Abstract
Glioblastoma (GBM) is an aggressive primary brain tumor with an average survival of approximately 1 year. A recently recognized subtype, glioblastoma with oligodendroglioma component (GBM‐O), was designated by the World Health Organization (WHO) in 2007. We investigated GBM‐Os for their clinical and molecular characteristics as compared to other forms of GBM. Tissue samples were used to determine EGFR, PTEN, and 1p and 19q status by fluorescence in situ hybridization (FISH); p53 and mutant IDH1 protein expression by immunohistochemistry (IHC); and MGMT promoter status by methylation‐specific polymerase chain reaction (PCR). GBM‐Os accounted for 11.9% of all GBMs. GBM‐Os arose in younger patients compared to other forms of GBMs (50.7 years vs. 58.7 years, respectively), were more frequently secondary neoplasms, had a higher frequency of IDH1 mutations and had a lower frequency of PTEN deletions. Survival was longer in patients with GBM‐Os compared to those with other GBMs, with median survivals of 16.2 and 8.1 months, respectively. Most of the survival advantage for GBM‐O appeared to be associated with a younger age at presentation. Among patients with GBM‐O, younger age at presentation and 1p deletion were most significant in conferring prolonged survival. Thus, GBM‐O represents a subset of GBMs with distinctive morphologic, clinical and molecular characteristics.
Keywords: glioblastoma, glioblastoma with oligodendroglioma component, IDH1 mutation, LOH 1p 19q, molecular characteristics
Introduction
Diffusely infiltrative gliomas, which include astrocytomas, oligodendrogliomas and oligoastrocytomas 2, 3, account for approximately 30% of all primary intracranial tumors 4. Glioblastoma (GBM), a World Health Organization (WHO) grade IV astrocytoma, is the most frequent tumor in this category, comprising approximately 60% of the infiltrative gliomas 4. With an average survival of just 1 year following standard therapy 17, GBMs are highly aggressive and carry a dismal prognosis. GBMs can be characterized by their clinical presentation as either primary, arising de novo or secondary, progressing from a lower grade glioma. Primary GBMs account for greater than 90% and tend to occur in older patients (sixth to seventh decade) 17, 22. Secondary GBMs account for less than 10% and tend to occur in younger patients (fifth to sixth decade) and have a slightly better prognosis 17, 22. These pathways also have distinctive molecular genetic alterations: EGFR amplification and PTEN deletion are more common in primary GBMs, whereas TP53 and IDH1 mutations are more common in secondary GBMs 17, 20, 31.
As a group, GBM shows a tremendous degree of histologic variability and numerous morphologic subtypes have been recognized, including fibrillary, gemistocytic, granular cell, small cell, giant cell and gliosarcoma 13. Some of these subtypes are associated with specific genetic alterations or clinical behaviors, suggesting that subclassification may have diagnostic and clinical significance. The WHO recently introduced a new subtype of GBM, the glioblastoma with oligodendroglioma component (GBM‐O), which contains both astrocytic and oligodendroglial differentiation (Figure 1). The presence of an oligodendroglioma component within a diffuse glioma is generally associated with improved clinical outcomes, grade for grade. However, precise criteria for classifying and grading oligoastrocytomas are not firmly established and interobserver variability is often high within this diagnostic category. In a recent study of prognostic features that included the full spectrum of histologic classes and grades of diffuse gliomas, Miller et al found that patients with anaplastic oligoastrocytomas whose tumors contained necrosis had a much shorter survival than patients whose tumors did not have necrosis. Those high grade anaplastic oligoastrocytomas with necrosis had clinical behavior similar to GBM, WHO grade IV, whereas those without necrosis behaved similar to WHO grade III gliomas 16. This finding led to the designation of high grade oligoastrocytomas with necrosis as GBM‐O in the WHO 2007 2, 16.
Figure 1.
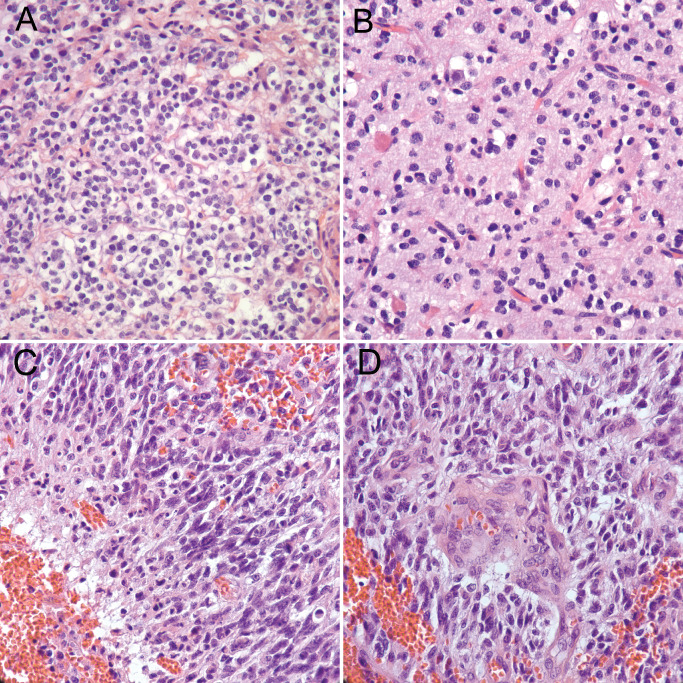
Glioblastoma with oligodendroglioma component. Typical features of oligodendroglial differentiation such as monotonous cells with round nuclei and perinuclear clearing or “halos” (A) are identified, along with “chicken wire” vasculature (B). In other areas of the tumor, elongated, hyperchromatic nuclei with irregular contours, indicative of astrocytic differentiation, are seen along with pseudopalisading necrosis (C) and microvascular proliferation (D), diagnostic features of glioblastoma.
Since GBM‐O is a recently recognized entity, its clinical and molecular genetic profile has not been clearly defined, and differences from other forms of GBM, if any, remain to be determined. The purpose of this study was to define the molecular genetic and clinical features of GBM‐Os, to determine if they differ from classic GBMs and to determine if there are prognostic factors within this category.
Materials and Methods
Patients in study
A retrospective analysis was performed on patients who underwent surgical resection and were diagnosed with a GBM at Emory University Hospitals from January of 2008 through December of 2011. A total of 236 GBMs were diagnosed during this period, with 210 (89%) of these being primary GBMs and 26 (11%) being secondary GBMs. GBMs were diagnosed as primary or secondary on the basis of information in the patients' electronic charts. Specifically, a GBM was considered secondary when there was a previous histopathological diagnosis of a lower grade glioma. Patients ranged from 18 to 87 years of age at the time of diagnosis, with an average age of 56.7 years. There were 142 males and 94 females in the study (1.5:1). Recurrent GBMs and GBMs from patients less than 18 years of age were excluded. Dates of death were obtained from medical records or the Social Security Death Index. Patients were treated with the standard of care for GBM, namely fractionated external beam radiation therapy with concurrent and adjuvant temozolomide. A subset of patients was entered into clinical trials. We did not factor these treatment differences into our study.
Histopathology
All hematoxylin and eosin (H&E)‐stained slides from each case were reviewed jointly by two neuropathologists using a multiheaded microscope. Classification and grading used WHO 2007 criteria. Tumors were considered to be of astrocytic differentiation if constituent cells were hyperchromatic and elongated, with irregular nuclear contours 3. A high grade astrocytic neoplasm was designated as a GBM if either microvascular proliferation or necrosis was identified 3. The presence of necrosis was necessary for a GBM to be diagnosed as a GBM‐O, along with a distinct area of classic oligodendroglial differentiation, at least of the size sufficient to fill a microscopic 100× field 5. Oligodendroglial differentiation was defined as monotonous cells with round, regular nuclei, often with perinuclear halos, accompanied by a delicate capillary vasculature 3. In making the diagnosis of GBM‐O over anaplastic oligodendroglioma, we required that the astrocytoma component have an anaplastic appearance, and also demonstrate astrocytic differentiation, namely elongated, hyperchromatic nuclei with irregular contours. Diagnoses used in this study were the original pathologic diagnoses and were rendered based on morphologic characteristics together with ancillary studies.
Immunohistochemistry
Immunoperoxidase staining for the p53 protein and for the IDH1 mutant protein was performed on formalin‐fixed, paraffin‐embedded tissue sections, according to the manufacturers' instructions. Representative blocks were selected from GBM‐Os and other GBMs for immunostaining. The p53 DO‐7 antibody (1:80; Dako North America, Inc., Carpinteria, CA, USA) was used to detect accumulation of the p53 protein. The R132H antibody (1:80; Dianova, Hamburg, Germany) was used to detect the IDH1 mutant protein. Expression of p53 was graded on a scale from 0 to 3+, with 0 and 1+ interpreted as negative. Tumors were considered to be p53 positive if ≥10% of cells stained with a 2 to 3+ intensity. Tumors were classified as being either positive or negative for IDH1.
Fluorescence in situ hybridization (FISH)
Dual‐color FISH was performed for EGFR amplification, PTEN deletion and 1p and 19q deletions. Slides were deparaffinized using CitriSolv for 10 minutes at room temperature, followed by dehydration with 100% ethanol at room temperature for 5 minutes and air drying in a slide warmer at 45–50°C. Slides were pretreated in solution (1 M NaSCN/1 M Tris base, pH 8.0) at 80°C for 30 minutes, followed by protease treatment involving immersion in protease solution for 10 minutes at 37°C. Slides were fixed in 10% buffered formalin for 10 minutes at room temperature. Denaturation was performed by immersing slides in a solution containing 70% formalin and 2XSSC at 72°C for 5 minutes. Hybridization was performed by applying 10 uL of the appropriate probe mixture to the slide and incubating at 37°C overnight. Tissues were counterstained with DAPI (10 uL).
For all FISH studies, 200 non‐overlapping nuclei were counted. EGFR amplification was considered present if >10 red signals were present in >10% of cells 9. PTEN deletion and 1p and 19q deletions were considered present if ≥10% of cells contained the respective deletions. The Vysis LSI EGFR/CEP 7 probe (Catolog # 05J48‐001; Abbott Molecular Inc., Des Plaines, IL, USA) was used to detect EGFR amplification. The Vysis LSI PTEN/CEP 10 probe (Catalog # 07J74‐001; Abbott Molecular Inc.) was used to detect the PTEN deletion. The Vysis LSI 1p36/1q25 and LSI 19p13/19q13 probe sets (Catalog # 07J73‐001; Abbott Molecular Inc.) were used to detect 1p and 19q deletions, respectively. FISH was performed on both GBM‐Os and GBMs of other types for EGFR amplification and PTEN deletion. FISH for 1p and 19q deletions was only performed on GBM‐Os. FISH testing for EGFR and PTEN as well as MGMT promoter analysis are performed for every newly diagnosed GBM at our institution. There are more cases with EGFR amplification and MGMT promoter methylation data because we implemented routine testing for these alterations earlier than for PTEN deletion.
O6‐methylguaninemethyltransferase methylation status
DNA was isolated from formalin‐fixed, paraffin‐embedded tissue and sent to Brigham and Women's Hospital for MGMT promoter analysis according to their protocol. Chemical (bisulfite) modification was performed to convert unmethylated cytosines to uracil. polymerase chain reaction (PCR) was performed using specific primers for either methylated or modified unmethylated DNA to determine the DNA methylation pattern in the CpG island of the MGMT gene.
Statistical analysis
Student's t‐test was used to analyze continuous variables. Fisher's exact test was used to calculate significance for categorical variables. Cox regression analysis, log‐rank test and Kaplan–Meier curves were used to analyze survival data. A result was interpreted as statistically significant if the P‐value was ≤0.05.
Results
Clinical features
There were a total of 236 GBMs diagnosed at our institution from January of 2008 through December of 2011. Of these, 28 (11.9%) were diagnosed as GBM‐O. Patients with GBM‐O tended to be younger, with an average age of 50.7 years, as compared to 58.7 years for patients with other types of GBMs (P = 0.00517; Table 1). GBM‐O patients tended to be male (21 male vs. 7 female; 3:1) and had a higher male: female ratio than did patients with other GBMs (121 male vs. 87 female; 1.4:1), but this difference was not significant (P = 0.102; Table 1). A larger percentage of GBM‐Os were secondary neoplasms, with 9 of 28 (32%) tumors arising from a lower grade glioma, as compared with only 17 of 208 (8%) other GBMs (P = 0.001; Table 1).
Table 1.
Clinical, molecular and immunohistochemical features of glioblastomas (GBMs) vs. glioblastomas with oligodendroglioma component (GBM‐Os)
| Variable | GBM | GBM‐O | P‐value |
|---|---|---|---|
| Average age | 58.7 (± 0.971) years | 50.7 (± 2.761) years | 0.00517 |
| Median age | 60 years | 50 years | |
| Male | 121 | 21 | 0.102 |
| Female | 87 | 7 | |
| MGMT methylated | 58 | 9 | 1 |
| MGMT unmethylated | 70 | 11 | |
| EGFR amplified | 59 | 6 | 0.193 |
| EGFR unamplified | 102 | 20 | |
| PTEN deleted | 93 | 10 | 0.05 |
| PTEN intact | 5 | 3 | |
| p53 positive (IHC) | 48 | 7 | 0.47 |
| p53 negative | 60 | 14 | |
| IDH1 positive (IHC) | 10 | 7 | 0.004 |
| IDH1 negative | 106 | 13 | |
| Primary neoplasm | 191 | 19 | 0.001 |
| Secondary neoplasm | 17 | 9 |
Molecular and immunohistochemical features
EGFR amplification, assessed by FISH, was noted in 6 of 26 (23%) GBM‐Os and in 59 of 161 (37%) other GBMs (P = 0.193; Table 1). PTEN deletion was less frequent in GBM‐Os, with 10 of 13 (77%) harboring the deletion compared with 93 of 98 (95%) other GBMs (P = 0.05; Table 1). The MGMT promoter was methylated at equal frequencies in the two groups, occurring in 9 of 20 (45%) GBM‐Os and in 58 of 128 (45%) other GBMs (P = 1; Table 1). Overexpression of p53 protein, as assessed by immunohistochemistry, was also less frequently seen in GBM‐Os (7 of 21, 33%) as compared to other GBMs (48 of 108, 44%) (P = 0.47; Table 1).
GBM‐Os were more frequently immunoreactive for mutant IDH1 protein (7 of 20, 35%) than other GBMs (10 of 116, 9%) (P = 0.004; Table 1). However, IDH1 positivity was also found to be significantly associated with secondary GBMs, with 10 of 18 (55.6%) secondary GBMs being IDH1 positive compared with only 7 of 118 (5.9%) primary GBMs (P = < 0.0001). Among primary neoplasms, 14.3% of GBM‐Os were immunoreactive for mutant IDH1, not significantly different from other GBMs (4.8% of primary GBMs; P = 0.194). Thus, while 32% of GBM‐Os in this study arose from a lower grade glioma compared with 8% of other GBMs, the higher frequency of secondary neoplasms among GBM‐O may account for the higher frequency of the IDH1 mutation in this group. No correlation was found between IDH1 mutation and p53 overexpression, with 7 of 16 (43.8%) IDH1‐mutated GBMs and 47 of 112 (42.0%) GBMs without the mutation being immunoreactive for p53 (P = 1).
FISH analysis for 1p and 19q was performed on all tumors with an oligodendroglioma component, but not on classic GBMs, since 1p/19q loss is rare in GBM and not prognostically significant 25. Of the 27 GBM‐Os that were tested for 1p and 19q status, eight (29.6%) were co‐deleted, 15 (55.6%) were intact at both loci, three (11.1%) contained only the 1p deletion, and one (3.7%) contained only the 19q deletion. Of the eight GBM‐Os that were co‐deleted for 1p and 19q, six (75%) were IDH1 mutant. Interestingly, none of the eight GBM‐Os that were intact at both 1p and 19q were IDH1 mutant, indicating that there is a strong positive correlation between 1p/19q co‐deletion and IDH1 mutation for this tumor type (P = 0.0070). We also found that deletion of 1p (regardless of 19q status) has a statistically significant inverse correlation with p53 overexpression, with 6 of 10 (60%) GBM‐Os with 1p and 19q intact demonstrating positivity for p53 compared with only 1 of 11 (9.1%) GBM‐Os that were 1p deleted (P = 0.0237). No significant correlation was found for 1p/19q co‐deletion and EGFR amplification. One of eight (12.5%) 1p/19q co‐deleted GBM‐Os was EGFR amplified, whereas 4 of 15 (26.7%) GBM‐Os without the co‐deletion were EGFR amplified (P = 0.6214).
Survival analysis
A univariate analysis of survival for all GBMs (primary and secondary) in this study showed that patients with GBM‐Os had longer survivals compared to patients with other types of GBMs, with median survivals of 16.2 and 8.1 months, respectively (P = 0.0028; Figure 2A and Table 2A). We next excluded all secondary GBMs and included only primary GBMs and GBM‐Os. Univariate analysis of primary tumors showed that patients with primary GBM‐Os also survived significantly longer (median = 16.8 months) than patients with primary GBMs of other types (median = 8.1 months) (P = 0.0148; Figure 2B and Table 2B). Within the GBM‐O group, no statistically significant difference in survival was seen between primary and secondary tumors, with median survivals of 16.8 and 15.3 months, respectively (P = 0.9439; Table 3).
Figure 2.

Kaplan–Meier survival curves for all glioblastomas (GBMs) and glioblastomas with oligodendroglioma component (GBM‐Os) (A) and primary GBMs and GBM‐Os (B).
Table 2.
Univariate survival analysis of all glioblastomas (GBMs) vs. glioblastomas with oligodendroglioma component (GBM‐Os) (A) and primary GBMs vs. GBM‐Os (B)
| Number dead | Number alive | Median survival | Mean survival | Log‐rank test P‐value | |
|---|---|---|---|---|---|
| A: Diagnosis (All GBMs) | |||||
| GBM | 172 | 31 | 8.1 | 11.05 | 0.0028 |
| GBM‐O | 18 | 8 | 16.2 | 20.71 | |
| B: Diagnosis (Primary GBMs) | |||||
| GBM | 156 | 30 | 8.10 | 11.11 | 0.0148 |
| GBM‐O | 12 | 6 | 16.80 | 22.01 | |
Table 3.
Univariate cox model results for glioblastoma with oligodendroglioma component (GBM‐O) group. HR = hazard ratio; CL = confidence limit; N/A = not applicable. Note: The number of PTEN intact GBM‐Os was too small to accurately determine the HR and CL.
| Variable | Univariate cox P‐value | HR | HR lower CL | HR upper CL |
|---|---|---|---|---|
| EGFR amplification | 0.4568 | 1.565 | 0.481 | 5.093 |
| Gender | 0.8583 | 1.109 | 0.355 | 3.462 |
| IDH1 mutation | 0.2267 | 0.387 | 0.083 | 1.804 |
| MGMT methylation status | 0.5342 | 1.519 | 0.406 | 5.679 |
| PTEN deletion | 0.9969 | N/A | N/A | N/A |
| Primary vs. secondary neoplasm | 0.9436 | 1.037 | 0.381 | 2.824 |
| p53 overexpression | 0.9224 | 1.068 | 0.282 | 4.046 |
| Age at diagnosis | 0.0480 | 1.037 | 1.000 | 1.075 |
To determine which variables had the largest impact on survival, stepwise variable selection was performed. Among all the GBMs in the study, the variables that were most strongly associated with a longer survival were younger age at diagnosis (P < 0.0001; Table 4), MGMT promoter methylation (P = 0.0383; Table 4) and secondary GBM status (P = 0.0002; Table 4). After multivariate analysis, adjusting for age, MGMT promoter methylation status and secondary GBM status, the presence of an oligodendroglioma component conferred only a marginal survival advantage to patients with GBM‐Os over patients with other types of GBMs (P = 0.0788; Table 4).
Table 4.
Multivariate Cox model results of all glioblastomas (GBMs) and glioblastomas with oligodendroglioma component (GBM‐Os) after stepwise variable selection
| Variable | Hazard ratio | 95% hazard ratio confidence limits | P‐value | |
|---|---|---|---|---|
| Diagnosis (GBM vs. GBM‐O) | 1.763 | 0.937 | 3.318 | 0.0788 |
| Age at diagnosis | 1.044 | 1.027 | 1.061 | <0.0001 |
| MGMT methylation status | 1.530 | 1.023 | 2.287 | 0.0383 |
| Primary vs. secondary neoplasm | 3.227 | 1.725 | 6.034 | 0.0002 |
Prognostic features in GBM‐O
Within the GBM‐O group, patients whose tumors contained the 1p deletion, regardless of the 19q status, were found to have a significant survival advantage over patients whose tumors had intact 1p and 19q chromosomal arms (P = 0.0476; Figure 3 and Table 5). Age was also a significant independent prognostic factor, with younger patients surviving longer than older patients with GBM‐Os (P = 0.0480; Table 3).
Figure 3.
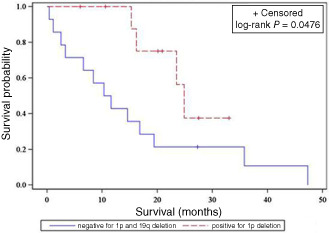
Kaplan–Meier survival curve of 1p deleted vs. 1p and 19q intact glioblastomas with oligodendroglioma component.
Table 5.
Survival analysis of 1p and 19q intact vs. 1p deleted glioblastomas with oligodendroglioma component
| 1p and 19q status | Number dead | Number alive | Median survival | Mean survival | Log‐rank test P‐value |
|---|---|---|---|---|---|
| Negative for 1p and 19q deletion | 13 | 1 | 10.95 | 15.69 | 0.0476 |
| Positive for 1p deletion | 4 | 6 | 24.90 | 22.35 |
Median survival rates were similar for patients with GBM‐Os on the basis of EGFR amplification (16.2 and 16.8 months; P = 0.4531), MGMT promoter methylation (15.3 and 16.8 months; P = 0.5312), IDH1 mutation (23.5 and 16.2 months; P = 0.2101), p53 expression (18.1 and 23.5 months; P = 0.9224), gender (16.2 and 17.0 months; P = 0.8583) or whether the patient's GBM was primary or secondary (16.8 and 15.3; P = 0.9439) (Table 3).
Discussion
GBM‐O is a recently recognized subtype of GBM, first designated by the WHO in 2007. Our study included tumors diagnosed as GBM‐O beginning in 2008, the first year after its classification by the WHO. As the criteria for diagnosis of GBM‐O may vary and as there are mimickers of oligodendroglial differentiation, such as small cell GBM, we rendered this diagnosis only if a GBM contained a distinct area of classic oligodendroglial differentiation 5. As such, GBM‐Os comprised a small subset of GBMs in our study (11.9%). As compared to other GBMs, we found that patients with GBM‐Os were younger at the time of diagnosis and were more likely to have had a GBM that progressed from a lower grade glioma (i.e., secondary GBM). EGFR amplification and PTEN deletion, two molecular alterations that are associated with primary GBMs, were seen less frequently in the GBM‐O group, albeit with only marginal statistical significance. However, it should be noted that the number of GBM‐Os with information on PTEN deletion was small and this may have had an impact on statistical significance.
GBM‐Os were more frequently positive for the IDH1 mutant protein than other GBMs. Mutation of IDH1 is a common event in lower grade gliomas, as well as the secondary GBMs that progress from them, and predicts a more favorable prognosis 21, 31. Conversely, IDH1 mutation is rarely found in primary GBMs 21, 31. Mutant IDH1 immunoreactivity was found to be strongly associated with secondary GBMs in our study, yet was not appreciably more frequent in primary GBM‐Os than other primary GBMs. Therefore, the higher frequency of IDH1 mutation in the GBM‐O group was likely because of its enrichment in secondary neoplasms. While the frequency of IDH1 mutation that we found in secondary GBMs by immunohistochemistry is lower than that found in most studies, there is a broad range of frequencies reported (50–84.2%) 10, 15, 19, 26. Most studies have shown that immunohistochemistry for mutant IDH1 protein is comparable in sensitivity to sequencing or PCR in detecting the IDH1 mutation 12, 14, 15, 27. Similar to our study, Wang et al found that 31% of GBM‐Os contained the IDH1 mutation, compared with less than 5% of conventional GBMs. GBM‐Os were also found to be p53 immunoreactive and have lower EGFR expression, thus resembling secondary GBMs in their immunophenotype 30. Hegi et al also found that GBM‐Os were enriched for IDH1 mutations. In their study, a second distinct subset of GBM‐Os was found to contain EGFR amplification, raising the possibility that IDH1 mutant GBM‐Os represent secondary GBMs, while EGFR‐amplified GBM‐Os represent primary GBMs with potential morphologic overlap with small cell variants of GBM 7. Our study did not uncover a statistically significant, mutually exclusive relationship between IDH1 mutation and EGFR amplification. Neither did we find a positive relationship between p53 overexpression and IDH1 mutation, although this has been reported previously and is thought to be typical of secondary GBMs 19, 20, 31. Similar to previous studies, however, we did find that IDH1 mutation was significantly associated with 1p/19q co‐deletion in GBM‐Os 20, 28, 31. Co‐deletion of 1p/19q was detected in 29.6% (8 of 27) of GBM‐Os, considerably lower than the 50–80% frequency seen in oligodendrogliomas, yet in the range of 20–30% reported for mixed oligoastrocytomas 1 and higher than most other studies of GBM‐Os 7, 8, 11, 18, 23, 30. The higher frequency of 1p/19q co‐deletion that we detected in GBM‐Os could be explained by differences in the techniques used [FISH vs. comparative genomic hybridization (CGH)] or because of the differences in the diagnostic inclusion criteria for each study. As we used 1p and 19q FISH probes directed at one locus on each of these respective chromosomal arms, we can only say for certain that the deletions we detected occur at these specific loci. However, current evidence suggests that, in oligodendrogliomas, 1p/19q loss is caused by an unbalanced translocation that results in loss of the whole arms of 1p and 19q 9. Prior studies have reached differing conclusions on the prognostic significance of 1p/19q deletion in GBM‐O. He et al detected 1p/19q co‐deletion in 7 of 25 GBM‐Os, but found no associated prognostic significance 6, whereas, Salvati et al identified co‐deletion in 7 of 36 GBM‐Os and uncovered a prognostic significance 24. Our findings are most consistent with those of Salvati et al because 1p deletion was found to confer a survival advantage to patients with GBM‐Os. We also found that deletion of 1p had a statistically significant inverse correlation with p53 overexpression. This finding, seen in previous studies 11, 20, may suggest that GBM‐Os arise by two separate pathways, one characterized by 1p deletion (or 1p/19q co‐deletion) and the other by TP53 mutation.
In our analysis of survival, we found that patients with GBM‐Os lived longer than patients with other GBMs. This survival advantage was also seen when secondary GBMs were excluded and only primary GBMs and GBM‐Os were considered. By univariate analysis, the difference in survival between GBM‐Os and other types of GBMs was statistically significant. However, by multivariate analysis, the presence of an oligodendroglioma component by itself showed only a trend toward longer survival. Among all GBMs in our study, multivariate analysis showed that younger age, MGMT promoter methylation, and secondary GBM status were independent favorable prognostic factors. It should be noted that we did not perform FISH for 1p and 19q on all GBMs, only those with an oligodendroglioma component, and therefore could not assess its prognostic significance within the larger group. Within the GBM‐Os, younger patient age and 1p deletion were significant predictors of longer patient survival. Previous studies have reached differing conclusions on the prognostic significance of GBM‐Os, with some finding that GBM‐Os have a better prognosis than other types of GBMs 11, 24, 29, 30, while others found no difference 6, 7, 8, 18, 23. Wang et al found that GBM‐Os had a better prognosis and also concluded that the presence of an oligodendroglial component was an independent favorable prognostic factor 30. The differing conclusions are most likely explained by a lack of standardized criteria for diagnosing GBM‐O. The incidence of GBM‐O in these studies varied widely, ranging from 4 to 27%, and suggest that inclusion criteria were similarly disparate 6, 7, 8, 23, 24, 29, 30.
In summary, GBM‐O represents a newly recognized subset of GBMs with a distinctive set of clinical and molecular correlates. We found that these tumors tend to occur in younger individuals, are enriched for IDH1 mutations and 1p/19q co‐deletion, and are frequently secondary neoplasms. As a group, GBM‐Os have a better prognosis, with younger age and 1p deletion conferring a favorable prognosis. While patients with GBM‐Os have a longer survival than patients with other GBMs, the presence of an oligodendroglioma component was not found to be an independent prognostic factor.
Acknowledgments
This work was supported by the Winship Cancer Institute Cancer Center Support Grant CA138292 and the Georgia Cancer Coalition. The Winship Cancer Tissue and Pathology Shared Resource, including Jennifer Shelton and Dianne Alexis, were instrumental in their assistance with this work.
References
- 1. Aldape K, Burger PC, Perry A (2007) Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch Pathol Lab Med 131:242–251. [DOI] [PubMed] [Google Scholar]
- 2. Brat DJ, Parisi JE, Kleinschmidt‐DeMasters BK, Yachnis AT, Montine TJ, Boyer PJ et al (2008) Surgical neuropathology update: a review of changes introduced by the World Health Organization (WHO) classification of tumors of the central nervous system, 4th edition. Arch Pathol Lab Med 132:993–1007. [DOI] [PubMed] [Google Scholar]
- 3. Brat DJ, Prayson RA, Ryken TC, Olson JJ (2008) Diagnosis of malignant glioma: role of neuropathology. J Neurooncol 89:287–311. [DOI] [PubMed] [Google Scholar]
- 4. CBTRUS (2012) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008 (March 23, 2012 Revision). Central Brain Tumor Registry of the United States, Hinsdale, IL. [Google Scholar]
- 5. Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK (1997) Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer 79:1381–1393. [DOI] [PubMed] [Google Scholar]
- 6. He J, Mokhtari K, Sanson M, Marie Y, Kujas M, Huguet S et al (2001) Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol 60:863–871. [DOI] [PubMed] [Google Scholar]
- 7. Hegi ME, Janzer RC, Lambiv WL, Gorlia T, Kouwenhoven M, Hartmann C et al (2012) Presence of an oligodendroglioma‐like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol 123:841–852. [DOI] [PubMed] [Google Scholar]
- 8. Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, Ohgaki H (2006) Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol 65:846–854. [DOI] [PubMed] [Google Scholar]
- 9. Horbinski C, Miller CR, Perry A (2011) Gone FISHing: clinical lessons learned in brain tumor molecular diagnostics over the last decade. Brain Pathol 21:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ichimura K, Pearson DM, Kocialkowski S, Bäcklund LM, Chan R, Jones DTW, Collins VP (2009) IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro‐Oncol 11:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kraus JA, Lamszus K, Glesmann N, Beck M, Wolter M, Sabel M et al (2001) Molecular genetic alterations in glioblastomas with oligodendroglial component. Acta Neuropathol 101:311–320. [DOI] [PubMed] [Google Scholar]
- 12. Lee D, Suh YL, Kang SY, Park TI, Jeong JY, Kim SH (2012) IDH1 mutations in oligodendroglial tumors: comparative analysis of direct sequencing, pyrosequencing, immunohistochemistry, nested PCR, and PNA‐mediated clamping PCR. Brain Pathol 23:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louis DN, Ohgaki H, Weistler OD, Cavenee WK (2007) In: WHO Classification of Tumours of the Central Nervous System, 4th edn. International Agency for Research: Lyon. [Google Scholar]
- 14. Loussouarn D, Le Loupp AG, Frenel JS, Leclair F, Von Deimling A, Aumont M et al (2012) Comparison of immunohistochemistry, DNA sequencing and allele‐specific PCR for the detection of IDH1 mutations in gliomas. Int J Oncol 40:2058–2062. [DOI] [PubMed] [Google Scholar]
- 15. Mellai M, Piazzi A, Caldera V, Monzeglio O, Cassoni P, Valente G, Schiffer D (2011) IDH1 and IDH2 mutations, immunohistochemistry and associations in a series of brain tumors. J Neurooncol 105:345–357. [DOI] [PubMed] [Google Scholar]
- 16. Miller CR, Dunham CP, Scheithauer BW, Perry A (2006) Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high grade gliomas. J Clin Oncol 24:5419–5426. [DOI] [PubMed] [Google Scholar]
- 17. Miller CR, Perry A (2007) Glioblastoma. Arch Pathol Lab Med 131:397–406. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura H, Makino K, Kuratsu J (2011) Molecular and clinical analysis of glioblastoma with an oligodendroglial component (GBMO). Brain Tumor Pathol 28:185–190. [DOI] [PubMed] [Google Scholar]
- 19. Nobusawa S, Watanabe T, Kleihues P, Ohgaki H (2009) IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 15:6002–6007. [DOI] [PubMed] [Google Scholar]
- 20. Ohgaki H, Kleihues P (2011) Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol 28:177–183. [DOI] [PubMed] [Google Scholar]
- 21. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perry A, Brat DJ (2010) Practical Surgical Neuropathology: A Diagnostic Approach. Churchill Livingstone: Philadelphia, PA. [Google Scholar]
- 23. Pinto LW, Araújo MB, Vettore AL, Wernersbach L, Leite AC, Chimelli LM, Soares FA (2008) Glioblastomas: correlation between oligodendroglial components, genetic abnormalities and prognosis. Virchows Arch 452:481–490. [DOI] [PubMed] [Google Scholar]
- 24. Salvati M, Formichella AI, D'Elia A, Brogna C, Frati A, Giangaspero F et al (2009) Cerebral glioblastoma with oligodendroglioma component: analysis of 36 cases. J Neurooncol 94:129–134. [DOI] [PubMed] [Google Scholar]
- 25. Smith JS, Perry A, Borell TJ, Lee HK, O'Fallon J, Hosek SM et al (2000) Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 18:636–645. [DOI] [PubMed] [Google Scholar]
- 26. Sonoda Y, Kumabe T, Nakamura T, Saito R, Kanamori M, Yamashita Y et al (2009) Analysis of IDH1 and IDH2 mutations in Japanese glioma patients. Cancer Sci 100:1996–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takano S, Tian W, Matsuda M, Yamamoto T, Ishikawa E, Kaneko MK et al (2011) Detection of IDH1 mutation in human gliomas: comparison of immunohistochemistry and sequencing. Brain Tumor Pathol 28:115–123. [DOI] [PubMed] [Google Scholar]
- 28. van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P et al (2010) IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the european organization for research and treatment of cancer brain tumor group. Clin Cancer Res 16:1597–1604. [DOI] [PubMed] [Google Scholar]
- 29. Vordermark D, Ruprecht K, Rieckmann P, Roggendorf W, Vince GH, Warmuth‐Metz M et al (2006) Glioblastoma multiforme with oligodendroglial component (GBMO): favorable outcome after post‐operative radiotherapy and chemotherapy with nimustine (ACNU) and teniposide (VM26). BMC Cancer 6:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Li S, Chen L, You G, Bao Z, Yan W et al (2012) Glioblastoma with an oligodendroglioma component: distinct clinical behavior, genetic alterations and outcome. Neuro‐Oncol 14:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]


