Abstract
Purpose
Antitumor activity of cancer immunotherapies may elicit immune responses to nontargeted (secondary) tumor antigens, or antigen spread. We evaluated humoral antigen spread after treatment with sipuleucel-T, an immunotherapy for asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC), designed to target prostatic acid phosphatase (PAP; primary antigen).
Experimental Design
Serum samples from patients with mCRPC enrolled in the placebo-controlled phase III IMPACT study (evaluable n = 142) were used to assess humoral antigen spread after treatment with sipuleucel-T. Immunoglobulin G (IgG) responses to self-antigens (including tumor antigens) were surveyed using protein microarrays and confirmed using Luminex xMAP. IgG responses were subsequently validated in ProACT (n = 33), an independent phase II study of sipuleucel-T. Association of IgG responses with overall survival (OS) was assessed using multivariate Cox models adjusted for baseline prostate-specific antigen (PSA) and lactate dehydrogenase levels.
Results
In patients from IMPACT and ProACT, levels of IgG against multiple secondary antigens, including PSA, KLK2/hK2, K-Ras, E-Ras, LGALS8/PCTA-1/galectin-8, and LGALS3/galectin-3, were elevated after treatment with sipuleucel-T (P < 0.01), but not control. IgG responses (≥2-fold elevation post-treatment) occurred in ≥25% of patients, appeared by 2 weeks after sipuleucel-T treatment, and persisted for up to 6 months. IgG responses to PSA and LGALS3 were associated with improved OS in sipuleucel-T–treated patients from IMPACT (P ≤ 0.05).
Conclusions
Sipuleucel-T induced humoral antigen spread in patients with mCRPC. IgG responses were associated with improved OS in IMPACT. The methods and results reported may identify pharmacodynamic biomarkers of clinical outcome after sipuleucel-T treatment, and help in clinical assessments of other cancer immunotherapies.
Introduction
Methods for the assessment of efficacy of cancer immunotherapies are critical in both clinical development and practice. Radiographic measures for objective responses (e.g., RECIST or WHO criteria) have limitations in their assessment of the effects of immunotherapies that are designed to stimulate immune responses against the tumor (1–8). Several clinical studies have now shown that immunotherapies can result in improved overall survival (OS), without frequent objective responses or improving disease progression as assessed by radiography (2, 3, 9). Therefore, appropriate modifications of existing methods or alternative biomarkers of clinical outcome are needed that are indicative of these agents’ immunologic mechanism of action (2, 3, 6–8, 10).
Evidence of immune responses to nontargeted (secondary) antigens following treatment with an immunotherapy, referred to as antigen (or epitope) spread, may enable the identification of novel biomarkers of clinical outcome (11–17). Originally described in autoimmune diseases, antigen spread is believed to play an important role in the progression and pathogenesis of immune-related disorders (18–21) and in the protection against infectious diseases (22, 23). In the context of antitumor immune responses, antigen spread to tumor-associated antigens (TAA) may be indicative of tumor cell killing, antigen release, and subsequent priming of self-reactive T and/or B lymphocytes against TAAs (21, 24, 25). It has been suggested that treatment-induced antigen spread may be associated with improved clinical outcomes (11–17), but evidence from controlled clinical studies is currently lacking. Here, we report an investigation of antigen spread and its association with OS following treatment with sipuleucel-T, an autologous cellular immunotherapy for the treatment of patients with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC; ref. 4).
Sipuleucel-T, designed to target the prostate antigen, prostatic acid phosphatase (PAP, the primary antigen), prolongs OS in patients with mCRPC, but without significant improvement in objective measures of disease progression (4). Immune responses to PAP have been shown to be associated with OS in patients who received sipuleucel-T (26), but antigen spread to TAAs following treatment may provide a more relevant measure of an effective antitumor immune response (13, 25). Here, we show that sipuleucel-T, but not control, elicited serum antibody [immunoglobulin G (IgG)] responses to nontargeted tumor antigens, including prostate-specific antigen (PSA; also known as KLK3), KLK2/hK2 (KLK2), K-Ras, E-Ras, LGALS8/PCTA-1/galectin-8 (LGALS8), and LGALS3/galectin-3 (LGALS3). These responses were observed at 2 weeks and up to 6 months after treatment with sipuleucel-T. Sipuleucel-T–induced IgG responses to PSA and LGALS3 were associated with improved OS in IMPACT.
These results further the understanding of the mechanism of action of sipuleucel-T and may help to identify biomarkers of clinical outcome for this therapy. The methods and results presented here may also facilitate the identification of serum bio-markers of clinical outcome for other cancer immunotherapies. Such easily accessible biomarkers of clinical outcome may meet a critical need for assessing the in vivo mechanism and efficacy of this class of cancer therapies (10, 11, 27). Antigen spread may also help in the identification of TAAs that can be targeted by immunotherapies in the future.
Materials and Methods
Treatments, clinical trials, and biologic specimens
Sipuleucel-T is prepared by culturing autologous peripheral blood mononuclear cells (PBMC) with PA2024, a fusion protein comprising PAP and granulocyte colony-stimulating factor (GM-CSF; ref. 4). Sipuleucel-T was administered as a total of three infusions (or doses) at approximately 2-week intervals (Supplementary Fig. S1). In IMPACT, three infusions of a control product were administered at the same time intervals as sipuleucel-T (4).
IMPACT (Clinicaltrials.gov identifier NCT00065442) was a multicenter, randomized, double-blind, phase III clinical trial of sipuleucel-T in 512 patients with asymptomatic or minimally symptomatic mCRPC (4), and randomized 2:1 to the sipuleucel-T and control arms. For the evaluation of immune responses, the original protocol was amended to allow collection of peripheral samples from patients who provided consent (4, 26). Median follow-up duration for the patients from whom serum samples were available was 33.7 months. ProACT (NCT00715078) is a multicenter, randomized, single-blind, phase II clinical trial of sipuleucel-T manufactured with three different concentrations of PA2024 and administered to a total of 122 patients with advanced mCRPC. All sipuleucel-T–treated patients included in the analyses reported here (IMPACT and ProACT) received sipuleucel-T manufactured using the antigen concentration approved by the FDA and EMA (European Medicines Agency).
Serum samples were collected from patients at time points specified under each study protocol (see Supplementary Methods). In both studies, a pretreatment or baseline sample was collected at the time of registration. In IMPACT, samples were collected at approximately 2, 10, and 22 weeks after treatment (6, 14, and 26 weeks after initiation of treatment, respectively). In ProACT, samples were collected at approximately 4, 12, and 20 weeks after treatment (8, 16, and 24 weeks after initiation of treatment). Other than pretreatment, all time points were post-treatment (e.g., “week 10” refers to week 10 after completion of treatment).
Pre- and posttreatment serum samples were available from a total of 224 patients from IMPACT (4, 26) who provided consent; 155 in the sipuleucel-T arm and 69 in the control arm. Serum samples were collected from patients only up to the time of objective disease progression (4); consequently, samples were available from more patients at earlier time points than at later time points. In IMPACT, pairs of serum samples were evaluable as follows: (i) pretreatment and week 2, n = 204 (142 sipuleucel-T and 62 control patients); (ii) pretreatment and week 10, n = 132 (93 sipuleucel-T, 39 control); and (iii) pretreatment and week 22, n = 76 (60 sipuleucel-T, 16 control).
In ProACT (all treated with sipuleucel-T), pairs of serum samples were evaluable as follows: (i) pretreatment and week 4, n = 33; (ii) pretreatment and week 12, n = 26; and (iii) pretreatment and week 20, n = 19.
Baseline demographic and clinical characteristics of the evaluated patients from IMPACT and ProACT are provided in Supplementary Tables S1 and S2 respectively.
Assessment of serum IgG responses using high-content protein microarray (ProtoArray)
ProtoArray v5.0 (Life Technologies Corporation) contains roughly 9,000 recombinant human proteins produced using a baculovirus expression system and printed on a glass slide under nondenaturing conditions (15, 28–30). ProtoArray was used to measure levels of IgG to these proteins in pretreatment and posttreatment serum samples from patients in IMPACT (Fig. 1 and Table 1). IgGs bound to the proteins on the array were detected using anti-human IgG conjugated to Alexa Fluor 647. Serum samples (30 μL) were assayed by Life Technologies Corporation at 1:500 dilution (see Supplementary Methods for experimental details, data acquisition, and normalization). Normalized signal intensities were log2-transformed before analysis.
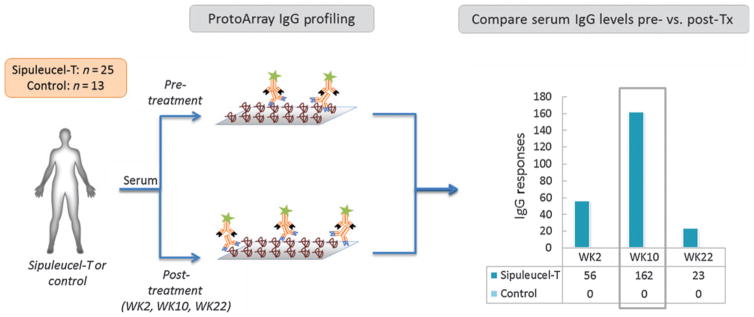
Figure 1.
Schematic for identification of serum IgG responses to secondary antigens in IMPACT, using ProtoArray. IgG levels in pre- and posttreatment (Tx) serum samples were compared with identify IgG responses against specific proteins (antigens) on the ProtoArray (≥2-fold average increase in serum IgG level posttreatment relative to pretreatment, with FDR ≤ 10%). The number of antigens against which IgG responses were observed (y-axis) at the three posttreatment time points (x-axis) are shown for patients in the sipuleucel-T and control arms.
Table 1.
Secondary antigen candidates identified in patients from IMPACT, using ProtoArray
| Antigen annotation
|
ProtoArray data
|
||||
|---|---|---|---|---|---|
| Antigen symbol | Antigen name | Average fold change | P | FDR (%) | Rank by average fold change |
| LGALS3 | Galectin-3 | 4.94 | 2.84E–10 | 2.05E–04 | 1 |
| CACNG1 | Calcium channel, voltage-dependent, gamma subunit 1 | 4.83 | 3.60E–07 | 2.69E–02 | 2 |
| ANPEP | Aminopeptidase N (CD13) | 4.73 | 5.69E–08 | 6.85E–03 | 3 |
| FBXO6 | F-box protein 6 | 3.88 | 1.62E–08 | 2.68E–03 | 4 |
| ECE1 | Endothelin converting enzyme 1 | 3.42 | 3.73E–06 | 3.26E–02 | 5 |
| ERAS | Embryonic stem-cell–expressed Ras | 2.72 | 4.16E–06 | 3.26E–02 | 15 |
| TSPAN13 | Tetraspanin 13 | 2.66 | 1.73E–06 | 3.26E–02 | 17 |
| PAP | Prostatic acid phosphatase | 2.55 | 1.18E–06 | 3.15E–02 | 23 |
| LGALS8/PCTA-1 | Galectin-8/prostate carcinoma tumor antigen 1 | 2.19 | 2.89E–05 | 5.09E–02 | 68 |
| KRAS | Kirsten rat sarcoma viral oncogene homolog | 2.10 | 3.20E–06 | 3.26E–02 | 99 |
| KLK2/hK2 | Kallikrien-related peptidase 2 | 2.04 | 3.67E–05 | 5.50E–02 | 138 |
NOTE: Increase in serum levels of IgGs against 10 candidate secondary antigens at week 10 after completion of sipuleucel-T treatment, as measured by ProtoArray. Data for PAP (primary antigen) are shown for reference. Columns indicate (for serum level of anti-antigen IgG posttreatment): average fold increase posttreatment, P value from moderated paired t test (limma), estimated FDR (% FDR, Benjamini and Hochberg procedure), and rank of antigens by average fold increase in corresponding serum IgG level.
At the time of the ProtoArray analysis, serum samples from pretreatment and all three posttreatment time points were available from 47 patients in the sipuleucel-T arm and 13 in the control arm in IMPACT. ProtoArray analyses were performed on samples from all 13 patients from the control arm, and a randomly chosen subset of 28 patients from the sipuleucel-T arm (to ensure successful analysis of roughly 25 samples from each time point). After eliminating failed assays and array quality control, Proto-Array data were available for analysis from the following patients: (i) pretreatment and 2 weeks, 25 sipuleucel-T and 12 control, (ii) pretreatment and 10 weeks, 24 sipuleucel-T and 11 control, (iii) pretreatment and 22 weeks, 24 sipuleucel-T and 13 control.
Assessment of serum IgG responses posttreatment using Luminex xMAP
IgGs levels against candidate antigens were evaluated by Life Technologies Corporation using Luminex xMAP, which uses multiplexed antigen-conjugated, spectrally distinguishable, fluorescent (31). All available pre- and posttreatment serum sample pairs from IMPACT and ProACT patients were evaluated. Samples (30 μL) were assessed at a 1:200 dilution (see Supplementary Methods). Normalized signal intensities were log2-transformed before analysis.
Statistical analyses
Statistical analyses were performed in the “R” computing environment (http://cran.us.r-project.org/). Statistical tests were two-sided, unless stated otherwise. Changes in serum IgG levels are reported relative to pretreatment (e.g., “fold change in IgG level at week 10” refers to the ratio of IgG level at week 10 to that at pretreatment).
Comparing pre- and posttreatment serum IgG levels from Proto-Array
To assess the statistical significance of pre- to posttreatment changes in levels of IgGs, normalized signal intensities (log2) from ProtoArray assays were tested using a moderated paired t test (limma, R/Bioconductor; ref. 32). The Benjamini and Hochberg procedure was used to perform multiple testing adjustment of P values and obtain estimated false discovery rates (FDR; percentage false discoveries estimated at a certain P value; ref. 33).
Comparing pre- and posttreatment serum IgG levels from Luminex xMAP
To evaluate increases in IgG levels after treatment, pre- and posttreatment signal intensities (log2) from Luminex xMAP were compared using a one-sided paired Wilcoxon signed-rank test. To evaluate whether the fold changes in IgG levels after treatment were higher in the sipuleucel-T group than in the control group, the values from the two groups were compared using a one-sided Wilcoxon rank-sum test. “IgG response” to an antigen was defined as ≥2-fold increase in signal intensity posttreatment compared with pretreatment (ad-hoc threshold).
Evaluating association of posttreatment changes in serum IgG levels (or IgG responses) with OS in IMPACT
The association between posttreatment changes in IgG levels (or IgG responses) and OS was evaluated in the sipuleucel-T–treated patients from IMPACT using a two-sided Wald test on the basis of Cox models. Associations were evaluated with: (i) fold change (log2) in serum IgG level posttreatment, and (ii) IgG response status (yes/no), using univariate as well as multivariate models adjusted for baseline PSA and lactate dehydrogenase (LDH) levels. Baseline PSA (log10) and LDH (log10) were selected for use in multivariate models following the modeling approach used in other analyses from the IMPACT study (4, 26). Unless stated otherwise, P values from multivariate models are given in the text, with additional information available in Supplementary Results. No adjustments to P values were made for multiplicity of variables or posttreatment time points.
Results
Identification of treatment-induced increases in serum IgGs to secondary antigens using protein microarray
We used an unbiased human protein microarray platform (ProtoArray v5.0, ≈9,000 proteins; refs. 15, 28–30, 34), representing a diversity of signaling pathways, oncogenes, and tumor antigens, to identify treatment-induced IgGs against self-antigens in IMPACT (Fig. 1; ref. 4). Because it was not established when the increases in levels of IgGs to secondary antigens may appear after treatment, we compared the pretreatment profiles to those from all available posttreatment time points in IMPACT, that is, weeks 2, 10, and 22 after completion of treatment (Supplementary Fig. S1; ref. 4). ProtoArray data from serum samples from 25 sipuleucel-T arm and 13 control arm patients were available for analysis (see Methods).
In the sipuleucel-T arm, the numbers of unique antigens on the ProtoArray with corresponding increases in IgG levels (with ≥2-fold average increase across the sample set, and estimated FDR ≤ 10%) were: 56 at week 2, 162 at week 10, and 23 at week 22 (Fig. 1). In the control arm, using the same thresholds, no increases in levels of IgGs against any of the antigens were observed on the ProtoArray at any of these time points. In the sipuleucel-T–treated patients, IgG against PAP (primary antigen) was highly induced at all the posttreatment time points (Table 1 and Supplementary Table S3). Because the number of antigens against which IgGs were elevated was highest at week 10, we focus the discussions below on further evaluation of the IgGs from this time point; IgGs from weeks 2 and 22 were also evaluated but are discussed only briefly (with supporting information in Supplementary Results).
We next examined whether the sipuleucel-T–induced IgGs were targeted against antigens that are overexpressed in prostate tumors. Target antigens of the 100 IgGs most highly induced at week 10 are encoded by genes that overlapped significantly (P = 0.01) with genes found to be overexpressed in prostate tumors, as identified by the largest study of gene expression in prostate tumor and normal tissues (see Supplementary Results for details of the analysis; ref. 35). This suggested that after treatment with sipuleucel-T, IgGs were induced against antigens that are overexpressed in prostate tumors.
Confirmation of serum IgG responses to secondary antigens with Luminex xMAP
Discoveries from high-content platforms (such as microarrays) can often contain false positives. Therefore, IgG responses to a subset of the secondary antigens identified using ProtoArray were confirmed using an independent analytic platform, Luminex xMAP (31). We chose Luminex xMAP because of its low sample volume requirements, high reported sensitivity, wide linear range, and capability for multiplex IgG detection. From the 162 secondary antigens to which IgG responses were observed with ProtoArray at week 10, we selected 10 for confirmation (Table 1). Of these 10 antigens, five corresponded to the IgGs that exhibited the highest-fold increases in level post-treatment (LGALS3, ANPEP, ECE1, FBXO6, and CACNG1); LGALS3 (36–38), ANPEP (39–41), ECE1 (42–45) are known to be expressed at high levels in prostate tumors or to have functional roles in prostate cancer development. We selected the remaining five antigens based on reported functional relevance in cancer and/or increased expression in prostate tumors, viz., KLK2 (46–48), E-Ras (49), K-Ras (35), TSPAN13 (50), and LGALS8 (36, 51). Descriptions of the 10 candidate antigens are provided in Supplementary Results. ProtoArray data showed that levels of IgGs to most of these 10 candidate secondary antigens were also elevated at the earlier (week 2) and later (week 22) time points (Supplementary Table S3).
Some well-known prostate tumor antigens were not present on the ProtoArray; consequently, no data were available from that platform regarding IgG responses to these antigens. We included two such antigens in the evaluations with Luminex xMAP: PSA and prostate-specific membrane antigen (PSMA [FOLH1]). Three additional antigens were evaluated for control purposes: tetanus toxoid (against which a majority of Americans are immunized; ref. 52), and PA2024 and PAP, the primary antigens in sipuleucel-T.
To consider IgG response to a candidate antigen as confirmed by Luminex xMAP in IMPACT, we required the following criteria to be satisfied (Table 2):
Table 2.
Confirmation of serum IgG responses to candidate secondary antigens at week 10 in IMPACT, using Luminex xMAP
| Antigens tested
|
Sipuleucel-T (n = 93)
|
Control (n = 39)
|
Sipuleucel-T vs. Control | |||||
|---|---|---|---|---|---|---|---|---|
| Selection source | Name or symbol | P (pre vs. post) | Patients with ≥2-fold upreg. (%) | Patients with ≥5-fold upreg. (%) | P (pre vs. post) | Patients with ≥2-fold upreg. (%) | Patients with ≥5-fold upreg. (%) | P (fold change) |
| Controls | PAP | 8.46E–16 | 69 (74.2) | 55 (59.1) | 0.107 | 4 (10.3) | 0 (0) | 2.38E–12 |
| PA2024 | 2.83E–17 | 86 (92.5) | 75 (80.6) | 0.256 | 10 (25.6) | 2 (5.1) | 4.81E–17 | |
| Tetanus Toxoid | 5.71E–05 | 10 (10.8) | 0 (0) | 0.100 | 6 (15.4) | 1 (2.6) | 1.93E–01 | |
| Known PCa antigens | PSA | 1.42E–10 | 36 (38.7) | 13 (14) | 0.066 | 5 (12.8) | 1 (2.6) | 2.15E–04 |
| PSMA | 1.48E–05 | 18 (19.4) | 4 (4.3) | 0.293 | 4 (10.3) | 2 (5.1) | 2.48E–02 | |
| ProtoArray candidates | LGALS3 | 2.83E–10 | 26 (28) | 3 (3.2) | 0.152 | 4 (10.3) | 2 (5.1) | 4.72E–04 |
| CACNG1 | 4.23E–04 | 9 (9.7) | 3 (3.2) | 0.425 | 2 (5.1) | 0 (0) | 3.07E–02 | |
| ANPEP | 8.14E–06 | 6 (6.5) | 1 (1.1) | 0.753 | 1 (2.6) | 0 (0) | 7.06E–04 | |
| FBXO6 | 4.69E–03 | 12 (12.9) | 4 (4.3) | 0.846 | 1 (2.6) | 0 (0) | 1.36E–02 | |
| ECE1 | 2.22E–04 | 25 (26.9) | 6 (6.5) | 0.302 | 4 (10.3) | 3 (7.7) | 5.22E–02 | |
| ERAS | 2.97E–10 | 39 (41.9) | 11 (11.8) | 0.148 | 5 (12.8) | 2 (5.1) | 1.92E–04 | |
| TSPAN13 | 1.41E–02 | 11 (11.8) | 4 (4.3) | 0.779 | 2 (5.1) | 0 (0) | 4.74E–02 | |
| LGALS8 | 3.57E–11 | 23 (24.7) | 5 (5.4) | 0.034 | 3 (7.7) | 0 (0) | 3.06E–04 | |
| KRAS | 1.82E–10 | 37 (39.8) | 14 (15.1) | 0.208 | 5 (12.8) | 0 (0) | 4.71E–05 | |
| KLK2 | 1.73E–09 | 41 (44.1) | 9 (9.7) | 0.079 | 5 (12.8) | 1 (2.6) | 3.94E–04 | |
NOTE: Columns indicate the antigens evaluated, antigen selection source (i.e., control antigens, known PCa antigens, and antigens identified from ProtoArray), P value for serum IgG level increase at week 10 vs. pretreatment, number (%) of patients in the sipuleucel-T and control arms with ≥2- or ≥5-fold increase in serum IgG level after treatment; the rightmost column lists P values for fold change in serum IgG levels with sipuleucel-T vs. control using a one-sided Wilcoxon rank-sum test (HA: fold change in serum IgG level posttreatment is higher in the sipuleucel-T arm). IgG responses against antigens that were confirmed by Luminex xMAP are in bold (PAP, PA2024, PSA, LGALS3, E-RAS, LGALS8, K-RAS and KLK2). See Supplementary Table S4 for information on the protein reagents used for Luminex assays. Abbreviation: PCa, prostate cancer.
In a comparison of patients from sipuleucel-T and control arms, higher fold change in IgG level posttreatment with sipuleucel-T (n =93) than with control (n =39), with P ≤0.01 for the comparison.
In sipuleucel-T–treated patients (n =93), a significant increase in IgG levels after treatment compared with pretreatment (P ≤ 0.01) and ≥10% of patients, demonstrating an IgG response (defined as ≥2-fold increase in IgG level posttreatment compared with pretreatment).
Sipuleucel-T–induced increases in levels of IgGs to the following antigens were confirmed by Luminex xMAP: PSA, KLK2, K-Ras, E-Ras, LGALS8, and LGALS3 (Table 2 and Fig. 2A). As expected, increases in levels of IgGs to PAP and PA2024 were also confirmed. IgG responses to several antigens, for example, PSA, KLK2, K-Ras, E-Ras, and LGALS3, were observed in ≥25% (range, 28%–44%) of the sipuleucel-T–treated patients from IMPACT. A 5-fold increase in anti-PSA, anti-K-Ras, or anti-E-Ras IgG level was observed in ≥10% (range, 12%–15%) of sipuleucel-T–treated patients. Levels of IgGs to these antigens were not significantly elevated after treatment in the control patients (P > 0.01; Table 2).
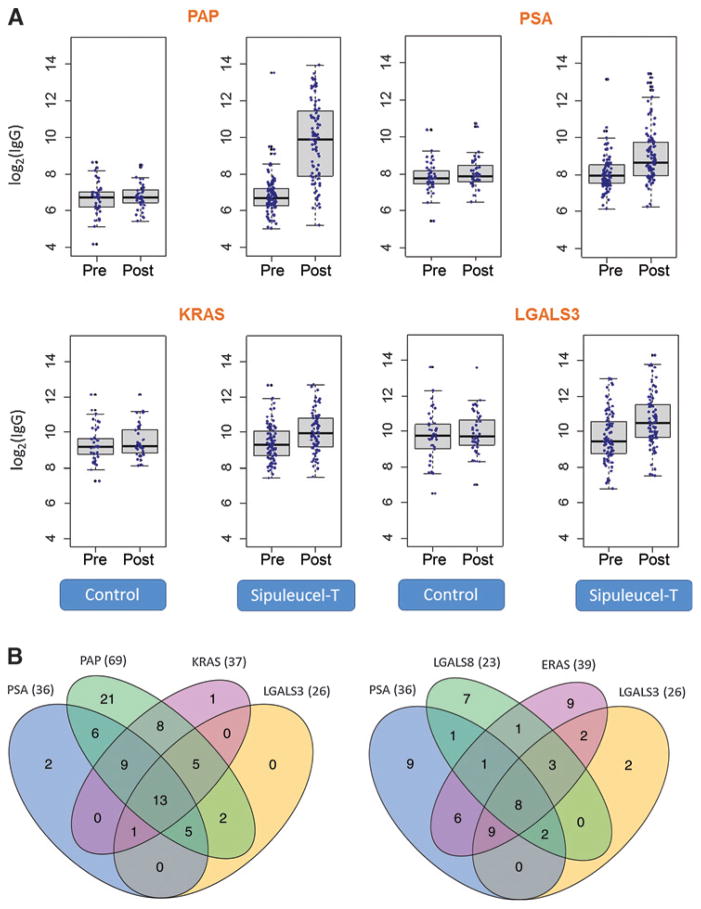
Figure 2.
Confirmation of serum IgG responses to secondary antigens in patients from IMPACT using Luminex xMAP, and analyses of overlaps between IgG responders. A, confirmation of IgG responses in IMPACT, 10 weeks after treatment, using Luminex xMAP. Log2 of serum IgG levels (y-axes) pre- and posttreatment in the control and sipuleucel-T arms are shown. Data for three secondary antigens (PSA, K-Ras, and LGALS3) are shown. Serum levels of IgG against PAP (primary antigen) are shown for reference. See Table 2 for details. B, overlap of IgG responses to different antigens in patients from the sipuleucel-T arm shown using Venn diagrams. The numbers of patients with overlapping IgG responses to different antigens at week 10 are shown. Left, overlaps include IgG responses to PAP (primary antigen). Right, overlaps include IgG responses to secondary antigens only. IgG responders are defined as patients with ≥2-fold increase in serum IgG level posttreatment versus pretreatment. Information on the significance of the overlaps between IgG responders to all antigens is given in Supplementary Table S5; representative examples are shown here.
In the evaluable patients treated with sipuleucel-T in IMPACT, changes in levels of IgGs at week 10 against the confirmed secondary antigens were not associated (P > 0.01) with the prognostic baseline characteristics reported by Halabi and colleagues (baseline serum PSA, LDH, albumin, or hemoglobin, total Gleason score, metastatic disease site or Eastern Cooperative Oncology Group [ECOG] performance status; refs. 53, 54), or prior therapeutic interventions (chemotherapy, radiotherapy, radical prostatectomy, or bisphosphonates; data not shown).
Overlap among serum IgG responders in the sipuleucel-T arm from IMPACT
To determine whether sipuleucel-T–treated patients shared IgG responses to the same secondary antigens, we evaluated overlaps among IgG responders (see Venn diagrams in Fig. 2B for representative examples, and Supplementary Table S5 for details). The majority of the sipuleucel-T–treated patients who had IgG responses to secondary antigens overlapped with those who had IgG responses to PAP (Fig. 2B, left). Although there were differences in the patterns of IgG responses among the patients, significant overlaps were also observed among IgG responders to a number of different secondary antigens (e.g., E-Ras and KLK2, or LGALS3 and K-Ras; P ≤ 0.01, hypergeometric test). For example, when IgG responses to PSA, E-Ras, LGALS8, and LGALS3 were considered, 25% (23 of 93) of sipuleucel-T–treated patients exhibited responses to three or more of the same antigens, and 9% exhibited responses to all four of these antigens (Fig. 2B, right). In this case, depending on the antigen, a minority (≤30%) of the IgG responses were unique (i.e., did not overlap with IgG responses to other antigens).
Validation of serum IgG responses in ProACT using Luminex xMAP
To determine whether the sipuleucel-T–induced IgG responses observed in IMPACT also occurred in patients from an independent clinical study of sipuleucel-T, we assessed IgG responses to PSA, LGALS3, E-Ras, LGALS8, K-Ras, and KLK2 in a phase II study, ProACT. Serum samples were available from ProACT patients (n = 26) for the week 12 time point, which was comparable with the week 10 time point in IMPACT. In these patients, levels of IgGs to PSA, KLK2, K-Ras, E-Ras, and LGALS3 were significantly elevated versus pretreatment (P ≤ 0.01), with 2-fold increase in ≥10% (range, 15%–31%) of patients (Supplementary Table S6). These results provided evidence of sipuleucel-T–induced IgG responses to multiple secondary antigens in an independent cohort.
Association of sipuleucel-T–induced changes in serum IgG levels with OS in IMPACT
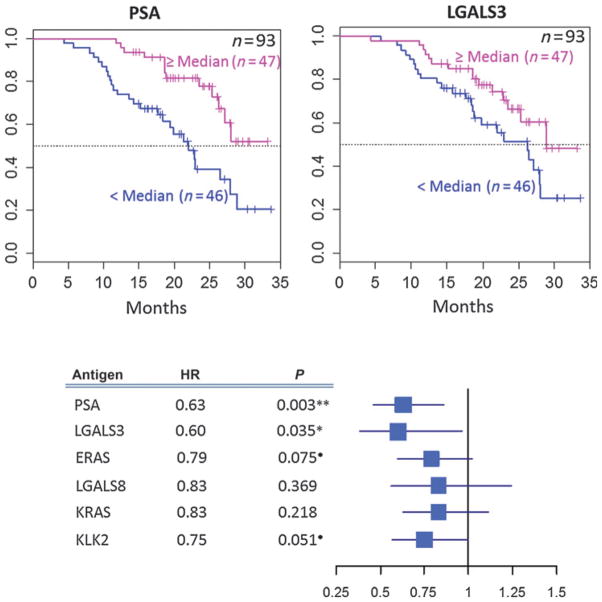
The association of sipuleucel-T–induced changes in IgG levels at week 10 with OS was evaluated in IMPACT using a multivariate Cox model, adjusted for baseline PSA and LDH levels. Sipuleucel-T–induced changes in levels of anti-PSA IgG [hazard ratio (HR), 0.63; 95% confidence interval (CI), 0.46–0.86; P < 0.01] and anti-LGALS3 IgG (HR, 0.60; 95% CI, 0.38–0.96; P = 0.04) were significantly associated with improved OS (Fig. 3 and Supplementary Table S7A). Sipuleucel-T–induced changes in levels of anti-KLK2 IgG and anti-E-Ras IgG showed a trend toward association with improved OS (0.05 < P < 0.1).
Figure 3.
Association of posttreatment changes in serum levels of IgG (log2) against specified secondary antigens with OS in the sipuleucel-T arm in IMPACT. The Kaplan–Meier plots for serum level of IgG to PSA (left) or LGALS3 (right) versus OS are shown with patients grouped by median fold change in serum IgG level at week 10 versus pretreatment (≥median, <median); the dotted horizontal line indicates estimated median OS. The total number of patients in the analyses is given at the top right of each plot; the numbers of patients in the ≥median and <median groups are also shown within each of the figures. The forest plot below the Kaplan–Meier plots shows HR and 95% CI for the associations of change in serum IgG levels (log2 scale) with OS (adjusted for baseline PSA and LDH) from a multivariate Cox model (Supplementary Table S7A for details); blue boxes indicate HR and whiskers indicate 95% CI. HRs and P values for each of the IgGs are to the left of the forest plot. P values are shown to 3 significant digits. **, P ≤ 0.01; *, P ≤ 0.05; ●, P ≤ 0.1.
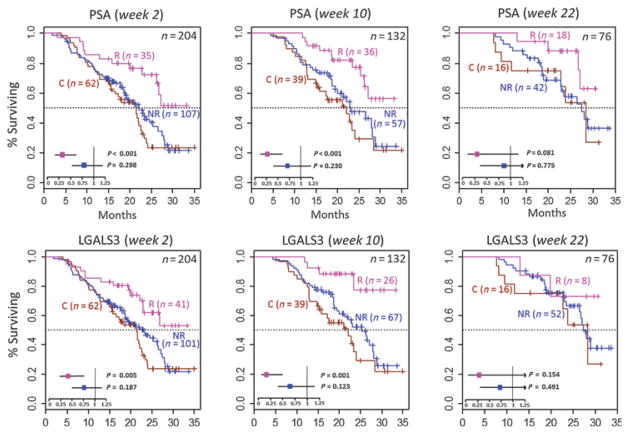
In the sipuleucel-T arm, IgG responses (≥2-fold increase at week 10) to PSA (responders, n = 36; nonresponders, n = 57; HR, 0.38; 95% CI, 0.19–0.80; P = 0.01) and LGALS3 (responders, n = 26; nonresponders, n = 67; HR, 0.25; 95% CI, 0.09–0.72; P = 0.01) were also significantly associated with improved OS (Supplementary Table S7B). Relative to patients in the control arm, patients in the sipuleucel-T arm who were anti-PSA IgG responders exhibited significantly improved OS (HR, 0.27; 95% CI, 0.12–0.58; P < 0.01; Fig. 4 and Supplementary Table S8), whereas OS in patients in the sipuleucel-T arm who were anti-PSA IgG nonresponders did not differ significantly from that in patients in the control arm (HR, 0.71; 95% CI, 0.41–1.23; P = 0.23). Similarly, patients in the sipuleucel-T arm who were anti-LGALS3 IgG responders exhibited significantly improved OS compared with those in the control arm (HR, 0.16; 95% CI, 0.06–0.49; P < 0.01; Fig. 4), whereas OS in patients in the sipuleucel-T arm who were anti-LGALS3 IgG nonresponders did not differ significantly from patients in the control arm (HR, 0.66; 95% CI, 0.38–1.12; P = 0.12). These results indicate that a relatively moderate increase in levels of IgGs to secondary antigens (≥2-fold) may identify patients who are more likely to have significant survival benefit after sipuleucel-T treatment than patients who do not show increase in these IgGs.
Figure 4.
The Kaplan–Meier plots of OS for anti-PSA and anti-LGALS3 IgG responders (R) and nonresponders (NR) in the sipuleucel-T arm and patients from the control arm (C) in IMPACT. These figures show the difference in OS between the control group and the IgG responder or nonresponder groups in the sipuleucel-T arm. Data for IgG responses at week 2 (left), week 10 (middle), and week 22 (right) are shown. Top, anti-PSA IgG; bottom, anti-LGALS3 IgG. IgG responders are defined as patients with ≥2-fold increase in serum IgG level posttreatment relative to pretreatment. The total number of patients in the analyses is given at the top right of each plot; the numbers of patients in the R, NR, and C groups are also shown within each of the figures. The P values and HRs (whiskers indicate 95% CI) in the forest plot inlays indicate the significance of the difference in OS in the R and NR groups relative to the C group using a multivariate Cox model (adjusted for baseline PSA and LDH levels) as described in the methods. Refer to Supplementary Table S8 (week 10) and S13 (weeks 2 and 22) for detailed information.
We next tested whether increasing numbers of IgG responses to secondary antigens was associated with progressively improved OS. Interestingly, as compared with patients with no IgG responses against the confirmed antigens (namely, PSA, LGALS3, E-Ras, LGALS8, K-Ras, and KLK2; n = 26), patients with ≥1 (n = 67; HR, 0.57; 95% CI, 0.29–1.10; P =0.09), ≥2 (n =52; HR, 0.51; 95% CI, 0.25–1.03; P = 0.06), ≥3 (n = 34; HR, 0.39; 95% CI, 0.17–0.92; P = 0.03), and ≥4 (n = 26; HR, 0.30; 95% CI, 0.11–0.82; P = 0.02) IgG responses showed progressively improved OS (Supplementary Fig. S2). The number of patients with IgG responses ≥5 was small (n ≤ 17), therefore, patients with ≥5 IgG responses were not analyzed. Overall, these data suggest that the total number of IgG responses (in other words, the extent of antigen spread) may be associated with improved OS.
Among patients from the sipuleucel-T arm in IMPACT, anti-PA2024 and anti-PAP IgG responders exhibited improved OS relative to control patients, whereas anti-PA2024 and anti-PAP IgG nonresponders did not (Supplementary Table S8). However, the increases in OS observed in the anti-PA2024 and anti-PAP IgG responders were not as significant as those in the anti-PSA or anti-LGALS3 IgG responders. A previous analysis of data from IMPACT, using different methodologies, showed that aggregated humoral (IgG and IgM combined) and cellular immune responses to PAP or PA2024 were associated with improved OS (26).
Serum IgG responses at earlier and later posttreatment time points
To determine whether the IgG responses to secondary antigens observed at the week 10 time point in IMPACT also occurred at other time points, we examined serum samples from the other available posttreatment time points in IMPACT (weeks 2 and 22) and ProACT (weeks 4 and 20; see Supplementary Table S9 for IMPACT and Supplementary Table S10 for ProACT results). In IMPACT, significant (P ≤ 0.01) increases in levels of IgGs against PSA, KLK2, K-Ras, E-Ras, LGALS8, and LGALS3 were observed in the sipuleucel-T group at week 2 (n = 142) and week 22 (n = 60). In ProACT, significant increases in levels of IgGs against PSA, KLK2, K-Ras, E-Ras, LGALS8, and LGALS3 were observed at week 4 (n = 33), but not at week 20 with sipuleucel-T (n = 19). The relatively small number of patients evaluable at week 20 in ProACT may have contributed to the lack of statistical significance.
Sipuleucel-T–induced IgG responses were largely consistent across the posttreatment time points. Patients from the sipuleucel-T arm in IMPACT with IgG responses to an antigen at week 10 frequently exhibited an IgG response to the same antigen at the earlier (week 2) and later (week 22) time points (P ≤ 0.01, hypergeometric test; Supplementary Table S11).
In the sipuleucel-T–treated patients from IMPACT, IgG responses to PSA at week 2 were significantly associated with improved OS (responders, n = 35; nonresponders, n = 107; HR, 0.42; 95% CI, 0.22–0.79; P < 0.01; Fig. 4; Supplementary Table S12B), and IgG responses to LGALS3 at week 2 showed a trend toward improved OS (responders, n = 41; nonresponders, n = 101; HR, 0.57; 95% CI, 0.31–1.02; P = 0.06). Patients in the sipuleucel-T arm who were anti-PSA or anti-LGALS3 IgG responders at week 2 (but not the corresponding nonresponders) exhibited significantly improved OS (P < 0.01) relative to the patients in the control arm (Fig. 4 and Supplementary Table S13). Patients in the sipuleucel-T arm with IgG responses to the primary antigen (PAP or PA2024) at week 2 also exhibited improved OS (P < 0.05; Supplementary Table S13) compared with patients in the control arm, whereas OS of patients without the IgG responses were not significantly different from patients in the control arm. IgG responses at week 22 were not significantly associated with OS; however, in the sipuleucel-T arm, patients with anti-PSA IgG responses showed a trend toward improved OS relative to nonresponders (responders, n = 18; nonresponders, n = 42; HR, 0.35; 95% CI, 0.12–1.05; P = 0.06; Supplementary Table S12B).
Thus, increases in levels of serum IgGs to multiple secondary antigens were observed as early as 2 weeks and up to 22 weeks (6 months) after completion of treatment with sipuleucel-T in patients from IMPACT. In the sipuleucel-T arm from IMPACT, IgG responses at multiple time points after treatment, spanning several months, were associated with improved OS.
Discussion
The results presented here show that humoral antigen spread occurred after treatment with sipuleucel-T. The IgG responses were not significantly associated with baseline prognostic characteristics or prior therapeutic interventions. Sipuleucel-T–induced IgG responses to PSA and LGALS3 were associated with improved OS in IMPACT.
Although antigen spread after treatment with cancer immunotherapies and its potential for association with clinical outcomes has been known for some time (13, 25), typically, such investigations have included relatively small cohorts or measured responses to a predefined set of antigens (11–15, 55, 56). To our knowledge, this is the most extensive demonstration yet of antigen spread and its relationship with clinical benefit in the context of a cancer immunotherapy, and the first such evaluation in a clinical study with a comparator control arm. The study provides insight into the temporal dynamic of the antigen spread induced by an immunotherapy using a broad platform (protein microarray) and demonstrates treatment-induced IgG responses to antigens against which immune responses had not been previously reported in prostate cancer (K-Ras and E-Ras). Finally, these data provide evidence in a relatively large population that IgG responses to secondary antigens can be associated with improved OS, and that such responses can be observed early and persist for several months after treatment.
A few other studies of cancer immunotherapies (15, 17, 30, 57) have reported unbiased or broad evaluations of humoral responses to self-antigens; some of these are of particular interest in light of the results presented here. Kwek and colleagues (15) reported a higher frequency (or breadth) of IgG responses against self-antigens in a small cohort of patients with malignant melanoma who responded clinically to ipilimumab and GM-CSF; the majority of these responses were patient-specific. In contrast, many patients shared responses to the same antigens (see Fig. 2B, right, for example) in our study. This may reflect differences in the modes of action of sipuleucel-T and ipilimumab/GM-CSF, or more generally, between antigen-directed [directed toward primary tumor antigen(s)] and nondirected (not directed toward specific tumor antigen[s]) immunotherapies. Compared with a nondirected immunotherapy (such as ipilimumab/GM-CSF), antigen spread after an antigen-directed immunotherapy (such as sipuleucel-T) may be limited to the antigens overexpressed or altered in tumor cells that express the primary antigen(s), and consequently, such responses may be shared among patients more often.
Using protein microarrays, Nguyen and colleagues (30) investigated antibody responses after treatment with GVAX, a vaccine for prostate cancer comprised two allogeneic prostate carcinoma cell lines, modified to secrete GM-CSF. Of the ≈30 candidate antigens against which antibody responses were observed after treatment (30), two (LGALS3 and LGALS8) were confirmed in our study; one (LGALS8) being a known prostate tumor antigen (51). Further studies may help determine whether immune responses to the galectins, which are highly expressed in prostate tumors (36), are common after treatment of prostate cancer with immunotherapies.
Another study used serologic analysis of expression cDNA libraries (SEREX) to investigate antigen spread in prostate cancer patients who received a viral vaccine encoding PSA (57). Of the vaccine-treated patients, 21% (7 of 30) developed antibody responses to a common set of ubiquitously expressed self-antigens, compared with none in the control groups. We did not observe responses to those antigens in our study. Patients with autoantibody responses after the vaccine treatment showed a trend toward decreased biochemical-free survival. The authors suggested that the nature of the secondary antigens may inform clinical outcome (57); immune responses to ubiquitously expressed self-antigens may reflect off-target activity and be associated with adverse effects, whereas responses to tumor antigens may reflect antitumor immune activity and therefore be associated with improved clinical outcomes (12–16). In this study, all of the confirmed IgG responses were associated with (or showed a trend toward) improved OS (Fig. 3).
The identification of posttreatment markers of an antitumor immune response is an important challenge in characterizing in vivo responses to cancer immunotherapies (7, 10). Measuring immune responses to the primary targets of antigen-directed immunotherapies (12, 26, 57) is a common practice. However, questions remain regarding the appropriate immunologic measures that provide the best information about clinical efficacy of immunotherapies (7, 11). Ideally, immunologic biomarkers should reflect the therapeutic mechanism of action and be quantifiable from easily accessed biospecimens (11). Humoral responses associated with antigen spread appear to have these properties and may hold potential utility as pharmacodynamic biomarkers for the assessment of clinical benefits of some cancer immunotherapies.
The associations of IgG responses with OS after sipuleucel-T treatment should be considered as hypotheses-generating observations. It will be important to establish the optimal methods for quantifying the IgG responses (e.g., with titrations of serum or antigen-conjugated Luminex beads) and to validate the associations with OS in additional clinical studies. The current results, along with previously published findings on sipuleucel-T (4, 26, 58), also point to opportunities to further characterize the observed IgG responses and to understand their origin and function. Some of these are discussed below.
Although our data do not reveal the mechanism of origin of the IgG responses (note that tumor specimens were not available for analyses in IMPACT or ProACT), a few observations argue in favor of a possible treatment-induced antitumor effect. First, in a neoadjuvant setting, sipuleucel-T increases the frequency of CD4+ and CD8+ T cells within prostate tissue 2 to 3 weeks after treatment, particularly at the tumor margin (58). Second, sipuleucel-T–induced antigen spread was a delayed manifestation; a higher number of IgG responses were observed on ProtoArray at week 10 than at week 2. Third, IgG responses were mounted against antigens that are overexpressed in prostate tumors (35), including intracellular oncogenes (e.g., Ras members). Taken together, these observations suggest a mechanism by which the initial immune responses mobilized by sipuleucel-T are responsible for some degree of tumor cell lysis and release of secondary antigens; these secondary antigens may have triggered the IgG responses observed here.
The induction of IgG responses suggests that CD4+ helper T cells (Th2) have been engaged. Both cellular and humoral responses to the primary antigen, PAP, have been reported (4, 26), and characterization of cytokine profiles in sipuleucel-T product indicated engagement of both Th1 and Th2 mechanisms (26). It will be useful to determine whether sipuleucel-T–treated patients exhibit cellular immune responses to the secondary antigens. Combined cellular and humoral responses may be more effective in identifying the patients with antigen spread after-treatment. It is of note here that in another study, after treatment with PSA-TRICOM, a PSA-targeted viral vaccine for prostate cancer (59), primarily T-cell responses to PSA were generated, with only rare instances of anti-PSA antibody responses. In contrast, we found that anti-PSA IgGs were frequently induced after treatment with sipuleucel-T.
To date, the development of active cancer immunotherapies has focused on induction of CD8+ cytotoxic T-cell responses; however, recent studies have shown the importance of CD4+ T cells, B cells and humoral immunity in mediating tumor control (60–62). In particular, infiltration of plasma B cells and immunoglobulin gene expression have been identified as important predictors of good prognosis and response to chemotherapy (61, 62). The ability of IgG antibodies to mediate tumor cell killing suggests that tumor-specific antibodies may play a role in the overall ability of the immune system to combat tumors. Although the magnitudes of sipuleucel-T–induced IgG responses to secondary antigens were modest compared with that observed to the primary antigen, multiple IgG responses could collectively mediate an additive or synergistic antitumor effect. Several of the confirmed IgG responses were against antigens known to be involved with PSA and Ras-related molecular functions: PSA, KLK2, LGALS3, and K-Ras. KLK2 generates the enzymatically active form of PSA from pro-PSA (48). LGALS3 is cleaved by PSA, potentially modulating its function (38), and is a binding partner and activator in K-Ras signaling (63, 64). Whether there is a functional significance to the appearance of IgG responses to multiple antigens in this molecular pathway is unknown. It is also unclear why a subset of the confirmed IgG responses was more significantly associated with OS, although all showed a trend toward improved OS. The association of a given IgG response with OS may be determined by particularities of the expression or biologic role of the given antigen, or the functionality of the IgGs generated. Investigation of the isotypes of these IgGs and their ability to mediate antibody-dependent cell-mediated cytotoxicity (ADCC) and other functions (binding or neutralization), individually and in combination, may help shed light on their relevance to antitumor responses. In this study, we have confirmed only a relatively limited number of IgG responses; it will be of interest in future studies to assess a wider set of IgGs than those characterized here. Specifically, additional candidates from the protein microarrays as well as known prostate cancer-related gene variants, such as the TMPRSS2–ERG fusion product may help in gleaning a more complete understanding of IgG responses and their functional relevance.
Finally, treatment-induced immune responses to secondary antigens may provide targets for developing future therapies (15). Therefore, it will be of interest to ask in preclinical studies if immunization against the particular antigens to which IgG responses were observed here is protective against tumor growth.
Supplementary Material
Translational Relevance.
Clinical benefits of cancer immunotherapies are often not evident with the commonly used radiographic measures of objective tumor response (e.g., RECIST or WHO criteria). Biomarkers that are indicative of immunologic antitumor activity may be useful in assessing the clinical benefit of this class of therapeutics. The development of immune responses to secondary tumor antigens not directly targeted by a therapy, or antigen spread, may indicate tumor cell destruction and provide biomarkers of clinical benefit. We show here that serum antibody (IgG) responses to secondary tumor antigens (humoral antigen spread) can be detected within weeks after treatment with sipuleucel-T, an immunotherapy for meta-static castration-resistant prostate cancer, and such responses can be associated with improved overall survival. These results may enable identification of pharmacodynamic bio-markers of clinical outcome after treatment with sipuleucel-T. More generally, they may constitute a useful tool for evaluating the efficacy of treatment regimens that include cancer immunotherapies.
Acknowledgments
Matt Harmon is thanked for providing the information from clinical trials. The authors thank Susan Veals and Johnathan Maher of Dendreon Corporation for suggestions on the article and medical writing support. Lawrence Fong and Frances Stewart are thanked for helpful discussions. Life Technologies Corporation is thanked for their expertise and consultation on the humoral assays and assay designs. This work would not be possible without the patients who enrolled in sipuleucel-T clinical trials and consented to contribute their biologic specimens for investigational purposes. Physicians and investigators of IMPACT and ProACT are thanked for their contribution in enrolling and treating patients.
Grant Support
Funding for the study was provided by Dendreon Corporation.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
D. GuhaThakurta, N.A. Sheikh, T. DeVries, and J.B. Trager have ownership interest (including patents) in Dendreon Corporation. P. Kantoff is a consultant/advisory board member for Dendreon Corporation. C.S. Higano reports receiving a commercial research grant from and is a consultant/advisory board member for Dendreon Corporation. C.G. Drake reports receiving commercial research grants from Aduro Biotech, Bristol-Myers Squibb, and Janssen; holds ownership interest (including patents) in Compugen and NexImmune; and is a consultant/advisory board member for Bristol-Myers Squibb, Compugen, Dendreon Corporation, ImmunExcite, NexImmune, Novartis, and Roche/Genentech. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: D. GuhaThakurta, N.A. Sheikh, M.W. Frohlich, J.B. Trager, C.G. Drake
Development of methodology: D. GuhaThakurta, L.-Q. Fan, M.W. Frohlich
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): D. GuhaThakurta, L.-Q. Fan, C.S. Higano, T.A. Gardner, K. Bailey, T. Vu
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): D. GuhaThakurta, N.A. Sheikh, H. Kandadi, T.C. Meagher, S.J. Hall, P.W. Kantoff, C.S. Higano, T.A. Gardner, T. DeVries, J.B. Whitmore, M.W. Frohlich, J.B. Trager, C.G. Drake
Writing, review, and/or revision of the manuscript: D. GuhaThakurta, N.A. Sheikh, H. Kandadi, T.C. Meagher, S.J. Hall, P.W. Kantoff, C.S. Higano, E.J. Small, T.A. Gardner, T. DeVries, J.B. Whitmore, M.W. Frohlich, C.G. Drake
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): N.A. Sheikh, H. Kandadi
Study supervision: D. GuhaThakurta, T.C. Meagher, C.S. Higano, M.W. Frohlich, J.B. Trager
Other (scientific interpretation of data (not necessarily computational)): C.G. Drake
References
- 1.Ribas A, Hersey P, Middleton MR, Gogas H, Flaherty KT, Sondak VK, et al. New challenges in endpoints for drug development in advanced melanoma. Clin Cancer Res. 2012;18:336–41. doi: 10.1158/1078-0432.CCR-11-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 3.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–75. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–97. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoos A. Evolution of end points for cancer immunotherapy trials. Ann Oncol. 2012;23(Suppl 8):viii47–52. doi: 10.1093/annonc/mds263. [DOI] [PubMed] [Google Scholar]
- 8.Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15:7116–8. doi: 10.1158/1078-0432.CCR-09-2376. [DOI] [PubMed] [Google Scholar]
- 10.Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, Ascierto PA, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9:214. doi: 10.1186/1479-5876-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60:433–42. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 13.Ribas A, Timmerman JM, Butterfield LH, Economou JS. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 2003;24:58–61. doi: 10.1016/s1471-4906(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 14.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwek SS, Dao V, Roy R, Hou Y, Alajajian D, Simko JP, et al. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189:3759–66. doi: 10.4049/jimmunol.1201529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbiere V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethe B, et al. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71:1253–62. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 17.Smith HA, Maricque BB, Eberhardt J, Petersen B, Gulley JL, Schlom J, et al. IgG responses to tissue-associated antigens as biomarkers of immunological treatment efficacy. J Biomed Biotechnol. 2011;2011:454861. doi: 10.1155/2011/454861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 20.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–5. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 21.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 22.Catterall WA. Structure and function of voltage-sensitive ion channels. Science. 1988;242:50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- 23.Powers PA, Liu S, Hogan K, Gregg RG. Molecular characterization of the gene encoding the gamma subunit of the human skeletal muscle 1,4-dihydropyridine-sensitive Ca2+ channel (CACNLG), cDNA sequence, gene structure, and chromosomal location. J Biol Chem. 1993;268:9275–9. [PubMed] [Google Scholar]
- 24.Markiewicz MA, Fallarino F, Ashikari A, Gajewski TF. Epitope spreading upon P815 tumor rejection triggered by vaccination with the single class I MHC-restricted peptide P1A. Int Immunol. 2001;13:625–32. doi: 10.1093/intimm/13.5.625. [DOI] [PubMed] [Google Scholar]
- 25.Gulley JL. Therapeutic vaccines: the ultimate personalized therapy? Hum Vaccin Immunother. 2013;9:219–21. doi: 10.4161/hv.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–47. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butterfield LH, Palucka AK, Britten CM, Dhodapkar MV, Hakansson L, Janetzki S, et al. Recommendations from the iSBTc-SITC/FDA/NCI Workshop on Immunotherapy Biomarkers. Clin Cancer Res. 2011;17:3064–76. doi: 10.1158/1078-0432.CCR-10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweitzer B, Meng L, Mattoon D, Rai AJ. Immune response biomarker profiling application on ProtoArray protein microarrays. Methods Mol Biol. 2010;641:243–52. doi: 10.1007/978-1-60761-711-2_14. [DOI] [PubMed] [Google Scholar]
- 29.Gnjatic S, Ritter E, Buchler MW, Giese NA, Brors B, Frei C, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci USA. 2010;107:5088–93. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen MC, Tu GH, Koprivnikar KE, Gonzalez-Edick M, Jooss KU, Harding TC. Antibody responses to galectin-8, TARP and TRAP1 in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy. Cancer Immunol Immunother. 2010;59:1313–23. doi: 10.1007/s00262-010-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickering JW, Martins TB, Schroder MC, Hill HR. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for auantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin Diagn Lab Immunol. 2002;9:872–6. doi: 10.1128/CDLI.9.4.872-876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth GK. Limma: linear models for microarray data. In: Gentleman VCR, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and computational biology solutions using {R} and bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 33.Bejamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Soc, Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 34.Hudson ME, Pozdnyakova I, Haines K, Mor G, Snyder M. Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc Natl Acad Sci USA. 2007;104:17494–9. doi: 10.1073/pnas.0708572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO, et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- 37.Califice S, Castronovo V, Bracke M, van den Brule F. Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 2004;23:7527–36. doi: 10.1038/sj.onc.1207997. [DOI] [PubMed] [Google Scholar]
- 38.Newlaczyl AU, Yu LG. Galectin-3—a jack-of-all-trades in cancer. Cancer Lett. 2011;313:123–8. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Fukasawa K, Fujii H, Saitoh Y, Koizumi K, Aozuka Y, Sekine K, et al. Aminopeptidase N (APN/CD13) is selectively expressed in vascular endothelial cells and plays multiple roles in angiogenesis. Cancer Lett. 2006;243:135–43. doi: 10.1016/j.canlet.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 40.Larkin SE, Holmes S, Cree IA, Walker T, Basketter V, Bickers B, et al. Identification of markers of prostate cancer progression using candidate gene expression. Br J Cancer. 2012;106:157–65. doi: 10.1038/bjc.2011.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorensen KD, Abildgaard MO, Haldrup C, Ulhoi BP, Kristensen H, Strand S, et al. Prognostic significance of aberrantly silenced ANPEP expression in prostate cancer. Br J Cancer. 2013;108:420–8. doi: 10.1038/bjc.2012.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert LA, Whyteside AR, Turner AJ, Usmani BA. Isoforms of endothelin-converting enzyme-1 (ECE-1) have opposing effects on prostate cancer cell invasion. Br J Cancer. 2008;99:1114–20. doi: 10.1038/sj.bjc.6604631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrmann E, Bogemann M, Bierer S, Eltze E, Hertle L, Wulfing C. The endothelin axis in urologic tumors: mechanisms of tumor biology and therapeutic implications. Expert Rev Anticancer Ther. 2006;6:73–81. doi: 10.1586/14737140.6.1.73. [DOI] [PubMed] [Google Scholar]
- 44.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–9. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 45.Nelson JB, Udan MS, Guruli G, Pflug BR. Endothelin-1 inhibits apoptosis in prostate cancer. Neoplasia. 2005;7:631–7. doi: 10.1593/neo.04787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darson MF, Pacelli A, Roche P, Rittenhouse HG, Wolfert RL, Saeid MS, et al. Human glandular kallikrein 2 expression in prostate adenocarcinoma and lymph node metastases. Urology. 1999;53:939–44. doi: 10.1016/s0090-4295(98)00637-2. [DOI] [PubMed] [Google Scholar]
- 47.Williams SA, Xu Y, De Marzo AM, Isaacs JT, Denmeade SR. Prostate-specific antigen (PSA) is activated by KLK2 in prostate cancer ex vivo models and in prostate-targeted PSA/KLK2 double transgenic mice. Prostate. 2010;70:788–96. doi: 10.1002/pros.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rittenhouse HG, Finlay JA, Mikolajczyk SD, Partin AW. Human Kallikrein 2 (hK2) and prostate-specific antigen (PSA): two closely related, but distinct, kallikreins in the prostate. Crit Rev Clin Lab Sci. 1998;35:275–368. doi: 10.1080/10408369891234219. [DOI] [PubMed] [Google Scholar]
- 49.Kubota E, Kataoka H, Aoyama M, Mizoshita T, Mori Y, Shimura T, et al. Role of ES cell-expressed Ras (ERas) in tumorigenicity of gastric cancer. Am J Pathol. 2010;177:955–63. doi: 10.2353/ajpath.2010.091056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arencibia JM, Martin S, Perez-Rodriguez FJ, Bonnin A. Gene expression profiling reveals overexpression of TSPAN13 in prostate cancer. Int J Oncol. 2009;34:457–63. [PubMed] [Google Scholar]
- 51.Su ZZ, Lin J, Shen R, Fisher PE, Goldstein NI, Fisher PB. Surface-epitope masking and expression cloning identifies the human prostate carcinoma tumor antigen gene PCTA-1 a member of the galectin gene family. Proc Natl Acad Sci USA. 1996;93:7252–7. doi: 10.1073/pnas.93.14.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McQuillan GM, Kruszon-Moran D, Deforest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med. 2002;136:660–6. doi: 10.7326/0003-4819-136-9-200205070-00008. [DOI] [PubMed] [Google Scholar]
- 53.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 54.Halabi S, Lin CY, Kelly WK, Fizazi KS, Moul JW, Kaplan EB, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–7. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol. 2004;24:571–8. doi: 10.1023/B:JOCI.0000040928.67495.52. [DOI] [PubMed] [Google Scholar]
- 56.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 57.Nesslinger NJ, Ng A, Tsang KY, Ferrara T, Schlom J, Gulley JL, et al. A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin Cancer Res. 2010;16:4046–56. doi: 10.1158/1078-0432.CCR-10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106:268–280. doi: 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gulley JL, Madan RA, Tsang KY, Jochems C, Marte J, Farsaci B, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2:133–41. doi: 10.1158/2326-6066.CIR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt M, Hellwig B, Hammad S, Othman A, Lohr M, Chen Z, et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin kappa C as a compatible prognostic marker in human solid tumors. Clin Cancer Res. 2012;18:2695–703. doi: 10.1158/1078-0432.CCR-11-2210. [DOI] [PubMed] [Google Scholar]
- 62.Lohr M, Edlund K, Botling J, Hammad S, Hellwig B, Othman A, et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013;333:222–8. doi: 10.1016/j.canlet.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 63.Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922–30. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 64.Shalom-Feuerstein R, Cooks T, Raz A, Kloog Y. Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 2005;65:7292–300. doi: 10.1158/0008-5472.CAN-05-0775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.