakage area defined as the area of CNV fluorescence at 600 seconds minus the area of CNV fluorescence at 60 seconds after dye injection.9
STUDY POPULATION
The inclusion criteria for the study were the following: 1) male or female 18 years or older; premenopausal women had to use two forms of birth control; 2) actively leaking subfoveal CNV secondary to any cause other than AMD demonstrated by fluorescein angiography with no substantial subfoveal blood or scarring; 3) BCVA of 20/30 or worse; and 4) no history or evidence of myocardial ischemia, infarction, or arrhythmia within 28 days of infusion.
Subjects were excluded from the study if they had: 1) evidence of irreversible loss of vision in the study eye; 2) any treatment for CNV within 12 weeks of study entry; 3) intraocular surgery or another treatment in the study eye within three months of study entry; 4) blood pressure > 150/100; 5) a positive pregnancy test; 6) any history or physical signs of peripheral vascular disease; 7) a history of thromboembolism or stroke; or (8) any history of significant gastrointestinal, oral, or nasal bleeding within three years of study entry.
INFUSIONS OF BEVACIZUMAB
Each patient received 5 mg/kg of bevacizumab diluted in 100 ml of 0.9% sodium chloride as continuous infusion over a period of 90 minutes. If the first infusion was well tolerated without infusion-related adverse events (fever or chills), subsequent infusions were delivered over 60 minutes. The infusions were administered by a registered nurse or physician's assistant in the Johns Hopkins Sidney Kimmel Cancer Center under the guidance of one of the investigators (R.P.) with extensive experience administering bevacizumab to oncology patients.
STUDY ACTIVITIES AND ASSESSMENTS
Subjects were monitored closely for safety and tolerability using the following assessments and procedures: slit-lamp biomicroscopy, indirect ophthalmoscopy, tonometry, measurement of BCVA, adverse event reporting, vital signs, physical examinations, serum electrolytes, creatinine, and quantitative protein determination in 24-hour urine specimens. Stereoscopic color fundus photography and FA were performed at baseline and weeks 2, 6, 8, 10, 12, 24, 36, and 48. OCT was performed at each study visit.
OPTICAL COHERENCE TOMOGRAPHY
OCT was performed by one of the investigators (S.M.S.) using StratusOCT (Carl Zeiss Meditec, Dublin, California, USA). Scans were obtained using two standard protocols (6-mm fast macular thickness map and 6 × 6-mm crosshair) and one previously described modified acquisition protocol (three-line 8-mm papillomacular axis scan).10 The three-line 8-mm papillomacular axis scans utilized the disk as a landmark to ensure reproducible placement of scan lines at each visit; one line was at the superior margin, one was at the inferior margin, and one passed through the center of the disk. The 6 × 6-mm crosshair was a high-resolution scan used to follow morphological changes in the macula. The fast macular thickness map performed six linear scans 6 mm in length centered on the patient fixation at equally spaced angular orientations in 1.96 seconds. Retinal thickness at any point was defined as the distance between outer and inner reflectivity bands of the OCT cross-section. Foveal thickness (defined as the mean height of the neurosensory retina in a central 1-mm–diameter area) and total macular volume were computed automatically by the StratusOCT software (version 4.0). Due to the advanced nature of the disease and extensive change in the RPE morphology, RetinaTomographer version 1.0 (Retinal Imaging Research and Reading Center [RIRRC], Baltimore, Maryland, USA) was used to re-run all analysis done by the StratusOCT software and any artifacts produced by the automated analysis algorithm were corrected.
FLUORESCEIN ANGIOGRAPHY
High-resolution digital FAs were performed using Zeiss FF4 fundus camera (Carl Zeiss Meditec, Oberkochen, Germany) attached to an MRP (Boston, Massachusetts, USA) capture station. Quantitative analysis of FA was done by two independent investigators based on an already-established technique,9 using EyeNAV software version 1.1 (RIRRC, Baltimore, Maryland, USA), which generates best fit area vs time curves using logarithmic transformation. We have used these logarithmic curves of best fit to interpolate values at 60 seconds and 600 seconds for all FAs. For the CNV lesions in this study, all of which exhibited classic leakage patterns, where the lesion is filled by one minute, the 60-second time point gives a measure of lesion size and the difference in area between 600 seconds and 60 seconds provides a measure of leakage area.
STATISTICAL METHODS
All statistical analyses were performed in Statistical Package for Social Sciences version 9.0 (SPSS Inc, Chicago, Illinois, USA). Attributable to the small number of patients, changes in continuous measures were assessed using the Wilcoxon signed-rank test.
RESULTS
BASELINE CHARACTERISTICS
Ten patients with CNV due to causes other than AMD including pathologic myopia (4), ocular histoplasmosis (2), punctate inner choroidopathy (PIC; 2), birdshot chorioretinopathy (BCR) (1), and angioid streaks (1) were studied. Five of the patients had received prior photodynamic therapy (PDT), two had received triamcinolone acetonide, one had received pegaptanib, one had received intraocular bevacizumab, and two had no prior treatment (Table 1). There were six women and four men with a mean age of 44.6 years. The median number of letters read at 4 meters on an ETDRS chart was 25.5 and the median foveal thickness was 346 μm. All patients had CNV lesions which exhibited classic leakage patterns.
TABLE 1.
Demographics and Functional Outcome: The Difference in Visual Acuity at Baseline vs Weeks 24 and 48
| Pt | Dx | Age (years) | Gender | Previous Treatments | Duration (months) | BL | Wk 24 | Wk 48 | ΔWk 24 | ΔWk 48 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PM | 57 | F | None | 26 | 3 | 27 | 30 | 24 | 27 |
| 2 | BCR | 50 | F | PDT × 3 + Pegaptanib | 16 | 33 | 47 | 39 | 14 | 6 |
| 3 | PM | 48 | M | PDT × 4 | 15 | 7 | 20 | 19 | 13 | 12 |
| 4 | OHS | 51 | M | Triamcinolone + PDT × 1 | <1 | 14 | 55 | 50 | 41 | 36 |
| 5 | AS | 37 | F | PDT × 6 | 14 | 26 | 60 | 53 | 34 | 27 |
| 6 | PM | 23 | F | Pegaptanib | 4 | 43 | 55 | 52 | 12 | 9 |
| 7 | PM | 48 | F | None | 1 | 25 | 42 | 47 | 17 | 22 |
| 8 | OHS | 58 | M | Intravitreous Bevacizumab | 4 | 38 | 49 | 47 | 11 | 9 |
| 9 | PIC | 41 | M | PDT × 3 | 3 | 23 | 63 | 55 | 40 | 32 |
| 10 | PIC | 33 | F | Triamcinolone | 6 | 31 | 40 | 50 | 9 | 19 |
| Mean | 24.30 | 45.80 | 44.20 | |||||||
| Median | 25.50 | 48.00 | 48.50 |
PM = pathologic myopia; BCR = birdshot chorioretinopathy; OHS = ocular histoplasmosis syndrome; AS = angioid streaks; PDT = photodynamic therapy; PIC = punctate inner choroidopathy. Demographic information is shown for each patient (Pt) including: the diagnosis age, gender, previous treatments, and duration of choroidal neovascularization at baseline. The functional outcome is the number of ETDRS letters read at 4 meters at baseline (BL), week 24 (Wk 24), and Wk 48, and the difference in letters read between Wk 24 and baseline (ΔWk 24; the primary outcome measure) or Wk 48 and baseline (ΔWk 48).
Boldface indicates the change in visual acuity from baseline.
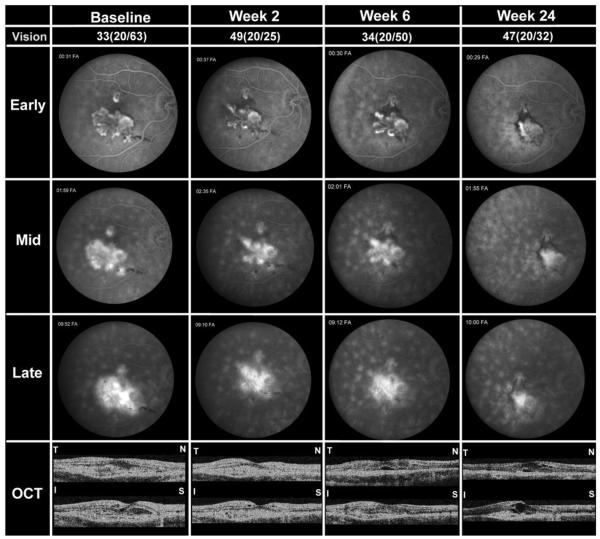
PATIENT 2
A 50-year-old Caucasian woman with BCR with worsening CNV despite three sessions of PDT and an injection of 0.3 mg of pegaptanib two months prior to study entry had VA of 33 letters (20/63) in the right eye and 53 letters (20/25) in left eye. The patient was enrolled into the study one month sooner than the three-month period after previous treatments as stated in the protocol, because there was documented progressive worsening of vision and increase in the size of the CNV. FA in the right eye was 49 letters (20/25). The size and leakage of the CNV and foveal thickness were reduced (Figure 1, week 2). A second infusion of bevacizumab was given and four weeks later VA in the right eye was 34 letters, FA showed persistent leakage, and OCT showed reduction in the subretinal mass, but new subretinal fluid on the horizontal cross-section (Figure 1, week 6). The patient was given a third infusion, and two weeks later a fourth infusion of bevacizumab because of persistent leakage. At the primary endpoint, week 24, VA was 47 letters (20/32) in the right eye, and the CNV was clearly reduced in size compared to baseline, but there was still some leakage and subretinal fluid (Figure 1, week 24). At week 36, 28 weeks after the fourth infusion, VA was 38 letters (20/50) in right eye and there was minimal fluorescein leakage, but OCT showed persistent intraretinal edema and a foveal thickness of 360 μm. Subsequently, the patient noted a decrease in vision; therefore, she was given an intraocular injection of 1.25 mg bevacizumab in the right eye at week 43, 10 months after the first infusion. At 48 weeks, VA was 39 letters (20/40) in the right eye and 57 letters (20/20) in the left eye, and foveal thickness was 355 μm in the right eye.
FIGURE 1.
Patient 2, a 50-year-old Caucasian woman with choroidal neovascularization (CNV) attributable to birdshot chorioretinopathy (BCR). Studies obtained at baseline and two, four, and 24 weeks after the first infusion are shown including best-corrected visual acuity (BCVA) in Early Treatment Diabetic Retinopathy Study (ETDRS) letters read at 4 meters and Snellen equivalent, frames from early, mid, and late phases of fluorescein angiograms (FA), and horizontal and vertical cross-sections of optical coherence tomography (OCT) scans labeled T-N (temporal-nasal) and I-S (inferior-superior), respectively. At the primary endpoint of 24 weeks after the first infusion, the patient showed improved BCVA, reduced CNV lesion size, and reduction in leakage and edema, but not complete resolution.
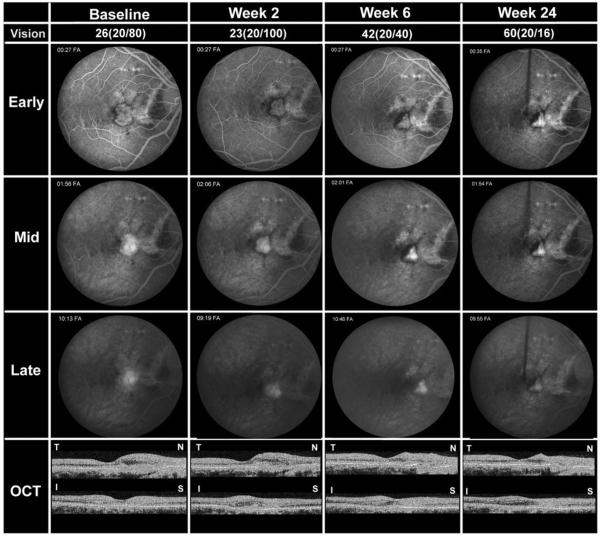
PATIENT 5
A 37-year-old Caucasian woman with angioid streaks and CNV in both eyes lost central vision in her left eye and, despite six PDT treatments, had progressive deterioration in the right eye. At baseline, VA was 26 letters (20/80) in the right eye and 1 letter at 4 meters/13 letters at 1 meter (20/40) in the left eye. FA showed a window defect from a large streak between the disk and fovea with a large leaking subfoveal CNV lesion (Figure 2, baseline). OCT showed subretinal fluid and thickening on the nasal side of the fovea. Two weeks after the first infusion of bevacizumab, VA in the right eye was 23 letters (20/100), fluorescein leakage was reduced, and OCT showed little change from baseline (Figure 2, week 2). A second infusion of bevacizumab was given and one month later VA was 42 letters (20/40), the CNV was smaller and did not leak, and OCT showed reduced thickening of the nasal part of the macula (Figure 2, week 6). No infusion was administered. At the week 12 visit, 10 weeks after the last infusion, the VA was 51 letters (20/25), but there was a question of mild recurrent fluorescein leakage; therefore, a third infusion of bevacizumab was given. At the primary endpoint, 24 weeks after study entry and 12 weeks after the last infusion, VA in the right eye was 60 letters (20/16); the CNV appeared smaller than at baseline and did not leak fluorescein, and there was no macular thickening (Figure 2, week 12). At 36 weeks, VA remained at 60 letters (20/16) at 4 meters in the right eye and two letters at 4 meters/22 letters at 1 meter (20/400) in the left eye. OCT showed minimal thickening in the right, and FA did not show evidence of leakage in either eye. The patient returned seven weeks later, at week 43, which was not a scheduled study visit, complaining of blurriness in the right eye; the VA was 20/40. FA revealed recurrent leakage at the edge of the CNV complex, and the patient was given an intraocular injection of 1.25 mg bevacizumab in the right eye as she was not eligible to receive additional intravenous bevacizumab. At the end-of-study visit (week 48), VA was 53 letters (20/25) in the right eye and 0 letters at 4 meters/19 letters at 1 meter (20/400) in the left eye. OCT showed foveal thickness of 220 μm in the right eye and 279 μm in the left eye.
FIGURE 2.
Patient 5, a 37-year-old Caucasian woman with CNV attributable to angioid streaks. Studies obtained at baseline and 2, 4, and 24 weeks after the first infusion are shown including BCVA in ETDRS letters read at 4 meters and Snellen equivalent, frames from early, mid, and late phases of FA, and horizontal and vertical cross-sections of OCT scans labeled T-N (temporal-nasal) and I-S (inferior-superior), respectively. At the primary endpoint of 24 weeks after the first infusion, the patient showed improved BCVA, reduced CNV lesion size, resolution of leakage, and resolution of subretinal and intraretinal fluid in the macula.
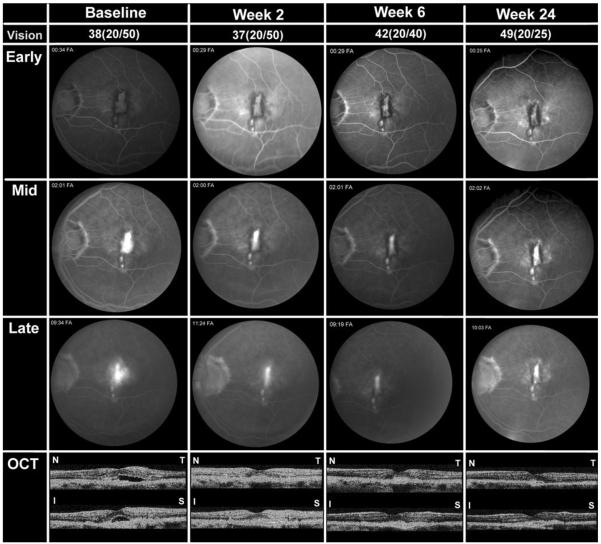
PATIENT 8
A 58-year-old Caucasian man with ocular histoplasmosis syndrome (OHS) and CNV in the left eye had persistent reduction in VA after an intraocular injection of bevacizumab and refused additional injections. Three months after the injection, he entered the study and VA was 54 letters read at 4 meters (20/20) in the right eye and 38 letters (20/50) in the left eye. FA showed leaking CNV in the left eye and OCT showed subretinal fluid and thickening with loss of the foveal pit (Figure 3, baseline). Two weeks after infusion of bevacizumab, the VA in left eye was 20/50, OCT showed resolution of subretinal fluid and reduced thickening in the fovea, and FA showed reduced leakage (Figure 3, week 2). A second infusion was given and one month later VA was 42 letters (20/40) in the left eye and FA showed minimal residual leakage, but a third infusion was given because OCT showed mild intraretinal edema and a small blister of subretinal fluid (Figure 3, week 6). Six weeks later the patient received the fourth infusion and three months later (week 24) VA was 49 letters (20/25) in the left eye and 60 letters (20/20) in right eye. OCT showed no fluid in the left eye and FA showed no leakage (Figure 3, week 24). Six months after the last infusion (week 36), VA was 52 letters (20/25), there was no evidence of leakage on FA, and foveal thickness was 233 μm in the left eye. Nine months after the last infusion (week 48), VA was 47 letters in the left eye (20/32), there was no leakage on FA, and foveal thickness was 220 μm.
FIGURE 3.
Patient 8, a 58-year-old Caucasian man with CNV attributable to ocular histoplasmosis. Studies obtained at baseline and two, four, and 24 weeks after the first infusion are shown, including BCVA in ETDRS letters read at 4 meters and Snellen equivalent, frames from early, mid, and late phases of FA, and horizontal and vertical cross-sections of OCT scans labeled T-N (temporal-nasal) and I-S (inferior-superior), respectively. At the primary endpoint of 24 weeks after the first infusion (followed by two subsequent infusions of 5 mg/kg bevacizumab), the patient showed improved BCVA, reduced CNV lesion size, resolution of leakage, and resolution of subretinal and intraretinal fluid in the macula.
INFUSIONS AND SAFETY
Eight patients received four infusions and two patients received three infusions. Infusions were well tolerated and there were no ocular or systemic adverse events. The median change from baseline in systolic and diastolic blood pressure was less than 5 mm Hg at all study visits. There was no proteinuria noted in any study subjects.
VISUAL ACUITY
There was rapid improvement in median VA to 35 letters after the first infusion, an increase of two lines (Figure 4). At the primary endpoint (24 weeks), the median number of letters read at 4 meters was 48.5, representing an improvement of 23 letters from baseline (P = .005). Visual acuity improved ≥1 line in all 10 patients, ≥2 lines in nine, ≥3 lines in six, ≥4 lines in four, ≥5 lines in four, and ≥6 lines in three. Eight patients had VA of 20/40 or better at 24 weeks compared with only one at baseline.
FIGURE 4.
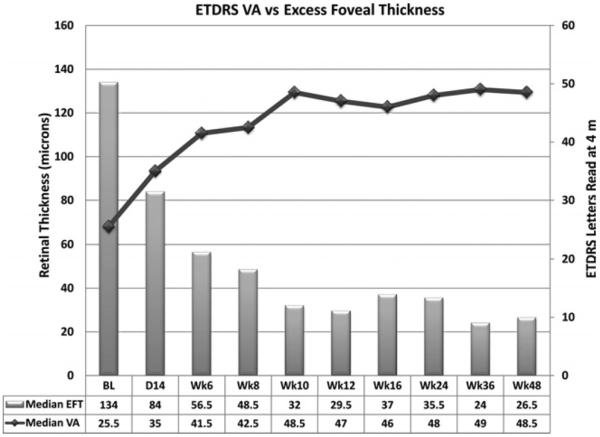
Visual acuity (VA) and excess foveal thickness and subretinal fluid between baseline and the primary endpoint at 24 weeks. Median VA in number of letters read on an ETDRS chart at 4 meters is shown by the line graph. The median VA at baseline was 25.5 letters compared with 48 letters at the primary endpoint, a gain of 23 letters. The median change in excess foveal thickness (EFT)/subretinal fluid (SRF), which is the thickness of the fovea combined with the height of SRF in the fovea – 212 μm, is an indication of the amount of excess fluid within and under the retina is shown by the bar at each time point. The excess fluid present at baseline was reduced by 72%.
FOVEAL THICKNESS
At baseline, the median excess foveal thickness was 134 μm due to subretinal and intraretinal fluid. This was reduced to 84 μm at two weeks after the first infusion, continued to decrease to 32 μm by week 10, and remained around that level through week 24 when it was 35 μm (Figure 4). At 24 weeks, four patients had complete resolution of all identifiable intraretinal and subretinal fluid, five patients were felt to have some residual thickening suggesting possible intraretinal fluid, and one patient had a small pocket of SRF.
FLUORESCEIN ANGIOGRAPHY
In a previous study,9 it was found that classic components of CNV lesions were completely filled by 60 seconds, and this was also true in the current study, providing a measure of CNV area. Dye spread from leakage was maximal at 600 seconds and the difference in area between 600 seconds and 60 seconds provided a measure of the area of retina to which leaked fluorescein had spread (leakage area). Areas of CNV and leakage at baseline and 24 weeks are presented in Table 2 for nine patients (one patient was found to have allergy to FA after entering the study).
TABLE 2.
Anatomical Outcomes: Excess Foveal Thickness and Areas of Choroidal Neovascularization and Leakage from Choroidal Neovascularization
| Leakage Area (disk areas) |
CNV Area (disk areas) |
Excess Foveal Thickness (microns) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | BL | Wk 24 | ΔWk 24 | % | BL | Wk 24 | ΔWk 24 | % | BL | Wk 24 | ΔWk 24 | % | Wk 48 | ΔWk 48 | % |
| 1 | 0.42 | 0.33 | −0.09 | −21 | 1.81 | 2.63 | 0.82 | +45 | 77 | −36 | −113 | −100 | 44 | −33 | −43 |
| 2 | 2.62 | 0.24 | −2.38 | −91 | 4.12 | 1.87 | −2.25 | −55 | 219 | 99 | −120 | −55 | 143 | −76 | −35 |
| 3 | 1.01 | 0.79 | −0.22 | −22 | 1.49 | 1.3 | −0.19 | −13 | 135 | 38 | −97 | −72 | 37 | −98 | −73 |
| 4 | NA | NA | NA | NA | NA | NA | NA | NA | 138 | −1 | −139 | −100 | −4 | −142 | −100 |
| 5 | 0.08 | 0.01 | −0.07 | −88 | 0.65 | 0.17 | −0.48 | −74 | 42 | −13 | −55 | −100 | −12 | −54 | −100 |
| 6 | 1.09 | 0 | −1.09 | −100 | 1.45 | 0.49 | −0.96 | −66 | 116 | 33 | −83 | −72 | 16 | −100 | −86 |
| 7 | 0.12 | 0 | −0.12 | −100 | 0.18 | 0.15 | −0.03 | −17 | 257 | −10 | −267 | −100 | −24 | −281 | −100 |
| 8 | 0.2 | 0 | −0.20 | −100 | 0.33 | 0.26 | −0.07 | −21 | 133 | 43 | −90 | −68 | 8 | −125 | −94 |
| 9 | 0.17 | 0 | −0.17 | −100 | 0.45 | 0.19 | −0.26 | −58 | 140 | 44 | −96 | −69 | 48 | −92 | −66 |
| 10 | 0.13 | 0.17 | 0.04 | +31 | 0.3 | 0.17 | −0.13 | −43 | 114 | 131 | 17 | +15 | 48 | −66 | −58 |
| Mean | 0.65 | 0.17 | −0.48 | −65.64 | 1.20 | 0.80 | −0.39 | −33.47 | 137.10 | 32.80 | −104.30 | −71.95 | 30.40 | −106.70 | −75.40 |
| Median | 0.20 | 0.01 | −0.17 | −90.84 | 0.65 | 0.26 | −0.19 | −43.33 | 134.00 | 35.50 | −96.50 | −71.70 | 26.50 | −95.00 | −79.40 |
Anatomic measures provided for each patient (Pt) are the area of leakage and area of choroidal neovascularization (CNV) determined from fluorescein angiograms as described in the Methods, and the excess foveal thickness calculated by subtracting the measured center subfield thickness obtained by optical coherence tomography from the upper limit of the normal range (212 μm) determined from measurements on a large population of subjects.18 Values for each of these three parameters are provided for baseline (BL) and the primary endpoint, week 24 (Wk 24). The change between baseline and week 24 (ΔWk 24) is shown for each parameter. The excess foveal thickness at Wk 48 and the change between baseline and Wk 48 (ΔWk 48) in excess foveal thickness is also shown.
Boldface indicates the median change from baseline.
Patient 8 with OHS, for whom FAs are shown in Figure 3, had a leakage area at baseline of 0.20 disk areas that was eliminated at 24 weeks, and CNV area was reduced by 21% from 0.33 to 0.26 disk areas. Patient 5 with angioid streaks, for whom FAs are shown in Figure 2, had fairly mild leakage at baseline (0.08 disk areas) that was reduced by 88% to 0.01 disk areas at 24 weeks accompanied by a 74% decrease in CNV area from 0.65 to 0.17 disk areas. The patient with BCR, for whom FAs are shown in Figure 1, had a 91% reduction in leakage area (2.62 to 0.24 disk areas) and a 55% reduction in CNV area (4.11 to 1.87 disk areas). The majority of patients had a dramatic reduction in leakage; elimination of leakage in four patients and reduction by roughly 90% in three patients. Two patients had a modest reduction in leakage area and one of these, Patient 1, is particularly interesting. She had chronic CNV in her right eye with long-standing poor vision and had new onset subfoveal CNV in her left eye with VA better than the eligibility cut-off. The right eye was eligible with regard to VA and had some active leakage, subretinal fluid, and blood, but was not ideal because there was considerable subretinal fibrosis. The patient was insistent that she had no other options, and because she had bilateral active disease and was eligible, she was allowed to enter the trial with the right eye as the study eye. Although there was only a 21% reduction in leakage area and an increase in CNV area at 24 weeks in the right eye, there was substantial resorption of subretinal fluid; foveal thickness was reduced from 289 μm to 176 μm and VA improved by 24 letters. The VA in the nonstudy left eye recovered to 20/20. Aside from this patient with longstanding CNV with a considerable fibrotic component that failed to decrease in size, all other patients showed reduction in area of CNV. The median CNV area at baseline was 0.65 disk areas and this was reduced by 43% to 0.26 disk areas at 24 weeks (Figure 5).
FIGURE 5.
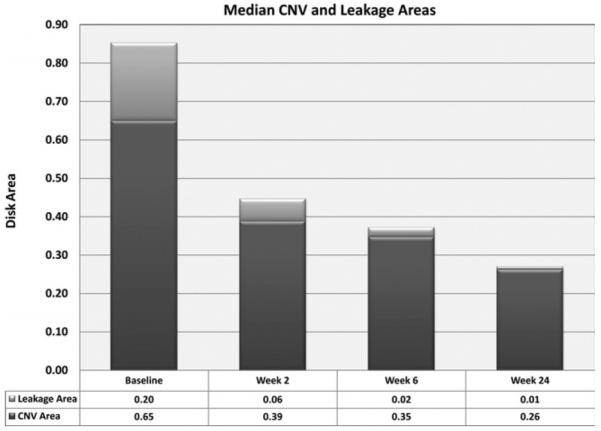
Median area of CNV and area of leakage at baseline and three time points after start of bevacizumab infusions. Each bar represents the median area of CNV (dark gray) and the median leakage area (light gray) calculated as described in the Methods section from measurements made on FAs obtained in nine patients at four time points. There is a sequential decrease in both parameters (total lesion size) at weeks 2 and 6.
Although Patient 10 showed a 43% decrease in CNV area, she was the only patient who did not show reduced leakage at 24 weeks compared with baseline and this was accompanied by a slight increase in foveal thickness on OCT. However, the patient had a nine-letter improvement in visual acuity and prior to the primary endpoint there had been evidence of a more complete response. At week 12, VA was 49 letters, an improvement of 18 letters from baseline, and foveal thickness was 289 μm, an improvement of 37 μm. Thus, the patient had evidence of imrovement during the treatment period, but the benefit was not completely sustained through the primary endpoint.
FOLLOW-UP BETWEEN THE PRIMARY ENDPOINT AND 48 WEEKS
The four patients who had elimination of leakage at 24 weeks remained stable with no additional treatment and no evidence of leakage through 48 weeks. One of the three patients who had reduction of leakage by 90% also remained stable with no additional treatment, but the other two patients complained of reduced vision about five months after the primary endpoint and were found to have mild fluorescein leakage and increased foveal thickness; each had an intraocular injection of 1.25 mg bevacizumab. Patient 1, who had five lines of improvement in VA and marked improvement in foveal thickness despite having a large, partially fibrotic CNV lesion that showed evidence of growth and persistent leakage at 24 weeks, maintained the improvement in VA between 24 and 48 weeks with no additional treatment. Patient 3, like Patient 1, had only a modest reduction in leakage and no substantial reduction in CNV area, but had a marked reduction in foveal thickness from 347 μm to 250 μm and improvement in VA of 27 letters and these improvements were maintained through week 48 without any additional treatment. Patient 10, the only patient who did not show reduced fluorescein leakage at 24 weeks, had an intraocular injection of 1.25 mg bevacizumab at week 27. No additional treatments were needed and at 48 weeks VA was 50 letters, a 19-letter improvement from baseline and a 10-letter improvement from 24 weeks.
DISCUSSION
ranibizumab is the first treatment to cause significant visual improvement in a substantial number of patients with CNV attributable to AMD, confirming that VEGF is a critical stimulus in that disease process.1,2 There is preliminary evidence suggesting that VEGF is also important in CNV attributable to pathologic myopia or angioid streaks. Intravenous infusions of 5 mg/kg bevacizumab caused dramatic improvement in two patients with CNV attributable to pathologic myopia that had not improved after PDT.8 Treatment with intraocular injections of bevacizumab has also been shown to cause improvements in patients with CNV attributable to pathologic myopia11–13 and in a patient with CNV attributable to angioid streaks.14 In this study, detailed prospective analysis of 10 patients with non AMD CNV treated with intravenous infusions of 5 mg/kg bevacizumab has provided additional evidence suggesting that VEGF is a critical stimulus for CNV attributable to pathologic myopia, angioid streaks, and choroiditis (BCR and PIC). Although several of the patients had relatively longstanding disease or had failed other treatments reducing visual prognosis, the median improvement in VA was 23 letters at 24 weeks, the primary endpoint. This was accompanied by anatomic evidence of improvement including a reduction in median foveal thickness from 346 μm to 248 μm (elimination of 72% of excess foveal thickness), and reductions in median fluorescein leakage area and CNV area of 91% and 43%, respectively. These results at the primary endpoint are impressive, but in addition several patients maintained the improvement for many months without additional treatment. Five patients showed no evidence of leakage at 48 weeks, the final visit, about nine months after the last infusion. Another patient showed no evidence of recurrent disease throughout the study, but was last seen at the month 9 visit, six months after the last infusion, because he moved away and refused to return for the one-year visit. Two other patients had disease-free periods of about eight months after the last infusion. Thus, eight of 10 patients had prolonged remissions. One of the two remaining patients had improvement in VA (nine letters) and a 47% reduction in CNV area at the primary endpoint, but required an intraocular injection of bevacizumab for persistent leakage, after which she experienced a disease-free period of at least six months and an improvement in VA at the week 48 visit of 19 letters. The only other patient in the study was the one with a large, partially fibrotic CNV lesion that experienced about a five line improvement in VA that was maintained at least through the week 48 visit without additional treatment, but this patient had evidence of smoldering disease as evidenced by an increase in CNV size, mild persistent leakage, and an increase in foveal thickness from 176 μm at the primary endpoint to 256 μm at 48 weeks.
This picture of stability after three or four bevacizumab infusions in the majority of patients suggests that the CNV was eliminated or made quiescent. One indication of quiescence is elimination of leakage; all of the patients who had a long disease-free period had elimination or near elimination of leakage at the primary endpoint. They also showed reductions in CNV area ranging from 13% to 74%; the amount of regression may not be critical, but its occurrence to any extent may be indicative of a fundamental change within the lesion that is associated with a favorable prognosis. Regression of CNV has not been well documented in treatment of CNV attributable to AMD with intraocular injections of VEGF antagonists, nor is it known if it occurs with intraocular injections of VEGF antagonists in patients with non AMD CNV. These are important questions for future investigations.
We noted beneficial effects of systemic bevacizumab in patients with CNV attributable to pathologic myopia, angioid streaks, or inflammatory eye diseases. Although heterogeneous with regard to primary insult, this population of patients shares certain key features that set the group as a whole apart from patients with neovascular AMD: 1) younger age; 2) lack of diffuse thickening of the Bruch membrane or widespread deposits of abnormal extracellular matrix; and 3) tendency toward small, classic CNV lesions rather than large lesions that frequently have substantial occult components. Perhaps most importantly, compared with neovascular AMD, they tend to share a better visual prognosis, although there are some differences (idiopathic CNV and CNV attributable to pathologic myopia or ocular histoplasmosis appear to have a somewhat better prognosis than CNV attributable to angioid streaks or multifocal choroiditis).
A series of 18 patients with neovascular AMD treated with systemic bevacizumab showed improvement in median VA of 14 letters and substantial reduction in foveal thickness over a period of 24 weeks.15 There was a significant elevation in blood pressure that required adjustment or institution of antihypertensive medicines in several patients. None of our patients showed elevation of blood pressure or any other complications, but the number of patients is too small to draw conclusions. Also, we cannot rule out the unlikely possibility that an adverse effect might become manifested more than seven to eight months after all bevacizumab from the infusions is eliminated from the body, which was the limit of our follow-up. However, taking our data and those obtained in AMD patients together, it seems that systemic anti-VEGF treatment may be more feasible in young patients with CNV than in AMD patients. This is consistent with the observation that most adverse events in cancer patients receiving a combination of bevacizumab and 5-fluorouracil occurred in patients older than 60.16 A large trial would be needed to adequately investigate safety in these patients with CNV attributable to diseases other than AMD in order to recommend systemic bevacizumab. There is unlikely to be strong motivation to conduct such a trial, because case reports and case series suggest that intraocular injections of bevacizumab may provide benefit in patients with CNV attributable to pathologic myopia.11–13,17 Enthusiasm for a large trial would be increased if there was some indication that results might be superior to those obtained after intraocular injections of bevacizumab. However, the results obtained after intraocular injections of bevacizumab are quite good, and there are not enough data at present to draw any conclusions as to how they compare to results obtained with systemic bevacizumab. A reasonable approach would be to offer intraocular injections of ranibizumab or bevacizumab to all young patients with subfoveal CNV and consider further study of systemic bevacizumab only if a leakage-free period of at least three months cannot be achieved, if there are frequent recurrences, or if there is bilateral disease with reasonable visual potential in each eye. Additional small studies may be warranted to determine if such an approach has merit. Also, the optimum systemic dose of bevacizumab for inducing CNV regression has not been investigated. Lower doses than those given in this trial may be adequate to treat CNV and investigation of this possibility should be considered. Finally, the results of this study are not just relevant to bevacizumab, but are also relevant to other orally active VEGF antagonists, and they suggest that the jury is still out on whether systemically administered VEGF antagonists will ever have a role in treatment of young patients with CNV.
Acknowledgments
THIS STUDY WAS SUPPORTED BY A SENIOR SCIENTIST AWARD FROM RESEARCH TO PREVENT BLINDNESS, NEW YORK, NEW York (Dr Campochiaro) and unrestricted grants (Dr and Mrs William Lake and Mr and Mrs Richard Heffner). The authors indicate no financial conflict of interest. Dr Nguyen is a recipient of a K23 Career Development Award (EY 13552) from the National Eye Institute. Involved in design and conduct of study (P.A.C., Q.D.N., G.H., D.V.D., J.A.H., R.P., I.E.Z.-G.); collection, management, analysis, and interpretation of the data (P.A.C., Q.D.N., G.H., S.M.S., K.J., A.S., I.E.Z.-G.); and preparation, review, or approval of the manuscript (P.A.C., Q.D.N., S.M.S., K.J., G.H., D.V.D., J.A.H., A.S., I.E.Z.-G., R.P.). The study was conducted in compliance with the Declaration of Helsinki, US Code 21 of Federal Regulations, the Harmonized Tripartite Guidelines for Good Clinical Practice (1996), and the institutional clinical trials review board of the Johns Hopkins University School of Medicine, and HIPAA. An Investigational New Drug (IND) application was filed with the Food and Drug Administration and an IND number was issued. The study is registered at clinicaltrials.gov (NCT00407719).
Dr Haller is the Robert Bond Welch, MD, Professor of Ophthalmology. Dr Campochiaro is the George S. and Dolores Dore Eccles Professor of Ophthalmology and Neuroscience.
Biographies

Quan Dong Nguyen, MD, MSc, is an Assistant Professor of Ophthalmology at Johns Hopkins, Baltimore, Maryland. A graduate of Phillips Exeter Academy, Yale University, and University of Pennsylvania School of Medicine, Dr Nguyen completed his residency and fellowships in Uveitis and Retina and Vitreous at the Massachusetts Eye and Ear Infirmary and the Schepens Eye Research Institute, and a fellowship in Ocular Immunology at Wilmer. Dr Nguyen focuses his research on early clinical trials of pharmacologic treatments for macular degeneration, macular edema, and ocular inflammatory diseases.

Syed Mahmood Ali Shah, MBBS, is a Research Scientist managing the Retinal Imaging Research and Reading Center (RIRRC) at the Wilmer Eye Institute of the Johns Hopkins Medical Institutions. A graduate of the Aga Khan University Medical College, Karachi, Pakistan, Dr Shah specializes in development of new and novel models of digital retinal imaging and efficacy analysis for clinical trials in ophthalmology.
REFERENCES
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 3.Amin R, Pulkin JE, Frank RN. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994;35:3178–3188. [PubMed] [Google Scholar]
- 4.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- 5.Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. [PubMed] [Google Scholar]
- 6.Mousa SA, Lorelli W, Campochiaro PA. Extracellular matrix-integrin binding modulates secretion of angiogenic growth factors by retinal pigmented epithelial cells. J Cell Biochem. 1999;74:135–143. [PubMed] [Google Scholar]
- 7.Green WR, Wilson DJ. Choroidal neovascularization. Ophthalmology. 1986;93:1169–1176. doi: 10.1016/s0161-6420(86)33609-1. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen QD, Shah SM, Tatlipinar S, Do DV, van Anden E, Campochiaro PA. Bevacizumab suppresses choroidal neovascularization due to pathologic myopia. Br J Ophthalmol. 2005;89:1368–1370. [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SM, Tatlipinar S, Quinlan E, et al. Dynamic and quantitative analysis of choroidal neovascularization by fluorescein angiography. Invest Ophthalmol Vis Sci. 2006;47:5460–5480. doi: 10.1167/iovs.06-0012. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Shah SM, Hafiz G, et al. A phase 1 trial of intravenously administered VEGF trap for treatment in patients with choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006;113:1522e1–1522e14. doi: 10.1016/j.ophtha.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 11.Laud K, Spaide RF, Freund KB, Slakter J, Klancnik JM., Jr Treatment of choroidal neovascularization in pathologic myopia with intravitreal bevacizumab. Retina. 2006;26:960–963. doi: 10.1097/01.iae.0000240121.28034.c3. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi H, Ikuno Y, Gomi F, et al. Intrvitreal injection of bevacizumab for choroidal neovascularization associated with pathological myopia. Br J Ophthalmol. 2007;91:161–165. doi: 10.1136/bjo.2006.099887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathological myopia. Br J Ophthalmol. 2007;91:157–160. doi: 10.1136/bjo.2006.096776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira A, Moraes N, Farah ME, Bonomo PP. Choroidal neovascularization treated with intravitreal injection of bevacizumab (Avastin) in angioid streaks. Acta Ophthalmol Scand. 2006;84:834–836. doi: 10.1111/j.1600-0420.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 15.Moshfeghi AA, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113:2002–2011. doi: 10.1016/j.ophtha.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 17.Tewari A, Dhalla MS, Apte RS. Intravitreal bevacizumab for treatment of choroidal neovascularization in pathologic myopia. Retina. 2006;26:1093–1094. doi: 10.1097/01.iae.0000254896.78766.74. [DOI] [PubMed] [Google Scholar]
- 18.Chan A, Duker JS, Ko TH, Fujimoto JG, Schuman JS. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006;124:193–198. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]