Mechanosensing of surfaces in bacteria is a process that often uses obstruction of flagellum rotation to trigger behaviors such as adhesion and surface-associated movement. In a recent publication, the PilY1 protein of Pseudomonas aeruginosa has been implicated as a novel mechanosensor that stimulates virulence in response to surface attachment.
Bacteria utilize diverse strategies for colonizing surfaces to form complex communities. In general, they respond to environmental signals, including nutritional, osmolar, and host-derived cues, activating regulatory circuits that control bacterial behaviors, such as adhesion and biofilm formation [1]. In addition, detection of mechanical stimuli through surface contact, termed mechanosensing, initiates multiple cellular responses that result in surface-associated behaviors, including attachment, movement across a surface, and cellular differentiation [2].
Mechanosensing is a ubiquitous instrument for translating environmental stimuli into biological responses. Organisms respond to gravity, contact with physical barriers, and flow. Some of the most common sensors of mechanical stimuli are ion channels that sense turgor pressure and mechanical tension [3]. In the animal kingdom, von Willebrand factor protein A (vWA) domains act as mechanosensors of flow by detecting shear force, which stimulates unraveling of the von Willebrand factor protein (Figure 1A). This conformational change allows platelets to rapidly bind vWA domains, promote coagulation and stop blood flow when epithelial integrity is breached [4]. In plants, an example of mechanosensation is the touch-based turgor response of the fern species Mimosa pudica. Motor organs at the leaf base, called pulvini, induce water loss to initiate leaflet folding upon physical stimulation [5]. However, mechanosensing is not restricted to specialized tissues of multicellular eukaryotes. Here we review what is known about how bacteria use mechanosensing of surfaces to regulate behavior, and we discuss new evidence provided in a recent publication by Siryaporn et al. [6] that offers support for another mechanism of surface sensing through mechanical stimulation.
Figure 1.
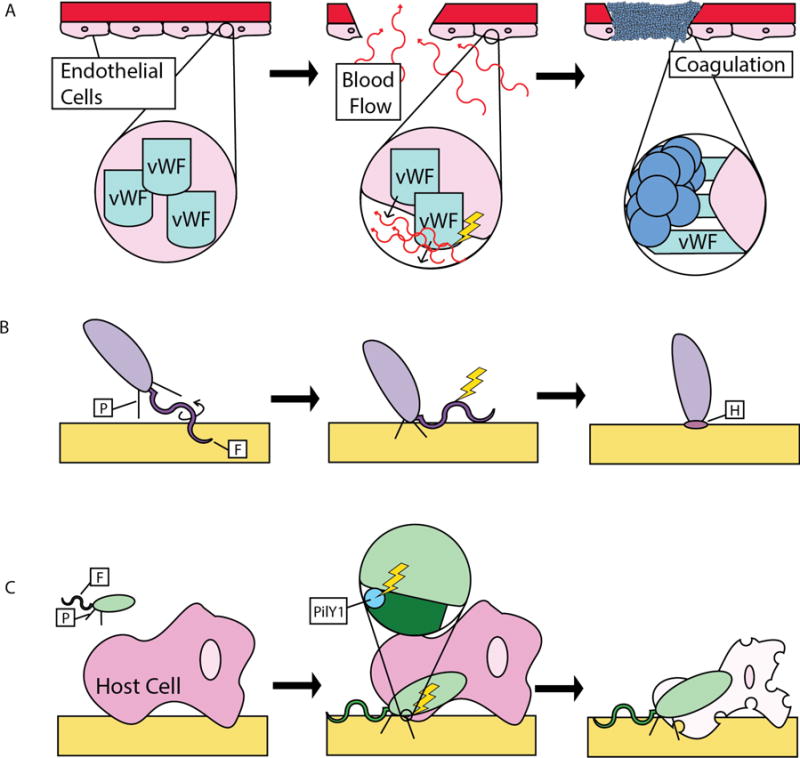
Various mechanisms of mechanosensing. (A) von Willebrand factor (vWF) is secreted from endothelial cells upon cell damage. It senses changes in shear force associated with blood flow toward cell damage and undergoes a conformational change that allows binding of platelets to cause coagulation. The lightning bolt represents the mechanical signal. (B) A Caulobacter crescentus swarmer cell detecting a surface via pili-mediated flagellum obstruction resulting in holdfast secretion. (C) A Pseudomonas aeruginosa cell sensing mechanical stimuli through the pilus-associated PilY1 protein to induce host cell death.
In order to generate appropriate behavioral and regulatory responses to surfaces, bacteria require the ability to sense surface contact. Bacteria can colonize and move across both biotic and abiotic surfaces, with many species using the flagellum as a surface sensor [2]. The flagellum is a rotating, membrane-embedded propeller that drives cell swimming. It obtains energy required for rotation through a proton- or sodium-gradient-generated motive force (P/SMF) that passes through a stationary motor complex called the stator [7]. Because the flagellum is a rotating structure, it provides an opportunity to sense obstruction of its rotation caused by surface contact. Also, an increase in environmental viscosity increases mechanical load on the flagellum independent of surface contact and has been shown to trigger transcription of genes involved in surface-associated functions, such as swarming in Vibrio parahaemolyticus [8,9]. Swarming is a complex type of multicellular surface-associated movement that is driven by flagellar rotation [10]. The deletion of the flagellum stator gene motB or a filament flagellin subunit gene flaC results in constant transcription of genes involved in swarming, indicating that mechanical or genetic perturbation of flagellum function mimics surface sensing [9]. Additionally, disruption of the SMF in V. parahaemolyticus using the drug phenamil blocks flagellar rotation and results in a dose-dependent increase in transcription of swarming-related genes in the absence of surface stimulation [11]. An increase in viscosity or the deletion of flagellar structural genes has also been shown to promote cellular differentiation into specialized swarmer cells in Proteus mirabilis [12].
The induction of swarming as a result of flagellar inhibition described above suggests a role of flagellar-mediated mechanosensing in regulating surface motility. Other research has implicated a role of flagellar-mediated surface sensing in bacterial adhesion. In Vibrio cholerae, chemical perturbation of membrane potential inhibits the transition from reversible to irreversible attachment [13]. In another example, surface contact stimulates secretion of a specialized adhesive polysaccharide involved in irreversible attachment called the holdfast in Caulobacter crescentus and the unipolar polysaccharide in Agrobacterium tumefaciens [14]. In C. crescentus, surface contact and tethering, mediated by both the flagellum and hair-like surface appendages called pili or fimbriae, rapidly inhibit flagellar rotation, resulting in stimulation of holdfast synthesis (Figure 1B). Viscous environments also inhibit C. crescentus flagellar rotation and stimulate holdfast production without surface contact and in a pili-independent manner, suggesting that the cell responds primarily to an increased load on the flagellum. In another example, antibody-tethering of the flagellum or deletion of the stator gene motB of Bacillus subtilis stimulates processes involved in biofilm formation, implicating the flagellum and stator in surface sensing [15]. Taken together, these studies indicate that surface contact is sensed by perturbation of flagellar function to control a range of surface-associated behaviors. However, the mechanisms by which signaling is induced upon mechanosensing remain unknown. Deletion of stator genes results in the induction of multiple surface-associated behaviors, suggesting involvement of the stator proteins and P/SMF in sensing flagellum obstruction. Interestingly, the number of stator units recruited to the flagellum increases when higher load force is applied to the flagellum in Escherichia coli, indicating that the flagellum stator complex can sense and respond to stresses placed on flagellar rotation [16]. Determining whether a change in proton flow is the signal for flagellar obstruction and subsequent induction of surface-associated behaviors, and how this signal might be sensed and transduced, are important questions for future investigation.
Although much remains to be elucidated about the mechanism of flagellar mechanosensing, a recent study conducted by Siryaporn et al. [6] reveals a role for the Pseudomonas aeruginosa protein PilY1 as a bacterial mechanosensor (Figure 1C). PilY1 is a cell surface-associated adhesin protein required for the biosynthesis of pili and attachment to the surface of host cells and has also been suggested to play a role in the regulation of swarming [17]. Along with the minor pilin proteins PilW and PilX, PilY1 was shown by Siryaporn et al. [6] to be required for surface-activated virulence. However, while PilY1 and the minor pilins are required for pilus synthesis, other P. aeruginosa mutants deficient in pilus assembly were still able to induce host cell death, indicating that PilY1 and the minor pilins play a role in regulating virulence that is independent of their role in pilus synthesis. Interestingly, PilY1 contains a vWA domain, the deletion of which resulted in hyperactive virulence. In contrast, deletion of other PilY1 domains led to complete lack of virulence activation. These results clearly implicate the PilY1 vWA domain in modulating virulence, but it is still unclear whether this regulation is directly due to mechanosensing.
Shear forces occur near surfaces in flowing systems, and other bacterial adhesins have previously been implicated in detecting shear stress. For example, pili and fimbriae are widely involved in surface attachment and bind to both biotic and abiotic surfaces through a variety of mechanisms [18,19]. The E. coli type 1 fimbrial adhesive subunit, FimH, has been implicated in sensing shear force, leading to increased surface adhesion [20]. The fact that PilY1 is a cell surface-exposed adhesin and contains a vWA domain suggests by analogy that it too may sense shear force rather than direct surface contact. However, several outstanding questions remain. How does PilY1 sense shear force, and what is the structural impact of the hypothesized conformational change in its vWA domain on the other domains of the protein? Is propulsion by the flagellum involved in generating the force sensed by PilY1? What are the mechanisms by which activated PilY1 regulates the virulence response? What is the role of the minor pilins? And finally, are the pilus biogenesis and mechanosensing functions truly separable in these proteins, or are the pili involved in enhancing or modulating the response?
Mechanosensing is an important and widespread biological process that allows organisms to perceive and respond to environmental changes. In bacteria, mechanosensing of surfaces is attributed to the regulation of surface-associated behaviors, including biofilm formation, movement, and now virulence. How surface sensing induces these and other biological processes remains subject to speculation. Clarification of the mechanisms behind surface-sensing pathways will provide valuable insight into understanding bacterial behaviors in response to environmental cues.
References
- 1.Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev. 2009;73:310–347. doi: 10.1128/MMBR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belas R. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol. 2014;22:517–527. doi: 10.1016/j.tim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124:1412–1425. doi: 10.1182/blood-2014-05-378638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monshausen GB, Gilroy S. Feeling green: mechanosensing in plants. Trends Cell Biol. 2009;19:228–235. doi: 10.1016/j.tcb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Siryaporn A, Kuchma SL, O’Toole GA, Gitai Z. Surface attachment induces Pseudomonas aeruginosa virulence. Proc Natl Acad Sci USA. 2014;111:16860–16865. doi: 10.1073/pnas.1415712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima S, Blair DF. International Review of Cytology. Academic Press; 2004. The bacterial flagellar motor: structure and function of a complex molecular machine; pp. 93–134. [DOI] [PubMed] [Google Scholar]
- 8.Belas R, Simon M, Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 10.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol Microbiol. 1996;20:693–699. doi: 10.1111/j.1365-2958.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- 12.Belas R, Suvanasuthi R. The ability of Proteus mirabilis to sense surfaces and regulate virulence gene expression involves FliL, a flagellar basal body protein. J Bacteriol. 2005;187:6789–6803. doi: 10.1128/JB.187.19.6789-6803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellen KLV, Houot L, Watnick PI. Genetic analysis of Vibrio cholerae monolayer formation reveals a key role for ΔΨ in the transition to permanent attachment. J Bacteriol. 2008;190:8185–8196. doi: 10.1128/JB.00948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Brown PJB, Tang JX, Xu J, Quardokus EM, Fuqua C, Brun YV. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol Microbiol. 2012;83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns LS, Marlow VL, Bissett E, Ostrowski A, Stanley-Wall NR. A mechanical signal transmitted by the flagellum controls signalling in Bacillus subtilis. Mol Microbiol. 2013;90:6–21. doi: 10.1111/mmi.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lele PP, Hosu BG, Berg HC. Dynamics of mechanosensing in the bacterial flagellar motor. Proc Natl Acad Sci USA. 2013;110:11839–11844. doi: 10.1073/pnas.1305885110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchma SL, Ballok AE, Merritt JH, Hammond JH, Lu W, Rabinowitz JD, O’Toole GA. Cyclic-di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa: the pilY1 gene and its impact on surface-associated behaviors. J Bacteriol. 2010;192:2950–2964. doi: 10.1128/JB.01642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isberg RR, Barnes P. Dancing with the host: flow-dependent bacterial adhesion. Cell. 2002;110:1–4. doi: 10.1016/s0092-8674(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 19.Mattick JS. Type iv pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 20.Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]


