Abstract
Humans are constantly exposed to mixtures, such as tobacco smoke, exhaust from diesel, gasoline or new bio-fuels, containing several thousand compounds, including many known human carcinogens. Covalent binding of reactive compounds or their metabolites to DNA and formation of stable adducts is believed to be the causal link between exposure and carcinogenesis. DNA and protein adducts are well established biomarkers for the internal dose of reactive compounds or their metabolites and are an integral part of science-based risk assessment. However, technical limitations have prevented comprehensive detection of a broad spectrum of adducts simultaneously. Therefore, most studies have focused on measurement of abundant individual adducts. These studies have produced valuable insight into the metabolism of individual carcinogens, but they are insufficient for risk assessment of exposure to complex mixtures. To overcome this limitation, we present herein proof-of-principle for comprehensive exposure assessment, using N-terminal valine adduct profiles as a biomarker. The reported method is based on our previously established immunoaffinity liquid chromatography-tandem mass spectrometry (LC-MS/MS) method with modification to enrich all N-terminal valine alkylated peptides. The method was evaluated using alkylated peptide standards and globin reacted in vitro with alkylating agents (1,2-epoxy-3-butene, 1,2:3,4-diepoxybutane, propylene oxide, styrene oxide, N-ethyl-N-nitrosourea and methyl methanesulfonate), known to form N-terminal valine adducts. To demonstrate proof-of-principle, the method was successfully applied to globin from mice treated with four model compounds. The results suggest that this novel approach might be suitable for in vivo biomonitoring.
Keywords: N-terminal valine adducts, multiple exposure detection, biomonitoring, mixtures, biomarkers
Introduction
Environmental and occupational exposures have been associated with increased risk for the development of numerous cancers (Davis et al. 2010; Swenberg et al. 2008). Accurate exposure assessment in humans, however, is challenging because humans are exposed to a variety of mixtures, such as tobacco smoke, exhaust from diesel, gasoline or new bio-fuels. Such exposures contain several thousand compounds, including many known or suspected human carcinogens. Covalent binding of reactive metabolites to DNA and the formation of stable adducts is believed to be the causal link between exposure and carcinogenesis. DNA adducts are well established biomarkers for the internal dose of reactive compounds or their metabolites and are an integral part of science-based risk assessment. Reactive compounds that form DNA adducts often also form protein adducts, such as with albumin and hemoglobin. The corresponding protein adducts are commonly used as surrogate biomarkers for DNA adducts and are well established biomarkers of exposures (Swenberg et al. 2008; Törnqvist et al. 2002; Wild 2009; Wild and Pisani 1998).
While globin adducts are not causally linked to mutagenesis, they have several advantages over DNA adducts: (i) protein adducts are recognized as good surrogate markers for the internal formation of the activated metabolites, (ii) in molecular epidemiology studies, blood samples are easier to obtain than tissue specimens, (iii) stable hemoglobin (Hb) adducts accumulate over the lifespan of the erythrocytes, which is about 30 days for mice, (iv) they are not removed by enzymatic repair systems like DNA adducts, and (v) due to their stability, protein adducts represent the cumulative exposure prior to sampling, which makes the timing of sample collection less critical. (vi) Lastly, they are excellent biomarkers that integrate in vivo metabolism over time and do not require invasive or time sensitive sampling (Törnqvist et al. 2002).
Extensive efforts have been made to quantitate adducts in albumin and hemoglobin. Of these, utilization of the modified Edman degradation method for analysis of N-terminal valine adducts is the most common (Boysen et al. 2007; Boysen and Hecht 2003; Osterman-Golkar et al. 2003; Törnqvist et al. 1986; Törnqvist et al. 2002). A comprehensive review of mass spectrometry study of protein adducts was recently published by Rubino et al. (Rubino et al. 2009). Unfortunately, technical limitations, such as low recovery, sensitivity and the need of adduct specific immunoaffinity chromatography, have prevented comprehensive exposure profiling of complex mixtures (“exposure-omics”), and the majority of studies report measurements of a single adduct or selected few adducts.
More recently Rappaport and colleagues reported progress in simultaneous monitoring of multiple adducts on cysteine in human serum albumin (Funk et al. 2010; Li et al. 2011). Further modifications of the Edman procedure for analysis of N-terminal valine adducts seem promising for future adduct profiling studies (Von Stedingk H. et al. 2010; Von Stedingk H. et al. 2011). These new approaches are aimed to establish a tool for multi-adduct profiling of reactive, and potentially genotoxic, compounds in mixtures. Such technology is expected to enable determination of the internal dose of numerous carcinogens simultaneously to (a) better understand the effects and fate of individual carcinogens in mixtures, (b) identify novel, until now, unknown adducts, and (c) investigate potential compound-compound-interactions.
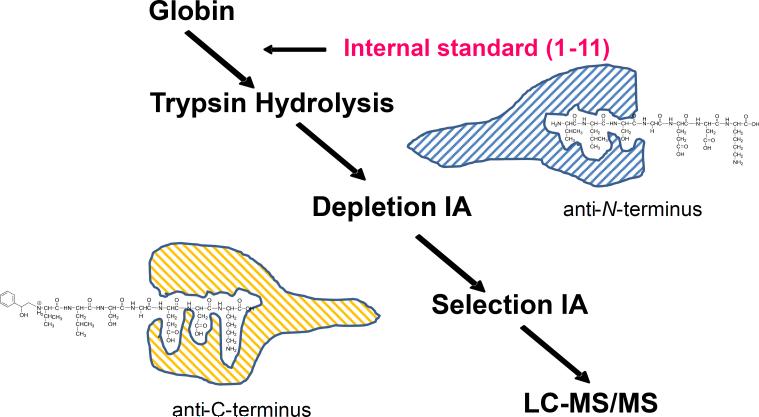
We report herein a novel proof-of-principle for a sensitive and specific method for qualitative profiling of exposure to a broad spectrum of reactive compounds or their metabolites using N-terminal valine adducts of hemoglobin as biomarkers. The reported method is based on our previously established immunoaffinity liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (Boysen et al. 2012; Boysen et al. 2004; Georgieva et al. 2007; Georgieva et al. 2010). To enable multi-adduct profiling, the adduct enrichment step has been modified to selectively isolate all alkylated N-terminal peptides of the globin α-chain, independent of adduct structure or chemical properties, prior to analysis by LC-MS/MS (Figure 1).
Figure 1.
Scheme of adduct profiling assay. The alkylated N-terminal peptides of the α-chain are isolated from trypsin hydrolyzed globin by series of depletion and selection IA chromatography, prior to analysis by LC-MS/MS as described in Materials and Methods.
Materials and Methods
Materials
Trypsin (biotin agarose, from bovine pancreas) was purchased from Sigma-Aldrich (St. Louis, MO). All reagents and solvents used were ACS grade or higher. Amicon 3 filters were obtained from Amicon Inc. (Beverly, MA) and Microspin filter tubes (regenerated cellulose, 0.2 μm) were from Altech Associates Inc. (Dearfield, IL). The non-alkylated (1-11) and methylated (1-11) peptide standards and [13C5] valine labeled non-alkylated (1-11) peptide used for synthesis of internal standards were purchased from Neo-Peptide, a subdivision of Neo-Group, Inc. (Cambridge, MA). The antibodies against the N-terminus (depletion) and C-terminus (selection) of the mouse α-Hb (1-7) peptides were raised by Open Biosystems Inc. (Huntsville, AL). Immunoaffinity (IA) depletion and selection columns were built using the respective antibody with the highest ELISA titers according to the procedure described in our earlier publication (Boysen, 2004).
Synthesis of standard peptides
The syntheses of analytical standard (AST) and internal standard (IST) peptides were performed by direct reaction of the non-alkylated (1-11) peptide with the reagents of interest in 0.1M NH4HCO3 buffer for 72 hours at the optimal molar ratio of 1:50 and pH 6.5. Full scan MS and MS/MS experiments of the products were used to confirm peptide sequence and site of alkylation.
In vitro alkylation of mouse (1-11) peptide or control globin
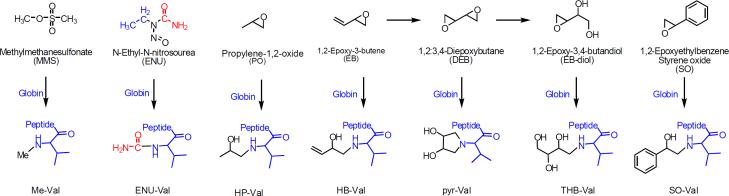
Globin was extracted from a female 12 week old B6C3F1 mouse as described previously (Mowrer et al. 1986). The reactions were performed at a molar ratio of peptide (globin): reagent = 1:1 in 0.1 M NH4HCO3 buffer for 72 hours at pH 6.5. Reagents, 1,2-epoxy-3-butene (EB), 1,2:3,4-diepoxybutane (DEB), propylene oxide (PO), styrene oxide (SO), N-ethyl-N-nitrosourea (ENU) and methyl methanesulfonate (MMS), were used alone or in a mixture (Figure 2). After trypsin hydrolysis, the samples were processed over depletion and selection IA columns, and elutes were analyzed as described below.
Figure 2.
Overview of selected model compounds and their corresponding N-terminal valine adducts.
Animal exposures
Female B6C3F1 mice were exposed in the UAMS Animal Facility to MMS, ENU, EB, and SO. MMS and ENU were given in saline by gavage once a day for 4 days and EB and SO were given once by i.p. injection on the fourth day. Mice were sacrificed on the fifth day and blood and tissues were harvested for further analyses. Globin from 2 samples of highly exposed mice (25 mg/kg body weight MMS and 100 mg/kg body weight ENU, plus 500 μmol/kg body weight both EB and SO) was extracted and analyzed for the expected adducts as described below.
Immunoaffinity enrichment and purification of in vitro reaction mixtures or globin samples from mice
Globin was first trypsinized as described previously (Boysen et al. 2004). After filtration on Amicon 3 columns and concentration in a speed-vac to about 0.3 mL, samples were loaded to the depletion IA columns, containing antibodies specific to the N-termini of trypsinized α-globin, in 0.5 mL PBS and incubated for 2 hrs. Then the samples from the depletion columns were slowly dripped to selection columns, containing antibodies specific to the C-termini of trypsinized α-globin, (rinsed 2 times with 0.5 mL PBS) incubated for an additional 2 hrs and washed five times with 2 mL water. The alkylated peptides were eluted in 3 mL freshly prepared 5% formic acid (FA) followed by drying overnight in a speed vac. Samples were dissolved in 150 μL water and filtered on Microspin filters (2 times 50 μL HPLC water washes), dried in a speed vac and stored at −20°C until MS analysis. Between sample analyses, both sets of depletion and selection IA columns were regenerated with 2 times 3 mL 5% FA, 2 times 5 mL water, 2 times 5 mL PBS and stored in PBS at 4°C.
Tandem Mass Spectrometry Analysis
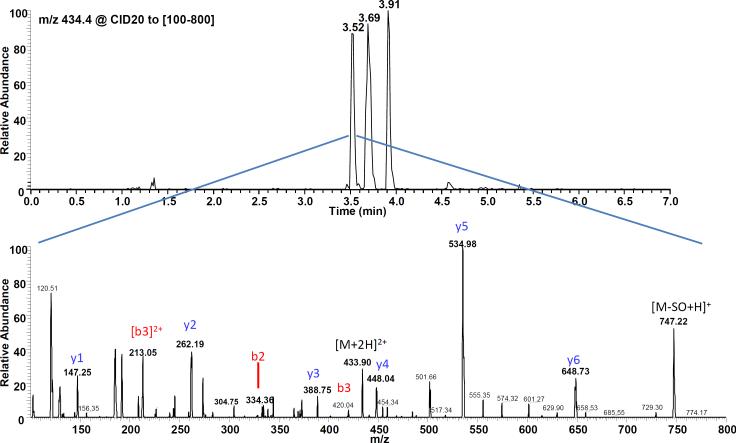
The qualitative analysis of the adducted N-terminal Val (1–7) peptides by LC–ESI–MS/MS was performed with an Acquity UPLC (Waters) coupled to a TSQ-Quantum Ultra triple quad mass analyzer (ThermoScientific). An Acquity UPLC BEH column C18 1.7μm 2.1 × 150 mm was operated with a linear gradient of 0.1% FA to 70% acetonitrile-0.1% FA in 10 min, at a flow rate of 0.4 mL/min. The retention times of N-terminal Val adducts were determined with synthesized peptide standards. The peptides were detected in single reaction monitoring (SRM) mode, monitoring the transition of the doubly charged precursor ions to the a1-or y2-fragment listed in Table 1. The MS conditions were as follows: column temperature 60°C, collision energy 20 eV. The IA purified samples were reconstituted in 20 μl of water and 1 to 2 μl were injected. Characterization of mouse SO-Val (1-7) was performed by monitoring the product ions derived from double charged precursor ion [M+2H]2+ m/z 434.4 at collision energies ranging from 10 to 40 eV.
Table 1.
Selected SRM transitions
| Adduct | Ion transitiona |
|---|---|
| H2N-Val | 374 → 262b |
| Me-Val | 381 → 262b |
| Et-Val | 396 → 116 |
| ENU-Val | 790 → 535c |
| HP-Val | 403 → 130 |
| HB-Val | 409 → 142 |
| pyr-Val | 417 → 158 |
| THB-Val | 426 → 176 |
| SO-Val | 434 → 192 |
doubly charged precursor ion to a1-fragment
doubly charged precursor ion to y2-fragment
singly charged precursor ion to y5-fragment
Results and Discussion
We report herein a modified version of our successfully established LC-MS/MS method for quantitation of BD-derived N-terminal valine adducts that allows simultaneous analysis of multiple adducts (Boysen et al. 2007; Boysen et al. 2012; Boysen et al. 2004; Georgieva et al. 2007; Georgieva et al. 2010). To enable adduct profiling, the adduct enrichment step has been modified to enable enrichment of all alkylated N-terminal peptides (Figure 1). Therefore, two sets of IA columns containing antibodies specific to N- or C-terminus of the target peptide (α-chain peptide 1-7) were prepared to generate depletion and selection IA chromatography columns, respectively. In the first enrichment step, non-alkylated peptides are depleted with depletion IA columns containing antibodies specifically raised against the N-terminus of the non-alkylated peptide. The unbound alkylated peptides, not retained on the depletion columns, are directly loaded onto the selection IA columns. Subsequently, the peptides of interest, the alkylated N-terminal peptides, are retained on the selection IA columns containing antibodies raised against the C-terminus. It was hypothesized that alkylation on the opposite N-terminus should not affect peptide enrichment. After washing with water, alkylated peptides are eluted and analyzed by LC-MS/MS. An advantage of this novel design is that peptides and antibodies are commercially available by various vendors. Therefore it is not necessary to synthesize standard peptides to raise adduct specific antibodies, a practice in our previous studies (Boysen et al. 2004). Prior to purification of real samples, IA columns were tested for recovery by loading AST and IST and performing the procedure for separation and enrichment (data not shown).
The method was evaluated using alkylated peptide standards and globin reacted in vitro with a few selected model alkylating agents. For this investigation, EB, DEB, PO, SO, ENU and MMS were chosen because they are known to form N-terminal valine adducts and represent a variety of compound classes (Kautiainen et al. 2000; Osterman-Golkar et al. 2003; Swenberg et al. 2001; Zhang et al. 2005). For the standards synthesis, optimal conditions for reactions of epoxides with peptide standards were determined experimentally (data not shown). It was found that reactions at pH 6.5 with a 1:50 molar ratio mainly produced the single valine alkylated peptide standards. The site of alkylation was confirmed by LC-MS and MS/MS analysis (Figure 3). The MS/MS spectrum suggests single alkylation at the N-terminal valine based on observed b- and y-fragments (Figure 3). Fragments indicative of alkylation at the lys7 or lys11 were not observed. The N-terminal valine group is the primary site of alkylation in reactions carried out at pH <7 because at higher pH all three NH2-goups are available to attack the epoxide ring. At pH<7 the ε-NH of lys7 and lys11 are protonated and are much less reactive. Therefore, all subsequent peptide reactions were carried out at pH 6.5 to specifically alkylate NH2-group of the N-terminal valine. These conditions were chosen to maximize standard synthesis. Under physiologic conditions the N-terminal valine has been shown to be the primary site of alkylation in the 1-7 peptide (Basile et al. 2001; Basile et al. 2002). In addition, for reactions with EB and SO, the expected positional (regional) isomers and diastereomers were resolved as separate peaks (Figures 4 and 5). This simple synthesis approach has been found suitable for synthesis of all peptide standards of the selected model compounds. This synthesis approach is more convenient compared to the previous strategy of synthesizing the alkylated valine and subsequent attachment to the 2-11 peptide as described by Jayaraj et al. (Jayaraj et al. 2003).
Figure 3.
Characterization of mouse SO-Val (1-11) standard. Mouse 1-11 peptide standard was reacted with SO in 0.1 M NH4HCO3 at pH 6.5 for 72 h. MS/MS fragmentation suggests single alkylation at the N-terminal valine.
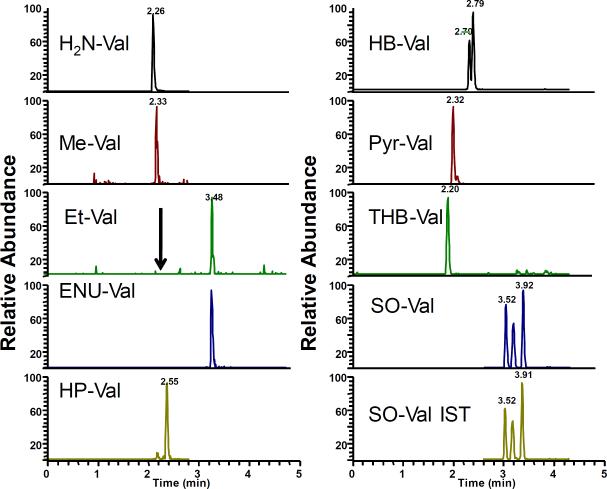
Figure 4.
Extracted ion chromatograms of mouse globin treated in vitro with a mixture of EB, DEB, PO and SO, MMS and ENU. The treated globin was processed as described in Materials and Methods and analyzed by LC-MS/MS. [13C5]SO-Val was utilized as internal standard.
Figure 5.
Representative extracted ion-chromatogram of exposed mouse globin. B6C3F1 mice were treated with EB, SO, MMS and ENU and globin was isolated and analyzed as described in Materials and Methods. [13C5]SO-Val was utilized as internal standard.
As a first step of method development, the overall recovery was determined by using SO-Val (1-11) peptide standard. The mean recovery of the SO-Val was 72.5 ± 23.7% (n=15) based on the peak area of peptide standard hydrolyzed and injected directly compared to the same amount hydrolyzed and processed over depletion and selection IA columns. Blank samples consisting of water, processed over both IA columns did not contain any detectable adducts. These results suggest that the modified adduct enrichment procedure effectively isolates alkylated N-terminal peptides.
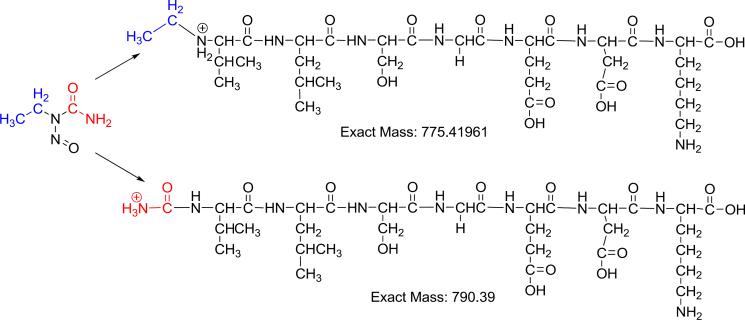
The investigation was expanded to analyses of 1-11 peptides that had been reacted with the selected model compounds, known to form N-terminal valine adducts, individually or as a mixture (Figure 2). Except for ENU, all other reactions of epoxide with the mouse peptide produced the corresponding alkylated peptide standards (data not shown). Surprisingly, the reaction of 1-11 mouse peptide with ENU did not result in the expected ethyl-Val (Et-Val). Instead after trypsin hydrolysis an ion with m/z of 790.4 was observed and further investigated by LC-MS/MS. The MS/MS spectrum suggests it is a carbamoylated-adduct (ENU-Val) (Figure 6 and Supplemental Figure S1). The formation of an ENU-Val adduct has been previously reported for methylnitrosourea (Zhang et al. 2005) and suggests that all nitrosourea derivatives may in fact form this type of adduct. Further validation and structural characterization of this specific adduct is under way.
Figure 6.
Proposed formation of the ENU-derived carbamoyl adduct (ENU-Val).
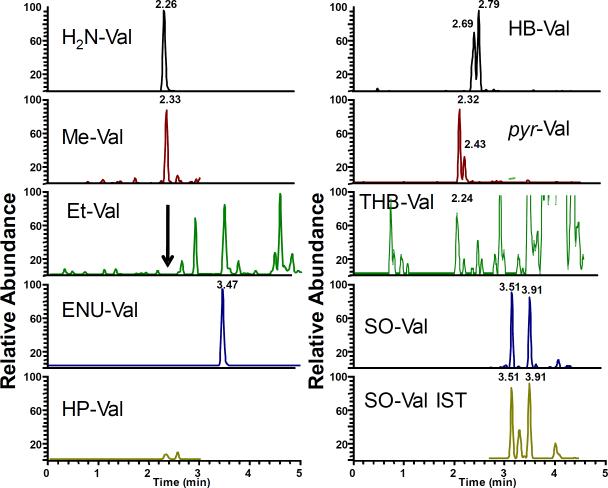
After synthesis and analyses of various model adduct standards, we extended our effort to analysis of in vitro alkylated globin. Therefore, control mouse globin was treated with several epoxides individually or as a mixture as described in Materials and Methods. The treated globin was analyzed and the expected adducts were readily detected (Figure 4). As with the peptide reaction, ENU did not produce detectable amounts of Et-Val. Instead a clear peak corresponding to the ENU-Val adduct was observed, demonstrating that it is also formed in globin. As with in vitro reacted peptides, HB- and SO-Val were detected as individually resolved peaks for corresponding isomers.
Subsequently, to demonstrate that the novel method is suitable for in vivo biomonitoring, we analyzed globin from two B6C3F1 mice that had been treated with MMS, ENU, EB and SO. Figure 5 shows the representative extracted ion chromatograms corresponding to the model adducts. Me-Val, ENU-Val, HB-Val, pyr-Val and SO-Val were clearly detected. Similar to in vitro results, positional isomers and diastereomers of SO-Val and HB-Val were resolved as separate peaks. No peaks were observed for HP-Val since the mice were not treated with PO. Surprisingly, no clear peaks were found for THB-Val, the main BD derived adduct. This is probably due to the fact that EB was given by i.p. injection and the time was insufficient to form significant amounts of EB-diol available for adduct formation. Interestingly, the ENU-Val-adducts from ENU were clearly detected, demonstrating its formation in vivo. The expected Et-Val adducts were not observed, suggesting that they either do not form or form at undetectable amounts. Overall, the results suggest that this novel approach might be suitable for in vivo biomonitoring and future studies to investigate physiologic exposure and dose-response are ongoing.
Unfortunately, we also observed some non-alkylated peptide, suggesting that the depletion step is not complete. Further computational investigation demonstrates that about 99.99% of non-alkylated peptides had been removed. However, the huge excess of non-alkylated peptides still leads to clearly detectable peaks. At this time it is not apparent whether further increase in depletion efficiency is needed, since the adducts of interest were clearly detected in both in vitro treated globin and in vivo exposed mice.
Conclusion
A proof of principle of a novel LC-MS/MS method for analysis of a broad spectrum of N-terminal valine adducts is presented. This method advances exposure monitoring by establishing methodology for qualitative profiling of exposures to complex mixtures and could lead to unprecedented insights into the biological importance of individual carcinogens in mixtures. The adduct-profiling method allows the monitoring of a variety of known endogenous alkylating agents in response to, and in addition to, environmental and occupational exposures. Here, we examined acute exposure to show proof-of-principle, and application to low and chronic exposures are needed to complete method validation. The long term goal of our research is to advance current adduct analysis from targeted to untargeted technology for human bio-monitoring.
ACKNOWLEDGEMENTS
Support has been provided in part by Health Effects Institute, NIEHS and the Arkansas Bioscience Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Abbreviations
- BD
1,3-butadiene
- DEB
1,2:3,4-diepoxybutane
- EB
1,2 epoxy-3-butene
- EB-diol
3,4-epoxy-1,2-butanediol
- ENU
N-ethyl-N-nitrosourea
- ENU-Val
carbamoylated-valine
- Et-Val
ethyl-valine
- FA
formic acid
- HB-Val
N-(2-hydroxy-3-buten-1-yl)-valine
- Hb
hemoglobin
- HP-Val
1-hydroxy (or 2-hydroxy)-propyl- valine
- IA
immunoaffinity
- LC-MS/MS
Liquid Chromatography-Tandem Mass Spectrometry
- Me-Val
methyl-valine
- MMS
methyl-methanesulfonate
- H2N-Val
non-alkylated-valine
- PO
propylene oxide
- pyr-Val
N,N-(2,3-dihydroxy-1,4-butadiyl)-valine
- SO
styrene oxide
- SO-Val
1-phenyl-2-hydroxyethyl-valine, or 2-phenyl-2-hydroxyethyl-valine
- THB-Val
2,3,4-trihydroxybutyl-valine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Basile A, Ferranti P, Mamone G, Manco I, Pocsfalvi G, Malorni A, Acampora A, Sannolo N. Structural analysis of styrene oxide/haemoglobin adducts by mass spectrometry: identification of suitable biomarkers for human exposure evaluation. Rapid Commun. Mass Spectrom. 2002;16(9):871–878. doi: 10.1002/rcm.655. [DOI] [PubMed] [Google Scholar]
- 2.Basile A, Ferranti P, Pocsfalvi G, Mamone G, Miraglia N, Caira S, Ambrosi L, Soleo L, Sannolo N, Malorni A. A novel approach for identification and measurement of hemoglobin adducts with 1,2,3,4-diepoxybutane by liquid chromatography/electrospray ionisation mass spectrometry and matrix-assisted laser desorption/ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2001;15(8):527–540. doi: 10.1002/rcm.263. [DOI] [PubMed] [Google Scholar]
- 3.Boysen G, Georgieva NI, Bordeerat NK, Sram RJ, Vacek P, Albertini RJ, Swenberg JA. Formation of 1,2:3,4-diepoxybutane-specific hemoglobin adducts in 1,3-butadiene exposed workers. Toxicol. Sci. 2012;125(1):30–40. doi: 10.1093/toxsci/kfr272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boysen G, Georgieva NI, Upton PB, Walker VE, Swenberg JA. N-terminal globin adducts as biomarkers for formation of butadiene derived epoxides. Chem Biol Interact. 2007;166(1-3):84–92. doi: 10.1016/j.cbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Boysen G, Georgieva NI, Upton PB, Jayaraj K, Li Y, Walker VE, Swenberg JA. Analysis of diepoxide-specific cyclic N-terminal globin adducts in mice and rats after inhalation exposure to 1,3-butadiene. Cancer Res. 2004;64(23):8517–8520. doi: 10.1158/0008-5472.CAN-04-3184. [DOI] [PubMed] [Google Scholar]
- 6.Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat. Res. 2003;543(1):17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 7.Davis ME, Laden F, Hart JE, Garshick E, Smith TJ. Economic activity and trends in ambient air pollution. Environ. Health Perspect. 2010;118(5):614–619. doi: 10.1289/ehp.0901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funk WE, Li H, Iavarone AT, Williams ER, Riby J, Rappaport SM. Enrichment of cysteinyl adducts of human serum albumin. Anal. Biochem. 2010;400(1):61–68. doi: 10.1016/j.ab.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgieva NI, Boysen G, Bordeerat N, Walker VE, Swenberg JA. Exposure-Response of 1,2:3,4-Diepoxybutane Specific N-Terminal Valine Adducts in Mice and Rats after Inhalation Exposure to 1,3-Butadiene. Toxicol. Sci. 2010;15(2):322–329. doi: 10.1093/toxsci/kfq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgieva NI, Boysen G, Upton PB, Jayaraj K, Gold A, Swenberg JA. Quantitative analysis of N-terminal valine peptide adducts specific for 1,2-epoxy-3-butene. Chem. Biol. Interact. 2007;166(1-3):219–225. doi: 10.1016/j.cbi.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaraj K, Georgieva NI, Gold A, Sangaiah R, Koc H, Klapper DG, Ball LM, Reddy AP, Swenberg JA. Synthesis and characterization of peptides containing a cyclic Val adduct of diepoxybutane, a possible biomarker of human exposure to butadiene. Chem. Res. Tox. 2003;16(5):637–643. doi: 10.1021/tx020099i. [DOI] [PubMed] [Google Scholar]
- 12.Kautiainen A, Fred C, Rydberg P, Törnqvist M. A liquid chromatography tandem mass spectrometric method for in vivo dose monitoring of diepoxybutane, a metabolite of butadiene. Rapid Commun. Mass Spectrom. 2000;14(19):1848–1853. doi: 10.1002/1097-0231(20001015)14:19<1848::AID-RCM106>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Grigoryan H, Funk WE, Lu SS, Rose S, Williams ER, Rappaport SM. Profiling Cys34 adducts of human serum albumin by fixed-step selected reaction monitoring. Mol Cell Proteomics. 2011;10(3):M110. doi: 10.1074/mcp.M110.004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mowrer J, Törnqvist M, Jensen S, Ehrenberg L. Modified edman degradation applied to hemoglobin for monitoring occupational exposure to alkylating-agents. Tox. Env. Chem. 1986;11(3):215–231. [Google Scholar]
- 15.Osterman-Golkar S, Czene K, Lee MS, Faller TH, Csanady GA, Kessler W, Perez HL, Filser JG, Segerback D. Dosimetry by means of DNA and hemoglobin adducts in propylene oxide-exposed rats. Toxicol. Appl. Pharmacol. 2003;191(3):245–254. doi: 10.1016/s0041-008x(03)00253-9. [DOI] [PubMed] [Google Scholar]
- 16.Rubino FM, Pitton M, Di FD, Colombi A. Toward an “omic” physiopathology of reactive chemicals: thirty years of mass spectrometric study of the protein adducts with endogenous and xenobiotic compounds. Mass Spectrom. Rev. 2009;28(5):725–784. doi: 10.1002/mas.20207. [DOI] [PubMed] [Google Scholar]
- 17.Swenberg JA, Fryar-Tita E, Jeong YC, Boysen G, Starr T, Walker VE, Albertini RJ. Biomarkers in Toxicology and Risk Assessment: Informing Critical Dose-Response Relationships. Chem Res Toxicol. 2008;21(1):253–265. doi: 10.1021/tx700408t. [DOI] [PubMed] [Google Scholar]
- 18.Swenberg JA, Koc H, Upton PB, Georgieva N, Ranasinghe A, Walker VE, Henderson R. Using DNA and hemoglobin adducts to improve the risk assessment of butadiene. Chem. Biol. Interact. 2001;135-136:387–403. doi: 10.1016/s0009-2797(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 19.Törnqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B. 2002;778(1-2):279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 20.Törnqvist M, Mowrer J, Jensen S, Ehrenberg L. Monitoring of environmental cancer initiators through hemoglobin adducts by a modified Edman degradation method. Analytical Biochemistry. 1986;154:255–266. doi: 10.1016/0003-2697(86)90524-5. [DOI] [PubMed] [Google Scholar]
- 21.Von Stedingk H, Rydberg P, Tornqvist M. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC-MS/MS. J Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2010;878(27):2483–2490. doi: 10.1016/j.jchromb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Von Stedingk H, Vikstrom AC, Rydberg P, Pedersen M, Nielsen JK, Segerback D, Knudsen LE, Tornqvist M. Analysis of hemoglobin adducts from acrylamide, glycidamide, and ethylene oxide in paired mother/cord blood samples from Denmark. Chem. Res Toxicol. 2011;24(11):1957–1965. doi: 10.1021/tx200284u. [DOI] [PubMed] [Google Scholar]
- 23.Wild CP. Environmental exposure measurement in cancer epidemiology. Mutagenesis. 2009;24(2):117–125. doi: 10.1093/mutage/gen061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wild CP, Pisani P. Carcinogen DNA and protein adducts as biomarkers of human exposure in environmental cancer epidemiology. Cancer Detect Prev. 1998;22(4):273–283. doi: 10.1046/j.1525-1500.1998.cdoa38.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Bartels MJ, Pottenger LH, Gollapudi BB. Differential adduction of proteins vs. deoxynucleosides by methyl methanesulfonate and 1-methyl-1-nitrosourea in vitro. Rapid Commun. Mass Spectrom. 2005;19(4):438–448. doi: 10.1002/rcm.1806. [DOI] [PubMed] [Google Scholar]