Abstract
Highly pathogenic avian influenza (HPAI), subtype H5N1, was first officially reported in Indonesia in 2004. Since then the disease has spread and is now endemic in large parts of the country. This study investigated the statistical relationship between a set of risk factors and the presence or absence of HPAI in Indonesia during 2006 and 2007. HPAI was evaluated through participatory disease surveillance (PDS) in backyard village chickens (the study population), and risk factors included descriptors of people and poultry distribution (separating chickens, ducks and production sectors), poultry movement patterns and agro-ecological conditions.
The study showed that the risk factors “elevation”, “human population density” and “rice cropping” were significant in accounting for the spatial variation of the PDS-defined HPAI cases. These findings were consistent with earlier studies in Thailand and Vietnam. In addition “commercial poultry population”, and two indicators of market locations and transport; “human settlements” and “road length”, were identified as significant risk factors in the models. In contrast to several previous studies carried out in Southeast Asia, domestic backyard ducks were not found to be a significant risk factor in Indonesia. The study used surrogate estimates of market locations and marketing chains and further work should focus on the actual location of the live bird markets, and on the flow of live poultry and poultry products between them, so that patterns of possible transmission, and regions of particular risk could be better inferred.
Keywords: Avian influenza, H5N1, Epidemiology, Spatial analysis, Indonesia
1. Introduction
Highly pathogenic avian influenza (HPAI), subtype H5N1, was first reported in Indonesia in 2004 (OIE, 2009). The first introduction is unknown, but it is suspected that infection might have originated from reservoirs of infection in wild water birds or illegal importation of infected poultry from neighbouring countries (Sims et al., 2005). Since then the disease has spread over most of the country (FAO, 2010). During this time, 163 human H5N1 cases have been reported, of which 135 have died according to the World Health Organization (WHO, 2010). These human cases were attributed to exposure to infected poultry, and so far no or limited human-to-human transmission appears to have occurred. Hypothetically, the genetic changes necessary to produce a virus which would be capable of transmitting from human-to-human and that could then result in a human influenza pandemic are most likely to occur in epidemiological systems where there is frequent interaction between the various infection reservoirs and humans. Indonesia provides such a system through its large smallholder poultry farming population and trading of almost all (>90%) poultry through traditional markets (McLeod et al., 2009). Trade through markets facilitates mixing of animals from different sources and involves frequent movements of live animals to and from markets, this may increase the chances of spreading disease (Barennes et al., 2007; Sims et al., 2003). Despite enormous effort by the Government of Indonesia to control HPAI, including participatory disease surveillance and response (Jost et al., 2007; Normile, 2007), preventive vaccination and culling of infected backyard flocks, the disease is endemic in large parts of the country. Outbreaks of the disease are detected frequently on the Islands of Java, Bali and Sumatra (FAO, 2010).
Previous studies carried out in Southeast Asia indicated that the disease spread and persistence associates to trading patterns, densities of poultry populations, poultry production structures, live bird markets (Sims, 2007) and ducks (Gilbert et al., 2006; Hulse-Post et al., 2005), although many of those association where not formally tested. Many if not all of the risk factors listed above may apply in Indonesia, but the specificities of the country in terms of environment, production structure and trading may impact the relative contribution of those risk factors, compared to previous work. The relative significance of the different components of this complex epidemiological system needs to be understood to help further refining policy and intervention measures to target the right poultry populations and disease spread pathways.
This study investigated the relationship between the spatial distribution of risk factors and evidence of HPAI presence in Indonesia during 2006 and 2007 detected through participatory disease surveillance (PDS). The analyses were conducted at district level and at village level in selected areas. Risk factors associated with HPAI H5N1 virus presence in Indonesia were compared with risk factors identified in Thailand and Vietnam.
2. Data and methods
2.1. Participatory disease surveillance (PDS) data
The PDS data were provided by the Directorate General of Livestock Services in the Ministry of Agriculture, Jakarta, Indonesia. The study was conducted on the Islands of Java and Bali, and in the provinces of Lampung (southern Sumatra) and North Sumatra. The veterinary PDS was first implemented in Indonesia in 2006 (Jost et al., 2007), and data collected from January 2006 to December 2007 in the study areas were used. In this period approximately 600 teams of Indonesia Government officers trained in PDS (Jost et al., 2007; Normile, 2007) visited households in villages over 168 districts and investigated possible HPAI outbreaks in backyard poultry (the study population). Active surveillance (the scheduled PDS visit found HPAI) and passive surveillance (the PDS team responded to notification of undiagnosed death in backyard poultry) was conducted. On occasion, multiple visits to the same (=geo-referenced boundaries (BPS, 2007)) village in the two year period were conducted and recorded. The diagnosis of a PDS-defined HPAI case event in backyard poultry was based upon detecting active outbreaks meeting the definition of “sudden death” combined with a positive rapid antigen test (Anigen©Influenza A test, Animal Genetics Incorporated) (Jost et al., 2007). The sensitivity of the rapid antigen test was low and up to three tests on three different sick or dead birds were conducted to reduce false negative tests to less than 4% (presuming all birds were shedding virus) (Loth et al., 2008). The exact location of the investigation and interview was recorded using a handheld Global Positioning System (GPS) receiver.
2.2. Geospatial datasets of risk factors
Risk factors considered in this analysis included agricultural production systems relevant to poultry farming and factors identified in earlier studies (Gilbert et al., 2008; Morris et al., 2005; Pfeiffer, 2006; Rushton et al., 2004; VSF, 2004). These risk factors were grouped, according to their role in disease outbreak, transmission and persistence. A list of all the variables used in the analysis is shown in Table 1.
-
-Human population related transmission:
- The Indonesian Population Census data of 2005, which recorded the number of human habitants per village, was used. Village-level human population data were aggregated to district level, using the district-level administration boundary map. The census data and the geo-referenced boundaries of villages and districts were obtained through BPS Statistics Indonesia (BPS, 2007).
- Human population densities at district and village levels were calculated, based on land areas at district and village levels, respectively.
- Urban and rural areas and the number of people living in these areas were estimated from the Global Rural Urban Mapping Project dataset produced by the Center for International Earth Science Information Network (CIESIN, 2004).
- Human population density is log-normally distributed, and therefore, a log-transformation of human population density was carried out, and used in the models. In addition, a quadratic term for population density was also added to explore the curvilinear relationship between human population and HPAI H5N1 in backyard poultry.
-
-Poultry trade and market related transmission:
- The centres of major human settlements (e.g., cities) were assumed to be related to poultry trade. The dataset for geo-referenced locations of major human settlements was obtained through CIESIN (2004).
- Poultry movement was regarded as a risk factor (Rappole and Hubalek, 2006). Total length of roads in a district was calculated and used for risk analysis at district level. For village level analysis, the distance from the village centre to the closest road was calculated and used for risk analysis.
-
-HPAI disease outbreaks in chickens and the amplification of H5N1 virus:
- Poultry dataset was comprised of (1) provincial-level poultry numbers for Indonesia and (2) district-level poultry data for the 168 districts in this study (Statistik Peternakan, 2006). The densities of backyard (rural, backyard), commercial broiler, and commercial layer chickens in a district were calculated based upon the surface area of that district. Total commercial poultry density was calculated by combining the layer and broiler densities. Poultry density was log-normally distributed, and a log transformation of poultry data was carried out and used in the models.
-
-HPAI disease and virus persistence in ducks:
- Duck populations are seen as a major risk for HPAI in chickens (Gilbert et al., 2006). District-level duck numbers and densities in 168 districts were available for this study (Statistik Peternakan, 2006).
-
-Agro-ecological and environmental risk factors:
- Free ranging ducks are associated with rice production as they feed on post-harvested rice fields. Geospatial datasets of paddy rice and cropping intensity (single-, double- and triple-cropping in a year) at 500-m spatial resolution, derived from analysis of satellite images from Moderate Resolution Imaging Spectroradiometer (MODIS) on board the NASA Terra satellite in 2005, were used (Gilbert et al., 2008; Xiao et al., 2005, 2006).
- Time-series satellite images were used to separate permanent (year-long standing) surface water bodies from seasonally flooded water bodies (including flooded rice fields) (Xiao et al., 2006). The percentages of permanent surface water and permanent vegetation (e.g., evergreen forests, scrubland) in a district were calculated and included in the risk analysis.
- Elevation had been identified as a significant risk factor in Thailand (Gilbert et al., 2006) and Vietnam (Pfeiffer et al., 2007). High elevation areas are usually dominated by forest and other natural vegetation types, while low altitude areas (e.g., flat plains, river deltas and coastal areas) are dominated by agriculture (Gilbert et al., 2004, 2008). The elevation data from the 90 m resolution SRTM dataset was used (CGIAR, 2007). The mean elevation and the range of elevation for individual districts and villages were calculated and included in the analysis. A quadratic term of elevation is used to explore a curvilinear relationship between elevation and H5N1 risks.
Table 1.
Variables at district and village level.
| Variable code | District | Village |
|---|---|---|
| PDS-defined H5N1 case | Ratio of PDS defined HPAI cases and non cases (positive and negative visits) | PDS defined HPAI cases and non cases |
| vill_pop | Number of people per district | Number of people per village |
| vill_pop_dens | Number of people per square km | Number of people per square km |
| hpop_rural_log | Log[number of people living rurally] | Log[number of people living rurally] |
| hpop_rural_log2 | Log[number of people living rurally]2 | Log[number of people living rurally]2 |
| hpop_urban_log | Log[number of urbanised people] | Log[number of urbanised people] |
| hpop_urban_log2 | Log[number of urbanised people]2 | Log[number of urbanised people]2 |
| settlement | The number of urban centres in the district | Distance to closest urban centre (km) |
| markets_km | The distance (km) from the middle of the village to the closest urban centre | |
| roadlen | Length of roads (km) | Distance to closest road (km) |
| native_denlog | Log[Backyard chickens per square km] | |
| native_log | Log[Total number of backyard chickens] | |
| brla_dens_log | Log [Commercial poultry (broilers and layers) per square km] | |
| brla_log | Log [Total number of commercial poultry] | |
| duck_denslog | Log[Ducks per square km] | |
| ducks_log | Log[Total number of ducks] | |
| rice_area | Area covered in rice fields (square km) | Area covered in rice fields (square km) |
| cropmax | Number of rice harvests per year | Number of rice harvests per year |
| water_area | Area covered by water (square km) | |
| water_pct | Percentage of water | Percentage of water |
| water_pct2 | [Percentage of water]2 | [Percentage of water]2 |
| vege_area | Area covered in evergreen vegetation (square km) | |
| vege_pct | Percentage of evergreen vegetation | Percentage of permanent vegetation |
| vege pct2 | [Percentage of evergreen vegetation]2 | [Percentage of permanent vegetation]2 |
| elev_avg | Average elevation | Average elevation |
| elev_avg2 | [Average elevation]2 | [Average elevation]2 |
| elev_max | Maximum elevation | Maximum elevation |
| elev_min | Minimum elevation | Minimum elevation |
| elev_range | Elevation Range | Elevation Range |
2.3. Statistical analysis methods
The statistical and spatial analysis techniques used were similar to the methods used to investigate the spatial distribution of HPAI outbreaks in relation to poultry, land use, and other variables in Thailand (Gilbert et al., 2006), Vietnam (Gilbert et al., 2008) and Indonesia (Pfeiffer, 2006).
The initial logistic model used all the variables shown in Table 1. Non-significant variables (P > 0.05) were removed using a backward selection procedure. In a multivariate model, colinearity between variability may influence the coefficient and significance of individual variables. In order to check that the sign and significance of the variable did not result from the presence of another variable in the model, each variable was tested in a simple model containing the variable alone, or the variable and its quadratic term. Linear spatial statistical models are affected by autocorrelation between response and predictor variables, i.e., the tendency for the value of neighbouring points to be more similar than those from distant points. This tendency, known as spatial autocorrelation, violates the assumption of independence among samples replicated through space. Spatial autocorrelation in the general model was accounted for by applying an autologistic approach (Augustin et al., 1996), where the extent of the autocorrelation of the outcome/response variable was first obtained from the range of the spatial correlogram ρ(h). This extent was then used to derive an autoregressive term that was added as predictor in the logistic model. The autoregressive term was also used in the models testing variables one by one.
Village level analysis was conducted on the islands of Java and Bali. Logistic regression, with the binary outcome of PDS-defined HPAI detection or absence in a village, was conducted. Applying logistic regression models to disease data with low prevalence values for the response variable (<10%) tends to bias model performance metrics (McPherson et al., 2004). To adjust for this, bootstrapping was applied at village level. All PDS-defined HPAI case positive villages were selected and an equivalent number of randomly selected HPAI negative villages. This operation was bootstrapped 500 times. This procedure was used twice. Firstly; to obtain the range of the spatial correlogram ρ(h) for the response variable: the presence or absence of HPAI at village level, a bootstrapped estimate of the semivariogram was used. Secondly; for each of the 500 village level auto-logistic models, the coefficient of each variable and the Akaike’s Information Criteria (AIC) (goodness of model fit) were estimated. The average of the 500 bootstraps were calculated and used in the final model. The performance of the village level models was assessed by determining the area under the curve (AUC) of the receiver operating characteristics (ROC) plots. AUC is a quantitative measure of the overall fit of the model that varies from 0.5 (chance event) to 1.0 (perfect fit) (Greiner et al., 2000).
District level analysis with as outcome the weighted ratio of the number of visits where PDS-defined HPAI case was detected and the number of PDS visits without detection in the district. In order to exclude districts with unreliable estimates of proportion of positives, and those very small district essentially composed of surface water, districts with less than 25 PDS visits recorded or with more than 90% permanent surface water were excluded from the analysis. The model used was a generalized linear model with a binomial response function. It included an autoregressive term to account for spatial autocorrelation, but did not need to account for strong bias in the proportion of positive, as the distribution of weighted ratios at the district level did not present such unbalances compared to the village level. In order to verify that the significance or sign of the risk factors was not influenced by the number of visits, this variable was included in a separate model.
The performance of the model was assessed by estimating the correlation of the outcome of the model with the actual PDS ratio. In addition, the predictive power of the regional model presented in Gilbert et al. (2008) against the PDS data was evaluated.
All statistical models were carried out using R software (R Development Core Team, 2008)
3. Results
In the study area and period (2006–2007), 126,488 households in 168 districts were visited and interviewed. During these investigations, a total of 4780 PDS-defined HPAI events were recorded by a matching case definition and a positive antigen test in the field. The overall disease detection rate (the number of PDS-defined HPAI cases divided by the total number of PDS visits) during the investigation period was 3.8% with a 95% confidence interval (CI) of 3.7% to 3.9%. The disease detection rates varied widely; the province of Yogyakarta recorded the highest detection rates (16.5%, 95% CI: 15.3–17.7%) and the province City of Jakarta the lowest (0.3%, 95% CI: 0.2–0.7%).
Exploratory data analysis of individual risk factors at the district level showed that strong co-linearity occurred among the poultry variables used in this study: “ducks density”, “broiler density”, “layer density”, “backyard chicken density” and “commercial poultry density” (combined broiler and layer). The correlation between the five variables was high (Pearson r commercial poultry – ducks = 0.61; Pearson r commercial poultry – backyard chicken = 0.66; Pearson r ducks – backyard chickens = 0.68). The commercial poultry density variable had the strongest association with the outcome at district level “PDS ratio”, and was the variable kept in the model. The other variables were either not significant when added to the model together with the “commercial poultry” variable, or changed from being a risk factor to being protective.
At the village level, evidence of PDS defined HPAI cases were detected in 2310 villages (8.8%) out of the 26,192 villages surveyed on the islands of Java and Bali. The bootstrapped estimate of the semivariogram of HPAI presence/absence data showed autocorrelation at distances <0.40 decimal degrees, which was set as the maximum distance of the autoregressive term. The significant variables (risk factors) identified by the autologistic regression models were “human population”, “commercial poultry population”, “movement (road length)”, “markets (settlements)”, “elevation” and “rice crop intensity” (Table 2). The autoregressive term was highly significant, which confirmed the presence of spatial autocorrelation in the data. The goodness-of-fit of the model of the (bootstrapped) AIC had a mean of 5265 with a standard deviation of 44. The average, 500 bootstrapped, AUC was 0.671 ± 0.00571.
Table 2.
Results of the autologistic regression model for villages. The outcome variable was the presence or absence of HPAI outbreaks at the village level.
| Variable | Mean | Standard deviation | Odds ratio | Ch-2LLa | Significance of change |
|---|---|---|---|---|---|
| (Intercept) | −2.428 | 0.1161 | – | – | – |
| brla_log | 0.0714 | 0.0289 | 1.0740 | 3.6 | 0.0572 |
| roads_len | 0.100 | 0.0198 | 1.1053 | 30.7 | <0.001 |
| elev_avg | 4.27E–04 | 1.91E–04 | 1.0004 | 2.5 | 0.113 |
| elev_avg2 | −8.80E–07 | 1.69E–07 | 1.0000 | 12.4 | <0.001 |
| markets_km | −0.0119 | 0.00337 | 0.9882 | 5.8 | 0.0159 |
| hpop_urbanlog | 0.0732 | 0.0183 | 1.0760 | 7.9 | 0.00485 |
| hpop_rural_log | 0.0886 | 0.0231 | 1.0927 | 8.2 | 0.00409 |
| cropmax | 0.0335 | 0.00621 | 1.0340 | 69.6 | <0.001 |
| Art | 49.272 | 1.9019 | – | 671.9 | <0.001 |
Average change in −2 Log-likelihood upon removal of variable.
At the district level, the analysis included 150 out of the 168 districts initially surveyed, where varying proportion of positives were observed (Fig. 1). The standardized semivariogram showed evidence of spatial autocorrelation in “PDS ratio” at distances <0.20 decimal degrees (latitude/longitude), and this distance was set as the maximum distance in estimating the autoregressive term (ArT). Significant variables (risk factors) identified by the model were “human population”, “commercial poultry population”, “movement (road length)”, “markets (settlements)”, “elevation” and the area of the district covered with rice paddies (Table 3). Again, the autoregressive term was highly significant. The goodness-of-fit of the model (AIC) was 4617. As measure of predictive power assessment the spearman correlation between predicted and observed ratios was 0.309 (P < 0.001).
Fig. 1.
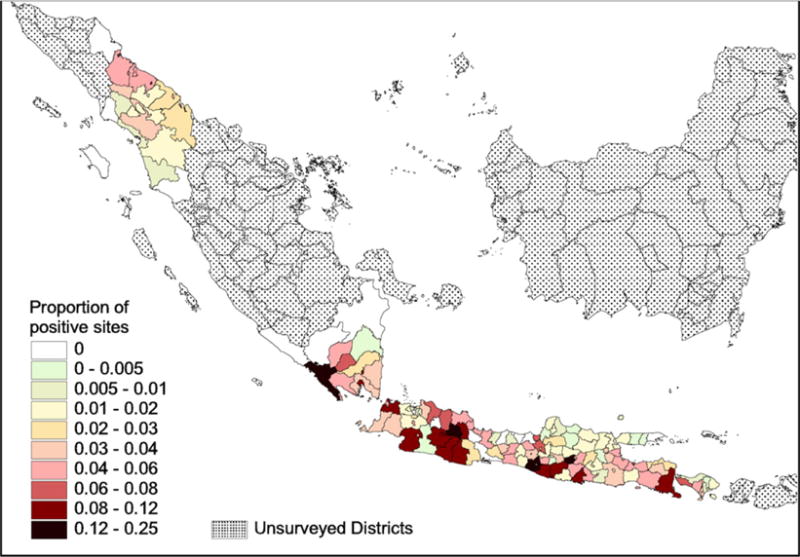
District level distribution of proportion of PDS positive sites on the islands of Bali, Java (all districts) and Sumatra.
Table 3.
Results of the autologistic regression model for districts. The outcome variable was the weighted ratio of the number of visits with outbreaks recorded and the number visits without outbreaks during the period.
| Variable | Mean | Standard deviation | Odds ratio | Ch-2LLa | Significance of change |
|---|---|---|---|---|---|
| (Intercept) | −4.372 | 0.0935 | – | – | – |
| brla_dens_log | 0.1245 | 0.02026 | 1.133 | 36.627 | <0.0001 |
| rice_area | 0.000546 | 0.000103 | 1.001 | 24.362 | <0.0001 |
| elev_avg | 1.53E–03 | 2.18E–04 | 1.002 | 48.074 | <0.0001 |
| elev_avg2 | −1.31E–06 | 2.18E–07 | 1.000 | 35.277 | <0.0001 |
| hpop_urban_log | 0.1242 | 0.04717 | 1.132 | 5.01 | 0.0252 |
| hpop_urban_log2 | −0.0476 | 0.00797 | 0.954 | 34.212 | <0.0001 |
| hpop_rural_log | 0.432 | 0.05709 | 1.540 | 53.827 | <0.0001 |
| hpop_rural_log2 | −0.07825 | 0.009753 | 0.925 | 59.961 | <0.0001 |
| settlement | 0.09334 | 0.008658 | 1.098 | 115.687 | <0.0001 |
| road_len | 2.62E–07 | 1.03E–07 | 1.000 | 4.408 | 0.0358 |
| ArT | 32.56 | 1.941 | – | 287.899 | <0.0001 |
Change in −2 Log-likelihood upon removal of variable.
Both at the village and district level, the effect of each variable included in the multivariate model remained significant, and with the same sign, when tested separately (Supplementary Tables 1a and 1b). In complement, including the number of visits as a covariate had little impact on the significance of the risk factors in the district multivariate model, with the exception of human population density (Supplementary Table 2).
The prediction of the proportion of PDS positive visits for surveyed districts is shown in Fig. 2 (top). Previous predictions of the probability of HPAI presence (Gilbert et al., 2008) did not correlate well to the prediction of our model, nor with the distribution of observed PDS ratios (Fig. 2 bottom map).
Fig. 2.
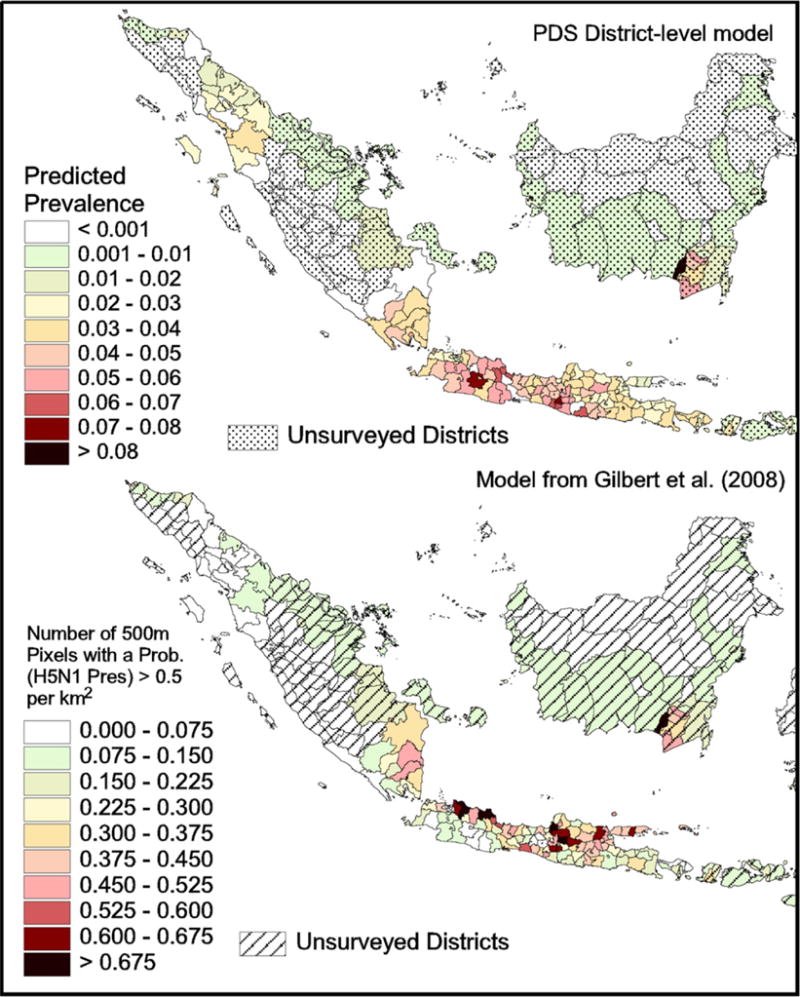
District-level predictions (top) of the proportion of PDS positives per district, and predictions from Gilbert et al. (2008) (bottom) expressed as numbers of 500-m pixels with a probability of H5N1 presence >0.5/km2 (Spearman ρ = 0.117; N.S. with observed PDS ratios).
4. Discussion
The results from this study showed that the risk factors “elevation”, “human population density” and “cropping intensity” were significant in accounting for the spatial variation of the PDS-defined HPAI cases in Java and Bali. “Elevation” and “human population density” were significant for the statistical models at both district and village levels, “cropping intensity” was significant for the statistical models at village levels. These findings were consistent with earlier studies in Thailand and Vietnam (Gilbert et al., 2008). In addition “human settlements” (Poultry markets have been identified as a major risk factor contributing to the spread of HPAI in poultry (Indriani et al., 2010; Wang et al., 2006), but no data on locations of approximately 13,500 markets in Indonesia were available) and “road length”, which could be indicators of market locations and transport, were identified as significant risk factors in the models at both district- and village levels. “Road length” was also identified as a significant risk factor in the province of West Java by Yupiana et al. (2010).
This study partitioned total population into rural and urban human populations, and explored their exposure risk to the H5N1 cases reported by PDS. The results showed that those areas with very densely populated urban areas and very low levels of rural human occupation tends to be associated to lower levels PDS-defined HPAI presence. In contrast, areas with lower dense urban areas (peri-urban) and densely populated rural areas tend to statistically associated to PDS-defined HPAI cases. This could be indicative of the high human and poultry movements, number of markets, low distance to markets and high commercial poultry production observed in peri-urban areas. “Road length” and “markets (centers of urban areas)” and “settlements” were all found to contribute to the models predictive power. Human population settlement was also correlated with elevation; high population density in low elevation areas, but low population density in high elevation areas.
This study used the HPAI outbreak data from HPAI surveillance in backyard poultry, but surprisingly, backyard poultry numbers as reported at the district level were not or not as significant as the number of commercial poultry in the statistical model at the district level. The strong correlations between commercial poultry and backyard poultry data in Indonesia suggested that the district-level poultry data needs to be further evaluated for its sources and quality. This may also explain why a smaller scale study identified poultry density negatively associated with HPAI outbreaks in poultry in West Java (Yupiana et al., 2010). In several previous studies, domestic backyard ducks had been identified as major risk factors (Martin et al., 2006; Tiensin et al., 2007), but this study showed that ducks were apparently comparatively less of an important risk factor compared to commercial poultry in Indonesia. It is also interesting to note that rice paddies and rice cropping were significant in district- and village-level analysis; as backyard duck production in rural families are closely coupled with paddy rice agriculture, the significance of paddy rice in this study may indicate that ducks did play a role in the spread HPAI infection that was not necessarily picked up through the duck census data, an observation that has already been made in previous studies (Gilbert et al., 2008). An alternative, and not exclusive, hypothesis is that high cropping intensity corresponds to regions with high irrigation network where transmission through water contamination would be facilitated.
Comparing the findings of this study with previous risk maps (Gilbert et al., 2008) did not show results with much agreement in the distribution of risk areas (Fig. 2). Those differences can largely be attributed to the result that duck distribution was the key variable predicting the distribution of risk in Thailand and Vietnam, while the distribution of PDS cases correlated much more strongly to the distribution of broilers and layers. The low statistical association between duck numbers and PDS-defined HPAI cases in Indonesia may be attributed to an actual comparatively lower role of the ducks in Avian Influenza epidemiology in Indonesia as compared to other countries. One cannot exclude, however, some possible discrepancies between duck census data and actual numbers in the field, as the relationship with rice cropping partly suggests.
Although many significant variables were identified in our models, considerable variability remained unexplained by both models, although the predictive power is not that far from that of previous studies that tried to understand spatial patterns of HPAI risk elsewhere. This may have multiple causes. First, the analysis relied on the assumption that PDS defined HPAI cases reflected the actual disease situation during the period, or at least that the omission error was homogeneously distributed over the surveyed spatial units. PDS was only conducted in villages (backyard poultry) while no information was available of HPAI outbreaks in commercial poultry flocks. Second, the study assumed that villages were randomly visited. If a significant number of inaccessible villages were not visited, randomised sampling could not be assumed, resulting in bias study outcomes. Third, several variables used in this model are only surrogate estimates of the true variable of epidemiological interest (e.g. assuming that the live bird markets distribution follows that of settlements, or that roads density is an indicator of poultry trade intensity). Having risk factors more closely related to the true epidemiological variable of interest would be better able to generate predictions closer to the observations. Fourth, in dynamic and seasonal market chains implying both commercial and backyard production, good census data are notoriously difficult to obtain, and one can expect a significant variability between the poultry census data used in this study and the reality of the numbers of poultry actually present in the field at the time of PDS surveys. Fifth, there may have been temporal disease detection rate variations and fluctuations of livestock numbers, both possibly caused by seasonal weather patterns during the study period and these were not accounted for. Finally, other factors that may be important to HPAI spread and persistence were simply not tested in the model. For example, the supply chain structure of the diverse poultry production systems, contacts with migratory and resident wild birds, and environmental conditions (humidity, temperature, sun hours, etc.) affecting persistence of the virus outside the host in the environment (Brown et al., 2007) were not accounted for. Given all these possible sources of variations, the fact that we found risk factors associated with PDS-defined cases is in itself encouraging. These findings could assist the Government of Indonesia to target surveillance and to concentrate response efforts in high priority areas and high priority poultry production systems.
This study used surrogate estimates of the distribution of markets and marketing chains. Further work should focus on collection of more detailed data on the distribution of the live bird markets themselves, and on the flow of live poultry and poultry products that connect these markets, so that patterns of possible transmission, and regions of particular risk could be better inferred.
Supplementary Material
Acknowledgments
The authors would like to thank John Weaver for his technical comments. This study was supported by the Food and Agriculture Organisation of the United Nations, and the National Institutes of Health Fogarty International Center grant (R01TW00786901; through the NIH and National Science Foundation Ecology of Infectious Diseases program), the Global Avian Influenza Network for Surveillance project from the Wildlife Conservation Society, the Australian Government Overseas Aid Program and the US Agency for International Development.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.prevetmed.2011.06.006.
References
- Augustin NH, Mugglestone MA, Buckland ST. An autologistic model for the spatial distribution of wildlife. J Appl Ecol. 1996;33:339–347. [Google Scholar]
- Barennes H, Martinez-Aussel B, Vongphrachanh P, Strobel M. Avian influenza risk perceptions, Laos. Emerg Infect Dis. 2007;13 doi: 10.3201/eid1307.061197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BPS. Badan Pusat Statistik Indonesia. 2007 Retrieved 20 July 2007, from http://www.bps.go.id/sector/population/
- Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 2007;51:285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- CGIAR. The CGIAR Consortium for Spatial Information. 2007 Retrieved Retrieved 6 November 2007, from http://srtm.csi.cgiar.org/SRTM/
- CIESIN. Cities, Center for International Earth Science Information Network, Columbia University; International Food Policy Research Institute (IPFRI), the World Bank; and Centro Internacional de Agricultura Tropical (CIAT), Global Rural-Urban Mapping Project (GRUMP): Settlement Points. Palisades, NY: CIESIN, Columbia University; 2004. Retrieved 12 June 2008, from http://sedac.ciesin.columbia.edu/gpw/global.jsp. [Google Scholar]
- FAO. Emergency Prevention System (EMPRES) for Transboundery Animal and Plant Pest and Diseases, EMPRESMAP. 2010 doi: 10.1196/annals.1307.003. Retrieved 1 May 2010, from http://www.fao.org/ag/againfo/programmes/en/empres/mapsnew.html. [DOI] [PubMed]
- Gilbert M, Chaitaweesub P, Parakamawongsa T, Premashthira S, Tiensin T, Kalpravidh W, Wagner H, Slingenbergh J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg Infect Dis. 2006;12:227–234. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Wint W, Slingenbergh J. The Ecology of Highly Pathogenic Avian Influenza in East and Southeast Asia: Outbreak Distribution, Risk Factors and Policy Implications. Consultancy report for the Animal Health Service of the Animal Production and Health Division of the Food and Agriculture Organization of the United Nations; Rome, Italy: 2004. p. 43. [Google Scholar]
- Gilbert M, Xiao X, Pfeiffer DU, Epprecht M, Boles S, Czarnecki C, Chaitaweesub P, Kalpravidh W, Minh PQ, Otte MJ, Martin V, Slingenbergh J. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc Natl Acad Sci USA. 2008;105:4769–4774. doi: 10.1073/pnas.0710581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Hulse-Post DJ, Sturm-Ramirez KM, Humberd J, Seiler P, Govorkova EA, Krauss S, Scholtissek C, Puthavathana P, Buranathai C, Nguyen TD, Long HT, Naipospos TS, Chen H, Ellis TM, Guan Y, Peiris JS, Webster RG. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci USA. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriani R, Samaan G, Gultom A, Loth L, Indryani S, Adjid R, Dharmayanti N, Weaver J, Mumford E, Lokuge K, Kelly P, Darminto Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerg Infect Dis. 2010;16 doi: 10.3201/eid1612.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost CC, Mariner JC, Roeder PL, Sawitri E, Macgregor-Skinner GJ. Participatory epidemiology in disease surveillance and research. Rev Sci Tech - Office International des Epizooties. 2007;26:537–549. [PubMed] [Google Scholar]
- Loth L, Prijono WB, Wibawa H, Usman TB. Evaluation of two avian influenza type A rapid antigen tests under Indonesian field conditions. J Vet Diagn Invest. 2008;20:642–644. doi: 10.1177/104063870802000519. [DOI] [PubMed] [Google Scholar]
- Martin V, Sims L, Lubroth J, Pfeiffer D, Slingenbergh J, Domenech J. Epidemiology and ecology of highly pathogenic avian influenza with particular emphasis on South East Asia. Dev Biol (Basel) 2006;124:23–36. [PubMed] [Google Scholar]
- McLeod A, Kobayashi M, Gilman J, Siagian A, Young M. The use of poultry value chain mapping in developing HPAI control programmes. World’s Poultry Sci J. 2009;65:217–224. [Google Scholar]
- McPherson JM, Jetz W, Rogers DJ. The effects of species’ range sizes on the accuracy of distribution models: ecological phenomenon or statistical artefact? J Appl Ecol. 2004;41:811–823. [Google Scholar]
- Morris R, Jackson R, Stevenson R, Benard H, Cogger N. Epidemiology of H5N1 Avian Influenza in Asia and Implications for Regional Control. 2005 Retrieved 12 July 2009, from http://www.fao.org/docs/eims/upload//246974/aj122e00.pdf.
- Normile D. Epidemiology. Indonesia taps village wisdom to fight bird flu. Science. 2007;315:30–33. doi: 10.1126/science.315.5808.30. [DOI] [PubMed] [Google Scholar]
- OIE WAHID Interface Animal Health Information. 2009 Retrieved 12 May 2009, 2007, from http://www.oie.int/wahidprod/public.php?page=home.
- Pfeiffer D. Assistance in the Geospatial Analysis of HPAI outbreaks in Indonesia. 2006 Retrieved 12 December 2007, from http://www.fao.org/docs/eims/upload/199669/Pfeiffer_Report_Indonesia_2005.pdf.
- Pfeiffer DU, Minh PQ, Martin V, Epprecht M, Otte MJ. An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. Vet J. 2007;174(2):302–309. doi: 10.1016/j.tvjl.2007.05.010. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: 2008. Retrieved 28 November 2008, from http://www.R-project.org. [Google Scholar]
- Rappole JH, Hubalek Z. Birds and influenza H5NI virus movement to and within North America. Emerg Infect Dis. 2006;12:1486–1492. doi: 10.3201/eid1210.051577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton J, Viscarra R, Guerne-Bleich E, Mcleod A. Impact of Influenza Outbreaks in the Poultry Sectors of Five South-east Asian Countries (Cambodia, Indonesia, Lao PDR, Thailand, Viet Nam) Outbreak Costs, Responses and Potential Long-term Control. Food and Agriculture Organization of the United Nations; 2004. Retrieved 18 July 2009, from www.fao.org/docs/eims/upload/214194/poultrysector_seasia_en.pdf. [Google Scholar]
- Sims LD. Lessons learned from Asian H5N1 outbreak control. Avian Dis. 2007;51:174–181. doi: 10.1637/7637-042806R.1. [DOI] [PubMed] [Google Scholar]
- Sims LD, Domenech J, Benigno C, Kahn S, Kamata A, Lubroth J, Martin V, Roeder P. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet Rec. 2005;157:159–164. doi: 10.1136/vr.157.6.159. [DOI] [PubMed] [Google Scholar]
- Sims LD, Ellis TM, Liu KK, Dyrting K, Wong H, Peiris M, Guan Y, Shortridge KF. Avian influenza in Hong Kong 1997–2002. Avian Dis. 2003;47:832–838. doi: 10.1637/0005-2086-47.s3.832. [DOI] [PubMed] [Google Scholar]
- Statistik Peternakan. Statistik Peternakan. Direktorat Jenderal Peternakan Departemen Pertanian RI; Jakarta: 2006. [Google Scholar]
- Tiensin T, Nielen M, Songserm T, Kalpravidh W, Chaitaweesub P, Amonsin A, Chotiprasatintara S, Chaisingh A, Damrongwatanapokin S, Wongkasemjit S, Antarasena C, Songkitti V, Chanachai K, Thanapongtham W, Stegeman JA. Geographic and temporal distribution of highly pathogenic avian influenza A virus (H5N1) in Thailand, 2004–2005: an overview. Avian Dis. 2007;51:182–188. doi: 10.1637/7635-042806R.1. [DOI] [PubMed] [Google Scholar]
- VSF. Organization of Avian Production and Description of HPAI Epidemiological Patterns in Vietnam - Intermediate Report. Veterinaires Sans Frontieres/World Bank; 2004. Retrieved 12 August 2009, from www.fao.org/docs/eims/upload//246973/aj121e00.pdf. [Google Scholar]
- Wang M, Di B, Zhou D, Zheng B, Jing H, Lin Y, Liu Y, Wu X, Qin P, Wang Y, Jian L, Li X, Xu J, Lu E, Li T, Xu J. Food markets with live birds as source of avian influenza. Emerg Infect Dis. 2006;12:1773–1775. doi: 10.3201/eid1211.060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Avian influenza—situation in Indonesia—update 12 February 2010. 2010 Retrieved 1 May 2010, from http://www.who.int/csr/don/2010_02_12a/en/index.html.
- Xiao X, Boles S, Frolking S, Li C, Babu JY, Salas W, Moore B. Mapping paddy rice agriculture in South and Southeast Asia using multi-temporal MODIS images. Remote Sens Environ. 2006;100:95–113. [Google Scholar]
- Xiao X, Boles S, Liub J, Zhuangb D, Frolkinga S, Lia C, Salasc W, Moore B. Mapping paddy rice agriculture in southern China using multi-temporal MODIS images. Remote Sens Environ. 2005;95:480–492. [Google Scholar]
- Yupiana Y, de Vlas SJ, Adnan NM, Richardus JH. Risk factors of poultry outbreaks and human cases of H5N1 avian influenza virus infection in West Java Province, Indonesia. Int J Infect Dis. 2010;14:e800–e805. doi: 10.1016/j.ijid.2010.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


