Sometimes simple things are hard to handle. This is true for infectious diarrhea, which remains one of the leading causes of death for children worldwide and a significant factor in long-term morbidity.1 Some gut infections rapidly go systemic, with deadly impact even for adults, as evidenced most recently by the outbreak of Shiga toxin–producing E. coli in Europe this summer. Vaccines have long been sought to protect the intestine from such pathogens and their toxins. But the search has proved difficult, because conventional routes of immunization — by injection through the skin — usually generate systemic immunity but provide little or no protection at mucosal surfaces, while mucosal immunizations target immune responses to mucosal tissues, but are often hampered by insufficient potency or poor safety or both. And so a new study reported by Hammerschmidt and colleagues2 warrants attention.
Effective immunization requires processing and presentation of antigen by dendritic cells in juxtaposition to naïve T cells. This occurs only where the two cells meet in secondary lymphoid organs, such as lymph nodes draining the skin, or organized lymphoid aggregates that collect antigens from mucosal surfaces such as those found in the intestine (sub-epithelial Peyers patches and mesenteric lymph nodes), lung, and other sites open to the environment. In lymph nodes, dendritic cells migrating from the periphery loaded with exogenous antigens and activated by “danger signals” identify naïve T cells uniquely capable of recognizing the antigen and induce them to proliferate and to differentiate into one of several effector T cell phenotypes. Dendritic cells also provide directions to newly-minted “effector” T cells so that they can migrate, establish local immunity and fight infection where required.
We now know how naïve T cells are “imprinted” by dendritic cells to target the small intestine.3,4 Unlike dendritic cells in other lymphoid tissues, those in Peyer’s patches and mesenteric lymph nodes possess enzymes that convert dietary vitamin A into retinoic acid (RA). Thus, the presence of RA is a characteristic feature of the lymphoid environment of the intestine. The presence of RA during antigen presentation induces activated B and T cells to express on their surfaces the α4β7 integrin that binds MAdCAM-1 – an adhesion receptor specific to mucosal microvascular endothelial cells – and the chemokine receptor CCR9 that senses CCL25, a chemokine that is constitutively expressed in small intestine. When the activated lymphocytes return from the mucosal lymphoid tissues into the blood, these two trafficking molecules (termed “homing receptors”) enable effector cells to migrate to the intestinal mucosa (see Figure). If RA is not present, however, such as when dendritic cells and naive T cells meet in lymph nodes draining the skin, the T cells are induced to express other “homing receptors” that target the T cell back to the skin (see Figure). The resulting effector cells can accumulate in the skin but traffic poorly to the gut, which is why percutaneous vaccination often generates inadequate mucosal protection.
Figure.
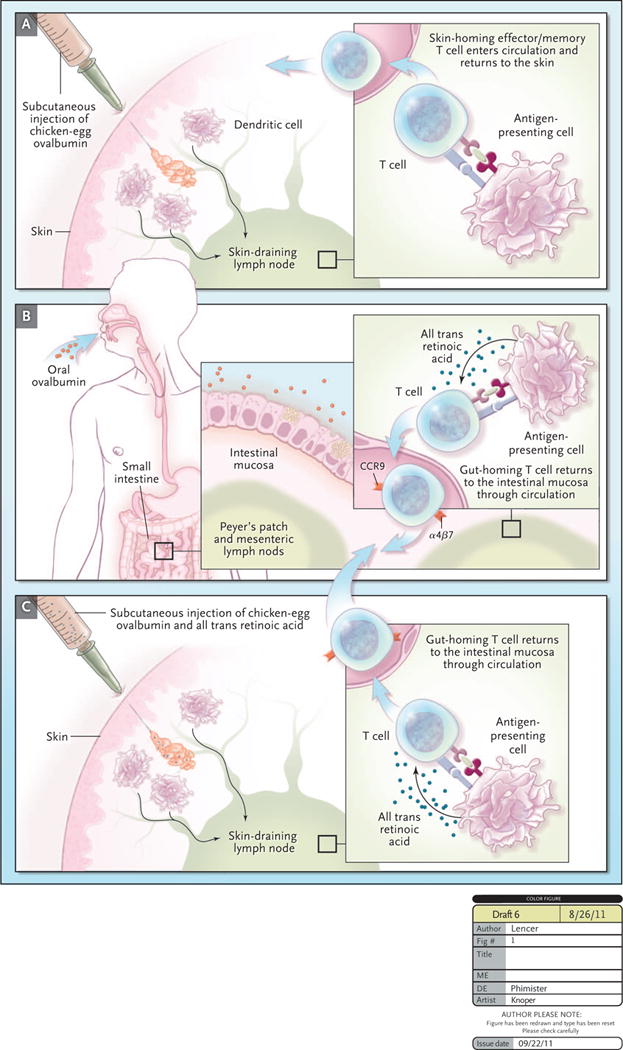
While seemingly a simple proposition, immunization via the gut has been profoundly problematic because the intestine is organized to promote immune tolerance, not immunity toward most orally administered antigens. This is why we are usually not allergic to the food we eat. To get around this problem, Hammerschmidt et al. reasoned that immunizations applied by subcutaneous injection might induce intestinal lymphocyte homing (and thus intestinal immunity) if the injection also contained RA. This is exactly what they found. They immunized mice with chicken egg ovalbumin applied subcutaneously with or without RA or by oral gavage of ovalbumin alone, administered directly to the gut mucosa (Figure). To monitor antigen-specific lymphocyte trafficking, the mice were infused with fluorescently-tagged naïve T cells. RA applied subcutaneously, together with ovalbumin, caused the transferred T cells to express the “homing receptors” for the intestine and to migrate there and function in numbers comparable to those induced by oral immunization with ovalbumin alone. Remarkably, subcutaneous immunizations against cholera toxin and salmonella, when applied with RA, induced significant protection against “disease” as modeled in the mouse. In short, the skin-draining lymph node was converted from a site specifying dermal immune defense to a site specifying intestinal (mucosal) immunity.
This is an important discovery, coincidently shared by Tan et al.6 Both groups have elegantly applied knowledge of how RA operates in antigen presentation,3,4 to demonstrate a strategy for clinical application. This approach is attractive because immune responses in skin-draining lymph nodes are better defined and more vigorous compared with those of mucosal lymphoid tissues. On the other hand, the administration of RA carries known mutagenic toxicities,7 of concern when considering in the context of vaccine for healthy children and women at child-bearing age. Another potential limitation is that RA targets activated lymphocytes to the small intestine, but not necessarily to other mucosal surfaces, such as the colon.5 Although approach demonstrated by Hammerschmidt et al.2 and Tan et al.6 and warrants further investigation, it would be premature to abandon efforts to develop technology for oral delivery of antigen and adjuvant for mucosal vaccination.
Making More of Mucosal Immunity.
Hammerschmidt et al. recently described an approach to elicit mucosal immunity. They immunized mice against chicken ovalbumin by subcutaneous injection (Panel 1), by oral gavage of ovalbumin administered directly to the gut mucosa (Panel 2), or by subcutaneous injection of ovalbumin administered together with retinoic acid (RA) (Panel 3). Dendritic cells processed ovalbumin and presented ovalbumin peptides to T cells in the peripheral skin-draining lymph nodes (Panel 1) or the gut-draining peyers patch/mesenteric lymph nodes (Panel 2). Immunization with RA resulted in antigen processing in the skin-draining lymph node and T cells “programmed” to home to the gut, rather than back to the skin.
References
- 1.Moore SR, Lima AA, Guerrant RL. Infection: Preventing 5 million child deaths from diarrhea in the next 5 years. Nat Rev Gastroenterol Hepatol. 2011;8:363–4. doi: 10.1038/nrgastro.2011.103. [DOI] [PubMed] [Google Scholar]
- 2.Hammerschmidt SI, Friedrichsen M, Boelter J, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. The Journal of clinical investigation. 2011 doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 4.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 5.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews Immunology. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. Journal of virology. 2011 doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. Jama. 2002;287:3116–26. doi: 10.1001/jama.287.23.3116. [DOI] [PubMed] [Google Scholar]


