Abstract
How contraceptives affect women’s sexual well-being is critically understudied. Fortunately, a growing literature focuses on sexual aspects of contraception, especially hormonal contraception’s associations with libido. However, a more holistic approach to contraceptive sexual acceptability is needed to capture the full range of women’s sexual experiences. We conducted a narrative literature review of this topic, working with an original sample of 3,001 citations published from 2005 to 2015. In Part 1, we draw from a subset of this literature (264 citations) to build a new conceptual model of sexual acceptability. Aspects include macro factors (gender, social inequality, culture, and structure), relationship factors (dyadic influences and partner preferences), and individual factors (sexual functioning, sexual preferences, such as dis/inhibition, spontaneity, pleasure, the sexual aspects of side effects, such as bleeding, mood changes, sexual identity and sexual minority status, and pregnancy intentions). In Part 2, we review the empirical literature on the sexual acceptability of individual methods (103 citations), applying the model as much as possible. Results suggest contraceptives can affect women’s sexuality in a wide variety of positive and negative ways that extend beyond sexual functioning alone. More attention to sexual acceptability could promote both women’s sexual well-being and more widespread, user-friendly contraceptive practices.
Access to safe, effective contraception is both a public health and feminist imperative. Family planning products and services are associated with a range of health benefits, including reduced unintended pregnancies, improved infant health, and lowered pregnancy-related morbidity and mortality (Kavanaugh & Anderson, 2013). Successful fertility control also leads to many social and economic benefits for women, from educational attainment and personal autonomy to relationship stability and satisfaction (Sonfield, Hasstedt, Kavanaugh, & Anderson, 2013). Thus, contraceptive access and acceptability are critical to both sexual and social health.
A severely understudied aspect of contraceptives is their sexual acceptability, or how methods influence the user’s sexual experiences, which can in turn influence family planning preferences and practices. Though contraception is expressly designed for sexual activity, we know little about how contraceptives affect women’s sexual functioning and well-being. This “pleasure deficit” (Higgins & Hirsch, 2007) is even more striking when compared to research on male-based methods (Oudshoorn, 2003) or even newer multiprevention technologies for women, such as microbicides (Jones et al., 2009; Martin et al., 2010; Mathenjwa & Maharaj, 2012; Sobze Sanou et al., 2013; Tanner et al., 2009; Woodsong & Alleman, 2008; Zubowicz et al., 2006). Researchers and policymakers have recognized that limited uptake of these latter methods will result unless they are sexually acceptable (i.e., do not hinder or interfere with sexual pleasure) for both partners. In comparison, portrayals of female-based contraceptives in the scientific, media, and public policy spheres are almost entirely de-eroticized.
Researchers have documented a number of reasons why we consider contraceptives more a medical than a sexual good (Granzow, 2007; Hensel, Newcamp, Miles, & Fortenberry, 2011). For example, advocates from the late 19th through the end of the 20th century sought medical and legal respectability for birth control, thus downplaying its potentially sexually revolutionary aspects—especially for women (Tone, 2006). Even today, while advertisements for male condoms and erectile dysfunction medications highlight sexual pleasure and enjoyment as the products’ main selling points, few erotic scripts of contraceptives used by women exist in mainstream culture, illustrated in both contraceptive advertisements (Medley-Rath & Simonds, 2010) and pornographic films (Shachner, 2014). The state can also devalue women’s sexuality in place of narratives around motherhood—as evidenced, for example, in laws surrounding health care reform and over-the-counter access to emergency contraception (Burkstrand-Reid, 2013). 1 School-based sexuality education similarly focuses on the harms versus the pleasures of sex (Connell, 2009; Goldman, 2008), especially for girls and young women (Fine, 1988). Clinically, care providers may lack both tools and time to discuss sexual issues with patients (Akers, Gold, Borrero, Santucci, & Schwarz, 2010; Bombas et al., 2012), and providers may be especially unlikely to inquire about sexuality in relationship to new contraceptive methods (versus, say, menopause) (Kottmel, Ruether‐Wolf, & Bitzer, 2014). Public health programs and policies can also both reflect and perpetuate dominant gendered assumptions about women’s sexuality—for example, with female condom programs focusing on reproductive health outcomes versus sexual rights (Peters, van Driel, & Jansen, 2013), or with adolescent pregnancy prevention policies that emphasize “sex is not for fun” and that young women should be sexually uninterested (Goicolea, Wulff, Sebastian, & Öhman, 2010). All these phenomena underscore the notion that contraception is a medical versus a sexual good; they also contribute to mixed messages about whether contraceptives should be sexually acceptable at all for women.
Despite these reasons, while contraception certainly helps people maximize their health, women do not have sex in order to use contraception. Rather, women engage in sexual activity for a range of recreational, relational, and personal reasons. Overlooking these reasons will not only fail to recognize women as full sexual agents but also limit people’s willingness to use contraception (Gomez & Clark, 2014; Lessard et al., 2012).
After all, though contraceptives need to be effective to prevent pregnancy, they also need to be acceptable so women will use them. However, despite decades of research, we still face questions about how to make these products as acceptable and appealing as possible, and many women are unsatisfied with their method and/or are using their method inconsistently. For example, in a study of 1,840 new contraceptive users in the United States, after 12 months of use only 41% of pill users were “very satisfied” with their method, and 45% of women using oral contraceptives at baseline discontinued using this method at some point during the year (Peipert et al., 2011). The limited success of contraception is also affected by personal use practices that contribute to a large gap between perfect-use and typical-use failure rates for many methods. 2 For example, the typical-use failure rate of combined oral contraceptives (9%), the most popular method of contraception in the United States (Daniels, Daugherty, & Jones, 2014), is 30 times worse than its perfect-use failure rate of 0.3% (Trussell, 2011). The overwhelming majority of “contraceptive failures” attributed to methods such as oral contraceptive pills (Jones, Darroch, & Henshaw, 2002) and male condoms (Sanders et al., 2012) are due to behavior on the part of the users, not malfunctions of the products themselves (Sanders et al., 2012). Better documenting sexual acceptability could potentially help explain why women dislike, discontinue, and/or use certain methods inconsistently. Ultimately, attending to sexual acceptability could also improve contraceptive practices—and women’s lives—by matching women with contraceptive methods that they will like and use over time.
Encouragingly, in recent years, reproductive health researchers have shown progressive interest in how contraception affects women’s sexual functioning and how sexuality may influence people’s contraceptive choices and practices. Scholars have published several reviews on this topic, particularly in terms of hormonal contraception and sexual functioning (Burrows, Basha, & Goldstein, 2012; Schaffir, 2006; Stuckey, 2008; Welling, 2013). This growing body of research suggests significant associations between contraceptives’ sexual acceptability—particularly with regards to the domain of sexual function—and how consistently and continuously women use these methods. However, given the relatively nascent state of this research, significant knowledge gaps remain.
First, this literature could benefit from a review of the larger method mix. Though there are scant reviews of hormonal contraception (Burrows et al., 2012), intrauterine devices (IUDs) and implants (Sanders, Smith, & Higgins, 2014), and oral contraceptives (Davis & Castano, 2004), comparatively few reviews examine a wider range of methods—including male condoms and their influences on women’s sexual experiences (and not men’s alone). Condoms are one of the most commonly used contraceptive methods in the world and are the only currently available method that protects against both pregnancy and sexually transmitted infections (STIs). Although a systematic review of all studies on all methods of contraception is beyond the scope of any one article, we endeavor to capture at least a wider swath of the method mix. Doing so will enable us to compare and contrast the range of methods available to individuals or couples seeking contraception.
A second critical gap is existing studies’ relatively narrow approach to sexual acceptability—sometimes with only a single question (Graesslin et al., 2009; Wiebe, 2010). When researchers have measured contraceptives’ sexual aspects, they most frequently use measures of classic sexual functioning such as arousal, desire (libido), lubrication, orgasm, satisfaction, and pain. The most common assessment tool is the Female Sexual Functioning Index (FSFI) (Rosen et al., 2000), which is used both in the United States and in studies all over the world. However, researchers use a large variety of scales and measures, many of which appear in Appendix A (online). (Please refer to Appendix A for detailed outlines of studies, methodologies, and measures used in contemporary contraceptive research). Most of these sexuality measures were originally developed to help identify sexual dysfunction, usually in midlife women experiencing sexual or other health problems, and may not serve particularly useful in identifying contraceptive-related sexual outcomes in younger, healthy women. Most scales also fail to measure partner- or relationship-specific aspects of sexuality (Manuel, 2013; Puts & Pope, 2013). For example, though contraceptive acceptability studies tend to document how methods affect bleeding, cramping, mood, or breast tenderness, rarely are such effects considered for their specifically sexual repercussions. Nor do studies tend to assess how individual sexual preferences for dis/inhibition or spontaneity may contribute to contraceptive acceptability. After all, sexuality encompasses myriad factors, from physiological function and sensation to broader psychological well-being (Cobey & Buunk, 2012), and contraception could affect all these experiences. Moreover, the broader sociocultural context conditions virtually all the potential pathways through which contraception can influence women’s sexuality. Needed is a model of contraceptive sexual acceptability that incorporates a wider range of sexual aspects, experiences, and influences.
This narrative review addresses both these gaps. In Part 1, we draw on the literature to define and operationalize a socioecological model for the sexual acceptability of contraception. In Part 2, we apply this model as we review the past 10 years of literature on the sexual acceptability of specific methods.
METHODOLOGY
We employed a narrative review approach, which provides a broad overview of the topic area (Bettany-Saltikov, 2010a, 2010b). This approach is apt for our goal of defining and then applying a novel concept versus methodically investigating a narrow, clearly defined question (per systematic reviews). The narrative approach is particularly well suited for topics in which vast evidence is lacking, as investigators can make recommendations or conjectures based on their work with the broader literature.
Though explicit search protocols are more common in traditional systematic reviews than narrative reviews, we nonetheless used specific search criteria and terms to create boundaries for our endeavor. Our search terms in PubMed, Web of Science, CINAHL, and PsycINFO were as follows: (contraception OR contraceptive OR contraceptive device OR contraceptive devices) AND (pleasure OR libido OR “sexual function” OR “sexual functioning” OR sexuality NOT [“sexual behavior” OR “sexual health”]). Since we wanted to conduct a review of all contraceptive methods that have been approved by the Food and Drug Administration (FDA), not merely one type of method (as is more common), we elected to limit our search to a particular time frame. We focused on the past decade (2005–2015) given both the relatively rapid pace at which contraceptive technology evolves and the fact that second-generation IUDs and implants have only made a resurgence in the last five to 10 years. We thus limited the search to English language articles published in the past 10 years (2005–2015). We identified several other sources from earlier years given their particular relevance to the topic. After removal of duplications, the original search located 3,001 references (1,819 in PubMed, 438 in Web of Science, 661 in CINAHL, and 418 in PsycINFO).
We enlisted a third, doctoral-level colleague with expertise in this area to help with the first review of these 3,001 references. Two people initially reviewed each title and abstract (sometimes the entire piece as well) to identify articles, chapters, commentaries, and other works that spoke to or informed some knowledge of the relationship between sexual experience and contraception use. Based on these assessments, each piece was placed into a yes, maybe, or no file. The first author then reviewed all those labeled as maybe; she placed nine of these additional references in the yes category, for a total of N = 485 citations identified for review in the next stage. The second author then categorized each citation based on the contraceptive method(s) covered and/or the section(s) of the review for which it was relevant. During the final review stage, we read each article to identify which best informed our understanding and conceptualization of sexual acceptability. For Part 1, we worked more closely with a subset of 264 citations, including articles, commentaries, book chapters, and dissertations; for Part 2, we identified 226 sources that referenced sexuality in relation to contraception use. Of those, 103 peer-reviewed articles explicitly measured sexual outcomes. We summarize these 103 studies in Table 1. (For a more detailed outline of each of these 103 articles, please see our Supplemental Table online.)
TABLE 1.
SUMMARY OF STUDIES REVIEWED IN PART 2: Positive and negative sexual aspects of contraception documented by peer-reviewed research, 2005-2015
| Methods | Citations | Select Positive Sexual Impacts | Select Negative Sexual Impacts | Comments and Commonalities |
|---|---|---|---|---|
|
Male Condom1 (n=54 identified in the review) Reviewed here: Male condom research that addresses women’s experiences (n=21) |
(Bolton et al., 2010) (Braun, 2013) (Crosby et al., 2008) (Crosby et al., 2013) (Crosby et al., 2010) (Deardorff, Tschann, et al., 2013) (Fennell, 2014) (Free et al., 2007) (Garcia et al., 2006) (Gebhardt et al., 2006) (Higgins et al., 2009) (Higgins & Wang, 2015a) (Kaneko, 2007) (Randolph et al., 2007) (Sanders et al., 2010) (Sunmola, 2005) (Træen & Gravningen, 2011) (Tung et al., 2012) (Versteeg & Murray, 2008) (Wang, 2013) (Widdice et al., 2006) |
Among women, condom use was positively associated with feeling comfortable communicating about sex and about sex in general. Young women expressing higher levels of sexual self-acceptance were less likely to report a dislike of condoms as a strategy to avoid using them (Deardorff, Tschann, et al., 2013). Female sex workers described skills in applying condoms in sexually arousing ways in order to increase client acceptance of condom use (Free et al., 2007). Men and women perceived condoms as hygienic and as providing sense of security and protection (Garcia et al., 2006). Among women who always used condoms, 28% agreed that condoms reduce sexual sensation; this figure was significantly larger (53%) among women who did not always use condoms (Kaneko, 2007). In a nationally representative survey of young adults in Norway, of the 20% who used a condom at most recent sex, 15% of men and 9% of women reported they did so to feel more clean; 18% of men and 23% of women reported a condom was used to avoid mess; 2% of both men and women stated they used a condom for fun; 9% of men and 7% of women used a condom to make sex last longer; and 2% of men and 7% of women reported using a condom to facilitate more penetration (Træen & Gravningen, 2011). |
Many participants in a qualitative study invoked a narrative of how “natural” or “proper” heterosex does not involve condoms and use of a common metaphor of “condom-as-killer” highlighted the tension between condoms and sexual pleasure (Braun, 2013). Identifying the most common condom “turn-offs”, Crosby et al. (2008) found:
Almost one-third of both women and men reported problems with condom “feel” during sex (Crosby et al., 2013). For both young women and men, pleasure-related attitudes were more strongly associated with lack of condom use at last PVI than all other socio-demographic or sexual history variables (Higgins & Wang, 2015a). |
Research overwhelmingly associated condoms (versus other methods) with reduced physical pleasure (Fennell, 2014). Though popular discourses about “proper” sex may mean that many young adults find condoms sexually unacceptable, one author argued that common anti-condom sentiments are socially constructed and thus possible to change (Braun, 2013). Pleasure-related reasons and feelings of increased intimacy with skin-to-skin contact influence condom discontinuation and non-use among women (Bolton et al., 2010). Studies including both women and men suggest a number of commonalities by gender. For example, the most common turn-offs relate to loss of pleasure for both men and women (Crosby et al., 2008) . Both women and men report condoms can reduce sexual spontaneity. Associations between women’s and men’s pleasure attitudes and their use/non-use patterns are similar if not identical (Higgins & Wang, 2015a). However, some findings illustrate gender-specific findings. Women were more likely than men to report that their partner experienced sexual discomfort with condom use. Many women also report an inability to negotiate condoms with partners due to reduced pleasure for their male partners, perceived and/or actualized side effects, and trust (Versteeg & Murray, 2008). Findings suggest that more emotional, affective motivations for sex can undermine condom use. More research is needed on how to normalize condom use within sexual contexts of expressing love and pleasing one’s partner (Gebhardt et al., 2006). |
|
Oral Contraception (n=24 here, n=38 in the table; please see “multiple methods” section below for more information) |
(Avellanet et al., 2009) (Battaglia et al., 2012) (Bishop et al., 2009) (Caruso et al., 2011) (Caruso et al., 2013) (Caruso et al., 2009) (Davis et al., 2013) (Di Carlo, Gargano, et al., 2014) (Gardella et al., 2011) (Goldstein et al., 2010) (Goretzlehner et al., 2011) (Guzick et al., 2011) (Kucuk et al., 2012) (Lee et al., 2011) (Machado et al., 2012) (Nappi et al., 2014) (Pastor et al., 2013) (Shahnazi et al., 2015) (Skrzypulec & Drosdzol, 2008b) (Strufaldi et al., 2010) (Wallwiener, Wallwiener, Seeger, Muck, et al., 2010) (Warnock et al., 2006) (Wonglikhitpanya & Taneepanichskul, 2006) (Zimmerman et al., 2015) |
Pooled results from a systematic review show that 85% of women using combined OCs reported an increase or no change in libido (Pastor et al., 2013). In a study of women using Klaira®, a combined multiphasic (4 phases) combined OC pill containing estradiol valerate (E2V)/dienogest (DNG), found significant improvements in Quality of Life (QoL), sexual enjoyment, desire, and pain after 3 and 6 cycles of OC use while adhering to a reduced hormone-free interval (26/2 regimen). This regimen was also associated with improvements in QoL, sexual function, PMS symptoms, and reduced bleeding (Caruso et al., 2011). Another study of Klaira® showed that after 6 months of use, younger women reported significant improvements in sexual pain and older women reported significant improvement in desire and overall sexual function compared to their baseline measures (Di Carlo, Gargano, et al., 2014). A study on continuous cycling regimens (OCs unspecified) reported positive sexual function (mainly improvement in orgasm, satisfaction, and pain) and QoL outcomes related to body pain, general health, and social function after 5 months (2 cycles of 72/4 regimen). Reports of desire, arousal, and lubrication did not change (Caruso et al., 2013). In a study of women diagnosed with PCOS, after 6-9 cycles of Belara® (combined OC containing 30 ug EE/2 mg chlormadinone acetate (CMA) used for 9 cycles), 81% reported significant increases in frequency of partnered sex, orgasm during intercourse, and a significant decrease in masturbation frequency (Caruso et al., 2009). A separate study of Belara® using an extended cycle regimen showed the pill was associated with improvement in: skin problems, symptoms of dysmenorrhea, headache, breast tenderness, withdrawal bleeding, bleeding duration, and libido (Goretzlehner et al., 2011). Results from a randomized, prospective study of women using either a combined OC containing 30 mcg EE/150 mcg LNG or a combined OC containing 20 mcg EE/100 mcg LNG, found significant increases in sexual desire, but this increase was statistically significant only for women using EE20/LNG100 (compared to EE30/LNG150) (Strufaldi et al., 2010). |
Yasmin® use was related to increased pain during sex, decreased libido, issues with spontaneous arousal, and reductions in frequency of weekly sex and orgasm during sex. However, no women in this study met clinical criteria for sexual dysfunction (Battaglia et al., 2012). In a study assessing genetic biomarkers, women with the ll genotype were almost 8 times as likely to be classified as having sexual dysfunction if they used OC (OC type unspecified) (Bishop et al., 2009). Breast tenderness after 1 cycle of combined OC use ranged from 10% (Caruso et al., 2013) to 16% (Wonglikhitpanya & Taneepanichskul, 2006), with a majority reporting that the symptom had resolved by 6 months of use. One study of Italian women found that all 102 women reported a significant reduction in vaginal lubrication after 6 months of using Klaira® (Di Carlo, Gargano, et al., 2014). The use of combined OCs containing ethinylestradiol (EE) may have long-lasting effects on sex hormone binding globulin (SHBG) production even after method discontinuation. This may help explain why genital pain disorders do not always resolve with discontinued use of OCs (Goldstein et al., 2010). Pooled results from a systematic review show that 15% of women reported a decrease in sexual desire (libido) after OC use; decreased libido was significantly associated with using pills that containined 15ug ethinylestradiol (EE) (Pastor et al., 2013). Results from a large cross-sectional survey found no significant differences in sexual function based on women using combined OCs containing either androgenic or antiandrogenic progestins, or between different doses of EE. However, women using any type of combined OC reported significantly poorer sexual function scores compared to women not using combined OCs (Wallwiener, Wallwiener, Seeger, Muck, et al., 2010). One randomized, double-blind study provided good evidence that women who suffer from reduced desire and arousal attributed to their birth control pill may find significant improvement in sexual function if they switch to a different pill formulation, particularly those containing E2V/DNG or EE/levonorgestrel (LNG) (Davis et al., 2013). |
Though some women report positive or negative sexual impacts in relation to their use of combined OC, the large majority of women report no impact in sexual function or frequency of sex related to their OC use. Nonetheless, women who do report decreases in sexual function may wish to switch to another OC formulation. Differences in aspects of sexual function such as experiences with sexual pain and levels of sexual desire vary by age and should be contextualized and accounted for in contraceptive research and clinical care. In terms of OCs and sexual pain, combined OCs may be a beneficial treatment for endometriosis-related pelvic pain (Guzick et al., 2011). However, the effects of OCs on experiences with genital pain and interstitial cystitis (Gardella et al., 2011) are not well understood. More research is needed on potential long-term, long-lasting impacts of OC use on women’s sexual pain. The sexual repercussions of seemingly non-sexual side effects of OC use should also be considered; examples include body/facial hair growth changes, and breast tenderness. Aesthetic changes, for example, may be related to improving women’s sexual and social self-esteem as evidenced by increased frequency of partnered sex and reductions in masturbation (Caruso et al., 2009). Genetic differences may influence experiences of depression and sexual function in women taking both SSRIs and OCs. More research is needed to better understand how genetics may play a role in sexual function, particularly in the context of hormonal contraception use (Bishop et al., 2009). Though results remain inconclusive as to the effects of a combined OC pill containing DHEA, the potential for positive sexual function improvements in some women with androgen-sensitivity and/or oral contraceptive-associated sexual dysfunction show promise and requires further investigation (Zimmerman et al., 2015). Women using 3rd generation combined OCs (containing 0.03 mg EE/0.15 mg desogestrel) reported significantly better improvements in sexual function compared to women using 2nd generation combined OCs (low-dose estrogen pills containing 0.03 mg EE/0.15 mg LNG); even though both groups demonstrated higher sexual function after 4 months compared to baseline (Shahnazi et al., 2015). |
|
IUD/IUC/IUS (n=7 here, n=12 in the table; please see “multiple methods” section below for more information) |
(Bastianelli et al., 2011) (Enzlin et al., 2012) (Gomez & Clark, 2014) (Gorgen et al., 2009) (Higgins et al., 2015) (Panchalee et al., 2014) (Skrzypulec & Drosdzol, 2008a) |
In one study of the levonorgestrel (LNG) IUS, women reported a significant decrease in sexual pain and a significant increase in sexual desire after one year of use (Bastianelli et al., 2011). A cross-sectional study comparing women who had used either the LNG IUS or copper IUD for at least 6 months found that most women using both IUDs reported changes in menstrual bleeding after IUC placement, though LNG-IUS users were significantly more likely to report shorter menses and less blood flow. Women using either LNG IUS or copper IUD reported similar rates of self-perceived sexual satisfaction (58-60%), sex more than twice per week (48-49%), desire for sex more than twice per week (50-53%), ease in reaching physical arousal (47-54%), and ease in achieving orgasm (76-78%) (Enzlin et al., 2012). A qualitative study described both IUD users’ and non-users’ perceptions of the sexual aspects of IUDs. Sexual benefits included security, or enhanced sexual disinhibition thanks to IUDs’ efficacy, spontaneity, or improved sexual flow, and scarcity of hormones, which meant no/low hormonal influences on libido (Higgins et al. 2015). |
Breast tenderness was reported by 35% of women using the LNG IUS, but resolved by 6 months of use (Bastianelli et al., 2011). Compared to women using copper IUDs, women using the LNG-IUS perceived their method to have a greater negative impact on aspects of their sex life (frequency of sex, arousal and desire); however, orgasm and overall satisfaction with sex did not change with LNG IUS use (Enzlin et al., 2012). One study reported that, after 6 months of use, 12% of women using the LNG IUS reported decreased libido and 35% reported no change in libido. 13% reported experiences with pelvic pain (Gorgen et al., 2009). A qualitative study described both IUD users’ and non-users’ perceptions of the sexual aspects of IUDs. Sexual detractions included string, or negative sexual effects on partner, and sexual aspects of bleeding and cramping, which could affect sexual experiences (Higgins et al., 2015). |
In general, women using copper IUDs reported neither positive nor negative changes in sexual function related to their method. Women who reported existing sexual distress while using either type of IUD were more likely to attribute negative sexual function changes to their IUD usage rather than to other factors in their lives (Enzlin et al., 2012). Most studies show sexual improvements and/or no sexual changes among women using IUC. Studies indicate potential improvements to women’s sexual well-being through IUD/IUS use. For example, decreases in bleeding associated with IUDs for many users are likely to increase sexual acceptability of these methods. Young women also report psycho-sexual benefits of the IUC’s efficacy and no/low hormones (Higgins et al., 2015). Prospective research is needed to better understand the extent to which positive sexual function outcomes in women using a hormonal IUS can be attributed to the method itself versus particular characteristics of the women who choose to use this method (Witting, Santtila, Jern, et al., 2008). |
|
Vaginal Ring (n=4 here n=12 in the table; please see “multiple methods” section below for more information) |
(Caruso et al., 2014) (Merkatz et al., 2014) (Roumen, 2008) (Terrell et al., 2011) |
One study found that women reported an increase in desire, arousal, lubrication, orgasm, satisfaction, and improvement in dyspareunia during 2 extended use cycles (approximately 4.5 months of use). No changes in sexual frequency were observed. FSFI scores increased and sexual distress (FSDS) scores decreased at both follow-up assessments (after 63 days and 126 days of use) (Caruso et al., 2014). In one study, male partners reported never feeling the ring during sex (72%), no change in sexual sensations (92%), and never feeling the ring move during coitus (87%). Though 16% of male partners experienced ring expulsion during sex, only 2 men found this experience disruptive. Most women and their partners found the ring to be highly sexually acceptable and women using the ring expressed fewer issues with vaginal dryness compared to combined OC users. (Roumen, 2008). Adolescent women most willing to try the ring reported more comfort with their genitals and greater knowledge of positive ring attributes (month-long protection, covert use) (Terrell et al., 2011). In one study, over 91% of women using the ring reported a steady increase or no change in sexual desire over 12 cycles (Sabatini & Cagiano, 2006) (see citation in “Multiple Methods” section). |
2% of women using ring reported vaginal discomfort and 4% reported device-related events such as ring slipping out. The most common sexual “problems” with this method pertain to the mechanics of the ring during sexual activity and discomfort with touching their own genitals. Ring-related events (feeling the ring inside vagina, interference with sex, and expulsion) were associated with higher rates of discontinuation (Roumen, 2008). Women less willing to try the ring reported concerns of the ring getting lost inside or falling out of the vagina (Terrell et al., 2011). Mild adverse outcomes included bleeding, nausea, headache, and breast tenderness (Caruso et al., 2014; Roumen, 2008). |
Some women using the ring have reported improvements in sexual function and quality of life and decreases in sexual distress. A small minority of users have reported adverse sexual outcomes such as vaginal discomfort. Higher user satisfaction with the ring is related to ease of removal, not being able to feel the ring during normal use, and either no change or an increase in sexual pleasure and/or sexual frequency. Women who were more satisfied with the method (including positive sexual attributes of the method) were more likely to adhere to correct use and to continue use over time (Merkatz et al., 2014). Among women less comfortable touching own genitals, providing alternative strategies such as wearing gloves or using an applicator to insert/remove the ring may facilitate willingness to try the method (Terrell et al., 2011). The extent to which ring users enjoy and/or find bothersome vaginal wetness associated with use should be explored to better understand sexual acceptability (Battaglia et al., 2014) (see citation in “Multiple Methods” section). |
|
Implant (n=5 here n=7 in the table; please see “multiple methods” section below for more information) |
(Aisien & Enosolease, 2010) (Di Carlo, Sansone, et al., 2014) (Duvan et al., 2010) (Gezginc et al., 2007b) (Visconti et al., 2012) |
Participants in one study exhibited significantly increased FSFI scores at 3 months, showing improvement in domains measuring arousal, orgasm, satisfaction and pain; no changes were observed at 6 months compared to 3 months (Di Carlo, Sansone, et al., 2014). Visconti et al. (2012) found that by 3 months, women reported statistically significant improvements in frequency and intensity of orgasm, better sexual satisfaction, and less sexual anxiety. By 6 months, scores measuring sexual pleasure, personal initiative, orgasm frequency, sexual satisfaction, discomfort and anxiousness had all improved significantly from baseline. Weekly frequency of sex increased significantly by 6 months compared to baseline (Visconti et al., 2012). |
A small minority (2%-9%) of women using the implant reported reduced libido (Aisien & Enosolease, 2010) (Duvan et al., 2010) (Gezginc et al., 2007b). Bleeding profiles associated with implant use after one year of use were variable. The range from several studies is as follows:
Other reported side effects that may have sexual repercussions included the following:
(Aisien & Enosolease, 2010) (Duvan et al., 2010) (Gezginc et al., 2007b) (Visconti et al., 2012) |
Several studies indicate improvements in women’s sexual functioning and satisfaction with implant use. The authors of one study (Visconti et al., 2012) attributed an increased sense of security from pregnancy as leading factor in increased frequency of sex and improvements in sexual function associated with this method. A minority (<10%) of users report libido reductions. A minority of women also report a number of bleeding changes and/or side effects such as breast tenderness, weight gain, or headaches that could decrease women’s sexual well-being. 88% of women in one study reported no negative feelings about the method (Aisien & Enosolease, 2010), though 25% of women in another study discontinued Implanon® within the first year of use; 35% discontinued due to bleeding irregularities and 10% stopped due to interference with sexual function (Gezginc et al., 2007b). Tolerability of irregular bleeding patterns associated with the implant should be further explored in regards to sexual acceptability. |
|
Injectable (n=2 here, n=8 in the table; please see “multiple methods” section below for more information) |
(Gubrium, 2011) (Wanyonyi et al., 2011) |
After 6 months of Depo® use, women reported marginally significant improvements in physical health, which could have sexual repercussions. Women reported no significant changes in either mental health or sexual function after 6 months of use of injectable contraception (Wanyonyi et al., 2011). | Results from a qualitative study illustrate decreased libido (sexual desire) as a key theme associated with Depo® use. Participants linked this libido decrease with emotional and body image changes (Gubrium, 2011). 33% of women reported menstrual irregularities. Main reasons for discontinuation in one study included: menstrual irregularity (27%); reduced libido (13%); and weight gain (20%) (Wanyonyi et al., 2011). |
Few studies report improvements to women’s sexual well-being with use of injectable contraception. Studies suggest that a minority of women experience libido reductions on this method, which may also be related to factors such as weight gain and changes in body image, unpredictable bleeding, and emotionality. Side effects associated with the shot are not experienced singly, but as a constellation of factors (examples: weight gain leads to changes that, taken together, contribute to the sex-acceptability of the method). |
|
Female Condom (n=7 here, n=8 in the table; please see “multiple methods” section below for more information) |
(Latka et al., 2008) (Mack et al., 2010) (Mathenjwa & Maharaj, 2012) (Okunlola et al., 2006) (Sobze Sanou et al., 2013) (Telles Dias et al., 2006) (van Dijk et al., 2013) |
Studies have documented a number of positive sexual aspects of the female condom, including the following: high level of sexual comfort due to sufficient lubricant and better lubrication compared to male condom; low risk of breakage, especially during rough sex; ability to accommodate all penis sizes; reduced interruption of sexual encounter due to ability to insert before intercourse; greater protection of the outer labia; preferred the smell to the male condom; lack of side effects; increased protection from pregnancy & STIs/HIV; female-controlled use; lower likelihood of allergic reaction compared to male condom; increased ability to relax and enjoy sex; increased sensation; clitoral stimulation through external ring; and massage of head of penis with internal ring (Latka et al., 2008) (Mack et al., 2010) (Mathenjwa & Maharaj, 2012) (Telles Dias et al., 2006) (van Dijk et al., 2013). | Initially, women in one study reported that the method’s design, particularly the internal ring, made it difficult (and painful) to insert and remove. However, after several uses, more than half of participants preferred the female to the male condom (Mack et al., 2010). Among female condom users, the most common complaint (30%) was poor sexual satisfaction associated with use. 22% reported difficulties with insertion, and 5% experienced pain during intercourse when using the female condom (Okunlola et al., 2006). Common complaints included noise during intercourse, stiffness of internal ring, resistance of partners to use, and excessive lubrication (Telles Dias et al., 2006). |
The preconceived notion that female condoms decrease sexual pleasure can be a barrier to use among both men and women (Sobze Sanou et al., 2013). Women in a number of studies discussed difficulties with insertion and/or aesthetic detractions such as noise and stiffness of the internal ring. However, women in a variety of studies reported myriad sexual advantages to female condoms, especially compared to male condoms. Pleasure-related aspects experienced by both men and women increased acceptability and long-term use of this method. Though women’s first impressions of the female condom may be negative, particularly regarding insertion and large size, perceptions are likely to improve with time and practice. Women with greater personal autonomy were more likely to report sustained use (Telles Dias et al., 2006). |
|
Female Sterilization (n=3 here, n=7 in the table; please see “multiple methods” section below for more information) |
(Dias et al., 2014) (Schaffir, Fleming, et al., 2010) (Smith et al., 2010) |
92% of women were satisfied with the procedure and would recommend it to friends (Dias et al., 2014). After controlling for age and other socio-demographic characteristics, women with a tubal ligation were significantly less likely than non-sterilized women to experience negative sexual outcomes such as a lack in sexual desire, issues with vaginal lubrication, or taking too long to orgasm. Sterilized women reported significantly higher levels of sexual and relationship satisfaction and sexual pleasure compared to non-sterilized women (Smith et al., 2010). |
After tubal ligation, women reported significantly more bleeding, premenstrual symptoms, dysmenorrhea, and noncyclic pelvic pain; they also reported significantly reduced libido and fewer sex acts per week (Dias et al., 2014). 37% of women undergoing sterilization agreed with a statement that they would have less sexual desire after procedure (Schaffir, Fleming, et al., 2010). |
Studies report both positive and negative impacts of sterilization on aspects of sexual function, yet women report overwhelmingly high rates of satisfaction with the method, highlighting the aspect of safety and freedom from pregnancy as important aspects of method acceptability. Many women report sexual concerns in anticipation of gynecological procedures. We recommend that physicians address sexual concerns with women in more detail before surgery and continue to address concerns as needed post-procedure. |
|
Vasectomy (n=3 here, n=5 in the table, please see “multiple methods” section below for more information) |
(Al-Ali et al., 2014) (Bunce et al., 2007) (Shih et al., 2013) |
Female partners in one study reported significantly more positive sexual function after vasectomy. No significant changes were reported in men’s sexual function (Al-Ali et al., 2014). Men often cited seeking vasectomy to allow their female partners to discontinue hormonal methods (Bunce et al., 2007). |
Loss of manhood and misconceptions around negative impacts on men’s sexual function, desire, and performance were cited by men and their partners as reasons for not selecting vasectomy. Both male and female participants cited potential for infidelity as both a positive and negative aspect of vasectomy (Shih et al., 2013). Partner influence, including partner’s approval, was an important factor in men seeking vasectomy (or not) (Bunce et al., 2007). |
Though vasectomies are performed on male bodies, women may experience positive sexual effects from this method, potentially related to security against unwanted pregnancy and/or no longer having to take contraceptive responsibility. Cultural constructions of gender relating to infidelity and manhood and sexuality may deter some men and their partners from vasectomy. Highlighting the rapid return to prior sexual function is an important component in vasectomy counseling and could increase knowledge and acceptability of the procedure (Shih et al., 2013). |
|
Withdrawal (n=4 here, n=5 in the table, please see “multiple methods” section below for more information) |
(Higgins & Wang, 2015b) (Ortayli et al., 2005) (Rahnama et al., 2010) (Sirkeci & Cindoglu, 2012) |
For both women and men, those who felt condoms could diminish sexual pleasure were significantly more likely to have used any/only withdrawal at last sexual intercourse (Higgins & Wang, 2015b). Iranian women who use withdrawal cited dissatisfaction with sexual sensation associated with condom use and partners’ unwillingness as reasons for not using modern (more highly effective) methods of contraception (Rahnama et al., 2010) |
Turkish men who did not use withdrawal reported anxiety, decreased sexual pleasure, and dislike of coital-dependent methods as reasons for non-use. Almost all current withdrawal users cited reductions in sexual pleasure with the method, but less so than with male condom use (Ortayli et al., 2005). 34% of women reported decreased sexual enjoyment when using withdrawal; 42% perceived their partner to experience decreased enjoyment as well (Rahnama et al., 2010). Participants acknowledged female sexual pleasure as a consideration for using withdrawal as well as difficulties with climax control for some men (Ortayli et al., 2005). |
Findings suggest that sexual acceptability issues may play a larger role in shaping withdrawal and other contraceptive practices than acknowledged by prior research. Withdrawal reduced both women’s and men’s sexual well-being in a number of studies; other studies suggested that couples were more likely to use withdrawal when they experienced pleasure-reductions with other methods (e.g., male condoms). Authors acknowledge the need for climax awareness and control for male partners in order for withdrawal to work successfully (Freundl et al., 2010). |
|
Diaphragm (n=2) |
(Sahin-Hodoglugil et al., 2011) (Thorburn et al., 2006) |
Current users described the diaphragm as valuable to women’s autonomy with a female-initiated method and the ability to use covertly. Women and men enjoyed the increased sexual pleasure when using the diaphragm with a gel (Sahin-Hodoglugil et al., 2011). 66% of women who had never used a diaphragm perceived that it “does not decrease sexual pleasure” (Thorburn et al., 2006). |
Less than 25% of women in one study felt confident in using the method correctly when sexually excited or in the heat of the moment. 17% of women preferred methods that require no genital touching (Thorburn et al., 2006). Current diaphragm users reported the need for partner negotiation as an attribute that contributed to overall acceptability (Sahin-Hodoglugil et al., 2011). |
Few study participants reported negative sexual attributes of diaphragms, though (like condoms and other coitus-dependent methods) this method may hinder sexual flow and spontaneity. Women acknowledge the difficulty of stopping to insert one’s diaphragm in the heat of the sexual moment. The ability to insert the diaphragm before sexual activity increased its acceptability. Along these lines, comfort with genital touching will impact acceptability. |
|
Natural Family Planning (NFP) (n=1 here, n=3 in the table; please see “multiple methods” section below for more information) |
(Freundl et al., 2010) | Results from a systematic literature review highlighted sexual self-control and increased body awareness as positive attributes of NFP reported by users (Freundl et al., 2010). | Natural family planning methods may disallow spontaneity as most methods require abstaining from PVI during peak periods of fertility (Freundl et al., 2010). | Couple-focused research may enlighten effective sexual communication strategies of couples who successfully use NFP or other methods negotiated by both partners. Highlighting and promoting strategies for intimacy and other sexual activities that don’t involve PVI may increase the sexual acceptability of NFP methods; more research is needed. |
|
EC Pills (n=1) |
(Escajadillo-Vargas et al., 2011) | N/A | Regression analyses from a nested case-control study indicate that women using oral EC in the past 3 months had significantly greater odds for increased risk of sexual dysfunction (Escajadillo-Vargas et al., 2011). | More research is needed to better understand the characteristics of women who use EC and how oral EC use might be related to sexual function. |
|
Multiple Methods Measured in the Same Study (n=19) |
(Battaglia et al., 2014) (Davison et al., 2008) (Elaut et al., 2012) (Fataneh et al., 2013) (Gabalci & Terzioglu, 2010) (Guida et al., 2014) (Halmesmaki et al., 2007) (Higgins, Hoffman, et al., 2008) (Mohamed et al., 2011) (Nishtar et al., 2013) (Ott et al., 2008) (Roumen, 2007) (Sabatini & Cagiano, 2006) (Sanders, Smith, et al., 2014) (Schaffir, Isley, et al., 2010) (Smith et al., 2014) (Stewart et al., 2007) (Tabari et al., 2012) (Witting, Santtila, Jern, et al., 2008) |
In a genetic study using a within-subject, crossover study design with random-order use of 3 contraceptive methods (combined OCs, progestin-only pills, and the ring), both partners’ level of sexual desire was statistically significantly higher among women using the vaginal ring (Elaut et al., 2012). Compared to women using the ring, pill, or no method (control group), after 6 months, implant users reported the most significant improvements in sexual discomfort, anxiousness, personal initiative, and fantasy. All method users reported significantly more sexual pleasure, satisfaction and higher orgasm frequency at 6 months compared to controls (Guida et al., 2014) Women reported that the ring was more likely to interfere with sex compared to the pill and significantly more women reported that their sex partners preferred the pill (Stewart et al., 2007). A systematic review found either improvement or no change in sexual function and sexual experience in women using both implants and IUD/IUS (Sanders, Smith, et al., 2014). |
Results from a prospective study found that women using drospirenone-containing OC (Yasmin®) reported significant reductions in sexual frequency and orgasm during sex, and reported more pain during sex after 6 months of use compared to baseline measures. After 6 months of use, women using Yasmin® and vaginal ring demonstrated significant decreases in sexual function scores compared to baseline assessment (Battaglia et al., 2014). Results from a case-controlled study found that, compared to controls, women using any contraceptive method reported significantly poorer scores in the domains measuring desire, arousal, lubrication, orgasm, pain, and satisfaction (Fataneh et al., 2013). Controlling for a number of socio-demographic and relationship characteristics, results of one cross-sectional study demonstrated that male condoms, either used alone or in conjunction with hormonal methods (dual use), were most strongly associated with decreased sexual pleasure (Higgins, Hoffman, et al., 2008). In a randomized, prospective trial conducted from method initiation to 12 months or method discontinuation, findings show that, compared to pill users, ring users reported significantly more experiences with vaginitis, decreased libido, and ring-related problems. Conversely, compared to ring users, women using combined OCs reported significantly more experiences with increased weight, acne, and emotional lability (Mohamed et al., 2011). Results from a large cross-sectional study show that a regression controlling for a number of socio-demographic and relationship characteristics indicated that women using hormonal contraception experienced significantly less frequent sex, and significantly more problems with arousal, pleasure, orgasm, and vaginal lubrication compared to women using non-hormonal methods (Smith et al., 2014). |
Studies comparing sexual outcomes for multiple methods show mixed findings. However, studies of multiple methods do highlight that contraceptives can affect a wide range of sexual domains for women, from inference with sexual flow to partner preference to sexual functioning and pleasure to more general sexual satisfaction. Most studies of multiple methods compare and contrast various formulations of hormonal methods. Studies with sufficient sample sizes to compare a wider range of methods are warranted. Researchers have paid especially little attention to women’s sexual experiences with long-acting reversible contraception, or LARC (implants and IUDs) in the past 10 years. With the recent public health focus on LARC, more research is needed, especially in the US context (Sanders, Smith, et al., 2014). In one study, the authors note that dual users are likely “erotizing safety” associated with doubling up on pregnancy and STI prevention methods. (This group reported higher sexual satisfaction levels than pill-only or condom-only users (Higgins, Hoffman, et al, 2008).) Adolescents and young women often report frequent method switching, starting, and stopping, all of which reflect their dynamic lives and intimate experiences. More research is needed on how mood influences interest in sex and reasons for method switching or discontinuation among young women (Ott, Shew et al, 2008). |
Methods are presented in descending order per number of citations, with those methods most commonly cited appearing first and articles assessing multiple methods (n=19) located at the end of the table.
For the purpose of this review, our focus pertains to currently available contraceptive methods that people use primarily for pregnancy prevention. We acknowledge that many women use hormonal methods in particular for a variety of noncontraceptive benefits, from relieving severe menstrual pain to reducing acne or excess hair growth. One nationally representative survey found that upward of 14% of oral contraceptive users in the United States reported using the pill for noncontraceptive purposes only (Jones, 2011). However, given that the overwhelming majority of contraceptive methods are used at least in part to prevent pregnancy, we have focused our attention here on healthy populations of reproductive age and—to the best of our knowledge, based on the source—of reproductive capacity. Examples of articles we thus reviewed but ultimately excluded comprised studies of oral contraceptive use among postmenopausal women, acceptability of rectal microbicides among gay men, and contraceptive practices among women with special health issues (e.g., epilepsy, cancer, or endometriosis), though certainly these populations require tailored, patient-centered contraceptive care. However, we acknowledge that relatively healthy, reproductive-age people often use contraception for a wide variety of purposes, not only to prevent pregnancy, and that these purposes may well affect sexual acceptability.
Although we highlight men’s roles when appropriate, we focus primarily on women’s sexual experiences of contraceptive methods given the dearth of women’s perspectives in the existing literature. Moreover, though condoms (and some of the other multipurpose technologies) can prevent transmittal of human immunodeficiency virus (HIV) and STIs for a variety of sexual acts, and can affect both partners involved, we focus here on penile–vaginal intercourse—and primarily on women’s experiences. That said, we make no assumptions about the sexual identities of women who have penile-vaginal intercourse and/or who use contraception. A growing body of research suggests that a large proportion of unintended pregnancies occur among sexual minority women (Charlton et al., 2013; Rosario et al., 2014), and many sexual minority women thus face situations in which they wish to prevent pregnancy in a sexually acceptable way.
Finally, this review does not rigorously evaluate the methodological quality of all available studies; interested readers should refer to such critiques elsewhere (Bancroft, Hammond, & Graham, 2006; Graham & Bancroft, 2013a, 2013b; Hong, Wu, & Fan, 2008). Rather, our purposes here are, first, to enlighten and broaden the concept of how contraceptives can influence sexuality and, second, to use the wider literature to overview method-by-method sexual acceptability when considered in a context more comprehensive than sexual physiology alone.
PART 1: BUILDING A CONCEPTUAL MODEL OF SEXUAL ACCEPTABILITY OF CONTRACEPTION
In their 2005 piece, “Critical Issues in Contraceptive and STI Research,” Severy and Newcomer defined acceptability as “the voluntary sustained use of a method in the context of alternatives” (p. 47). They argued that especially since most women and men would rather not have to use a contraceptive method during sex, contraception decisions really involve finding “the least bad alternative.” Contraceptive methods are only effective if used, and thus they must be designed to fit people’s needs rather than vice versa. A variety of factors will shape acceptability for both partners, including effectiveness, aesthetics (viscosity, taste, smell), how one obtains and applies/uses the method, its presence or “absence” during sex (including ability to be used covertly), and its actual or perceived effect on sexual intimacy. “Sexual pleasure,” they wrote, “plays a central role in determining user perspectives regarding new methods” (p. 45).
Severy and Newcomer’s eroticized, user-first version of acceptability serves as a cornerstone of our own model (Severy & Newcomer, 2005). We are similarly guided by other scholars who have described various pathways through which contraceptives may affect sexuality and sexual well-being (Bitzer, 2010; Higgins & Hirsch, 2008), including hormonal pathways (Bancroft et al., 2006; Graham & Bancroft, 2013a, 2013b). Here, we attempt to build an even broader, more ecological approach to the sexual acceptability of contraception, exploring three major levels of influence/pathways: macro (gender, cultural, race/ethnicity, place, inequality, and structure), social (relationship and partner factors), and individual. We argue that the best research on this topic will contextualize contraceptives within the sexual, social, and cultural settings in which they are selected and used (Woodsong & Alleman, 2008). Please refer to Figure 1 for a visual rendering of the model, particularly how the various layers interact and/or embed within one another.
Figure 1.
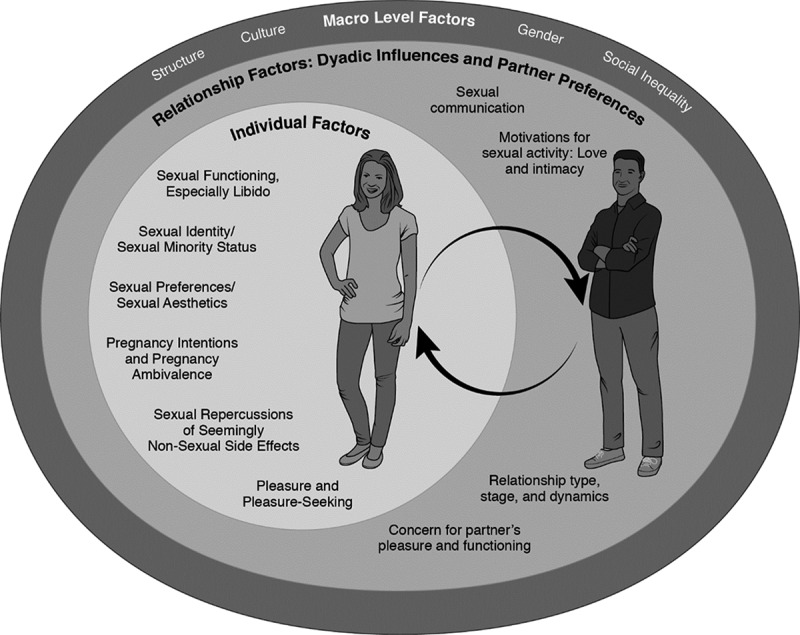
Conceptual model of the sexual acceptability of contraception.
Macro-Level Factors: Gender, Social Inequality, Location, Culture, and Structure
The outermost layer of the model, macro-level factors, serves as the context in which individuals and couples experience contraceptives in their lives.
Gender
All women’s experiences of sexuality and contraception will be filtered through gender. Here, we briefly highlight three relevant aspects: sexual and reproductive autonomy, sexual scripts, and sexual empowerment.
Sexual and Reproductive Autonomy
Though a key objective of this review is to address “the pleasure deficit” in family planning research, we of course acknowledge that many women face challenges to sexual autonomy, let alone pleasure. At a basic level of sexual rights, gendered power imbalances may undermine sexual autonomy and contraceptive use. Women may not be able to have only consensual, desired sexual experiences (Billy, Grady, & Sill, 2009; Vannier & O’Sullivan, 2010) and/or be able to regulate their fertility as they wish (Chacham, Maia, Greco, Silva, & Greco, 2007; East, Jackson, O’Brien, & Peters, 2011). As a result of these gendered power imbalances, one group of scholars has advocated for the replacement of the concept of safe sex (i.e., sexual activity that’s protected from unintended pregnancy and/or STIs) with woman-controlled safe sex (Alexander, Coleman, Deatrick, & Jemmott, 2012). Along those lines, the call for women-controlled dual-prevention technologies, such as female condoms and microbicides, consistently highlights the notion that women need access to methods that they can use covertly, regardless of their male partners’ knowledge or participation (Mathenjwa & Maharaj, 2012). Though woman-controlled safe sex is a powerful and necessary feminist concept, it may be less relevant to those situations in which women’s heterosexual activity is consensual and, ideally, mutually pleasurable. However, for at least some women, gender-based power differences may mean that using contraceptives to enhance well-being is of less immediate salience than, say, maintaining sexual safety or minimizing sexual harm.
Gender inequality may affect sex and contraception in more subtle ways, too. For example, women are more likely to be depressed than men (Piccinelli & Wilkinson, 2000); they may also face more stress and fatigue, particularly with young children in the household (Bird, 1997). Depression, stress, and use of psychotropic drugs can undermine both sexual functioning (Echeverry, Arango, Castro, & Raigosa, 2010; Garbers, Correa, Tobier, Blust, & Chiasson, 2010) and effective contraceptive use (Stidham Hall, Moreau, Trussell, & Barber, 2013).
Sexual Scripts
Gendered sexual scripts serve as another strong influence on women’s sexual and contraceptive experiences (Levin, 2010). Strongly rooted cultural norms contrast gender expectations in sexual desire and pleasure, the degree to which one’s sexuality and sex “drive” are controllable, and who bears primary responsibility for sexual protection and pregnancy prevention (Borges & Nakamura, 2009; Brown, 2015; Campo-Engelstein, 2013; Gubrium & Torres, 2013; Hust, Brown, & L’Engle, 2008). The stage is set for women to enact a gendered responsibility for contraception that is often disconnected from pleasure seeking—traditionally viewed as men’s domain. For example, in an analysis of direct-to-consumer contraceptive advertising, investigators concluded that “[t]he viewer of these websites comes away with the impression that for men sex is supposed to be fun and feel good and for women sex is risky and not to be done without taking precautions” (Medley-Rath & Simonds, 2010). Others have documented the tension women may feel between, on the one hand, assuming bodily control and independently making contraceptive decisions and, on the other hand, wanting to share responsibility as a couple (Lowe, 2005). Women may feel greater social pressure to maximize their independence through contraception than to achieve sexual empowerment (Lowe, 2005). One scholar analyzed young women’s struggle for/with the needs of two different bodies: the “sexy body” versus the “fertile body” (Keogh, 2006). Most cultural scripts of masculinity and sexuality do not involve these latter tensions. While both women and men may describe how condoms, withdrawal, and/or vasectomy can decrease men’s pleasure (Marchi, De Alvarenga, Osis, & Bahamondes, 2008), fewer scripts exist on how contraceptive methods may potentially diminish or improve women’s pleasure and sexual well-being.
Sexual Empowerment
Scholarship also draws ties between sexual empowerment and contraceptive practices, with women who are more socially and sexually empowered more likely to use the contraceptives they wish (Crissman, Adanu, & Harlow, 2012; Do & Kurimoto, 2012; Gipson & Hindin, 2007). 3 Hensel et al. (2011) argued that even though women may not think of contraception as a “sexy” part of their lives, various studies link more successful contraceptive use with women’s ability to express sexual desire and to advocate on their own behalf for pleasure. Having an enriched view of one’s sexuality and sexual health have also been associated with oral contraceptive use (versus nonuse) in Spain (Carrasco‐Garrido et al., 2011) and contraceptive use more generally among young women in the United Kingdom (Free, Ogden, & Lee, 2005). In sum, endeavoring to improve women’s sexual perceptions of themselves is not only an important goal in its own right; it is also good for increasing (consensual, acceptable) contraceptive use.
Social Location: Race/Ethnicity, Social Class, and Other Axes of Inequality
Although gender is surely an influential shaper of contraceptive and sexual experiences, so too are other axes of inequality, including but not limited to race/ethnicity, social class, sexual identity, dis/ability, and nation. Furthermore, as feminist scholars of intersectionality have argued, gender varies according to one’s social location (Andersen & Hill Collins, 2013). For example, constructions of inner-city, low-income, Black women’s sexuality may differ considerably from suburban, middle-class, White women’s sexuality. Along these lines, gender is not the only or even the primary framework through which women experience their sexuality or use contraception. For example, in one qualitative study of contraceptives’ sexual acceptability, poor women described trying to minimize painful side effects in light of other chronic health conditions, such as diabetes, arthritis, and depression; in comparison, socially advantaged respondents reported fewer chronic health conditions and could focus more on maximizing sexual pleasure through contraceptive use (Higgins & Hirsch, 2008). Though all women have a right to safe and pleasurable sexual experiences, sexual enjoyment may be less of an immediate goal for women in resource-deprived and/or conflict-torn settings.
Unfortunately, histories of racism, classism, and “stratified reproduction” (Ginsburg & Rapp, 1995) mean that contraception is not always a tool of individual feminist empowerment; it can also be used as a means of social control and disempowerment. A disturbing number of both prior and current reproductive injustices have used contraceptives to limit the fertility of socially disadvantaged women, especially poor women of color (Roberts, 1997). In the 1990s, U.S. policymakers supported numerous programs to encourage or coerce socially disadvantaged women to use Norplant, an implantable, five-year contraceptive method associated with a number of serious side effects. Proposed legislation and judicial decisions included cash incentives for Norplant insertion, welfare benefits contingent upon insertion, and even Norplant insertion to avoid jail time (Gold, 2014). In the contemporary United States, African American women are still significantly more likely than White women to have been sterilized, even after accounting for age, insurance status, number of children, and education (Borrero et al., 2007). Women with public or no insurance are also significantly more likely to have been sterilized compared to women with private insurance (Borrero et al., 2007). Another recent study documented that providers were more likely to recommend long-acting methods such as IUDs to women of color and poor women than to White women and middle-class women (Dehlendorf et al., 2010). In sum, socially disadvantaged women have been and currently are discouraged and/or prevented from having (more) children. Acknowledging these injustices does not mean we have to dispense with contraceptive promotion and/or a focus on sexual acceptability. On the contrary, recognizing these histories can help us understand the potential for such abuses, and develop counseling methods and policies that are more client centered.
One framework we can use to consider and address the unique needs of socially disadvantaged women is reproductive justice, a term dating back to 1994, when African American women activists combined the terms reproductive rights and social justice (Luna & Luker, 2013). According to one reproductive justice organization, “[R]eproductive justice exists when women and girls and all people have the social, political, and economic power and resources to make health decisions about our gender, bodies, and sexuality for ourselves, our families, and our communities” (Reproductive Justice Collective, 2015). At a fundamental level, a reproductive justice approach means giving all women knowledge and access to all contraceptive methods but also valuing women’s own needs over the desire to decrease certain groups’ birthrates and/or the need to meet certain institutional thresholds or metrics, such as local strategies to reduce teen pregnancies. Moreover, contraceptive services should be only one small part of a larger host of efforts to improve the lives of socially disadvantaged women—from reducing institutionalized racism and sexism to improving educational opportunities, community safety, and employment possibilities.
Culture
While gender and other social inequalities exist in every country in the world, widespread and meaningful cultural differences influence the sexual acceptability of contraception. For example, compelling comparative analyses of the United States and the Netherlands document how differences in sexual cultures may help explain large disparities in adolescent contraceptive use and teen pregnancy in these countries (Brugman, Caron, & Rademakers, 2010; Schalet, 2011). Sexual norms taken for granted by most North Americans—for example, the idea of adolescents’ “raging hormones”—may not exist within the Dutch setting (Brugman et al., 2010; Schalet, 2011). In one study, U.S. girls mentioned themes of driven by hormones and peers, unprepared, satisfying him, and uncomfortable and silent parents; in contrast, prominent themes among Dutch girls were motivated by love, control of my own body, and parents as supporters and educators (Brugman et al., 2010). Such research helps illustrate how sex is “not a natural act” (Tiefer, 2004) but a series of socially constructed scripts—scripts into which contraceptives may or may not fit. A number of researchers have also documented the multitude of sexual and contraceptive scripts within countries, with variation by race/ethnicity, social class, and age cohort (Chen, 2008; Gillmore, Chen, Haas, Kopak, & Robillard, 2011; Sandberg, 2011). For example, cultural differences exist in the degree to which people uphold the virgin/whore dichotomy for women (Gubrium & Torres, 2013) or the degree to which women are sexually stigmatized for using contraception or carrying condoms (Oyedeji & Cassimjee, 2006). Taken cumulatively, these studies remind us that various layers of the sexual acceptability model will take on different profiles for people in different circumstances, either within or across cultures. We thus must endeavor for synthesis with local ideologies when promoting sexually acceptable contraception (Gilley, 2006).
Structure
In our review process, we located few articles documenting how structural factors may shape contraception’s sexual acceptability. However, levels of global and local social advantage are likely to affect both sexual well-being and contraceptive practices. For example, poverty and structural disadvantage may mean that women have more pressing concerns (e.g., finding stable housing and employment, establishing safety—either sexual or otherwise) than having contraceptives that optimize their sex lives (Higgins & Hirsch, 2008). In many settings, young women may be motivated to sexually engage with older men in exchange for goods, income, and/or school fees, and these motivations could undermine contraceptive use in general, let alone pleasurable contraceptive use (Cockcroft et al., 2010). Two studies of withdrawal use in Turkey suggest that education, employment, and social class—that is, one’s place in the social milieu—will affect not only sexuality but also preferred contraceptive methods. More educated, liberal Turkish women tend to use withdrawal less frequently than women from lower socioeconomic strata (Cindoglu, Sirkeci, & Sirkeci, 2008), a finding that led authors of a similar study to conclude that “women with better social and physical resources prefer withdrawal less” (Sirkeci & Cindoglu, 2012, p. 614). In sum, women’s contraceptive choices and practices are not haphazard, nor do they reflect the users’ contraceptive knowledge and access alone. Rather, they are shaped by a variety of structural, cultural, and gendered variables and processes, many of which affect sexual experiences and practices.
Relationship Factors: Dyadic Influences and Partner Preferences
Even though contraceptives are used within sexual and relational interactions, contraceptive acceptability research largely has been remiss in assessing partnership factors. In some ways, this omission has been purposeful: Research on women-controlled technologies has deliberately tried to provide women with contraceptives they could use independently of their partners’ knowledge or participation (Mathenjwa & Maharaj, 2012; Naidu, 2013). In other ways, this omission has come with a cost, particularly in terms of fully understanding the sexual aspects of contraceptives. One scholar argued that inattention to partner influences, particularly gendered power dynamics, could help explain why the theory of planned behavior has been good at predicting intentions to use contraceptives but less adequate in predicting their actual use (McCave, 2010). And many women want to choose and use contraceptive methods in consultation and/or collaboration with their partners. As Woodsong and Alleman (2008) have argued, “Although woman-initiated use is an important goal, […] the need for men’s cooperation or agreement must be addressed” (p. 171). Unfortunately, we do not have adequate space to wholly review the literature on associations between intimate relationships, partner factors, and contraceptive use. However, we wish to highlight at least a few relationship-level factors that connect directly to contraceptive practices and contraception’s sexual acceptability. In the conceptual figure (Figure 1), these relationship-level factors are nested within the larger macro-level factors and work interactively with women’s individual sexual experiences and preferences.
Motivations for Sexual Activity: Love and Intimacy
One’s motivations for engaging in sexual activity, particularly those related to love and intimacy, can strongly affect contraceptive behaviors, especially nonuse (Bjelica, 2008). In a qualitative study with African American women, participants listed a variety of reasons people might engage in sex (e.g., love/feelings, for fun, curiosity, pressured, for money, for material things), and they linked lack of condom use most strongly with love and intimacy motivations (Deardorff, Suleiman, et al., 2013). In a U.S. focus group study with adult women, participants gave 146 reasons for having unprotected sex (Nettleman, Brewer, & Ayoola, 2007). In a follow-up survey study, the investigators found that 56% of family planning client respondents were having unprotected intercourse, and “being in a long term or strong relationship” was the second most common reason for nonuse of contraception (the first was “lack of thought/preparation”) (Nettleman, Brewer, & Ayoola, 2009). In a study of Norwegian young adults, “being in love” helped explain reasons for engaging in unprotected intercourse (Træen & Gravningen, 2011). A U.S. daily diary study of young adult women showed that feelings of love and sexual interest can work interchangeably to lower odds of any contraceptive use on any given day (Tanner, Hensel, & Fortenberry, 2010). Taken cumulatively, these studies suggest a common phenomenon in which contraceptive use can be undermined by sexual motivations of expressing and experiencing love and intimacy with a partner. For these reasons, methods that can be used outside of the sexual moment may be a more sexually acceptable choice to some women.
Sexual Communication
It’s easier for most people to actually engage in sexual activity than to talk about it (Norman, 2013). However, a couple’s (in)ability to communicate, sexually and otherwise, can affect contraceptive behaviors. For example, a U.S. study of African American adults found that many couples lacked communication skills and were thus more likely to nonverbally communicate about methods such as condoms (Bowleg, Valera, Teti, & Tschann, 2010). Young adult Latinos in one U.S. study reported communicating about pleasure and satisfaction (often nonverbally) before talking about contraception (including condoms)—and that all these conversations could be difficult (Alvarez & Villarruel, 2013). Not surprisingly, couples with stronger verbal sexual communication skills have been found to use condoms more often (Norman, 2013). In sum, contraceptive researchers and practitioners should stay mindful of the actual contexts in which couples use and communicate about pregnancy prevention, remembering that many people may need to build sexual communication skills (DiClemente, Wingood, Rose, Sales, & Crosby, 2010).
Relationship Type, Stage, and Dynamics
In their analysis of the 2006 National Couples Survey, Grady, Klepinger, Billy, and Cubbins (2010) found that men’s and women’s contraceptive method preferences are both significantly related to the couples’ method choice. They found no gender difference in the magnitude of these relationships, although women in married and cohabiting relationships appeared to have greater power over method choice than women in dating relationships. In other words, relationship type may moderate the ways in which relationship influences and gendered power dynamics affect contraceptive use—a finding upheld in a study of adolescent Finnish women who were more likely to be active agents in both sexual activity and their own contraceptive use in the context of longer-term rather than more casual relationships (Kuortti & Lindfors, 2014). Relationship length is also closely related to sexuality and contraception, with studies across the world documenting more common condom use at the beginning of a partnership, then waning as trust grows and the desire for more intimate sex waxes (Braun, 2013; Osei, Harding Mayhew, Biekro, & Collumbien, 2014; Tsuyuki, 2014). Regardless of relationship type or stage, relationship dynamics can play a role in contraceptive practices, with both individual and couple preferences sustaining an important influence. A U.S. longitudinal study of young adult couples showed that less “dominant” individuals were more likely to cater to their partners’ preferences, and the intentions of those with more relationship power (often, but not always, men) were more likely to exert a direct influence on condom (non)use (VanderDrift, Agnew, Harvey, & Warren, 2013).
Concern for Partners’ Pleasure and Functioning
Closely related to the latter theme, many women may make contraceptive decisions to try to maximize their partners’ pleasure, if not their own (Higgins & Hirsch, 2008). While both women and men have reported how condoms, withdrawal, and/or vasectomy can decrease men’s pleasure (Bunce et al., 2007; Marchi et al., 2008; Rahnama et al., 2010), few studies document men’s reports of how contraceptive methods may potentially diminish or improve women’s pleasure. Moreover, to help protect men’s sexual self-esteem, women may not suggest condoms if they worry their partner will not be able to sustain an erection (Ekstrand, Tydén, & Larsson, 2011). In a U.S. qualitative study of the sexual acceptability of IUDs, when asked how they thought IUDs might affect sexuality, young women overwhelmingly first mentioned the potential for the string to poke the penis; that is, of all the possible sexual aspects of IUDs, the one that was most top-of-mind was the one that could affect their partners, not themselves (Higgins, Ryder, Skarda, Koepsel, & Bennett, 2015). This theme is in keeping with feminist sexuality research, which has described how women are socialized to be more partner oriented in sex, as well as how women are less culturally encouraged to develop their own sexual subjectivity or perceived right to sexual pleasure. Thus, although researchers could do far more to document and address contraceptives’ sexual acceptability, larger cultural shifts are also needed to increase women’s sexual selfhood and empowerment.
Individual Factors
Women have individual sexual experiences, profiles, and preferences that will directly affect their experiences with contraceptives. In Figure 1, these factors work interactively with partner and relationship factors, all of which are nested within the broader cultural and structural context.
Sexual Functioning, Especially Libido
Per Part 2 of the review, a substantial amount of research on the sexual acceptability of contraception perhaps understandably has focused on physiologic sexual functioning, which can, to some extent, be measured in a more straightforward and seemingly objective way than other aspects of sexuality. Most sexual functioning measures assess arousal, genital response (including vaginal lubrication), libido/desire/interest in sexual activity, orgasm, and satisfaction, as well as sexual dysfunction, including pain. 4
Measures such as the FSFI (Rosen et al., 2000) assess such functioning domains and have been employed by a number of contraceptive researchers. Extensively used and validated (though not without significant critique; Forbes, Baillie, & Schniering, 2014; Meyer-Bahlburg & Dolezal, 2007), measures such as the FSFI were originally developed to help identify sexual dysfunction in midlife women experiencing sexual or other health problems. The field would benefit from sexual function measures developed specifically for contraceptive-seeking women, who tend to be younger and healthier than midlife populations. Another inherent limitation of some of the sexual functioning studies reviewed in this article is their cross-sectional nature, which disallows investigations of sexual changes over time in concert with contraception use. Furthermore, many clinical studies in particular lack the sample sizes to account for variation in age or weight (Esposito et al., 2007), relationship type and length (Witting, Santtila, Alanko, et al., 2008), stress, depression, fatigue (Cyranowski et al., 2004), or even the presence of young children in the household (Fataneh, Marjan, Nasrin, & Taraneh, 2013; Smith, Jozkowski, & Sanders, 2014)—all of which are associated with sexual functioning and satisfaction. Finally, readers will note that no ideal control group exists for this type of research. For example, women using no method, sterilized women, and/or women using natural family planning methods are likely to differ meaningfully from women using (other) reversible methods. We thus consider these studies in light of these limitations.
We happily leave in-depth endocrinologic examinations of women’s sexual functioning to others (for example, see Bancroft & Graham, 2011), and we refer the readers to reviews and/or concept papers about the potential effects of hormonal contraceptives on sexuality (Burrows et al., 2012; Carey, 2006; Schaffir, 2006; Stuckey, 2008; Welling, 2013). However, it is important to note that the standard—albeit controversial—explanation for how hormonal methods influence sexual functioning primarily involves the role of androgens. Given that combined hormonal methods inhibit luteinizing hormone (LH) and prevent ovulation, they also decrease ovarian production of testosterone. 5 Contraceptive-related increases in estrogen levels raise the amount of sex hormone binding globulin (SHBG), which also leads to declines in levels of free testosterone (Devineni et al., 2007). Some researchers have thus concluded that lower levels of free testosterone contribute to lowered libido in women, particularly given that non-contraception-focused trials suggest that testosterone may improve libido significantly more than placebo (Braunstein, 2006). As a result, some researchers have been studying the effects of “androgen-restored contraception,” which supplements combined oral contraceptives with testosterone (Zimmerman et al., 2015).
However, research is extremely mixed on the exact nature of the relationship between serum testosterone levels, sexual functioning, and women’s sexual well-being. While research has established that combined oral contraceptives lead to reductions in plasma testosterone levels, other studies suggest that these lowered levels may not actually affect women’s sexual outcomes (Bancroft & Graham, 2011; Graham, Bancroft, Doll, Greco, & Tanner, 2007). As Bancroft and Graham (2011) have argued, a majority of women may “experience substantial reductions in their testosterone levels with no adverse effects on their sexual desire” (p. 723).
Along these lines, significant debate surrounds these pathways, including which hormones (estrogen, progesterone, testosterone, and/or oxytocin) (Salonia et al., 2005) exert the greatest influence on sexual functioning and how (Stuckey, 2008), or even if, hormonal changes are really that important relative to women’s subjective experiences of their sexuality. And though testosterone decreases are perhaps the most popular explanation for how hormonal contraceptives affect women’s sexual functioning, testosterone research itself may be marred by possible confounders that bias the interpretation of findings (van Anders, Goldey, & Bell, 2014). No single androgen level is predictive of “low” female sexual function (Davis, Davison, Donath, & Bell, 2005), and women vary in the degree to which they are sensitive to these hormonal changes (Warnock, Clayton, Croft, Segraves, & Biggs, 2006) or the degree to which lowered testosterone has any clear effect on sexual interest (Graham et al., 2007). Some argue that while hormones and neurotransmitters (e.g., dopamine, serotonin) do influence women’s receptivity to sexual stimuli, they do not directly influence sexual responses (Zimmerman et al., 2015). In other words, hormonal levels may influence the body’s responsiveness to sexual stimuli, but they do not directly cause sexual response. Along these lines, the context of sexual activity matters just as much as, if not more than, the relative amount of hormones present in the body. In addition, emerging scientific inquiry is beginning to investigate the role of genetics in sexual well-being (Bishop, Ellingrod, Akroush, & Moline, 2009), particularly in relation to androgen sensitivity and sexual desire (Elaut et al., 2012). Our understanding of how our genetics may predispose susceptibility to sexual dysfunction and how hormonal contraception may confound these effects is in its infancy; however, existing research shows exciting promise in this regard. In sum, we urge readers to use caution in assuming that androgens always contribute directly to increased sexual desire in women—and/or that hormonal contraceptive methods thus result in universal changes to sexual libido.
Libido, Desire, and Sexual Interest
Despite the mixed research on hormonal pathways mentioned, not a single contraceptive method exists that women have not associated, at least on some level, with detrimental effects on sexual desire, sex drive, or libido—after all, per Severy and Newcomer’s (2005) concept of acceptability, most people would choose not to use contraception if they could. Since the 1970s, research has provided evidence of the link between oral contraceptive (OC) use and diminished libido experienced by a small but notable minority of users (Herzberg, Draper, Johnson, & Nicol, 1971). Research reports a range of 5% to 48% of women using OCs exhibiting a decrease in sexual interest or desire (Burrows et al., 2012; Graham, Ramos, Bancroft, Maglaya, & Farley, 1995; Herzberg et al., 1971; Wallwiener, Wallwiener, Seeger, Mueck, Zipfel, et al., 2010). Researchers have most strongly associated reductions in desire with hormonal methods, with their decrease in testosterone level cited as a plausible (but controversial) biological mechanism of action (Zimmerman et al., 2014). However, women’s experiences with sexual interest in relation to their contraceptive use are variable. Reviews of OCs and libido published in 2004 and 2012 have reported both potential for increased or decreased libido with pill use (Burrows et al., 2012; Davis & Castano, 2004). A U.S. prospective study followed 100 new OC users for one year; among the 47% who discontinued the pill over the course of the study, decreased sexual thoughts and decreased psychosexual arousability were the strongest predictors of discontinuation (Sanders, Graham, Bass, & Bancroft, 2001). In one study, the levonorgestrel IUD was associated with significant improvements in sexual desire after one year of use compared to baseline (Bastianelli, Farris, & Benagiano, 2011), yet another found the copper IUD superior to the hormonal IUD when assessing improvement in sexual interest prospectively (Enzlin et al., 2012). For women using the injectable contraceptive Depo-Provera®, reduced libido is one of the most consistently reported and unacceptable side effects associated with its use (Gubrium, 2011; Wanyonyi, Stones, & Sequeira, 2011).
We should remember that many influences on libido do not involve hormone levels. For example, per this article’s section on dis/inhibition, women who feel strongly protected against pregnancy may be better able to “let go” and enjoy sex, which could help explain libido increases in some OC users. Perhaps due to these individual differences, other studies show no or mixed effects between OCs and libido. A review of diary studies did not find associations between hormonal contraceptives and young women’s sexual interest (Fortenberry & Hensel, 2011). The authors also pointed out that, due to the importance of sociocultural influences, levels of sex hormones have been linked only inconsistently with women’s sexual behavior (Fortenberry & Hensel, 2011).
Sexual Thoughts
We know that women’s sexual thoughts and behaviors can change over the menstrual cycle, especially around ovulation, and for women both in and out of relationships (Brown, Calibuso, & Roedl, 2011). (Notably, sexual thoughts and fantasies can lead to increases in testosterone in men and women (Goldey, Avery, & van Anders, 2014); so it’s not that hormonal levels directly determine sexual thoughts but rather that a feedback loop can occur between ovulation, thoughts, and testosterone levels.) Hormonal contraception has been shown to decrease the number of sexual thoughts in some women (Davison, Bell, LaChina, Holden, & Davis, 2008). Sexual thoughts can change testosterone, but hormonal contraception use may change the testosterone response to sexual thoughts (Goldey & van Anders, 2011). Goldey and van Anders (2011) concluded that although the precise mechanism of action is still unclear, hormonal contraception is a “variable of interest” in influencing hormonal and sexual responses.
Pain and Dysfunction
Any discussion of sexual functioning would be remiss without acknowledging sexual pain and dysfunction (Smith et al., 2014). One study found that, compared to women using nonhormonal methods, hormonal contraceptive users experienced significantly higher proportions of sex acts in the past four weeks that were categorized as painful or uncomfortable (Smith et al., 2014). At least two clinical studies demonstrated more frequent pain reported among pill than ring users (Battaglia et al., 2014), or compared to baseline measures (Battaglia et al., 2012), though a number of other studies showed improvement in genital pain with pill (Caruso et al., 2011; Caruso et al., 2013; Di Carlo, Gargano, et al., 2014), implant (Di Carlo, Sansone, et al., 2014; Visconti et al., 2012), or IUD use (Bastianelli et al., 2011; Gorgen, Api, Akça, & Cetin, 2009). Other research suggests hormonal contraceptives may dull both pleasure and pain postorgasm (Paterson, Amsel, & Binik, 2013). Some women also describe experiencing vaginal pain with male condom use, most likely due to insufficient lubrication (Fennell, 2014; Sunmola, 2005). Although we take a positive approach to sexuality in this review and argue that contraceptives should, ideally, promote sexual pleasure and well-being, we also acknowledge that pain and discomfort are common sexual experiences for women and that contraceptives can both reduce or exacerbate such discomfort.
Sexual Identity, Sexual Minority Status, and Contraceptive Use
Research often oversimplifies relationships between sexual identity, sexual orientation, and sexual behavior; contraceptive researchers may also assume that most if not all contraceptive-using women identify as heterosexual. However, emerging research explores how women’s sexual identity, attraction, and lived sexual experiences relate to their contraceptive use (or nonuse) patterns. For example, Saewyc, Bearinger, Blum, and Resnick (1999) illustrated that self-identified bisexual and lesbian adolescents reported similar rates of penile–vaginal intercourse as their heterosexually identified counterparts, yet they reported higher rates of physical and sexual abuse and lower rates of effective contraceptive use. Several studies document that, compared to teens with solely heterosexual behaviors, behaviorally bisexual teens demonstrate disparately high rates of unintended pregnancy (Charlton et al., 2013; Lindley & Walsemann, 2015; Saewyc, Poon, Homma, & Skay, 2008) and STI acquisition (Everett, 2013; Mojola & Everett, 2012; Xu, Sternberg, & Markowitz, 2010). In other words, many sexual minority girls and women—as well as transgender men—face situations in which they wish to prevent pregnancy in a sexually acceptable way.
However, obstacles thwart sexual minority women’s and trans men’s access to contraceptives in general, let alone in ways that promote sexual acceptability or acknowledge the complexity of their sexual lives. The stigma associated with sexual minority status and transgender status may result in such clients delaying and/or forgoing preventive reproductive health care, including contraceptive care (Charlton et al., 2011). Sexual minority women often report negative or even discriminatory interactions with their providers (Hutchinson, Thompson, & Cederbaum, 2006; Johnson, Mimiaga, & Bradford, 2008), and such patients may be reluctant to disclose their need for contraception. In addition, women who primarily have sex with women may not anticipate their need for contraception and STI protection. Efforts to address system-, provider-, and individual-level stigma surrounding sexual minority and non-gender-conforming identities are needed to enhance patient-centered contraception care for behaviorally bisexual women and transgender populations.
Sexual Preferences/Sexual Aesthetics
The most common sexual functioning measures usually involve objective cutoff points to indicate levels of sexual well-being. However, individuals have subjective preferences regarding sexuality (for example, the levels to which individuals find pain sexually pleasurable or distressing, or one’s degree of comfort with inserting the contraceptive ring into the vagina) that also have implications for contraception’s sexual acceptability. Ideally, contraceptive clients would be able to match methods with their own preferences and aesthetics.
Wetness and Vaginal Effects
One such individual preference pertains to vaginal wetness or lubrication. Women’s ideal amount of vaginal lubrication during sex varies at the individual level and by partner preferences. Broader cultural norms and local vaginal practices will also likely affect the sexual acceptability of any prophylactic substance or product placed in or affecting the vagina (Scorgie et al., 2011; Veldhuijzen et al., 2006). Though most U.S. women report that both they and their male partners prefer sex to feel more wet (Jozkowski et al., 2013), many South African women and their partners desire comparatively drier sex, and 18% of South African women in one study declared that increased vaginal wetness associated with the progestin-only injectable was the most distressing side effect of this method (Smit, McFadyen, Zuma, & Preston-Whyte, 2002). Research on dual-prevention microbicides has been especially likely to underscore how changes to wetness and lubrication can affect women’s pleasure, usually positively (Jones et al., 2009; Martin et al., 2010; Tanner et al., 2009; Woodsong & Alleman, 2008; Zubowicz et al., 2006). Both male and female study participants have indicated preferences for a microbicide that lubricates (Veldhuijzen et al., 2006) but not too much (Martin et al., 2010; Short, Succop, Rupp, & Rosenthal, 2008; Zubowicz et al., 2006).
Few studies have inquired about sexual pleasure in relation to vaginal wetness and contraception use, though Smith et al. (2014) found that using hormonal contraception is related to increased reports of feeling “[my] vagina was drier than I would have liked” compared to women using nonhormonal methods. Moreover, the condom section in Part 2 indicates that some women experience a vaginal drying out with the use of male condoms and may need encouragement to use external lubricants with these methods. The vaginal ring has been associated with fewer issues of vaginal dryness compared to the pill (Roumen, 2008). In fact, using the ring is linked to improvements in prospective measures assessing satisfaction with vaginal lubrication during sexual activity (Caruso et al., 2014). Though the issue of vaginal dryness is relatively easily overcome with the use of exogenous lubricants, women in many cultures may eschew such products if they feel a need to be “naturally” lubricated to demonstrate desire and/or youthful sexuality. Exogenous lubricants may also prove an uncomfortable topic to discuss with partners or health care providers (Akers et al., 2010). Vaginal lubrication is closely linked with genital and sexual pain, so acknowledging its importance in relation to contraceptive sexual acceptability and facilitating more open dialogue between women, their partners, and their health care providers may grease the wheels, so to speak, for addressing this important concern.
Aesthetics
How methods smell, taste, feel, and otherwise appeal to the senses can greatly affect their sexual acceptability, particularly for methods used at the time of sexual activity (Severy & Newcomer, 2005). For example, women have described a number of sensory detractions of male condoms (Fennell, 2014; Higgins & Hirsch, 2008), including irritation (Tafuri, Martinelli, Germinario, & Prato, 2010), smell, or the way they make fingers or penises taste. One of the most frequently cited barriers to using the female condom is its large size, an intimidating factor for some, though likely resolvable with practice and use (Latka, Kapadia, & Fortin, 2008). A number of microbicide studies have wisely gathered users’ ratings on color, smell, consistency, and feeling in the vagina, as well as their preferences for a gel over other formulations (e.g., a vaginal moisturizer) (Zubowicz et al., 2006). A growing body of research on male condom fit and feel suggests that the aesthetics of condoms’ textures, sizes, and shapes may affect sexual acceptability for both women and men (Crosby et al., 2013). In attending to such sexual aesthetics, both private and public organizations have attempted to provide thinner, more “sensitive” male condoms in a variety of sizes, including the NYC Condom brand promoted by that city’s health department (Burke et al., 2011).
Sexual Timing of Method Used
Women vary in their preferences about whether ideal contraceptive methods are used during the time of sexual activity. One U.S. study of 574 women seeking abortions asked participants to select their contraceptive preferences from a list. In terms of those selecting “extremely important,” about twice as many women wanted a contraceptive not used at the time of sex (65%) as women who wanted to use a method at the time of sex (35%) (Lessard et al., 2012). Infrequent sexual activity could help explain some people’s preferences for these latter coital-dependent methods. Some participants in microbicide acceptability studies have expressed preferences for a product that could be used after sexual activity (Tanner et al., 2008), while others would like a product that could be used before (van der Straten et al., 2012). In a study of the diaphragm as a dual-prevention technology in Zimbabwe and South Africa, the ability to insert it before sexual activity increased its acceptability among both women and men (Sahin-Hodoglugil et al., 2011).
Spontaneity
Closely related to sexual timing of using a method, several studies suggest many people prefer fluid, free-flowing sexual encounters that, ideally, are uninterrupted by the application of a contraceptive method (Shiely & Saifuddin, 2014). Participants in a number of studies, including both women and men, dislike that condoms can interrupt sexual spontaneity and the heat of the moment (Braun, 2013; Ekstrand et al., 2011; Fennell, 2014; Higgins & Hirsch, 2008). (Microbicides that can be applied before sexual activity may be preferable to condoms in this regard; see Montgomery et al., 2010).) Studies of withdrawal use also suggest that coitus interruptus can undermine both men’s and women’s sexual experience by curtailing the rhythm of intercourse (Rahnama et al., 2010). Comparatively, hormonal methods and IUDs offer far less interference with sexual spontaneity (Higgins et al., 2015), though of course they offer no STI protection.
Dis/Inhibition
Qualitative research in the United States has explored how taking procreative risks may be sexual turn-ons (Higgins, Hirsch, & Trussell, 2008), while others cannot fully enjoy sex unless properly protected against pregnancy (Higgins & Hirsch, 2008). Women in one qualitative study of IUD and implant use in the United States described “sexual security,” or enhanced sexual disinhibition due to the unparalleled efficacy of these methods (Higgins et al., 2015). Another study of Austrian couples, interviewed both before and again six months after vasectomy, revealed that women (but not men) demonstrated improved sexual functioning, suggesting the psychosexual benefits to women of no longer having to worry about—or take responsibility for—pregnancy prevention (Al-Ali et al., 2014).
Pleasure and Pleasure Seeking
Though pleasure is clearly connected to the sections previously discussed, some studies of this topic have focused on a more general, diffuse definition of pleasure (Fennell, 2014) and/or have used qualitative methods to document the contraceptive aspects of pleasure in users’ own words (Fennell, 2014; Higgins & Hirsch, 2008). In one U.S. Internet study of 382 women, the most frequently desired contraceptive feature was “does not interfere with the pleasure of sex” (87%) (Gomez & Clark, 2014), although this criterion did not specify whose pleasure might be interfered with—women’s, men’s, or both. This feature was even more commonly desired than the criterion about efficacy: “is 99% effective without user having to do anything” (74%). In a similar study of preferred contraceptive features, 64% of women reported a contraceptive that “does not reduce woman’s sexual enjoyment” was extremely important, compared to 84% reporting a contraceptive should be “very effective” (Lessard et al., 2012). Dual-prevention research has also emphasized the importance of women’s pleasure in their potential uptake of new technologies (Higgins & Hirsch, 2007). Microbicides have been shown to increase some women’s pleasure through vaginal wetness and lubrication (Jones et al., 2009; Martin et al., 2010; Tanner et al., 2009; Woodsong & Alleman, 2008; Zubowicz et al., 2006), and female condoms have been documented to enhance women’s pleasure through both heat transfer and its outer ring, which can stimulate the clitoris (Mathenjwa & Maharaj, 2012; Sobze Sanou et al., 2013). Sex workers have also described the female condom as being more pleasurable (to themselves, not their partners) than male condoms (Mathenjwa & Maharaj, 2012).
Male condoms are the most commonly maligned method in terms of pleasure reduction. Most of this research has focused on men’s pleasure, but a growing body of work documents how condoms can reduce women’s pleasure too (Crosby et al., 2013; Fennell, 2014; Higgins & Fennell, 2013; Higgins & Wang, 2015a; Randolph, Pinkerton, Bogart, Cecil, & Abramson, 2007). At least one scholar has documented and criticized the anti-condom discourse so common in the general vernacular (Braun, 2013). Research does suggest that these anti-condom pleasure narratives, while socially influenced, can be powerful in shaping contraceptive choice and behavior: Many people believe that condoms reduce sexual pleasure, and those who do are less likely to use them (Higgins, Tanner, & Janssen, 2009; Randolph et al., 2007). One recent nationally representative study in the United States found not only that pleasure attitudes were more strongly associated with young adults’ condom nonuse than any other sociodemographic or sexual history factors but also that the associations were virtually identical for both women and men (Higgins & Wang, 2015a). Those with negative pleasure attitudes about condoms may also be more likely to use withdrawal instead (Higgins & Wang, 2015b).
Wanting more pleasurable or “natural” sexual experience is also a major reason many women provide for having unprotected intercourse (Biggs, Karasek, & Foster, 2012; Foster, Higgins, Karasek, Ma, & Grossman, 2012; Træen & Gravningen, 2011). “Seeking pleasure” was a common justification provided for unprotected intercourse among Norwegian young adults (Træen & Gravningen, 2011). In other words, many people may believe that using nothing is more sexually enjoyable than using contraception. Most couples would rather not have to use a birth control method during sex (Severy & Newcomer, 2005), a phenomenon that has led Severy and Silver (1993) to suggest that any selected contraceptive method is “the least bad alternative” (p. 225).
Sexual Repercussions of Seemingly Nonsexual Side Effects
Women’s concerns about side effects loom large in their experiences of and preferences for contraceptive methods (Frost, Singh, & Finer, 2007a, 2007b; Lessard et al., 2012; O’Sullivan, Cooper-Serber, Kubeka, & Harrison, 2007; Osei et al., 2014), whether or not these perceptions of side effects are misperceptions (Küçük, Aksu, & Sezer, 2012). “Lack of side effects” was one of the top contraceptive features preferred by women at high risk for unintended pregnancy (Lessard et al., 2012). Gynecologists also perceive side effects as important reasons behind women’s discontinuation and method switching (Bombas et al., 2012). Side effect concerns have been associated with unprotected intercourse (Ayoola, Nettleman, & Brewer, 2007; Nettleman et al., 2009), women’s use of withdrawal (Ong, Temple-Smith, Wong, McNamee, & Fairley, 2013), and women’s interest in trying new technologies, such as a vaginal microbicide surrogate (Tanner et al., 2009). Concerns about sexual side effects from hormonal methods (including bleeding and low libido) were a reason cited for vasectomy in Tanzania (Bunce et al., 2007). Here, we consider the sexual aspects of a few common contraceptive side effects.
Bleeding
We know that bleeding and sexual activity can be related, as many women avoid coital activity during menstruation. Though more research in this area is needed, one daily diary study of young women in the United States found an almost complete cessation of vaginal intercourse during menses (Fortenberry & Hensel, 2011). An older study from Chile found that 70% of women and 72% of men avoided sexual activity during menstruation, and 54% of women and 60% of men did so during vaginal spotting (Barnnart, Furman, & Devoto, 1995). Thus, contraceptive-related changes in menstrual bleeding (amount of blood, days of bleeding, unscheduled bleeding) could all affect the sexual acceptability of certain contraceptive methods.
Many women appreciate contraceptive-induced menstrual elimination or reduction. A Canadian web-based assessment of contraceptive preferences from several thousand respondents found that 61% of adolescents and 52% of adults said they would prefer to avoid menses if possible (Nguyen & Jamieson, 2011). Some clinicians recommend continuous use of oral contraceptives (that is, skipping the seven placeholder pills at the end of each 28-day pack) to eliminate menstrual bleeding and reduce menstrual-related symptoms (Archer, 2006), a regimen that could have implications for the method’s sexual acceptability. Conversely, in a study in Finland, a regular menstrual cycle was cited by women as one of the top benefits of oral contraceptives (though mood swings, lowered libido, and weight gain were cited as the biggest drawbacks, all of which could directly or indirectly affect sexual acceptability) (Tiihonen, Leppänen, Heikkinen, & Ahonen, 2008). In a global survey of attitudes toward monthly bleeding among 4,039 women in eight countries, 67% of all contraceptive users said menstrual bleeding “severely or very severely interferes with my sexual life” (Szarewski, Von Stenglin, & Rybowski, 2012). Women using nonhormonal methods in this study were significantly more likely than hormonal users to agree strongly that bleeding had a negative impact on their lives. Research also suggests that methods such as the copper IUD can increase bleeding and cramping in some users, particularly compared to the levonorgestrel IUD (Andersson, Odlind, & Rybo, 1994). In one qualitative study of potential IUD and implant users, participants noted possible detractions to one’s sex life through increased bleeding and cramping (Higgins et al., 2015). While research rarely considers the sexual repercussions of such bleeding patterns, they seem likely to affect these methods’ sexual acceptability.
However, the relationship between contraception, bleeding, and sexual well-being is complex, and both cultural factors and individual preferences are likely to play a role in this relationship. Some women may feel such suppression or elimination of menstrual cycles challenges their notions of “natural,” healthy bodies (Granzow, 2014). A study of women in Brazil found that while many women disliked menstrual bleeding, some preferred monthly bleeds to establish them as healthy and nonpregnant (Makuch, Osis, Petta, dePádua, & Bahamondes, 2011). Similarly, a study in the Dominican Republic found that anticipated menstrual changes (from irregular bleeding to amenorrhea) were a major obstacle to women’s uptake of hormonal family planning methods (McKinney et al., 2013). Some women are partial to menstrual bleeding as an indicator of not being pregnant, while others have negative associations with menstruation (Makuch et al., 2011) and want to use contraception as a way to control and manage bleeding (Nguyen & Jamieson, 2011; Tiihonen et al., 2008). Counseling women on the common bleeding profiles associated with particular methods and aligning women’s bleeding preferences with a contraceptive method may result in greater satisfaction and sustained method use.
Though the literature located for this review less frequently documented the sexual aspects of cramping, breast tenderness, or weight changes, these side effects will undoubtedly shape sexual acceptability and contraceptive continuation in some women—regardless of whether these side effects are “real” or perceived. In terms of mood changes, Wiebe, Kaczorowski, and Mackay (2012) conducted a survey of practicing physicians in Canada, who reported that many of their patients described mood and sexual side effects of hormonal contraception. In fact, physicians cited these mood and sexual changes as the main reasons their patients discontinued OC use.
Pregnancy Intentions and Pregnancy Ambivalence
A small but growing body of work links pregnancy intentions to both sexuality and contraceptive behavior. Per the dis/inhibition theme, women with clear desires to avoid pregnancy are likely to benefit sexually from effective methods that help them feel strongly secure about preventing conception. However, many women are unclear or ambivalent about their pregnancy intentions, and some may feel happy about an unintended pregnancy, even if they were not trying to have a child (Aiken & Potter, 2013). Perhaps more directly, other women (and men) have described the pleasures involved in conceiving a baby with one’s partner, even if a child is not truly planned (Higgins, Hirsch, et al., 2008). For some, the “risk” of pregnancy—that procreative potential—can serve as a turn-on in the sexual moment; for others, passively romanticizing about a pregnancy with a partner can undermine consistent or effective contraceptive use (Higgins, Hirsch, et al., 2008).
Along those lines, a surprising proportion of women may be willing to dispense with contraception entirely, especially if they feel ambivalent about pregnancy (Foster, Higgins, Biggs, et al., 2012). In one U.S. study of contraceptive clients, upward of 50% reported willingness to have unprotected sex in the future, and wanting a child sometime in the next three years was strongly associated with such willingness (Foster, Higgins, Biggs, et al., 2012). Such research reminds us that women have multiple goals and “projects” in their sexual lives (e.g., testing for or playing with fertility), some of which may undermine effective contraceptive use.
PART 2. A METHOD-BY-METHOD REVIEW OF RESEARCH ON SEXUAL ACCEPTABILITY
In this part, we review the literature on individual contraceptive methods, drawing whenever possible from the model of sexual acceptability. We identified a total of 103 peer-reviewed journal articles that assessed sexual outcomes associated with contraceptive use. Table 1 summarizes the positive and negative sexual aspects of contraception that have been documented by peer-reviewed research in the past decade. For a more detailed table with studies’ sample characteristics, measures, methods, and relevant findings as they pertain to our model of sexual acceptability, readers may refer to the online appendix. Given both space restrictions and the review’s goals, we do not provide a methodological critique of each study. Instead, we draw on cumulative findings to inform our understanding of sexual acceptability and identify areas where more attention is needed to enhance this model.
Here, we briefly present tallies from the articles contained in Table 1. The 103 references included seven reviews, for a total denominator of 96 empirical studies. Of these latter 96 studies, 61% (n = 59) were cross-sectional, 29% (28) were prospective, 18% (17) were randomized controlled trials, 28% (27) were qualitative, and 44% (42) had sample sizes of more than 200 participants. The text that follows summarizes the main findings from the methods in Table 1 and suggests implications for the larger sexual acceptability of each method.
As we note in our concluding section, the large majority of the studies reviewed in Part 2 focused primarily on sexual functioning and not on other aspects of the model. Very few approached sexual acceptability using the kind of broader ecological approach we proposed in Part 1. However, even in the absence of findings from the literature, we do comment on other aspects of the model that are likely to play a role with particular methods—for example, partner and couple dynamics, sexual aspects of side effects such as bleeding and cramping, and women’s perceptions.
Male Condoms
Though male condoms help prevent STIs, they also play an enormous role in pregnancy prevention. Population-level research indicates that at least one in three sex acts among 15- to 44-year-old adults in the United States involves the use of male condoms (Higgins, Smith, et al., 2014). Prior studies have examined the sexual acceptability of male condoms for men, with findings that condoms can reduce men’s pleasure and sensation, and that men who report these condom-related sexual detractions are less likely to use them than men who do not report them as strongly (Crosby, Milhausen, Yarber, Sanders, & Graham, 2008; Hensel, Stupiansky, Herbenick, Dodge, & Reece, 2012). A novel vein of research has also documented men’s experiences of sexual condom-use problems, including condom-associated erection loss (Crosby, Sanders, Yarber, & Graham, 2003; Sanders, Hill, Crosby, & Janssen, 2014; Sanders et al., 2012).
Research has been slower to document women’s sexual experiences with condoms (Higgins & Fennell, 2013). However, the winds may be shifting: Our review identified 21 articles from the past 10 years that deal in some way with women’s sexual experiences of condoms. Taken cumulatively, these studies confirm prior findings that some women perceive condoms as a barrier to intimacy and trust, which can lead to a waning of condom use as a relationship ensues (Bolton, McKay, & Schneider, 2010; Sanders et al., 2010; Versteeg & Murray, 2008; Wang, 2013), and that sexual motivations pertaining to love (versus, say, pleasure or fun) can undercut the likelihood of condom use (Gebhardt, Kuyper, & Dusseldorp, 2006). However, the reviewed studies also convincingly demonstrate that women, too, can experience reductions of both pleasure and sensation with condom use (Crosby et al., 2013; Crosby et al., 2008). For example, especially when used in the absence of exogenous lubricants, condoms may reduce vaginal lubrication, which can cause some women discomfort or pain (Fennell, 2014; Higgins & Hirsch, 2008). In fact, these studies exhibit far more gender similarities than differences, with reporting of condom “turn-offs” similar for women and men (Crosby et al., 2013; Crosby et al., 2008). (Exceptions from one study include the following: Men were more likely than women to report “fit and feel” issues with male condoms (Crosby et al., 2013); women were more likely than men to report that their partner experienced sexual discomfort due to condoms (Crosby et al., 2013); and women were more likely than men to report pain associated with condom use (Fennell, 2014).) Most notably, although men were proportionally more likely than women in these studies to say that condoms diminish their physical pleasure (Kaneko, 2007; Randolph et al., 2007; Træen & Gravningen, 2011; Widdice, Cornell, Liang, & Halpern-Felsher, 2006), at least three studies showed that the associations between such pleasure attitudes/reductions and actual condom practices may be just as strong, if not stronger, for women (Fennell, 2014; Higgins et al., 2009; Higgins & Wang, 2015a).
The 21 reviewed studies did not document solely negative aspects of male condoms’ (lack of) sexual acceptability. One nationally representative study from the United Sttes showed no associations between (reduced) sexual functioning and condom use at last penile–vaginal intercourse (Sanders et al., 2010). Young adults in one study associated condoms with a number of sexual benefits, such as reduced mess, longer-lasting sexual intercourse, and personal feelings of cleanliness and sexual hygiene (Træen & Gravningen, 2011). Sex workers in two studies reported that being able to apply condoms in an erotic, sexually pleasing way was particularly effective at enlisting male partners’ support for condom use (Free, Roberts, & McGuire, 2007; Garcia, Yam, & Firestone, 2006). And in one analysis of the “condom as killer” discourse among young adults, one researcher pointed out that anti-condom narratives are socially constructed, and thus can—and should—be shifted to narratives in which condoms can be better integrated into the sexual experience (Braun, 2013). Given that successful condom strategies have been associated with sexual communication skills and women’s sexual self-comfort (Deardorff, Tschann, et al., 2013), we should endeavor to increase these latter phenomena as well.
Contraceptive researchers and practitioners should start with the assumption that many women, like many men, experience reductions in pleasure and sensation when using male condoms; moreover, women may uniquely experience condom-related pain and reductions in vaginal lubrication. Future research and interventions should assess contraceptive users’ beliefs about and experiences with condom-related pleasure reduction, as failure to do so could mean overlooking one of the largest predictors of condom use/nonuse. Sexual health professionals might also wish to share ideas on how to better integrate condoms into the sexual experience with both male and female clients. Young men might struggle with condoms’ fit and feel, whereas young women might dislike texture or lubrication attributes; such clients should be encouraged to try a variety of condom types, sizes, and lubricants.
Oral Contraception Pills
Oral contraception has recently surpassed female sterilization as the most commonly used method among women in the United States (Daniels et al., 2014). OCs are often the first hormonal method used by young women for the treatment and regulation of painful periods and as they become sexually active; many women remain satisfied OC users for decades. The birth control pill recently celebrated its 50th birthday. Thanks to technological and medical innovation, the pills of today are much different from those used by its earliest adapters. Though the link between OC use and diminished libido is well documented, little clinical guidance exists to help women or their providers identify which pills may be better suited for those dealing with adverse sexual side effects. Encouragingly, preliminary findings suggest that women experiencing issues with desire or arousal may find relief when switching to a different hormonal formulation (Bednarczyk, Davis, Ault, Orenstein, & Omer, 2012; Shahnazi et al., 2015; Strufaldi et al., 2010). Specific recommendations for clinicians are needed to ensure optimal patient-centered care for women who wish to use the pill.
In the past 10 years, several specific branded pills and hormonal combinations have received attention in the literature due to their potential impact on women’s sexual experiences. Studies of Yasmin® (Battaglia et al., 2012; Battaglia et al., 2014; Drosdzol & Skrzypulec, 2008; Goldstein, Burrows, & Goldstein, 2010; Mabrouk et al., 2012), Q(K)laira® (Caruso et al., 2011; Di Carlo, Gargano, et al., 2014), and Belara® (Caruso, Rugolo, Agnello, Romano, & Cianci, 2009; Göretzlehner, Waldmann-Rex, & Schramm, 2011) make up almost one-third (n = 10) of all combined OC articles identified in the review. Yasmin, containing 30 μg ethinylestradiol (EE) and 3 mg drospirenone (DRSP), has received a particularly high level of attention (n = 5). Research consistently finds Yasmin related to increased pain during sex, decreased libido, issues with spontaneous arousal and orgasm during sex, and reduced frequency of sex (Battaglia et al., 2012; Battaglia et al., 2014; Drosdzol & Skrzypulec, 2008). However, the extent of these symptoms does not meet the level for clinical sexual dysfunction in most women (Battaglia et al., 2012).
As the fine-tuning of hormonal components continues to evolve, we see improvements in many symptoms alongside the unfortunate emergence of new issues (e.g., dyspareunia) related to OC use, particularly with low-dose combined OCs. Studies are beginning to investigate how OC induces changes in labial thickness and pulsatility index of arteries located in the clitoris and labia minora (Battaglia et al., 2012). One study found that, compared to baseline measurements, after three months of Yasmin use labia minora thickness and the vaginal introitus (canal) area had decreased significantly. The pulsatility index of the dorsal clitoral artery and the posterior labial artery significantly increased after three months of use. These biomarkers show physical and physiological changes in women’s genitals with Yasmin use that may be related to sexual function (Battaglia et al., 2012).
Our understanding of the long-term impacts related to genital pain and dyspareunia is conflicting, with some studies showing resolution of symptoms after discontinuing OC use (Avellanet, Patricia-Ortiz, Pando, & Romaguera, 2009), though others reveal long-lasting effects (Gardella et al., 2011; Goldstein et al., 2010). Goldstein et al. (2010) hypothesized that newer, low-dose combined OCs chosen for their anti-androgenic characteristics, in addition to the use of decreased hormone-free intervals, may be associated with an increased risk for experiencing vestibulodynia. Combined OCs containing estrogen may have long-lasting impacts on SHBG production even after pill discontinuation, which may help explain why genital pain disorders do not always resolve with discontinued use (Goldstein et al., 2010). Though levels of free testosterone are likely to be reduced in women using combined OCs (Bancroft & Graham, 2011), this reduction does not appear to be associated with changes in clitoral sensations as measured by heat, cold, and vibrations or with pain thresholds in the vulvar vestibule (Baeten et al., 2012). Zimmerman et al. (2015) have experimented with adding dehydroepiandrosterone (DHEA) to DRSP-containing pills in an attempt to counteract the possibility for sexual impairment that can result from reduced free and total testosterone. Though results of “androgen-restored contraception” remain inconclusive with regard to the effects of a combined OC pill containing DHEA, the potential for positive sexual function improvements in some women with androgen sensitivity and/or OC-associated sexual dysfunction shows promise and requires further investigation. More research is needed to understand if and how low androgen levels and/or individual sensitivity to androgens are related to genital sensations and sexual desire in women using hormonal contraception, particularly combined OCs (Warnock et al., 2006).
Obviously not all women using OCs experience negative sexual function outcomes (Hoga, Rodolpho, Penteado, Borges, & Alvarez, 2013; Machado, De Melo, Prota, Lopes, & Megale, 2012; Witting, Santtila, Jern, et al., 2008; Wonglikhitpanya & Taneepanichskul, 2006). A randomized study showed positive improvement in sexual pleasure, orgasm frequency, and orgasm intensity compared to no-method controls (Farrell et al., 2014). Birth control pills also relieve dysmenorrhea and lighten menstrual bleeding (Di Carlo, Gargano, et al., 2014; Wonglikhitpanya & Taneepanichskul, 2006), two potential sexual benefits for many women and couples. OCs can mitigate potentially sexual, negative aspects of health conditions such as endometriosis and polycystic ovarian syndrome (PCOS). For example, OCs have been associated with reductions in endometriosis-related pelvic pain (Guzick, Huang, Broadman, Nealon, & Hornstein, 2011) and unwanted aesthetic aspects of PCOS such as hirsutism (excessive facial and body hair) (Caruso et al., 2009) and acne (Caruso et al., 2009; Wonglikhitpanya & Taneepanichskul, 2006). Extended cycle regimens, or continuous pill use without a hormone-free week for several cycles, have also been associated with positive changes across a variety of sexual acceptability factors, from sexual functioning to aesthetics. These include improvements in sexual function and libido, reductions in dysmenorrhea, duration and volume of withdrawal bleeds, and breast tenderness, and improvements in skin problems (Caruso et al., 2011; Caruso et al., 2013; Göretzlehner et al., 2011). However, other aspects of combined OC use, such as breakthrough bleeding, breast tenderness (Mabrouk et al., 2012; Roumen, 2007), and vaginal dryness (Sabatini & Cagiano, 2006), are likely to detract from sexual acceptability and should be further explored.
Intrauterine Device/Intrauterine System
IUDs have made a major resurgence in the family planning field in recent years, especially under the banner of long-acting, reversible contraception (LARC). LARC traditionally includes both intrauterine contraception (typically abbreviated IUC, as well as IUD or IUS, for intrauterine system) and subdermal contraceptive implants. LARC’s popularity has increased in the past decade, and public health practitioners are keen to increase LARC use among young, nulliparous women in particular. Though significant research explores provider- and financial-level barriers to IUDs, fewer studies employ a patient-centered approach among potential users themselves, especially in terms of how sexuality may shape client satisfaction with their method. These studies are particularly lacking in the United States, despite the current intensity of IUD promotion efforts there. However, we hypothesize that strong overall user satisfaction with IUDs may be influenced at least in part by their facilitation of enjoyable, spontaneous sex.
A recent systematic review of women’s sexual experiences using an IUD/IUS located only 10 studies published in the past 30 years, with all research occurring outside of the United States (Sanders, Smith, & Higgins, 2014). The few studies that do exist consistently find that both hormonal and nonhormonal IUDs are associated with either no change or improvement in sexual function (Gorgen et al., 2009). After approximately six months of use, women report reductions in sexual and pelvic pain (Bastianelli et al., 2011; Gorgen et al., 2009; Witting, Santtila, Jern, et al., 2008), improvement in sexual desire (Bastianelli et al., 2011; Drosdzol & Skrzypulec, 2008; Gorgen et al., 2009; Witting, Santtila, Jern, et al., 2008), and arousal, specifically in women using the levonorgestrel (LNG) IUD (Drosdzol & Skrzypulec, 2008). Both copper and “hormonal” LNG IUDs work by creating an environment in the uterus that is hostile to sperm and impairs implantation; LNG IUDs also contain progestin to help thicken cervical fluid and suppress the endometrium, thereby reducing blood loss (Hatcher, 2011). Although LNG IUDs release only a small amount of localized hormones and contain no estrogens, hormonal-related side effects are not unheard of with this method: in one study, 35% of women using LNG IUDs reported breast tenderness, though this effect resolved within six months (Bastianelli et al., 2011). A study comparing sexual function and satisfaction among women using either the hormonal or nonhormonal IUDs found few differences between the groups (Enzlin et al., 2012). However, LNG IUD users were significantly more likely to report being “very satisfied” with their method compared to copper IUD users, perhaps due in part to reductions in menstrual bleeding. Of note, women in both IUD groups reported similar rates of self-perceived sexual satisfaction (58% to 60%), sexual activity more than twice per week (48% to 49%), desired sex more than twice per week (50% to 53%), ease in reaching physical arousal (47% to 54%), and ease in achieving orgasm (76% to 78%) (Enzlin et al., 2012).
While these studies document more objective sexual outcomes in relationship to IUD use, other studies have assessed less sexually direct and/or more sexually subjective aspects of this method. A recent qualitative study in the United States of IUDs’ sexual aspects revealed mostly positive themes, such as increased sexual disinhibition due to strong efficacy, greater allowance for sexual spontaneity compared to condoms, and psychosexual benefits of no or fewer synthetic hormones compared to other hormonal methods (Higgins et al., 2015). These sexual themes connected to users’ method satisfaction and nonusers’ openness to the method. Another study documented that the women least interested in using an IUD wanted a method that they could see, highlighting the complexity of the relationship between contraceptive features and psychosexual well-being (Gomez & Clark, 2014). Illustrative of this point, Bastianelli et al. (2011) noted that recruitment of Italian women into their study took much longer than anticipated, as very few (9% of women seeking contraception in their clinic) were willing to try the IUD. In the U.S. qualitative study mentioned earlier, nonusers’ most frequently cited sexual concern about IUDs was the potential for their partner to feel or be “poked” by the string (Higgins et al., 2015)—even though little evidence from clinical literature upholds this phenomenon. Nonetheless, counseling for sexual concerns could increase women’s willingness to try IUDs.
Bleeding is another aspect that shapes IUDs’ sexual acceptability. For women preferring little to no menstrual bleeding, an IUD may be particularly enticing, as blood flow decreases significantly in many users. However, bleeding profiles differ by IUD type. Though both hormonal and nonhormonal IUDs are associated with lighter bleeding, LNG IUD users were significantly more likely than women using a nonhormonal IUD to report shorter menses and less blood flow (Enzlin et al., 2012), and approximately one-third of hormonal IUD users experienced amenorrhea within first six months of use (Bastianelli et al., 2011). The bleeding profile associated with IUD use is likely to increase sexual acceptability of this method, though it is important to note that preferences for monthly withdrawal bleeds may influence discontinuation for some women (Bastianelli et al., 2011). On the other hand, the sexual acceptability of IUDs may be thwarted for women who experience increases in amount of blood, unscheduled bleeding, and/or cramping with the device.
Though the studies reviewed here demonstrate mostly positive effects of IUDs on women’s sexuality, this method’s sexual acceptability will vary according to individual user characteristics, including bleeding preferences, comfort with an object inside the body, and tolerance for hormonal-related side effects for women using hormone-containing devices. Women desiring “green” methods, free from hormones, may find the copper IUD particularly appealing, especially if amenorrhea is not a priority. IUDs may also be extremely desirable methods for women who have trouble “letting go” in sex unless securely protected against pregnancy. On the other hand, they may be less appealing to women who want more hands-on, day-to-day control over their contraception.
Combined Hormonal Vaginal Ring
The vaginal ring provides a powerful and unique glimpse into the sexual acceptability of contraception. Routine use calls for inserting the pliable ring into the vagina for three to four weeks and then removing for a ring-free interval every month (women can also continuous cycle with the ring). The largest studies of this method strongly suggested that women who associated the method with positive sexual attributes were more likely to continue using it over time (Merkatz et al., 2014). More specifically, studies document several pathways through which the ring may affect sexual functioning. At the basic level of sexual self-comfort, the ring requires touching one’s genitals. In one U.S. study of adolescent women, those participants most willing to try the ring expressed more comfort with their genitals (Terrell, Tanner, Hensel, Blythe, & Fortenberry, 2011). Women less apt to try the method were concerned that the ring could get lost inside or easily fall out of the vagina. Providing women with alternative strategies, such as wearing gloves, using an applicator (Terrell et al., 2011), or enlisting a partner’s assistance to insert and remove the ring, may facilitate willingness to initiate the method among those women who are less comfortable touching their own genitals.
The mechanics of the ring also affect its sexual acceptability, for both women and their partners. In one review comparing the ring to combined OCs, women reported that the ring was more likely to interfere with sex, and a significantly larger proportion of women reported that their sex partners preferred the pill (Roumen, 2008). Ring-related events, such as feeling the ring inside the vagina, interference with sex, and expulsion, were associated with higher rates of discontinuation (Roumen, 2008). Conversely, the overwhelming majority of male partners reported that they never felt the ring during sex, expressed no change in sexual sensations, and did not report that the ring disrupted sex. Satisfied ring users in one U.S. study reported no or rare detection of the ring during normal use (Huang et al., 2014).
As with any hormonal method, hormonal influences affect the ring’s sexual acceptability in both negative (mostly) and positive ways. Mild, adverse, hormone-related outcomes include bleeding, nausea, headache, breast tenderness, and vaginal discomfort (Caruso et al., 2014; Roumen, 2008). However, compared to combined OC users, ring users experienced comparatively fewer issues with vaginal dryness in one study (Roumen, 2008). Extended cycle use (63 days followed by hormone-free interval) has been associated with improvements in Italian women’s dyspareunia, sexual function, sexual distress, and quality of life (Caruso et al., 2014). Highly satisfied ring users in one U.S. study reported fewer side effects than with pills, no detection of the ring during normal use, and either no change or an increase in sexual pleasure and/or sexual frequency; these satisfied women were more likely to continue ring use (Huang et al., 2014). One study involved 130 sexually active U.S. women (ages 15 to 21) who were randomly assigned to the ring or pill for three cycles and then switched to the other method for an additional three cycles; women reported significantly higher rates of liking the ring, greater ease of use, and more inclination to recommend it to friends (Balkus et al., 2007).
Progestin-Only Transdermal Implant
As with the IUD, the transdermal implant is receiving increased attention within the family planning community as a highly effective, reversible method that is acceptable to women of all ages and parity levels. In 2014, the American Academy of Pediatrics (AAP) recommended that implants and IUDs be promoted as “first line” methods of contraception for adolescents. Sometimes called “get it and forget it” contraception, the implant requires no attention for up to three years. Although its extremely strong efficacy is likely to enhance sexual disinhibition in many users, the implant may also decrease sexual acceptability through its associated unpredictable and highly irregular bleeding profile (Aisien & Enosolease, 2010; Duvan, Gozdemir, Kaygusuz, Kamalak, & Turhan, 2010; Gezginc, Balci, Karatayli, & Colakoglu, 2007; Visconti et al., 2012). Studies suggest that 21% to 50% of implant users report heavier or prolonged bleeding within the first year of use (Duvan et al., 2010; Visconti et al., 2012). However, at least one-third (32% to 42%) of users experience amenorrhea (Duvan et al., 2010; Gezginc et al., 2007)—which may be welcome to some women but concerning and undesirable to others (Gezginc et al., 2007). A variety of other common implant side effects may also indirectly decrease sexual acceptability, including breast tenderness, headaches, acne (Duvan et al., 2010; Gezginc et al., 2007), weight gain, and hirsutism (Duvan et al., 2010).
Worth celebrating, however, are the positive sexual health outcomes documented with implant use. Compared to ring and pill users, as well as a no-method control group, Nexplanon® users in one study reported the most significant improvements in sexual discomfort, anxiousness, personal initiative, and sexual fantasy over a six-month period (Farrell et al., 2014). Another study reported significantly higher FSFI scores measuring arousal, orgasm, satisfaction, and pain after three months of Nexplanon® use compared to baseline assessments, and these higher scores persisted at six months (Di Carlo, Sansone, et al., 2014). Though 2% to 9% of new implant users across several studies reported libido loss (Aisien & Enosolease, 2010; Duvan et al., 2010; Gezginc et al., 2007), these figures are smaller than those seen in studies of OC and other hormonal methods.
Tolerability of irregular bleeding patterns and other side effects should be further explored in regard to the sexual acceptability of the contraceptive implant. Particularly among adolescent women, whose menstrual cycles are likely not yet regular, the unpredictable bleeding profile could play a small role in sexual acceptability and may be far outweighed by sexual satisfaction, safety, and user-independent properties associated with the method.
Progestin-Only Injectable Contraceptive
Sexual acceptability of the injectable contraceptive Depo-Provera (DMPA) will likely vary by a woman’s experiences with and tolerance for irregular bleeding, the most commonly cited reason for discontinuation (Wanyonyi et al., 2011). However, one study found that, compared to pill users, women using the shot for a minimum of six months reported fewer days of bleeding per month, and the investigators observed no differences in sexual function or relationship satisfaction between the two groups (Schaffir, Fleming, & Waddell, 2010). Several studies have documented a decrease in libido with DMPA use (Gubrium, 2011; Sedigheh, Maryam, Ali, & Mehdi, 2012; Wanyonyi et al., 2011), with one study reporting that up to 21% of women reported reductions in sexual desire (compared to only 8% reporting increases) (Tabari, Moslemi, Esmaelzadeh, & Bijani, 2012). The latter study also found that 20% of women using DMPA reported breast tenderness and 9% experienced sexual pain (Sedigheh et al., 2012). DMPA has also been associated with negative changes in mood (Abbott & Dalla, 2008) and weight gain (Gubrium, 2011; Wanyonyi et al., 2011), both of which could shape women’s sexual well-being. In an attempt to better understand the “lived experiences” of Depo-Provera use, Gubrium’s (2011) qualitative interview findings from 34 U.S. women highlighted that side effects are not experienced in isolation but rather as a constellation of factors including weight gain, changes in body image, unpredictable bleeding, and emotionality. Taken together, these factors can enact changes in sexual desire or libido, thus contributing in important and understudied ways to the sexual acceptability of this contraceptive method.
Female Condom
Outside of male condoms, female condoms are the only currently available contraceptive method that helps prevent both pregnancy and STIs, including HIV. Though used less commonly than male condoms, they provide vital woman-controlled prophylaxis (Mathenjwa & Maharaj, 2012; Naidu, 2013)—especially for female sex workers, among whom their use has been the most promoted by public health officials and policymakers (Peters et al., 2013). 6 Most of the eight studies reviewed took place in developing countries and/or higher HIV-prevalence settings. Findings suggest that female condoms can be sexually acceptable to many women (especially sex workers) by way of enhanced level of lubrication (Mack, Grey, Amsterdam, Williamson, & Matta, 2010; Mathenjwa & Maharaj, 2012), greater pleasure and sensation than the male condom (Mathenjwa & Maharaj, 2012), clitoral stimulation through the outer ring (Mathenjwa & Maharaj, 2012), capacity to comfortably accommodate all penis sizes (Mack et al., 2010), ability to insert prior to sexual activity (Mathenjwa & Maharaj, 2012), and better smell than male condoms (Mack et al., 2010). Indeed, women’s experiences of greater pleasure and sexual sensations helped predict long-term use of female condoms among 255 women in Brazil (Telles Dias, Souto, & Page-Shafer, 2006).
However, not all women’s sexual experiences with this device are positive, and—just as with male condoms—a barrier to more widespread female condom use are women’s perceptions that it interferes with sexual satisfaction and pleasure (Okunlola, Morhason-Bello, Owonikoko, & Adekunle, 2006; Sobze Sanou et al., 2013). In a study of 850 Nigerian undergraduate students who had any history of female condom use, 30% reported reduced sexual satisfaction with this method (Okunlola et al., 2006). Women in smaller studies reported pain during intercourse (Okunlola et al., 2006), and a number of women who had not used it were concerned about insertion—both in terms of difficulty (Latka et al., 2008; Okunlola et al., 2006) and in touching one’s genitals (Latka et al., 2008). However, women can grow more comfortable with insertion through practice, and several studies show that the more women use female condoms, the more they like them and/or prefer them to male condoms (Mack et al., 2010; Telles Dias et al., 2006; Van Dijk, Pineda, Grossman, Sorhaindo, & García, 2013). In sum, female condoms can be a sexually acceptable option to many women and a unique woman-controlled dual-prevention technology. Women who struggle with sexual aspects of male condoms should be encouraged to try female condoms—and urged to try them several times before making up their mind about the method.
Female Sterilization
Female sterilization is one of the most widely used contraceptive methods in the world, with more than 180 million women across the globe using this highly effective procedure—most of whom live in China and India (EngenderHealth, 2002). Female sterilization was also the most commonly used contraceptive method in the United States for decades, only recently surpassed in popularity by OCs (in 2014, 15.5% versus 16.0%, respectively) (Daniels et al., 2014). Despite the widespread prevalence of this method, few recent studies assessed its sexual acceptability. However, the research reviewed here suggests that, for most women, there is no negative impact (Fakoya et al., 2008; Schaffir, Fleming, & Waddell, 2010), and there is potential for a positive impact (American College of Obstetricians and Gynecologists [ACOG], 2008) on women’s sexual function after the procedure. One study conducted after sterilization found that the irregular bleeding, dysmenorrhea, and pelvic pain experienced by some women resulted in less frequent sexual activity and/or reduced libido, even among younger women; yet despite these changes 92% of study participants reported satisfaction with the sterilization process and would recommend it to a friend (Dias et al., 2014). Before undergoing sterilization, some women report concerns about losing their femininity and/or their desire for or ability to enjoy sex, though few women actually experience these outcomes after the procedure (Schaffir, Fleming, & Waddell, 2010). Though not documented by the studies reviewed here, subsequent sexual disinhibition due to strong contraceptive efficacy may counteract negative sexual changes. Especially after a reproductive lifetime of trying to prevent unintended pregnancy, many women may enjoy the psychological and sexual freedoms of no longer having to actively “practice” contraception. Celebrating the high sexual acceptability of female sterilization, particularly if done in tandem with in-depth counseling for sexual concerns, may prove beneficial for women seeking permanent contraception.
Vasectomy
Besides the male condom, vasectomy is the only contraceptive option currently available for men in the United States. (Though some other reversible and permanent methods are available to men in other countries, none of those methods arose in our review.) Compared to tubal ligations, vasectomies are cheaper, more cost-effective, and associated with fewer postoperative complications; however, the ratio of male to female sterilizations in the United States is 1:3 (Daniels et al., 2014). In a large number of countries, from Bangladesh to Morocco to Zimbabwe, vasectomy is rarely if ever carried out (EngenderHealth, 2002). This underutilization of vasectomy pertains at least in part to widespread concerns, documented in the United States and abroad, related to “loss of manhood” and potential interference with erection and ejaculation (Bunce et al., 2007; Shih, Dube, Sheinbein, Borrero, & Dehlendorf, 2013). Dispelling these misconceptions and highlighting the rapid return to sexual function after the procedure may result in increased use among men and couples seeking permanent contraception. Moreover, vasectomy is associated with few sexual side effects and may even promote better couple-level sexuality. In a study of Austrian couples, while men reported no changes in sexual function after six month, their female partners reported improved sexual functioning (Al-Ali et al., 2014). Findings from this study underscore the psychosexual benefits that many women feel when protected against pregnancy.
Withdrawal
The family planning community often dismisses withdrawal as a method of contraception. For example, the U.S. Centers for Disease Control and Prevention (2015) excludes coitus interruptus from its list of contraceptive methods. However, withdrawal is commonly practiced the world over, both as a sole or extra method. In a study of 4,634 adult women in the United States, Jones et al. (2014) found that 33% reported any use of withdrawal in the past 30 days, and 13% reported withdrawal exclusively. For a variety of cultural reasons, certain countries, such as Turkey, have particularly large rates of withdrawal use, with estimates of one-quarter to one-third of all Turkish couples reporting some recent use of withdrawal, with rates holding steady since the 1990s (Çiftçioğlu & Erci, 2009; Cindoglu et al., 2008). The few studies located for this review suggest several sexual implications of the method—primarily that although withdrawal interrupts the moment, requires climax awareness and control for effective use (Freundl, Sivin, & Batár, 2010), and can interfere with pleasure for both partners, it may still be sexually preferable to male condoms (Higgins & Wang, 2015a; Ortayli, Bulut, Ozugurlu, & Çokar, 2005; Rahnama et al., 2010; Whittaker, Merkh, Henry-Moss, & Hock-Long, 2010). In one nationally representative study of U.S. young adults, both women and men who reported that condoms can reduce their pleasure had significantly greater odds of having used withdrawal at their last penile–vaginal episode (Higgins & Wang, 2015b). A similar finding emerged from studies of withdrawal in both Turkey (Ortayli et al., 2005) and Uganda (Higgins, Gregor, et al., 2014): Withdrawal use was associated with perceptions that condoms can reduce pleasure in both these settings. Particularly if couples want to engage in additional pregnancy prevention (that is, dual- or even triple-method use), withdrawal may provide a more sexually acceptable “double-up option” than condoms if STI risk is not present.
Other Barrier Methods
Our review resulted in two articles that assessed the sexual acceptability of the diaphragm—a method that has received increased research attention in recent years as a potential dual-prevention method for both pregnancy and STIs/HIV. Using a diaphragm may promote sexual autonomy for women, as it can be used covertly when inserted before sex (Sahin-Hodoglugil et al., 2011). Even women who do not want or need covert use report liking the notion that the diaphragm can be inserted before sexual activity, thereby being less sexually disruptive (Sahin-Hodoglugil et al., 2011). Other women feel confident they could use a diaphragm while sexually excited without breaking the mood (Kraft et al., 2007). Women in one study described the importance of having partner support when using the diaphragm, which some obtained by erotizing safety and highlighting the pleasurable, lubricating aspects associated with using microbicidal/spermicidal gel along with the diaphragm (Sahin-Hodoglugil et al., 2011). Barrier methods such as the diaphragm, sponge, and cervical cap require comfort with inserting the object inside the vagina; Thorburn, Harvey, and Tipton (2006) found that almost 20% of U.S. college-based women preferred a method that does not require genital touching, so these methods may not be well suited for everyone (Kraft et al., 2007; Thorburn et al., 2006). Enlisting a partner’s help with insertion of barrier methods could increase intimacy when used during foreplay and encourages both partners’ active engagement in family planning. More research is needed on how couples erotically incorporate barrier methods, including technologies such as dental dams, microbicides, and spermicides, into their sexual routine.
Natural Family Planning
Recent research has paid scant attention to natural family planning methods in the contraceptive literature, despite the United Nations’ estimate that 41 million couples worldwide used some form of natural family planning (Freundl et al., 2010). We located only three articles that referenced sexual outcomes associated with natural family planning (Badcock et al., 2014; Fataneh et al., 2013), one of which was a systematic review of fertility awareness–based methods (Freundl et al., 2010). The review highlighted increased bodily awareness and self-control as common positive experiences among natural family planning users (Freundl et al., 2010). Particularly given the necessity of couple participation with these methods, couple factors are likely to influence uptake and sexual acceptability. Many couples may choose natural family planning methods due to their “naturalness,” facilitation of skin-to-skin contact, and/or lack of hormonal influences. However, these methods do not allow for spontaneous sexual activity during periods of peak fertility—a phenomenon that could serve as a sexual turn-off for many potential users. Another turn-off could be these methods’ relatively high typical-use failure rates, which could stymie sexual disinhibition. On the other hand, couples who feel ambivalent about pregnancy intentions and/or who want a pregnancy to “just happen” could deliberately select natural family planning methods for the positive attributes listed.
SUMMARIZED FINDINGS, TRENDS, AND RECOMMENDATIONS FOR FUTURE RESEARCH AND PROGRAMS
This review has documented an almost dizzying array of ways that contraceptives affect women’s sexual well-being in both positive and negative ways, from direct hormonal or even genetic impacts on sexual functioning and indirect psychosexual impacts of efficacy or side effects, to more subjective assessments of contraceptive-related pleasure and satisfaction for/among both women and their partners. The model proposed in Part 1 groups these factors into several socioecological layers: gender, cultural and structural factors, relationship factors, and individual factors. Though a few findings are unidirectional across studies (e.g., no women reported that condoms enhance sensation or that breast tenderness improves one’s sex life), many other themes show strong variability—with the very contraceptive feature sexually desired by one woman disdained by another (e.g., timing/method of application, effect on menstrual bleeding). However, several trends, take-home messages, and recommendations for future research stand out. Here, we review four.
First, the array of studies documented here provides clear evidence that the way contraceptive methods affect women’s well-being matters to their overall happiness with their methods, which in turn influences continuation and, over time, exposure to unintended pregnancy. Moreover, these effects matter regardless of whether methods are “truly” or solely responsible for any sexual changes; even if stress, fatigue, and/or relationship factors are more likely the causes of sexual detractions, women’s perceptions of what caused these changes will ultimately shape contraceptive acceptability and behavior (Enzlin et al., 2012). Along the same lines, women’s concerns about contraceptive side effects can be more influential than actual side effects in shaping women’s willingness to try to continue using certain contraceptive methods (Frost, Lindberg, & Finer, 2012; Gilliam, Warden, Goldstein, & Tapia, 2004; Westhoff et al., 2007). Similarly, women’s perceptions of how contraceptives might affect their—or their partners’—sexual well-being will influence their contraceptive decisions (Higgins et al., 2015). Furthermore, though contraceptive efficacy is important to women, “not interfering with sexual pleasure” may be an equally if not more important contraceptive criterion (Gomez & Clark, 2014). After all, women do not engage in sexual activity to use contraception; they do so for a host of other sexual, psychological, and relational reasons, and it makes sense that they want their contraceptives to align with these reasons and goals.
However, a second trend that emerges is that the overwhelming majority of the literature we reviewed for Part 2 in particular—that is, analyses that included sexual outcomes in relationship to contraceptive use—focused overwhelmingly on sexual functioning and not other aspects of the model, including women’s sexual perceptions or preferences. A focus on sexual functioning was especially common with methods involving hormonal configurations, particularly OCs—the most commonly used contraceptives in the United States (Daniels et al., 2014). The majority of these studies on hormonal methods employed more classic measures of sexual functioning (such as the FSFI) and/or biomarkers such as serum hormone levels, blood flow, or physical changes to women’s genitalia. 7 Such research is undoubtedly important, especially given the minority of women who do experience negative effects on sexual functioning, particularly libido (Bancroft et al., 2006), and who discontinue as a result (Sanders et al., 2001). On the other hand, while the concept of women’s physiologic sexual functioning should be vital to all contraceptive research, a narrow concentration on this one outcome fails to capture the other psychological, relational, or cultural factors that matter to actual contraceptive users and/or that influence contraceptive preferences and sexual practices. Extremely few studies in this review approached sexual acceptability using the kind of broader ecological approach we proposed in Part 1. Even if studies are not able to collect data on all levels of the model, researchers could at least comment in their discussion sections about how findings must be considered within the context of the couple, cultural constructions of sexuality, and/or social location, all of which may moderate results. Even at the individual level, we recommend the development and use of a more multifaceted measure of sexual acceptability that collects information on more than physiologic sexual functioning—work that we are engaging in ourselves and encourage others to do as well. We also argue that more research is needed on the interplay between solo and dyadic aspects of sexuality among couples using contraception (Elaut et al., 2012).
Third, our review uncovered a number of limitations in study design. 8 (Many of these limitations have been discussed in a variety of places in the literature; for a small sampling, see (Bancroft & Graham, 2011; Bastianelli et al., 2011; Davis & Castano, 2004; Graham et al., 2007).) For example, many of the studies reviewed in Part 2 were cross-sectional. Cross-sectional study designs are unable to document sexual changes over time with continued contraceptive use; neither do they allow researchers to control for baseline characteristics that might influence both contraceptive choices and sexual outcomes. Furthermore, reports of negative sexual outcomes may be underestimated given that many of those women whose sexuality was negatively affected by a particular method will have already discontinued using it. Another limitation of some studies is insufficient sample size to compare methods and/or offer an appropriate comparison or “control” group. We acknowledge that no ideal, noninterventional control group exists for this type of research: women using no method, sterilized women, and/or women using natural family planning methods are likely to differ meaningfully from women using (other) reversible methods and thus are not a useful comparator (Higgins & Davis, 2014). On the other hand, researchers should at least consider recruiting new-start contraceptive users and collecting baseline data on factors such as functional health, relationship length and satisfaction, and other factors that could affect both contraceptive choice and sexual outcomes. On a related note, only a few studies in this review (e.g., Sanders et al., 2001) have been able to follow women for several months, a year, or longer periods of time to assess whether sexual outcomes at one time point can help predict method satisfaction and continuation at another time point. If the ultimate goal is to help direct women to methods they will like and use over time, thereby realizing the full benefits of contraception, we also need to document the degree to which sexual acceptability affects overall continuation and satisfaction compared to factors such as efficacy, access and cost, (nonsexual) side effects, method of administration, and other contraceptive features (Gomez & Clark, 2014).
A fourth and final observation worth noting from our review is the absence of research on how to integrate sexual acceptability into educational, medical, or other program settings. Although we surely need more clinical studies that document methods’ sexual acceptability for women and their partners, we also need much more research on how to practically apply the outcomes of sexual acceptability studies to serve actual contraceptive clients. Due to the wide array of what women find sexually acceptable about contraception, the field (and women!) would be well served by investing research efforts in devising broader, more individualized measures of contraceptive sexual acceptability, with the ultimate goal of creating an algorithm that better links individual women with methods that align with their individual sexual preferences, lives, and relationships. This new scale or index of sexual acceptability could be useful in web-based tools and educational, clinical, and promotional programs. We also urge reproductive health researchers to devise and evaluate tools to help clinicians, educators, counselors, and other practitioners avail themselves of the larger sexual acceptability literature and amend their contraceptive counseling practices as a result.
We conclude by advocating for a sex-positive approach in both research and programs and policies relating to contraception. Although we argue that sexual acceptability measures should be a standard part of contraceptive research and development, as well as all contraceptive acceptability studies, we also encourage incorporating and valuing women’s sexual pleasure, satisfaction, and well-being in public health discourse or even commercial efforts that promote contraceptive methods. Some researchers and practitioners have similarly advocated for the benefits of using pleasure-philic, sex-positive messages to promote condoms and other contraceptives (Philpott, Knerr, & Maher, 2006). Along similar lines, sex workers have described how they can increase successful use of both male and female condoms by emphasizing their pleasure for both partners, or by applying condoms in sexually appealing ways (Free et al., 2007). Positive sexual aspects of methods could greatly strengthen our counseling, marketing, and educational efforts. For example, by highlighting the sexual disinhibition benefits associated with very effective methods, counselors could help encourage use of IUDs and implants. Acknowledging that contraceptive users want methods that enhance their sexual well-being (or at least do not undermine it), we are more likely to take a patient-centered approach in our research and programs and, more specifically, our efforts to promote contraception and women’s well-being.
Supplementary Material
Acknowledgments
Jenny Higgins gratefully acknowledges support from an NIH K12 award (K12HD055894) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) as well as a NICHD Population Research Infrastructure grant (P2CHD047873). Nicole Smith acknowledges NICHD grant P2CHD047879. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The authors also wish to thank librarians Rhonda Sager and Lia Vellardita at the University of Wisconsin–Madison and Elana Broch at Princeton University for their instrumental help with the literature search and extraction process. The authors are indebted to Libby Wagner for her artistic skills in designing our Conceptual Model. Finally, Dr. Jessica Sanders of University of Utah School of Medicine helped us with the initial review of abstracts; thank you, Jess.
Footnotes
In the words of one legal scholar: “A core aspect of conceptualizing women as mothers in the law is viewing them—and treating them legally—as people who should engage in sexual activity for the purpose of parenthood, not pleasure: this is desexualization” (Burkstrand-Reid, 2013, pp. 223–224).
There is no such gap for LARC, such as IUDs and implants, which allow for no “user error.” When combined with LARC’s extremely strong effectiveness in preventing pregnancy (Trussell, 2011), this lack of gap has made LARC “first-line” methods recommended by organizations such as the American College of Obstetricians and Gynecologists (Committee on Adolescent Health Care, 2012).
Of course, these relationships are complex and hardly universal. In one study using data from the National Longitudinal Study of Adolescent to Adult Health, or Add Health (Harris, 2013), the investigator found that U.S. girls who did well in school were more confident about their ability to use contraception but were also more likely to associate sex with guilt and shame (Pearson, 2009).
A robust feminist literature deconstructs the “objectivity” of sexual dysfunction diagnostic criteria in particular, demonstrating how social constructions of gender directly influence what we consider ideal and problematic in terms of sexual functioning and desire in particular (Tiefer, 2004; Tolman & Diamond, 2001).
Ovulation inhibition is not the primary mode of action for most progestin-only contraceptives (Rivera, Yacobson, & Grimes, 1999). However, the majority of the research in this area has focused on combined hormonal methods, which contain both estrogen and progestin.
Peters et al. (2013) reviewed 16 policy papers on female condoms. None of them advocated making female condoms available for all women; most focused on their importance to female sex workers in particular. The authors contrasted this focus with male circumcision, which public health officials promptly wanted to roll out to all men in Sub-Saharan Africa. The authors argued that “[n]ormalizing female condom use for commercial sex workers rather than for other categories of women who are sexually active, implicitly links HIV to sexually immoral behaviour, thus stigmatizing HIV-positive women in their local communities” (Peters et al., 2013, p. 10).
Even in studies that do measure functioning, more research is needed to determine whether standard cutoff points for determining “sexual dysfunction” are accurately identifying women who are distressed with their sexual life or sexual function (e.g., see Gabalci & Terzioglu, 2010).
Although it is a limitation directly related to study design, readers of this literature should also be mindful of studies that are sponsored by pharmaceutical companies versus nonsponsor funders. Pharmaceutical-sponsored research may be less likely to report contraceptive methods’ negative outcomes (Kemmeren, Algra, & Grobbee, 2001; Lexchin, Bero, Djulbegovic, & Clark, 2003), including those related to sexuality.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Abbott D. A., Dalla R. L. “It’s a choice, simple as that”: Youth reasoning for sexual abstinence or activity. Journal of Youth Studies. 2008;11:629–649. doi: 10.1080/13676260802225751. [DOI] [Google Scholar]
- Aiken A. R., Potter J. E. Are Latina women ambivalent about pregnancies they are trying to prevent? Evidence from the Border Contraceptive Access Study. Perspectives on Sexual and Reproductive Health. 2013;45:196–203. doi: 10.1363/4519613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisien A. O., Enosolease M. E. Safety, efficacy, and acceptability of Implanon, a single rod implantable contraceptive (etonogestrel) in University of Benin Teaching Hospital. Nigerian Journal of Clinical Practice. 2010;13:331–335. [PubMed] [Google Scholar]
- Akers A. Y., Gold M. A., Borrero S., Santucci A., Schwarz E. B. Providers’ perspectives on challenges to contraceptive counseling in primary care settings. Journal of Women’s Health. 2010;19:1163–1170. doi: 10.1089/jwh.2009.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ali B. M., Shamloul R., Ramsauer J., Bella A. J., Scrinzi U., Treu T., Jungwirth A. The effect of vasectomy on the sexual life of couples. Journal of Sexual Medicine. 2014;11:2239–2242. doi: 10.1111/jsm.2014.11.issue-9. [DOI] [PubMed] [Google Scholar]
- Alexander K. A., Coleman C. L., Deatrick J. A., Jemmott L. S. Moving beyond safe sex to women-controlled safe sex: A concept analysis. Journal of Advanced Nursing. 2012;68:1858–1869. doi: 10.1111/jan.2012.68.issue-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez C. P., Villarruel A. Sexual communication among young adult heterosexual Latinos: A qualitative descriptive study. Hispanic Health Care International. 2013;11:101–110. doi: 10.1891/1540-4153.11.3.101. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics AAP updates on teen pregnancy. 2014 https://www.aap.org/en-us/about-the-aap/aap-press-room/pages/AAP-Updates-Recommendations-on-Teen-Pregnancy-Prevention.aspx Retrieved from.
- American College of Obstetricians and Gynecologists Treatment of menorrhagia and sexual function. ACOG Clinical Review. 2008;13:7. [Google Scholar]
- Andersen M. L., Hill Collins P. Race, class, and gender: An anthology. 8th ed. Belmont, CA: Wadsworth Cengage Learning; 2013. [Google Scholar]
- Andersson K., Odlind V., Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: A randomized comparative trial. Contraception. 1994;49:56–72. doi: 10.1016/0010-7824(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Archer D. F. Menstrual-cycle-related symptoms: A review of the rationale for continuous use of oral contraceptives. Contraception. 2006;74:359–366. doi: 10.1016/j.contraception.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Avellanet Y. R., Patricia-Ortiz A., Pando J. R., Romaguera J. Dyspareunia in Puerto Rican middle-aged women. Menopause: Journal of the North American Menopause Society. 2009;16:742–747. doi: 10.1097/gme.0b013e31819724f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola A. B., Nettleman M., Brewer J. Reasons for unprotected intercourse in adult women. Journal of Women’s Health. 2007;16:302–310. doi: 10.1089/jwh.2007.0210. [DOI] [PubMed] [Google Scholar]
- Badcock P. B., Smith A. M. A., Richters J., Rissel C., De Visser R. O., Simpson J. M., Grulich A. E. Characteristics of heterosexual regular relationships among a representative sample of adults: The Second Australian Study of Health and Relationships. Sexual Health. 2014;11:427–438. doi: 10.1071/SH14114. [DOI] [PubMed] [Google Scholar]
- Baeten J. M., Donnell D., Ndase P., Mugo N. R., Campbell J. D., Wangisi J., Bumpus N. N. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkus J., Bosire R., John-Stewart G., Mbori-Ngacha D., Schiff M. A., Wamalwa D., Farquhar C. High uptake of postpartum hormonal contraception among HIV-1-seropositive women in Kenya. Sexually Transmitted Diseases. 2007;34:25–29. doi: 10.1097/01.olq.0000218880.88179.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J., Graham C. A. The varied nature of women’s sexuality: Unresolved issues and a theoretical approach. Hormones and Behavior. 2011;59:717–729. doi: 10.1016/j.yhbeh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Bancroft J., Hammond G., Graham C. Do oral contraceptives produce irreversible effects on women’s sexuality? Journal of Sexual Medicine. 2006;3(3):567. doi: 10.1111/jsm.2006.3.issue-3. author reply, 568–570. [DOI] [PubMed] [Google Scholar]
- Barnnart K., Furman I., Devoto L. Attitudes and practice of couples regarding sexual relations during the menses and spotting. Contraception. 1995;51:93–98. doi: 10.1016/0010-7824(94)00014-N. [DOI] [PubMed] [Google Scholar]
- Bastianelli C., Farris M., Benagiano G. Use of the levonorgestrel-releasing intrauterine system, quality of life and sexuality. Experience in an Italian family planning center. Contraception. 2011;84:402–408. doi: 10.1016/j.contraception.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Battaglia C., Battaglia B., Mancini F., Busacchi P., Paganotto M. C., Morotti E., Venturoli S. Sexual behavior and oral contraception: A pilot study. Journal of Sexual Medicine. 2012;9:550–557. doi: 10.1111/jsm.2012.9.issue-2. [DOI] [PubMed] [Google Scholar]
- Battaglia C., Morotti E., Persico N., Battaglia B., Busacchi P., Casadio P., Venturoli S. Clitoral vascularization and sexual behavior in young patients treated with drospirenone-ethinyl estradiol or contraceptive vaginal ring: A prospective, randomized, pilot study. Journal of Sexual Medicine. 2014;11:471–480. doi: 10.1111/jsm.2014.11.issue-2. [DOI] [PubMed] [Google Scholar]
- Bednarczyk R. A., Davis R., Ault K., Orenstein W., Omer S. B. Sexual activity-related outcomes after human papillomavirus vaccination of 11–12-year-olds. Pediatrics. 2012;130:798–805. doi: 10.1542/peds.2012-1516. [DOI] [PubMed] [Google Scholar]
- Bettany-Saltikov J. Learning how to undertake a systematic review: Part 1. Nursing Standard. 2010a;24:47–55. doi: 10.7748/ns2010.08.24.50.47.c7939. quiz 56. [DOI] [PubMed] [Google Scholar]
- Bettany-Saltikov J. Learning how to undertake a systematic review: Part 2. Nursing Standard. 2010b;24:47–56. doi: 10.7748/ns2010.08.24.50.47.c7939. quizzes 58, 60. [DOI] [PubMed] [Google Scholar]
- Biggs M. A., Karasek D., Foster D. G. Unprotected intercourse among women wanting to avoid pregnancy: Attitudes, behaviors, and beliefs. Women’s Health Issues. 2012;22:e311–e318. doi: 10.1016/j.whi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Billy J. O. G., Grady W. R., Sill M. E. Sexual risk-taking among adult dating couples in the United States. Perspectives on Sexual and Reproductive Health. 2009;41:74–83. doi: 10.1363/4107409. [DOI] [PubMed] [Google Scholar]
- Bird C. E. Gender differences in the social and economic burdens of parenting and psychological distress. Journal of Marriage and the Family. 1997;59:809–823. doi: 10.2307/353784. [DOI] [Google Scholar]
- Bishop J. R., Ellingrod V. L., Akroush M., Moline J. The association of serotonin transporter genotypes and selective serotonin reuptake inhibitor (SSRI)-associated sexual side effects: Possible relationship to oral contraceptives. Human Psychopharmacology: Clinical and Experimental. 2009;24:207–215. doi: 10.1002/hup.v24:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer J. Contraception and female sexual function: A conceptualization of a complex interaction. Journal of Sexual Medicine. 2010;7:132–133. [Google Scholar]
- Bjelica A. Socio-demographic factors influence contraception use among female students of the University of Novi Sad (Serbia) European Journal of Contraception and Reproductive Health Care. 2008;13:422–430. doi: 10.1080/13625180802296747. [DOI] [PubMed] [Google Scholar]
- Bolton M., McKay A., Schneider M. Relational influences on condom use discontinuation: A qualitative study of young adult women in dating relationships. Canadian Journal of Human Sexuality. 2010;19:91–104. [Google Scholar]
- Bombas T., Costa A. R., Palma F., Vicente L., Sá J. L., Nogueira A. M., Andrade S. Knowledge-attitude-practice survey among Portuguese gynaecologists regarding combined hormonal contraceptives methods. European Journal of Contraception and Reproductive Health Care. 2012;17:128–134. doi: 10.3109/13625187.2011.631622. [DOI] [PubMed] [Google Scholar]
- Borges A. L., Nakamura E. Social norms of sexual initiation among adolescents and gender relations. Revisto Revista Latino-Americana de Enfermagem. 2009;17:94–100. doi: 10.1590/S0104-11692009000100015. [DOI] [PubMed] [Google Scholar]
- Borrero S., Schwarz E. B., Reeves M. F., Bost J. E., Creinin M. D., Ibrahim S. A. Race, insurance status, and tubal sterilization. Obstetrics and Gynecology. 2007;109:94–100. doi: 10.1097/01.AOG.0000249604.78234.d3. [DOI] [PubMed] [Google Scholar]
- Bowleg L., Valera P., Teti M., Tschann J. M. Silences, gestures, and words: Nonverbal and verbal communication about HIV/AIDS and condom use in Black heterosexual relationships. Health Communication. 2010;25:80–90. doi: 10.1080/10410230903474019. [DOI] [PubMed] [Google Scholar]
- Braun V. “Proper sex without annoying things”: Anti-condom discourse and the “nature” of (hetero)sex. Sexualities. 2013;16:361–382. doi: 10.1177/1363460713479752. [DOI] [Google Scholar]
- Braunstein G. D. Androgen insufficiency in women. Growth Hormone and IGF Research. 2006;16:S109–S117. doi: 10.1016/j.ghir.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Brown S. “They think it’s all up to the girls”: Gender, risk and responsibility for contraception. Culture, Health, and Sexuality. 2015;17:312–325. doi: 10.1080/13691058.2014.950983. [DOI] [PubMed] [Google Scholar]
- Brown S. G., Calibuso M. J., Roedl A. L. Women’s sexuality, well-being, and the menstrual cycle: Methodological issues and their interrelationships. Archives of Sexual Behavior. 2011;40:755–765. doi: 10.1007/s10508-010-9630-3. [DOI] [PubMed] [Google Scholar]
- Brugman M., Caron S. L., Rademakers J. Emerging adolescent sexuality: A comparison of American and Dutch college women’s experiences. International Journal of Sexual Health. 2010;22:32–46. doi: 10.1080/19317610903403974. [DOI] [Google Scholar]
- Bunce A., Guest G., Searing H., Frajzyngier V., Riwa P., Kanama J., Achwal I. Factors affecting vasectomy acceptability in Tanzania. International Family Planning Perspectives. 2007;33:13–21. doi: 10.1363/3301307. [DOI] [PubMed] [Google Scholar]
- Burke R. C., Wilson J., Kowalski A., Murrill C., Cutler B., Sweeney M., Begier E. M. NYC condom use and satisfaction and demand for alternative condom products in New York City sexually transmitted disease clinics. Journal of Urban Health. 2011;88:749–758. doi: 10.1007/s11524-011-9597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkstrand-Reid B. A. From sex for pleasure to sex for parenthood: How the law manufactures mothers. Hastings Law Journal. 2013;65:211–258. [Google Scholar]
- Burrows L. J., Basha M., Goldstein A. T. The effects of hormonal contraceptives on female sexuality: A review. Journal of Sexual Medicine. 2012;9:2213–2223. doi: 10.1111/jsm.2012.9.issue-9. [DOI] [PubMed] [Google Scholar]
- Campo-Engelstein L. Raging hormones, domestic incompetence, and contraceptive indifference: Narratives contributing to the perception that women do not trust men to use contraception. Culture, Health, and Sexuality. 2013;15:283–295. doi: 10.1080/13691058.2012.752106. [DOI] [PubMed] [Google Scholar]
- Carey J. C. Pharmacological effects on sexual function. Obstetrics and Gynecology Clinics of North America. 2006;33:599–620. doi: 10.1016/j.ogc.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Carrasco‐Garrido P., De Andrés A. L., Hernández Barrera V., Jiménez‐Trujillo I., Santos‐Sancho J., Jiménez‐García R. Predictors of contraceptive methods among adolescents and young women residing in Spain. Journal of Sexual Medicine. 2011;8:2431–2438. doi: 10.1111/jsm.2011.8.issue-9. [DOI] [PubMed] [Google Scholar]
- Caruso S., Agnello C., Romano M., Cianci S., Lo Presti L., Malandrino C., Cianci A. Preliminary study on the effect of four‐phasic estradiol valerate and dienogest (E2V/DNG) oral contraceptive on the quality of sexual life. Journal of Sexual Medicine. 2011;8:2841–2850. doi: 10.1111/jsm.2011.8.issue-10. [DOI] [PubMed] [Google Scholar]
- Caruso S., Cianci S., Malandrino C., Cicero C., Lo Presti L., Cianci A. Quality of sexual life of women using the contraceptive vaginal ring in extended cycles: Preliminary report. European Journal of Contraception and Reproductive Health Care. 2014;19:307–314. doi: 10.3109/13625187.2014.914488. [DOI] [PubMed] [Google Scholar]
- Caruso S., Malandrino C., Cicero C., Ciancio F., Cariola M., Cianci A. Quality of sexual life of women on oral contraceptive continued-regimen: Pilot study. Journal of Sexual Medicine. 2013;10:460–466. doi: 10.1111/jsm.2013.10.issue-2. [DOI] [PubMed] [Google Scholar]
- Caruso S., Rugolo S., Agnello C., Romano M., Cianci A. Quality of sexual life in hyperandrogenic women treated with an oral contraceptive containing chlormadinone acetate. Journal of Sexual Medicine. 2009;6:3376–3384. doi: 10.1111/jsm.2009.6.issue-12. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Contraception. 2015 http://www.Cdc.Gov/reproductivehealth/unintendedpregnancy/contraception.Htm Retrieved from.
- Chacham A. S., Maia M. B., Greco M., Silva A. P., Greco D. B. Autonomy and susceptibility to HIV/AIDS among young women living in a slum in Belo Horizonte, Brazil. AIDS Care. 2007;19(Suppl. 1):S12–S22. doi: 10.1080/09540120601114402. [DOI] [PubMed] [Google Scholar]
- Charlton B. M., Corliss H. L., Missmer S. A., Frazier A. L., Rosario M., Kahn J. A., Austin S. B. Reproductive health screening disparities and sexual orientation in a cohort study of U.S. adolescent and young adult females. Journal of Adolescent Health. 2011;49:505–510. doi: 10.1016/j.jadohealth.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton B. M., Corliss H. L., Missmer S. A., Rosario M., Spiegelman D., Austin S. B. Sexual orientation differences in teen pregnancy and hormonal contraceptive use: An examination across 2 generations. American Journal of Obstetrics and Gynecology. 2013;209(204):e201–208. doi: 10.1016/j.ajog.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Z. Contraceptive use: A bio-psycho-cultural and relational method of protection for second-generation Chinese American women. 2008 http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=psyh&AN=2008-99200-548&site=ehost-live&scope=site ProQuest Information and Learning, U.S. (69). Retrieved from. Available from EBSCOhost Psych database.
- Çiftçioğlu S., Erci B. Coitus interruptus as a contraceptive method: Turkish women’s perceptions and experiences. Journal of Advanced Nursing. 2009;65:1686–1694. doi: 10.1111/jan.2009.65.issue-8. [DOI] [PubMed] [Google Scholar]
- Cindoglu D., Sirkeci I., Sirkeci R. F. Determinants of choosing withdrawal over modern contraceptive methods in Turkey. European Journal of Contraception and Reproductive Health Care. 2008;13:412–421. doi: 10.1080/13625180802255719. [DOI] [PubMed] [Google Scholar]
- Cobey K. D., Buunk A. P. Conducting high-quality research on the psychological impact of oral contraceptive use. Contraception. 2012;86:330–331. doi: 10.1016/j.contraception.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Cockcroft A., Kunda J. L., Kgakole L., Masisi M., Laetsang D., Ho-Foster A., Andersson N. Community views of inter-generational sex: Findings from focus groups in Botswana, Namibia, and Swaziland. Psychology, Health, and Medicine. 2010;15:507–514. doi: 10.1080/13548506.2010.487314. [DOI] [PubMed] [Google Scholar]
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists Committee opinion no. 539: Adolescents and long-acting reversible contraception: Implants and intrauterine devices. Obstetrics and Gynecology. 2012;120:983–988. doi: 10.1097/AOG.0b013e3182723b7d. [DOI] [PubMed] [Google Scholar]
- Connell E. E. Expelling pleasure? School-based sex education and the sexual regulation of youth. 2009 http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=psyh&AN=2009-99090-466&site=ehost-live&scope=site ProQuest Information and Learning, U.S. (69). Retrieved from. Available from EBSCOhost Psych database.
- Crissman H. P., Adanu R. M., Harlow S. D. Women’s sexual empowerment and contraceptive use in Ghana. Studies in Family Planning. 2012;43:201–212. doi: 10.1111/sifp.2012.43.issue-3. [DOI] [PubMed] [Google Scholar]
- Crosby R., Milhausen R., Mark K. P., Yarber W. L., Sanders S., Graham C. A. Understanding problems with condom fit and feel: An important opportunity for improving clinic-based safer sex programs. Journal of Primary Prevention. 2013;34:109–115. doi: 10.1007/s10935-013-0294-3. [DOI] [PubMed] [Google Scholar]
- Crosby R., Milhausen R., Yarber W. L., Sanders S., Graham C. A. Condom “turn offs” among adults: An exploratory study. International Journal of STD and AIDS. 2008;19:590–594. doi: 10.1258/ijsa.2008.008120. [DOI] [PubMed] [Google Scholar]
- Crosby R., Sanders S., Yarber W. L., Graham C. A. Condom-use errors and problems: A neglected aspect of studies assessing condom effectiveness. American Journal of Preventive Medicine. 2003;24:367–370. doi: 10.1016/S0749-3797(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Crosby R., Yarber W. L., Graham C. A., Sanders S. Does it fit okay? Problems with condom use as a function of self-reported poor fit. Sexually Transmitted Infections. 2010;86:36–38. doi: 10.1136/sti.2009.036665. [DOI] [PubMed] [Google Scholar]
- Cyranowski J. M., Bromberger J., Youk A., Matthews K., Kravitz H. M., Powell L. H. Lifetime depression history and sexual function in women at midlife. Archives of Sexual Behavior. 2004;33:539–548. doi: 10.1023/B:ASEB.0000044738.84813.3b. [DOI] [PubMed] [Google Scholar]
- Daniels K., Daugherty J., Jones J. NCHS data brief, no. 173) Hyattsville, MD: National Council for Health Statistics; 2014. Current contraceptive status among women aged 15–44: United States, 2011–2013 ( [PubMed] [Google Scholar]
- Davis A. R., Castano P. M. Oral contraceptives and libido in women. ANNUAL REVIEW OF SEX RESEARCH. 2004;15:297–320. [PubMed] [Google Scholar]
- Davis S. R., Bitzer J., Giraldi A., Palacios S., Parke S., Serrani M., Nappi R. E. Change to either a nonandrogenic or androgenic progestin-containing oral contraceptive preparation is associated with improved sexual function in women with oral contraceptive-associated sexual dysfunction. Journal of Sexual Medicine. 2013;10:3069–3079. doi: 10.1111/jsm.2013.10.issue-12. [DOI] [PubMed] [Google Scholar]
- Davis S. R., Davison S. L., Donath S., Bell R. J. Circulating androgen levels and self-reported sexual function in women. Journal of the American Medical Association. 2005;294:91–96. doi: 10.1001/jama.294.1.91. [DOI] [PubMed] [Google Scholar]
- Davison S. L., Bell R. J., LaChina M., Holden S. L., Davis S. R. Sexual function in well women: Stratification by sexual satisfaction, hormone use, and menopause status. Journal of Sexual Medicine. 2008;5:1214–1222. doi: 10.1111/j.1743-6109.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- Deardorff J., Suleiman A. B., Dal Santo T. S., Flythe M., Gurdin J. B., Eyre S. L. Motivations for sex among low-income African American young women. Health Education and Behavior. 2013;40:646–650. doi: 10.1177/1090198112473112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff J., Tschann J. M., Flores E., De Groat C. L., Steinberg J. R., Ozer E. J. Latino youths’ sexual values and condom negotiation strategies. Perspectives on Sexual and Reproductive Health. 2013;45:182–190. doi: 10.1363/psrh.2013.45.issue-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlendorf C., Ruskin R., Grumbach K., Vittinghoff E., Bibbins-Domingo K., Schillinger D., Steinauer J. Recommendations for intrauterine contraception: A randomized trial of the effects of patients’ race/ethnicity and socioeconomic status. American Journal of Obstetrics and Gynecology. 2010;203(319):e311–e318. doi: 10.1016/j.ajog.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni D., Skee D., Vaccaro N., Massarella J., Janssens L., LaGuardia K. D., Leung A. T. Pharmacokinetics and pharmacodynamics of a transdermal contraceptive patch and an oral contraceptive. Journal of Clinical Pharmacology. 2007;47:497–509. doi: 10.1177/0091270006297919. [DOI] [PubMed] [Google Scholar]
- Dias D. S., Dias R., Nahás-Neto J., Nahás E. A., Leite N. J., Bueloni-Dias F. N., Modotti W. P. Clinical and psychological repercussions of videolaparoscopic tubal ligation: Observational, single cohort, retrospective study. Sao Paulo Medical Journal. 2014;132:321–331. doi: 10.1590/1516-3180-2014-1326687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo C., Gargano V., De Rosa N., Tommaselli G. A., Sparice S., Nappi C. Effects of estradiol valerate and dienogest on quality of life and sexual function according to age. Gynecological Endocrinology. 2014;30(12):925–928. doi: 10.3109/09513590.2014.975688. [DOI] [PubMed] [Google Scholar]
- Di Carlo C., Sansone A., De Rosa N., Gargano V., Tommaselli G. A., Nappi C., Bifulco G. Impact of an implantable steroid contraceptive (etonogestrel-releasing implant) on quality of life and sexual function: A preliminary study. Gynecological Endocrinology. 2014;30:53–56. doi: 10.3109/09513590.2013.848851. [DOI] [PubMed] [Google Scholar]
- DiClemente R. J., Wingood G. M., Rose E., Sales J. M., Crosby R. A. Evaluation of an HIV/STD sexual risk-reduction intervention for pregnant African American adolescents attending a prenatal clinic in an urban public hospital: Preliminary evidence of efficacy. Journal of Pediatric and Adolescent Gynecology. 2010;23:32–38. doi: 10.1016/j.jpag.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do M., Kurimoto N. Women’s empowerment and choice of contraceptive methods in selected African countries. International Perspectives on Sexual and Reproductive Health. 2012;38:23–33. doi: 10.1363/3802312. [DOI] [PubMed] [Google Scholar]
- Drosdzol A., Skrzypulec V. Quality of life and sexual functioning of Polish infertile couples. European Journal of Contraception and Reproductive Health Care. 2008;13:271–281. doi: 10.1080/13625180802049187. [DOI] [PubMed] [Google Scholar]
- Duvan C. I., Gozdemir E., Kaygusuz I., Kamalak Z., Turhan N. Etonogestrel contraceptive implant (Implanon): Analysis of patient compliance and adverse effects in the breastfeeding period. Journal of the Turkish-German Gynecological Association. 2010;11:141–144. doi: 10.5152/jtgga. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East L., Jackson D., O’Brien L., Peters K. Condom negotiation: Experiences of sexually active young women. Journal of Advanced Nursing. 2011;67:77–85. doi: 10.1111/jan.2010.67.issue-1. [DOI] [PubMed] [Google Scholar]
- Echeverry M. C., Arango A., Castro B., Raigosa G. Study of the prevalence of female sexual dysfunction in sexually active women 18 to 40 years of age in Medellín, Colombia. Journal of Sexual Medicine. 2010;7:2663–2669. doi: 10.1111/(ISSN)1743-6109. [DOI] [PubMed] [Google Scholar]
- Ekstrand M., Tydén T., Larsson M. Exposing oneself and one’s partner to sexual risk-taking as perceived by young Swedish men who requested a chlamydia test. European Journal of Contraception and Reproductive Health Care. 2011;16:100–107. doi: 10.3109/13625187.2010.549253. [DOI] [PubMed] [Google Scholar]
- Elaut E., Buysse A., De Sutter P., De Cuypere G., Gerris J., Deschepper E., T’Sjoen G. Relation of androgen receptor sensitivity and mood to sexual desire in hormonal contraception users. Contraception. 2012;85:470–479. doi: 10.1016/j.contraception.2011.10.007. [DOI] [PubMed] [Google Scholar]
- EngenderHealth . Contraceptive sterilization: Global issues and trends. New York, NY: EngenderHealth; 2002. [Google Scholar]
- Enzlin P., Weyers S., Janssens D., Poppe W., Eelen C., Pazmany E., Amy J. J. Sexual functioning in women using levonorgestrel‐releasing intrauterine systems as compared to copper intrauterine devices. Journal of Sexual Medicine. 2012;9:1065–1073. doi: 10.1111/jsm.2012.9.issue-4. [DOI] [PubMed] [Google Scholar]
- Escajadillo-Vargas N., Mezones-Holguín E., Castro-Castro J., Córdova-Marcelo W., Blümel J. E., Pérez-López F. R., Chedraui P. Sexual dysfunction risk and associated factors in young Peruvian university women. Journal of Sexual Medicine. 2011;8:1701–1709. doi: 10.1111/j.1743-6109.2011.02259.x. [DOI] [PubMed] [Google Scholar]
- Esposito K., Ciotola M., Giugliano F., Bisogni C., Schisano B., Autorino R., Giugliano D. Association of body weight with sexual function in women. International Journal of Impotence Research. 2007;19:353–357. doi: 10.1038/sj.ijir.3901548. [DOI] [PubMed] [Google Scholar]
- Everett B. G. Sexual orientation disparities in sexually transmitted infections: Examining the intersection between sexual identity and sexual behavior. Archives of Sexual Behavior. 2013;42:225–236. doi: 10.1007/s10508-012-9902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakoya A., Lamba H., Mackie N., Nandwani R., Brown A., Bernard E., Gazzard B. British HIV Association, BASHH, and FSRH guidelines for the management of the sexual and reproductive health of people living with HIV infection 2008. HIV Medicine. 2008;9:681–720. doi: 10.1111/j.1468-1293.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Farrell R. M., Metcalfe J. S., McGowan M. L., Weise K. L., Agatisa P. K., Berg J. Emerging ethical issues in reproductive medicine: Are bioethics educators ready? Hastings Center Report. 2014;44:21–29. doi: 10.1002/hast.354. [DOI] [PubMed] [Google Scholar]
- Fataneh G., Marjan M. H., Nasrin R., Taraneh T. Sexual function in Iranian women using different methods of contraception. Journal of Clinical Nursing. 2013;22:3016–3023. doi: 10.1111/jocn.12289. [DOI] [PubMed] [Google Scholar]
- Fennell J. “And isn’t that the point?”: Pleasure and contraceptive decisions. Contraception. 2014;89:264–270. doi: 10.1016/j.contraception.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Fine M. Sexuality, schooling, and adolescent females: The missing discourse of desire. Harvard Educational Review. 1988;58:29–54. doi: 10.17763/haer.58.1.u0468k1v2n2n8242. [DOI] [Google Scholar]
- Forbes M. K., Baillie A. J., Schniering C. A. Critical flaws in the Female Sexual Function Index and the International Index of Erectile Function. Journal of Sex Research. 2014;51:485–491. doi: 10.1080/00224499.2013.876607. [DOI] [PubMed] [Google Scholar]
- Fortenberry J. D., Hensel D. J. The association of sexual interest and sexual behaviors among adolescent women: A daily diary perspective. Hormones and Behavior. 2011;59:739–744. doi: 10.1016/j.yhbeh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. G., Higgins J. A., Biggs M. A., McCain C., Holtby S., Brindis C. D. Willingness to have unprotected sex. Journal of Sex Research. 2012;49:61–68. doi: 10.1080/00224499.2011.572307. [DOI] [PubMed] [Google Scholar]
- Foster D. G., Higgins J. A., Karasek D., Ma S., Grossman D. Attitudes toward unprotected intercourse and risk of pregnancy among women seeking abortion. Women’s Health Issues. 2012;22:e149–e155. doi: 10.1016/j.whi.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Free C., Ogden J., Lee R. Young women’s contraception use as a contextual and dynamic behaviour: A qualitative study. Psychology and Health. 2005;20:673–690. doi: 10.1080/08870440412331337110. [DOI] [Google Scholar]
- Free C., Roberts I., McGuire M. Sex workers’ accounts of condom use: Implications for condom production, promotion and health policy. Journal of Family Planning and Reproductive Health Care. 2007;33:107–111. doi: 10.1783/147118907780254006. [DOI] [PubMed] [Google Scholar]
- Freundl G., Sivin I., Batár I. State-of-the-art of non-hormonal methods of contraception: IV. Natural family planning. European Journal of Contraception and Reproductive Health Care. 2010;15:113–123. doi: 10.3109/13625180903545302. [DOI] [PubMed] [Google Scholar]
- Frost J. J., Lindberg L. D., Finer L. B. Young adults’ contraceptive knowledge, norms, and attitudes: Associations with risk of unintended pregnancy. Perspectives on Sexual and Reproductive Health. 2012;44:107–116. doi: 10.1363/psrh.2012.44.issue-2. [DOI] [PubMed] [Google Scholar]
- Frost J. J., Singh S., Finer L. B. Factors associated with contraceptive use and nonuse, United States, 2004. Perspectives on Sexual and Reproductive Health. 2007a;39:90–99. doi: 10.1363/3909007. [DOI] [PubMed] [Google Scholar]
- Frost J. J., Singh S., Finer L. B. U.S. Women’s one-year contraceptive use patterns, 2004. Perspectives on Sexual and Reproductive Health. 2007b;39:48–55. doi: 10.1363/3904807. [DOI] [PubMed] [Google Scholar]
- Gabalci E., Terzioglu F. The effect of family planning methods used by women of reproductive age on their sexual life. Sexuality and Disability. 2010;28:275–285. doi: 10.1007/s11195-010-9161-9. [DOI] [Google Scholar]
- Garbers S., Correa N., Tobier N., Blust S., Chiasson M. A. Association between symptoms of depression and contraceptive method choices among low-income women at urban reproductive health centers. Maternal and Child Health Journal. 2010;14:102–109. doi: 10.1007/s10995-008-0437-y. [DOI] [PubMed] [Google Scholar]
- Garcia S. G., Yam E. A., Firestone M. “No party hat, no party”: Successful condom use in sex work in Mexico and the Dominican Republic. Reproductive Health Matters. 2006;14:53–62. doi: 10.1016/S0968-8080(06)28259-4. [DOI] [PubMed] [Google Scholar]
- Gardella B., Porru D., Nappi R. E., Daccò M. D., Chiesa A., Spinillo A. Interstitial cystitis is associated with vulvodynia and sexual dysfunction: A case-control study. Journal of Sexual Medicine. 2011;8:1726–1734. doi: 10.1111/j.1743-6109.2011.02251.x. [DOI] [PubMed] [Google Scholar]
- Gebhardt W. A., Kuyper L., Dusseldorp E. Condom use at first intercourse with a new partner in female adolescents and young adults: The role of cognitive planning and motives for having sex. Archives of Sexual Behavior. 2006;35:213–219. doi: 10.1007/s10508-005-9003-5. [DOI] [PubMed] [Google Scholar]
- Gezginc K., Balci O., Karatayli R., Colakoglu M. C. Contraceptive efficacy and side effects of Implanon. European Journal of Contraception and Reproductive Health Care. 2007a;12:362–365. doi: 10.1080/13625180701548040. [DOI] [PubMed] [Google Scholar]
- Gilley B. J. “Snag bags”: Adapting condoms to community values in Native American communities. Culture, Health, and Sexuality. 2006;8:559–570. doi: 10.1080/13691050600891917. [DOI] [PubMed] [Google Scholar]
- Gilliam M. L., Warden M., Goldstein C., Tapia B. Concerns about contraceptive side effects among young Latinas: A focus-group approach. Contraception. 2004;70:299–305. doi: 10.1016/j.contraception.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Gillmore M. R., Chen A. C., Haas S. A., Kopak A. M., Robillard A. G. Do family and parenting factors in adolescence influence condom use in early adulthood in a multiethnic sample of young adults? Journal of Youth and Adolescence. 2011;40:1503–1518. doi: 10.1007/s10964-011-9631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg F. D., Rapp R. Conceiving the New World Order: The global politics of reproduction. Berkeley: University of California Press; 1995. [Google Scholar]
- Gipson J. D., Hindin M. J. “Marriage means having children and forming your family, so what is the need of discussion?”: Communication and negotiation of childbearing preferences among Bangladeshi couples. Culture, Health, and Sexuality. 2007;9:185–198. doi: 10.1080/13691050601065933. [DOI] [PubMed] [Google Scholar]
- Goicolea I., Wulff M., Sebastian M. S., Öhman A. Adolescent pregnancies and girls’ sexual and reproductive rights in the Amazon Basin of Ecuador: An analysis of providers’ and policy makers’ discourses. BMC International Health and Human Rights. 2010;10:12. doi: 10.1186/1472-698X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R. B. Guarding against coercion while ensuring access: A delicate balance. Guttmacher Policy Review. 2014;17:8–14. [Google Scholar]
- Goldey K. L., Avery L. R., van Anders S. M. Sexual fantasies and gender/sex: A multimethod approach with quantitative content analysis and hormonal responses. Journal of Sex Research. 2014;51:917–931. doi: 10.1080/00224499.2013.798611. [DOI] [PubMed] [Google Scholar]
- Goldey K. L., van Anders S. M. Sexy thoughts: Effects of sexual cognitions on testosterone, cortisol, and arousal in women. Hormones and Behavior. 2011;59:754–764. doi: 10.1016/j.yhbeh.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Goldman J. D. G. Responding to parental objections to school sexuality education: A selection of 12 objections. Sex Education: Sexuality, Society, and Learning. 2008;8:415–438. doi: 10.1080/14681810802433952. [DOI] [Google Scholar]
- Goldstein A., Burrows L., Goldstein I. Can oral contraceptives cause vestibulodynia? Journal of Sexual Medicine. 2010;7:1585–1587. doi: 10.1111/j.1743-6109.2009.01685.x. [DOI] [PubMed] [Google Scholar]
- Gomez A. M., Clark J. B. The relationship between contraceptive features preferred by young women and interest in IUDs: An exploratory analysis. Perspectives on Sexual and Reproductive Health. 2014;46:157–163. doi: 10.1363/psrh.2014.46.issue-3. [DOI] [PubMed] [Google Scholar]
- Göretzlehner G., Waldmann-Rex S., Schramm G. A. Extended cycles with the combined oral contraceptive chlormadinone acetate 2 mg/ethinylestradiol 0.03 mg: Pooled analysis of data from three large-scale, non-interventional, observational studies. Clinical Drug Investigation. 2011;31:269–277. doi: 10.2165/11586720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gorgen H., Api M., Akça A., Cetin A. Use of the levonorgestrel-IUS in the treatment of menorrhagia: Assessment of quality of life in Turkish users. Archives of Gynecology and Obstetrics. 2009;279:835–840. doi: 10.1007/s00404-008-0834-x. [DOI] [PubMed] [Google Scholar]
- Grady W. R., Klepinger D. H., Billy J. O., Cubbins L. A. The role of relationship power in couple decisions about contraception in the U.S. Journal of Biosocial Science. 2010;42:307–323. doi: 10.1017/S0021932009990575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graesslin O., Barjot P., Hoffet M., Cohen D., Vaillant P., Clerson P. The EVAPIL scale, a new tool to assess tolerance of oral contraceptives. Contraception. 2009;80:540–554. doi: 10.1016/j.contraception.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Graham C. A., Bancroft J. Hormonal contraceptives and women’s sexuality: A comment on Burrows et al. Journal of Sexual Medicine. 2013a;10:611–612. doi: 10.1111/jsm.2013.10.issue-2. [DOI] [PubMed] [Google Scholar]
- Graham C. A., Bancroft J. Oral contraceptives and women’s sexuality: Commentary on Roberts, Cobey, Klapilová, and Havlíček (2013) Archives of Sexual Behavior. 2013b;42:1377–1378. doi: 10.1007/s10508-013-0157-2. [DOI] [PubMed] [Google Scholar]
- Graham C. A., Bancroft J., Doll H. A., Greco T., Tanner A. Does oral contraceptive-induced reduction in free testosterone adversely affect the sexuality or mood of women? Psychoneuroendocrinology. 2007;32:246–255. doi: 10.1016/j.psyneuen.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Graham C. A., Ramos R., Bancroft J., Maglaya C., Farley T. M. The effects of steroidal contraceptives on the well-being and sexuality of women: A double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception. 1995;52:363–369. doi: 10.1016/0010-7824(95)00226-X. [DOI] [PubMed] [Google Scholar]
- Granzow K. De-constructing “choice”: The social imperative and women’s use of the birth control pill. Culture, Health, and Sexuality. 2007;9:43–54. doi: 10.1080/13691050600963948. [DOI] [PubMed] [Google Scholar]
- Granzow K. The “nonmenstrual woman” in the new millennium? Discourses on menstrual suppression in the first decade of extended cycle oral contraception use in Canada. Culture, Health, and Sexuality. 2014;16:620–633. doi: 10.1080/13691058.2014.896475. [DOI] [PubMed] [Google Scholar]
- Gubrium A. “I’ve lost my mojo, baby”: A narrative perspective on the effect of Depo-Provera on libido. Sexuality Research and Social Policy: A Journal of the NSRC. 2011;8:321–334. doi: 10.1007/s13178-011-0055-0. [DOI] [Google Scholar]
- Gubrium A. C., Torres M. I. The message is in the bottle: Latino youth communicating double standard ideologies through PhotoVoice. American Journal of Health Education. 2013;44:146–155. doi: 10.1080/19325037.2013.767735. [DOI] [Google Scholar]
- Guida M., Cibarelli F., Troisi J., Gallo A., Palumbo A. R., Di Spiezio Sardo A. Sexual life impact evaluation of different hormonal contraceptives on the basis of their methods of administration. Archives of Gynecology and Obstetrics. 2014;290:1239–1247. doi: 10.1007/s00404-014-3323-4. [DOI] [PubMed] [Google Scholar]
- Guzick D. S., Huang L.-S., Broadman B. A., Nealon M., Hornstein M. D. Randomized trial of leuprolide versus continuous oral contraceptives in the treatment of endometriosis-associated pelvic pain. Fertility and Sterility. 2011;95:1568–1573. doi: 10.1016/j.fertnstert.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmesmäki K., Hurskainen R., Teperi J., Grenman S., Kivelä A., Kujansuu E., Paavonen J. The effect of hysterectomy or levonorgestrel-releasing intrauterine system on sexual functioning among women with menorrhagia: A 5-year randomised controlled trial. BJOG: An International Journal of Obstetrics and Gynaecology. 2007;114:563–568. doi: 10.1111/j.1471-0528.2007.01306.x. [DOI] [PubMed] [Google Scholar]
- Harris K. M. The Add Health Study: Design and accomplishments. Chapel Hill: Carolina Population Center, University of North Carolina at Chapel Hill; 2013. [Google Scholar]
- Hatcher R. A. Contraceptive technology. 20th ed. New York, NY: Ardent Media; 2011. [Google Scholar]
- Hensel D. J., Newcamp J., Miles J., Fortenberry J. D. Picturing sexual spaces in everyday life: Exploring the construction of sexuality and sexual behavior among early adult women. Sexuality Research and Social Policy. 2011;8:267–281. doi: 10.1007/s13178-011-0066-x. [DOI] [Google Scholar]
- Hensel D. J., Stupiansky N. W., Herbenick D., Dodge B., Reece M. Sexual pleasure during condom-protected vaginal sex among heterosexual men. Journal of Sexual Medicine. 2012;9:1272–1276. doi: 10.1111/jsm.2012.9.issue-5. [DOI] [PubMed] [Google Scholar]
- Herzberg B. N., Draper K. C., Johnson A. L., Nicol G. C. Oral contraceptives, depression, and libido. British Medical Journal. 1971;3:495–500. doi: 10.1136/bmj.3.5773.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Davis A. R. Contraceptive sex acceptability: A commentary, synopsis, and agenda for future research. Contraception. 2014;90:4–10. doi: 10.1016/j.contraception.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Fennell J. L. Providing for women’s pleasure in the next generation of condoms. Journal of Sexual Medicine. 2013;10:3151–3153. doi: 10.1111/jsm.2013.10.issue-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Gregor L., Mathur S., Nakyanjo N., Nalugoda F., Santelli J. S. Use of withdrawal (coitus interruptus) for both pregnancy and HIV prevention among young adults in Rakai, Uganda. Journal of Sexual Medicine. 2014;11:2421–2427. doi: 10.1111/jsm.2014.11.issue-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Hirsch J. S. The pleasure deficit: Revisiting the “sexuality connection” in reproductive health. Perspectives on Sexual and Reproductive Health. 2007;39:240–247. doi: 10.1363/3924007. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Hirsch J. S. Pleasure, power, and inequality: Incorporating sexuality into research on contraceptive use. American Journal of Public Health. 2008;98:1803–1813. doi: 10.2105/AJPH.2007.115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Hirsch J. S., Trussell J. Pleasure, prophylaxis and procreation: A qualitative analysis of intermittent contraceptive use and unintended pregnancy. Perspectives on Sexual and Reproductive Health. 2008;40:130–137. doi: 10.1363/4013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Hoffman S., Graham C. A., Sanders S. A. Relationships between condoms, hormonal methods, and sexual pleasure and satisfaction: An exploratory analysis from the women’s well-being and sexuality study. Sexual Health. 2008;5:321–330. doi: 10.1071/SH08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Ryder K., Skarda G., Koepsel E., Bennett E. A. The sexual acceptability of intrauterine contraception: A qualitative study of young adult women. Perspectives on Sexual and Reproductive Health. 2015;47:115–122. doi: 10.1363/47e4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Smith N. K., Sanders S. A., Schick V., Herbenick D., Reece M., Fortenberry J. D. Dual method use at last sexual encounter: A nationally representative, episode-level analysis of U.S. men and women. Contraception. 2014;90:399–406. doi: 10.1016/j.contraception.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Tanner A. E., Janssen E. Arousal loss related to safer sex and risk of pregnancy: Implications for women’s and men’s sexual health. Perspectives on Sexual and Reproductive Health. 2009;41:150–157. doi: 10.1363/psrh.2009.41.issue-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Wang Y. The role of young adults’ pleasure attitudes in shaping condom use. American Journal of Public Health. 2015a;105:1329–1332. doi: 10.2105/AJPH.2015.302567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Wang Y. Which young adults are most likely to use withdrawal? The importance of pregnancy attitudes and sexual pleasure. Contraception. 2015b;91:320–327. doi: 10.1016/j.contraception.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoga L. A. K., Rodolpho J. R. C., Penteado P. E. D., Borges A. L. V., Alvarez R. E. C. Religiosity and sexuality: Counseling provided by Brazilian Protestant pastors. Sexual and Reproductive Healthcare. 2013;4:57–63. doi: 10.1016/j.srhc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Hong L., Wu K., Fan D. Effect of testosterone therapy versus other factors on the self-reported sexual satisfaction of premenopausal women. Annals of Internal Medicine. 2008;149(5):361–362. doi: 10.7326/0003-4819-149-5-200809020-00020. 361; author reply. [DOI] [PubMed] [Google Scholar]
- Huang Y., Merkatz R., Zhu H., Roberts K., Sitruk-Ware R., Cheng L. The free perinatal/postpartum contraceptive services project for migrant women in Shanghai: Effects on the incidence of unintended pregnancy. Contraception. 2014;89:521–527. doi: 10.1016/j.contraception.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Hust S. J. T., Brown J. D., L’Engle K. L. Boys will be boys and girls better be prepared: An analysis of the rare sexual health messages in young adolescents’ media. Mass Communication and Society. 2008;11:3–23. doi: 10.1080/15205430701668139. [DOI] [Google Scholar]
- Hutchinson M. K., Thompson A. C., Cederbaum J. A. Multisystem factors contributing to disparities in preventive health care among lesbian women. Journal of Obstetric, Gynecological, and Neonatal Nursing. 2006;35:393–402. doi: 10.1111/j.1552-6909.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- Johnson C. V., Mimiaga M. J., Bradford J. Health care issues among lesbian, gay, bisexual, transgender, and intersex (LGBTI) populations in the United States: Introduction. Journal of Homosexuality. 2008;54:213–224. doi: 10.1080/00918360801982025. [DOI] [PubMed] [Google Scholar]
- Jones H. E., Chaikummao S., Van De Wijgert J. H., Friedland B. A., Manopaiboon C., Witwatwongwana P., Kilmarx P. H. Acceptability of a carrageenan-based candidate vaginal microbicide and matching placebo: Findings from a Phase II safety trial among women in Chiang Rai, Thailand. Journal of Women’s Health. 2009;18:1003–1010. doi: 10.1089/jwh.2008.0862. [DOI] [PubMed] [Google Scholar]
- Jones R. K. Beyond birth control: The overlooked benefits of oral contraceptive pills. New York, NY: Guttmacher Institute; 2011. [Google Scholar]
- Jones R. K., Darroch J. E., Henshaw S. K. Contraceptive use among U.S. women having abortions in 2000–2001. Perspectives on Sexual and Reproductive Health. 2002;34:294–303. doi: 10.2307/3097748. [DOI] [PubMed] [Google Scholar]
- Jones R. K., Lindberg L.D., Higgins J.A. Contraception. 2014;9:416–421. doi: 10.1016/j.contraception.2014.04.016. Pull and pray or extra protection? Contraceptive strategies involving withdrawal among US adult women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozkowski K. N., Herbenick D., Schick V., Reece M., Sanders S. A., Fortenberry J. D. Women’s perceptions about lubricant use and vaginal wetness during sexual activities. Journal of Sexual Medicine. 2013;10:484–492. doi: 10.1111/jsm.2013.10.issue-2. [DOI] [PubMed] [Google Scholar]
- Kaneko N. Association between condom use and perceived barriers to and self-efficacy of safe sex among young women in Japan. Nursing and Health Sciences. 2007;9:284–289. doi: 10.1111/nhs.2007.9.issue-4. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. L., Anderson R. A. Contraception and beyond: The health benefits of services provided at family planning centers. New York, NY: Guttmacher Institute; 2013. [Google Scholar]
- Kemmeren J. M., Algra A., Grobbee D. E. Third generation oral contraceptives and risk of venous thrombosis: Meta-analysis. British Medical Journal. 2001;323:131. doi: 10.1136/bmj.323.7305.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh L. A. Women’s contraceptive decision-making: Juggling the needs of the sexual body and the fertile body. Women’s Health. 2006;42:83–103. doi: 10.1300/J013v42n04_05. [DOI] [PubMed] [Google Scholar]
- Kottmel A., Ruether‐Wolf K. V., Bitzer J. Do gynecologists talk about sexual dysfunction with their patients? Journal of Sexual Medicine. 2014;11:2048–2054. doi: 10.1111/jsm.12603. [DOI] [PubMed] [Google Scholar]
- Kraft J. M., Harvey S. M., Thorburn S., Henderson J. T., Posner S. F., Galavotti C. Intervening with couples: Assessing contraceptive outcomes in a randomized pregnancy and HIV/STD risk reduction intervention trial. Women’s Health Issues. 2007;17:52–60. doi: 10.1016/j.whi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Küçük M., Aksu H., Sezer S. D. Misconceptions about the side effects of combined oral contraceptive pills. Gynecological Endocrinology. 2012;28:282–285. doi: 10.3109/09513590.2011.613502. [DOI] [PubMed] [Google Scholar]
- Kuortti M., Lindfors P. Girls’ stories about their first sexual intercourse: Readiness, affection, and experience-seeking in the process of growing into womanhood. Sexuality and Culture: An Interdisciplinary Quarterly. 2014;18:505–526. doi: 10.1007/s12119-013-9206-1. [DOI] [Google Scholar]
- Latka M. H., Kapadia F., Fortin P. The female condom: Effectiveness and convenience, not “female control,” valued by U.S. urban adolescents. AIDS Education and Prevention. 2008;20:160–170. doi: 10.1521/aeap.2008.20.2.160. [DOI] [PubMed] [Google Scholar]
- Lee M., Morgan M., Rapkin A. Clitoral and vulvar vestibular sensation in women taking 20 mcg ethinyl estradiol combined oral contraceptives: A preliminary study. Journal of Sexual Medicine. 2011;8:213–218. doi: 10.1111/jsm.2011.8.issue-1. [DOI] [PubMed] [Google Scholar]
- Lessard L. N., Karasek D., Ma S., Darney P., Deardorff J., Lahiff M., Foster D. G. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspectives on Sexual and Reproductive Health. 2012;44:194–200. doi: 10.1363/4419412. [DOI] [PubMed] [Google Scholar]
- Levin D. S. Let’s talk about sex education: Exploring youth perspectives, implicit messages, and unexamined implications of sex education in schools. 2010 http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=psyh&AN=2010-99220-286&site=ehost-live&scope=site ProQuest Information and Learning, U.S. (71). Retrieved from. Available from EBSCOhost Psych database.
- Lexchin J., Bero L. A., Djulbegovic B., Clark O. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. British Medical Journal. 2003;326:1167–1170. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley L. L., Walsemann K. M. Sexual orientation and risk of pregnancy among New York City high school students. American Journal of Public Health. 2015;105:1379–1386. doi: 10.2105/AJPH.2015.302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe P. Contraception and heterosex: An intimate relationship. Sexualities. 2005;8:75–92. doi: 10.1177/1363460705049575. [DOI] [Google Scholar]
- Luna Z., Luker K. Reproductive justice. Annual Review of Law and Social Science. 2013;9:327–352. doi: 10.1146/annurev-lawsocsci-102612-134037. [DOI] [Google Scholar]
- Mabrouk M., Montanari G., Di Donato N., Del Forno S., Frascà C., Geraci E., Seracchioli R. What is the impact on sexual function of laparoscopic treatment and subsequent combined oral contraceptive therapy in women with deep infiltrating endometriosis? Journal of Sexual Medicine. 2012;9:770–778. doi: 10.1111/jsm.2012.9.issue-3. [DOI] [PubMed] [Google Scholar]
- Machado R. B., De Melo N. R., Prota F. E., Lopes G. P., Megale A. Women’s knowledge of health effects of oral contraceptives in five Brazilian cities. Contraception. 2012;86:698–703. doi: 10.1016/j.contraception.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Mack N., Grey T. G., Amsterdam A., Williamson N., Matta C. I. Introducing female condoms to female sex workers in Central America. International Perspectives on Sexual and Reproductive Health. 2010;36:149–156. doi: 10.1363/3614910. [DOI] [PubMed] [Google Scholar]
- Makuch M. Y., Osis M. J., Petta C. A., dePádua K. S., Bahamondes L. Menstrual bleeding: Perspective of Brazilian women. Contraception. 2011;84:622–627. doi: 10.1016/j.contraception.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Manuel A. Filling in the gaps: The need for further investigation to understand the effects of oral contraceptives on female sexual satisfaction. Journal of Sexual Medicine. 2013;10:1897–1897. doi: 10.1111/jsm.2013.10.issue-7. [DOI] [PubMed] [Google Scholar]
- Marchi N. M., De Alvarenga A. T., Osis M. J. D., Bahamondes L. Contraceptive methods with male participation: A perspective of Brazilian couples. International Nursing Review. 2008;55:103–109. doi: 10.1111/inr.2008.55.issue-1. [DOI] [PubMed] [Google Scholar]
- Martin S., Blanchard K., Manopaiboon C., Chaikummao S., Schaffer K., Friedland B., Kilmarx P. H. Carraguard acceptability among men and women in a couples study in Thailand. Journal of Women’s Health. 2010;19:1561–1567. doi: 10.1089/jwh.2009.1362. [DOI] [PubMed] [Google Scholar]
- Mathenjwa T., Maharaj P. “Female condoms give women greater control”: A qualitative assessment of the experiences of commercial sex workers in Swaziland. European Journal of Contraception and Reproductive Health Care. 2012;17:383–392. doi: 10.3109/13625187.2012.694147. [DOI] [PubMed] [Google Scholar]
- McCave E. L. “It is not just about me”: A relational understanding of young women’s sexual negotiations. Smith College Studies in Social Work. 2010;80:53–69. doi: 10.1080/00377310903504924. [DOI] [Google Scholar]
- McKinney J. R., Halpern M., Levison J., Callender G., Lerebours-Nadal L., Fernandez-Esquer M. E. Factors influencing use of family planning services among HIV-positive women in the PMTCT program at Clínica de Familia la Romana in the Dominican Republic. Sexuality Research and Social Policy: A Journal of the NSRC. 2013;10:200–207. doi: 10.1007/s13178-013-0115-8. [DOI] [Google Scholar]
- Medley-Rath S. R., Simonds W. Consuming contraceptive control: Gendered distinctions in web-based contraceptive advertising. Culture, Health, and Sexuality. 2010;12:783–795. doi: 10.1080/13691058.2010.489240. [DOI] [PubMed] [Google Scholar]
- Merkatz R. B., Plagianos M., Hoskin E., Cooney M., Hewett P. C., Mensch B. S. Acceptability of the nestorone(r)/ethinyl estradiol contraceptive vaginal ring: Development of a model; implications for introduction. Contraception. 2014;90:514–521. doi: 10.1016/j.contraception.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg H. F., Dolezal C. The Female Sexual Function Index: A methodological critique and suggestions for improvement. Journal of Sex and Marital Therapy. 2007;33:217–224. doi: 10.1080/00926230701267852. [DOI] [PubMed] [Google Scholar]
- Mohamed A. M., El-Sherbiny W. S., Mostafa W. A. Combined contraceptive ring versus combined oral contraceptive (30-mug ethinylestradiol and 3-mg drospirenone) International Journal of Gynecology and Obstetrics. 2011;114:145–148. doi: 10.1016/j.ijgo.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Mojola S. A., Everett B. STD and HIV risk factors among U.S. young adults: Variations by gender, race, ethnicity, and sexual orientation. Perspectives on Sexual and Reproductive Health. 2012;44:125–133. doi: 10.1363/psrh.2012.44.issue-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery E. T., Woodsong C., Musara P., Cheng H., Chipato T., Moench T. R., van der Straten A. An acceptability and safety study of the Duet cervical barrier and gel delivery system in Zimbabwe. Journal of the International AIDS Society. 2010;13:30. doi: 10.1186/1758-2652-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu M. Perceptions around second generation female condoms: Reporting on women’s experiences. Anthropological Notebooks. 2013;19:25–34. [Google Scholar]
- Nappi R. E., Serrani M., Jensen J. T. Noncontraceptive benefits of the estradiol valerate/dienogest combined oral contraceptive: A review of the literature. International Journal of Women’s Health. 2014;6:711–718. doi: 10.2147/IJWH.S65481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleman M., Brewer J., Ayoola A. Reasons for unprotected intercourse in adult women: A qualitative study. Journal of Midwifery and Women’s Health. 2007;52:148–152. doi: 10.1016/j.jmwh.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Nettleman M., Brewer J., Ayoola A. Why women risk unintended pregnancy. Journal of Family Practice. 2009;58:E1–5. [PubMed] [Google Scholar]
- Nguyen L. N., Jamieson M. A. Adolescent users of an online contraception selection tool: How user preferences and characteristics differ from those of adults. Journal of Pediatric and Adolescent Gynecology. 2011;24:317–319. doi: 10.1016/j.jpag.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Nishtar N. A., Sami N., Faruqi A., Khowaja S., Ul-Hasnain F. Myths and fallacies about male contraceptive methods: A qualitative study amongst married youth in slums of Karachi, Pakistan. Global Journal of Health Science. 2013;5:84–93. doi: 10.5539/gjhs.v5n2p84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman L. R. Communication. In: Loue S., editor. Mental health practitioner’s guide to HIV/AIDS. New York, NY: Springer Science + Business Media; 2013. pp. 147–149. [Google Scholar]
- Okunlola M. A., Morhason-Bello I. O., Owonikoko K. M., Adekunle A. O. Female condom awareness, use, and concerns among Nigerian female undergraduates. Journal of Obstetrics and Gynaecology. 2006;26:353–356. doi: 10.1080/01443610600613516. [DOI] [PubMed] [Google Scholar]
- Ong J., Temple-Smith M., Wong W., McNamee K., Fairley C. Prevalence of and characteristics associated with use of withdrawal among women in Victoria, Australia. Perspectives on Sexual and Reproductive Health. 2013;45:74–78. doi: 10.1363/psrh.2013.45.issue-2. [DOI] [PubMed] [Google Scholar]
- Ortayli N., Bulut A., Ozugurlu M., Çokar M. Why withdrawal? Why not withdrawal? Men’s perspectives. Reproductive Health Matters. 2005;13:164–173. doi: 10.1016/S0968-8080(05)25175-3. [DOI] [PubMed] [Google Scholar]
- Osei I. F., Harding Mayhew S., Biekro L., Collumbien M. Fertility decisions and contraceptive use at different stages of relationships: Windows of risk among men and women in Accra. International Perspectives on Sexual and Reproductive Health. 2014;40:135–143. doi: 10.1363/4013514. [DOI] [PubMed] [Google Scholar]
- O’Sullivan L. F., Cooper-Serber E., Kubeka M., Harrison A. Body concepts: Beliefs about the body and efforts to prevent HIV and pregnancy among a sample of young adults in South Africa. International Journal of Sexual Health. 2007;19:69–80. doi: 10.1300/J514v19n02_06. [DOI] [Google Scholar]
- Ott M. A., Shew M. L., Ofner S., Tu W., Fortenberry J. D. The influence of hormonal contraception on mood and sexual interest among adolescents. Archives of Sexual Behavior. 2008;37:605–613. doi: 10.1007/s10508-007-9302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudshoorn N. The male pill: A biography of a technology in the making. Durham, NC: Duke University Press; 2003. [Google Scholar]
- Oyedeji O. A., Cassimjee R. A gendered study of young adult contraceptive use at one university in Kwazulu-Natal. Curationis. 2006;29:7–14. doi: 10.4102/curationis.v29i3.1088. [DOI] [PubMed] [Google Scholar]
- Panchalee T., Wongwananuruk T., Augsuwatana S., Sirimai K., Tammakunto M., Neangton C., Inthawong J. Prevalence and associating factors of sexual dysfunction in women who use intrauterine device (IUD) for contraception. Journal of the Medical Association of Thailand. 2014;97:20–27. [PubMed] [Google Scholar]
- Pastor Z., Holla K., Chmel R. The influence of combined oral contraceptives on female sexual desire: A systematic review. European Journal of Contraception and Reproductive Health Care. 2013;18:27–43. doi: 10.3109/13625187.2012.728643. [DOI] [PubMed] [Google Scholar]
- Paterson L. Q., Amsel R., Binik Y. M. Pleasure and pain: The effect of (almost) having an orgasm on genital and nongenital sensitivity. Journal of Sexual Medicine. 2013;10:1531–1544. doi: 10.1111/jsm.2013.10.issue-6. [DOI] [PubMed] [Google Scholar]
- Pearson J. D. Young women’s sexual agency in the transition to adulthood. 2009 http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=psyh&AN=2009-99050-061&site=ehost-live&scope=site ProQuest Information and Learning, U.S. (69). Retrieved from. Available from EBSCOhost Psych database.
- Peipert J. F., Zhao Q., Allsworth J. E., Petrosky E., Madden T., Eisenberg D., Secura G. Continuation and satisfaction of reversible contraception. Obstetrics and Gynecology. 2011;117:1105–1113. doi: 10.1097/AOG.0b013e31821188ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. J., van Driel F. T., Jansen W. H. Silencing women’s sexuality: Global AIDS policies and the case of the female condom. Journal of the International AIDS Society. 2013;16:18452. doi: 10.7448/IAS.16.1.18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott A., Knerr W., Maher D. Promoting protection and pleasure: Amplifying the effectiveness of barriers against sexually transmitted infections and pregnancy. The Lancet. 2006;368:2028–2031. doi: 10.1016/S0140-6736(06)69810-3. [DOI] [PubMed] [Google Scholar]
- Piccinelli M., Wilkinson G. Gender differences in depression: Critical review. British Journal of Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Puts D. A., Pope L. E. Moderators, mates, and matchmakers: Effects of oral contraceptives on sexual desire may also depend on partners’ behavior and the role of female choice. Archives of Sexual Behavior. 2013;42:1379–1380. doi: 10.1007/s10508-013-0165-2. [DOI] [PubMed] [Google Scholar]
- Rahnama P., Hidarnia A., Amin Shokravi F., Kazemnejad A., Ghazanfari Z., Montazeri A. Withdrawal users’ experiences of and attitudes to contraceptive methods: A study from eastern district of Tehran, Iran. BMC Public Health. 2010;10:779. doi: 10.1186/1471-2458-10-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph M. E., Pinkerton S. D., Bogart L. M., Cecil H., Abramson P. R. Sexual pleasure and condom use. Archives of Sexual Behavior. 2007;36:844–848. doi: 10.1007/s10508-007-9213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reproductive Justice Collective . Reproductive justice. Milwaukee, WI:: 2015. [Google Scholar]
- Rivera R., Yacobson I., Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. American Journal of Obstetrics and Gynecology. 1999;181:1263–1269. doi: 10.1016/S0002-9378(99)70120-1. [DOI] [PubMed] [Google Scholar]
- Roberts D. E. Killing the Black body: Race, reproduction, and the meaning of liberty. New York, NY: Vintage; 1997. [Google Scholar]
- Rosario M., Corliss H. L., Everett B. G., Reisner S. L., Austin S. B., Buchting F. O., Birkett M. Sexual orientation disparities in cancer-related risk behaviors of tobacco, alcohol, sexual behaviors, and diet and physical activity: Pooled youth risk behavior surveys. American Journal of Public Health. 2014;104:245–254. doi: 10.2105/AJPH.2013.301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen R., Brown C., Heiman J., Leiblum S., Meston C., Shabsigh R., D’Agostino R., Jr. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex and Marital Therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Roumen F. The contraceptive vaginal ring compared with the combined oral contraceptive pill: A comprehensive review of randomized controlled trials. Contraception. 2007;75:420–429. doi: 10.1016/j.contraception.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Roumen F. Review of the combined contraceptive vaginal ring, Nuvaring. Journal of Therapeutics and Clinical Risk Management. 2008;4:441–451. doi: 10.2147/tcrm.s1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini R., Cagiano R. Comparison profiles of cycle control, side effects, and sexual satisfaction of three hormonal contraceptives. Contraception. 2006;74:220–223. doi: 10.1016/j.contraception.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Saewyc E. M., Bearinger L. H., Blum R. W., Resnick M. D. Sexual intercourse abuse and pregnancy among adolescent women: Does sexual orientation make a difference? Family Planning Perspectives. 1999;31:127–131. [PubMed] [Google Scholar]
- Saewyc E. M., Poon C. S., Homma Y., Skay C. L. Stigma management? The links between enacted stigma and teen pregnancy trends among gay, lesbian, and bisexual students in British Columbia. Canadian Journal of Human Sexuality. 2008;17:123–139. [PMC free article] [PubMed] [Google Scholar]
- Sahin-Hodoglugil N. N., Montgomery E., Kacanek D., Morar N., Mtetwa S., Nkala B., van der Straten A. User experiences and acceptability attributes of the diaphragm and lubricant gel in an HIV prevention trial in Southern Africa. AIDS Care. 2011;23:1026–1034. doi: 10.1080/09540121.2010.543879. [DOI] [PubMed] [Google Scholar]
- Salonia A., Nappi R. E., Pontillo M., Smeraldi A., Briganti A., Fabbri F., Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Hormones and Behavior. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sandberg S. F. Embodying intimacy: Premarital romantic relationships, sexuality, and contraceptive use among young women in contemporary Tokyo. 2011 http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=psyh&AN=2011-99010-596&site=ehost-live&scope=site ProQuest Information and Learning, U.S. (71). Retrieved from. Available from EBSCOhost Psych database.
- Sanders J. N., Smith N. K., Higgins J. A. The intimate link: A systematic review of highly effective reversible contraception and women’s sexual experience. Clinical Obstetrics and Gynecology. 2014;57:777–789. doi: 10.1097/GRF.0000000000000058. [DOI] [PubMed] [Google Scholar]
- Sanders S. A., Graham C. A., Bass J. L., Bancroft J. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception. 2001;64:51–58. doi: 10.1016/S0010-7824(01)00218-9. [DOI] [PubMed] [Google Scholar]
- Sanders S. A., Hill B. J., Crosby R. A., Janssen E. Correlates of condom-associated erection problems in young, heterosexual men: Condom fit, self-efficacy, perceptions, and motivations. AIDS and Behavior. 2014;18:128–134. doi: 10.1007/s10461-013-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S. A., Reece M., Herbenick D., Schick V., Dodge B., Fortenberry J. D. Condom use during most recent vaginal intercourse event among a probability sample of adults in the United States. Journal of Sexual Medicine. 2010;7:362–373. doi: 10.1111/jsm.2010.7.issue-s5. [DOI] [PubMed] [Google Scholar]
- Sanders S. A., Yarber W. L., Kaufman E. L., Crosby R. A., Graham C. A., Milhausen R. R. Condom use errors and problems: A global view. Sexual Health. 2012;9:81–95. doi: 10.1071/SH11095. [DOI] [PubMed] [Google Scholar]
- Schaffir J. Hormonal contraception and sexual desire: A critical review. Journal of Sex and Marital Therapy. 2006;32:305–314. doi: 10.1080/00926230600666311. [DOI] [PubMed] [Google Scholar]
- Schaffir J., Fleming M., Waddell V. Patient perceptions regarding effect of gynecological surgery on sexuality. Journal of Sexual Medicine. 2010;7:826–831. doi: 10.1111/jsm.2010.7.issue-2pt1. [DOI] [PubMed] [Google Scholar]
- Schaffir J., Isley M. M., Woodward M. Oral contraceptives vs injectable progestin in their effect on sexual behavior. American Journal of Obstetrics and Gynecology. 2010;203(6):545.e1–545.e5. doi: 10.1016/j.ajog.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Schalet A. T. Not under my roof: Parents, teens, and the culture of sex. Chicago, IL: University of Chicago Press; 2011. [Google Scholar]
- Scorgie F., Smit J. A., Kunene B., Martin-Hilber A., Beksinska M., Chersich M. F. Predictors of vaginal practices for sex and hygiene in Kwazulu-Natal, South Africa: Findings of a household survey and qualitative inquiry. Culture, Health, and Sexuality. 2011;13:381–398. doi: 10.1080/13691058.2010.550321. [DOI] [PubMed] [Google Scholar]
- Sedigheh E., Maryam G. T., Ali B., Mehdi P. Comparison of complications and marital satisfaction in women taking contraceptive ampoules of Cyclofem and LD contraceptive pills. HealthMed. 2012;6:2689–2693. [Google Scholar]
- Severy L. J., Newcomer S. Critical issues in contraceptive and STI acceptability research. Journal of Social Issues. 2005;61:45–65. doi: 10.1111/josi.2005.61.issue-1. [DOI] [Google Scholar]
- Severy L. J., Silver S. E. Two reasonable people: Joint decision-making in fertility regulation. Advances in Population: Psychosocial Perspectives. 1993;1:207–227. doi: 10.1080/0907676x.1993.9961214. [DOI] [PubMed] [Google Scholar]
- Shachner J. Unwrapped: How the Los Angeles County Safer Sex in the Adult Film Industry Act’s condom mandate hurts performers and violates the First Amendment. Health Matrix. 2014;24:345–375. [PubMed] [Google Scholar]
- Shahnazi M., Bayatipayan S., Khalili A. F., Kochaksaraei F. R., Jafarabadi M. A., Banoi K. G., Nahaee J. Comparing the effects of the second- and third-generation oral contraceptives on sexual functioning. Iranian Journal of Nursing and Midwifery Research. 2015;20:47–55. [PMC free article] [PubMed] [Google Scholar]
- Shiely F., Saifuddin M. S. Contraceptive choice and acceptability: The future for STI risk in Kelantan, Malaysia. International Journal of STD and AIDS. 2014;25:219–227. doi: 10.1177/0956462413497699. [DOI] [PubMed] [Google Scholar]
- Shih G., Dube K., Sheinbein M., Borrero S., Dehlendorf C. He’s a real man: A qualitative study of the social context of couples’ vasectomy decisions among a racially diverse population. American Journal of Men’s Health. 2013;7:206–213. doi: 10.1177/1557988312465888. [DOI] [PubMed] [Google Scholar]
- Short M. B., Succop P. A., Rupp R., Rosenthal S. L. Adolescents’ reasons for using a microbicide-like product over time. International Journal of STD and AIDS. 2008;19:115–117. doi: 10.1258/ijsa.2007.007155. [DOI] [PubMed] [Google Scholar]
- Sirkeci I., Cindoglu D. Space, agency, and withdrawal: Birth control choices of women in Turkey. Health Care for Women International. 2012;33:614–630. doi: 10.1080/07399332.2012.655384. [DOI] [PubMed] [Google Scholar]
- Skrzypulec V., Drosdzol A. Evaluation of quality of life and sexual functioning of women using levonorgestrel-releasing intrauterine contraceptive system: Mirena. Collegium Antropolicum. 2008a;32:1059–1068. [PubMed] [Google Scholar]
- Skrzypulec V., Drosdzol A. Evaluation of the quality of life and sexual functioning of women using a 30-microg ethinyloestradiol and 3-mg drospirenone combined oral contraceptive. European Journal of Contraception and Reproductive Health Care. 2008b;13:49–57. doi: 10.1080/13625180701712406. [DOI] [PubMed] [Google Scholar]
- Smit J., McFadyen L., Zuma K., Preston-Whyte E. Vaginal wetness: An underestimated problem experienced by progestogen injectable contraceptive users in South Africa. Social Science and Medicine. 2002;55:1511–1522. doi: 10.1016/S0277-9536(01)00284-2. [DOI] [PubMed] [Google Scholar]
- Smith A., Lyons A., Ferris J., Richters J., Pitts M., Shelley J. Are sexual problems more common in women who have had a tubal ligation? A population-based study of Australian women. BJOG: An International Journal of Obstetrics and Gynaecology. 2010;117:463–468. doi: 10.1111/j.1471-0528.2009.02469.x. [DOI] [PubMed] [Google Scholar]
- Smith N. K., Jozkowski K. N., Sanders S. A. Hormonal contraception and female pain, orgasm, and sexual pleasure. Journal of Sexual Medicine. 2014;11:462–470. doi: 10.1111/jsm.2014.11.issue-2. [DOI] [PubMed] [Google Scholar]
- Sobze Sanou M., Fokam J., Guetiya Wadoum R., Russo G., Onohiol J. F., Djeunang D. B., Colizzi V. Condom perception and prevention of HIV/AIDS infection in Cameroon: Appraisal of knowledge, attitudes, and practices among level one students of the University of Dschang. Igiene E Sanità Pubblica. 2013;69:183–194. [PubMed] [Google Scholar]
- Sonfield A., Hasstedt K., Kavanaugh M. L., Anderson R. The social and economic benefits of women’s ability to determine whether and when to have children. New York, NY: Guttmacher Institute; 2013. [Google Scholar]
- Stewart F. H., Brown B. A., Raine T. R., Weitz T. A., Harper C. C. Adolescent and young women’s experience with the vaginal ring and oral contraceptive pills. Journal of Pediatric and Adolescent Gynecology. 2007;20:345–351. doi: 10.1016/j.jpag.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidham Hall K., Moreau C., Trussell J., Barber J. Young women’s consistency of contraceptive use: Does depression or stress matter? Contraception. 2013;88:641–649. doi: 10.1016/j.contraception.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strufaldi R., Pompei L. M., Steiner M. L., Cunha E. P., Ferreira J. A., Peixoto S., Fernandes C. E. Effects of two combined hormonal contraceptives with the same composition and different doses on female sexual function and plasma androgen levels. Contraception. 2010;82:147–154. doi: 10.1016/j.contraception.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Stuckey B. G. A. Female sexual function and dysfunction in the reproductive years: The influence of endogenous and exogenous sex hormones. Journal of Sexual Medicine. 2008;5:2282–2290. doi: 10.1111/j.1743-6109.2008.00992.x. [DOI] [PubMed] [Google Scholar]
- Sunmola A. M. Evaluating the sexual behaviour, barriers to condom use, and its actual use by university students in Nigeria. AIDS Care. 2005;17:457–465. doi: 10.1080/09540120412331319732. [DOI] [PubMed] [Google Scholar]
- Szarewski A., Von Stenglin A., Rybowski S. Women’s attitudes towards monthly bleeding: Results of a global population-based survey. European Journal of Contraception and Reproductive Health Care. 2012;17:270–283. doi: 10.3109/13625187.2012.684811. [DOI] [PubMed] [Google Scholar]
- Tabari M. G., Moslemi L., Esmaelzadeh S., Bijani A. Comparison of side effects and marital satisfaction between the women taking Cyclofem and depo-medroxyprogesterone contraceptive ampoules. African Journal of Pharmacy and Pharmacology. 2012;6:1933–1937. [Google Scholar]
- Tafuri S., Martinelli D., Germinario C., Prato R. Determining factors for condom use: A survey of young Italian adults. European Journal of Contraception and Reproductive Health Care. 2010;15:24–30. doi: 10.3109/13625180903427683. [DOI] [PubMed] [Google Scholar]
- Tanner A. E., Hensel D. J., Fortenberry J. D. A prospective study of the sexual, emotional, and behavioral correlates associated with young women’s first and usual coital events. Journal of Adolescent Health. 2010;47:20–25. doi: 10.1016/j.jadohealth.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. E., Katzenstein J. M., Zimet G. D., Cox D. S., Cox A. D., Fortenberry J. D. Vaginal microbicide preferences among Midwestern urban adolescent women. Journal of Adolescent Health. 2008;43:349–356. doi: 10.1016/j.jadohealth.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. E., Zimet G., Fortenberry J. D., Reece M., Graham C., Murray M. Young women’s use of a vaginal microbicide surrogate: The role of individual and contextual factors in acceptability and sexual pleasure. Journal of Sex Research. 2009;46:15–23. doi: 10.1080/00224490802398407. [DOI] [PubMed] [Google Scholar]
- Telles Dias P. R., Souto K., Page-Shafer K. Long-term female condom use among vulnerable populations in Brazil. AIDS and Behavior. 2006;10:S67–75. doi: 10.1007/s10461-006-9139-x. [DOI] [PubMed] [Google Scholar]
- Terrell L. R., Tanner A. E., Hensel D. J., Blythe M. J., Fortenberry J. D. Acceptability of the vaginal contraceptive ring among adolescent women. Journal of Pediatric and Adolescent Gynecology. 2011;24:204–210. doi: 10.1016/j.jpag.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Thorburn S., Harvey S. M., Tipton J. Diaphragm acceptability among young women at risk for HIV. Women’s Health. 2006;44:21–39. doi: 10.1300/J013v44n01_02. [DOI] [PubMed] [Google Scholar]
- Tiefer L. Sex is not a natural act and other essays. 2nd ed. Boulder, CO: Westview Press; 2004. [Google Scholar]
- Tiihonen M., Leppänen H.-M., Heikkinen A.-M., Ahonen R. Hormonal contraceptive usersʼ self-reported benefits, adverse reactions, and fears in 2001 and 2007. The Patient: Patient-Centered Outcomes Research. 2008;1:173–180. doi: 10.2165/1312067-200801030-00004. [DOI] [PubMed] [Google Scholar]
- Tolman D. L., Diamond L. M. Desegregating sexuality research: Cultural and biological perspectives on gender and desire. ANNUAL REVIEW OF SEX RESEARCH. 2001;12:33–74. [PubMed] [Google Scholar]
- Tone A. From naughty goods to Nicole Miller: Medicine and the marketing of American contraceptives. Culture Medicine and Psychiatry. 2006;30:249–267. doi: 10.1007/s11013-006-9015-1. [DOI] [PubMed] [Google Scholar]
- Træen B., Gravningen K. The use of protection for sexually transmitted infections (STIs) and unwanted pregnancy among Norwegian heterosexual young adults 2009. Sexuality and Culture: An Interdisciplinary Quarterly. 2011;15:195–212. doi: 10.1007/s12119-011-9090-5. [DOI] [Google Scholar]
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyuki K. Dual protection, relationship dynamics, and women’s empowerment in Brazil. 2014 http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=psyh&AN=2014-99180-014&site=ehost-live&scope=site ProQuest Information and Learning, U.S. (75). Retrieved from. Available from EBSCOhost Psych database.
- Tung W.-C., Cook D. M., Lu M. Sexual behaviors, decisional balance, and self-efficacy among a sample of Chinese college students in the United States. Journal of American College Health. 2012;60:367–373. doi: 10.1080/07448481.2012.663839. [DOI] [PubMed] [Google Scholar]
- van Anders S. M., Goldey K. L., Bell S. N. Measurement of testosterone in human sexuality research: Methodological considerations. Archives of Sexual Behavior. 2014;43:231–250. doi: 10.1007/s10508-013-0123-z. [DOI] [PubMed] [Google Scholar]
- VanderDrift L. E., Agnew C. R., Harvey S. M., Warren J. T. Whose intentions predict? Power over condom use within heterosexual dyads. Health Psychology. 2013;32:1038–1046. doi: 10.1037/a0030021. [DOI] [PubMed] [Google Scholar]
- van der Straten A., Montgomery E., Mavedzenge S., Musara P., Cheng H., Lutnick A., Woodsong C. Preference between precoital and daily use of Duet and BufferGel in Zimbabwe. AIDS and Behavior. 2012;16:1799–1807. doi: 10.1007/s10461-012-0256-4. [DOI] [PubMed] [Google Scholar]
- Van Dijk M. G., Pineda D. L., Grossman D., Sorhaindo A., García S. G. The female condom: A promising but unavailable method for Dominican sex workers, their clients, and their partners. Journal of the Association of Nurses in AIDS Care. 2013;24:521–529. doi: 10.1016/j.jana.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Vannier S. A., O’Sullivan L. F. Sex without desire: Characteristics of occasions of sexual compliance in young adults’ committed relationships. Journal of Sex Research. 2010;47:429–439. doi: 10.1080/00224490903132051. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen N., Nyinawabega J., Umulisa M., Kankindi B., Geubbels E., Basinga P., Van De Wijgert J. Preparing for microbicide trials in Rwanda: Focus group discussions with Rwandan women and men. Culture, Health, and Sexuality. 2006;8:395–406. doi: 10.1080/13691050600859302. [DOI] [PubMed] [Google Scholar]
- Versteeg M., Murray M. Condom use as part of the wider HIV prevention strategy: Experiences from communities in the North West Province, South Africa. SAHARA-J: Journal of Social Aspects of HIV/AIDS. 2008;5:83–93. doi: 10.1080/17290376.2008.9724905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti F., Zullo F., Marra M. L., De Masellis G., Caiazza M., Cibarelli F., Guida M. A new long-term reversible contraception method: Sexual and metabolic impact. Translational Medicine @ UniSa. 2012;4:86–89. [PMC free article] [PubMed] [Google Scholar]
- Wallwiener C. W., Wallwiener L.-M., Seeger H., Mück A. O., Bitzer J., Wallwiener M. Prevalence of sexual dysfunction and impact of contraception in female German medical students. Journal of Sexual Medicine. 2010;7:2139–2148. doi: 10.1111/j.1743-6109.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- Wallwiener M., Wallwiener L.-M., Seeger H., Mueck A. O., Zipfel S., Bitzer J., Wallwiener C. W. Effects of sex hormones in oral contraceptives on the Female Sexual Function score: A study in German female medical students. Contraception. 2010;82:155–159. doi: 10.1016/j.contraception.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Wang X. The role of attitude functions, efficacy, anticipated emotions, and relationship status on college students’ condom use intentions. Journal of Sex Research. 2013;50:704–714. doi: 10.1080/00224499.2012.687411. [DOI] [PubMed] [Google Scholar]
- Wanyonyi S. Z., Stones W. R., Sequeira E. Health-related quality of life changes among users of depot medroxyprogesterone acetate for contraception. Contraception. 2011;84:e17–e22. doi: 10.1016/j.contraception.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Warnock J. K., Clayton A., Croft H., Segraves R., Biggs F. C. Comparison of androgens in women with hypoactive sexual desire disorder: Those on combined oral contraceptives (COCs) vs those not on COCs. Journal of Sexual Medicine. 2006;3:878–882. doi: 10.1111/jsm.2006.3.issue-5. [DOI] [PubMed] [Google Scholar]
- Welling L. L. Psychobehavioral effects of hormonal contraceptive use. Journal of Evolutionary Psychology. 2013;11:718–742. [PubMed] [Google Scholar]
- Westhoff C. L., Heartwell S., Edwards S., Zieman M., Stuart G., Cwiak C., Kalmuss D. Oral contraceptive discontinuation: Do side effects matter? American Journal of Obstetrics and Gynecology. 2007;196(412):e411–e416. doi: 10.1016/j.ajog.2006.12.015. discussion,, e412 e416–e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker P. G., Merkh R. D., Henry-Moss D., Hock-Long L. Withdrawal attitudes and experiences: A qualitative perspective among young urban adults. Perspectives on Sexual and Reproductive Health. 2010;42:102–109. doi: 10.1363/psrh.2010.42.issue-2. [DOI] [PubMed] [Google Scholar]
- Widdice L. E., Cornell J. L., Liang W., Halpern-Felsher B. L. Having sex and condom use: Potential risks and benefits reported by young, sexually inexperienced adolescents. Journal of Adolescent Health. 2006;39:588–595. doi: 10.1016/j.jadohealth.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Wiebe E. Capturing the sexual side effects of hormonal contraception [Response to Graesslin et al., 2009, in Contraception, 80(6), 540–554]. Contraception. 2010;(6):581. doi: 10.1016/j.contraception.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Wiebe E., Kaczorowski J., Mackay J. Mood and sexual side effects of hormonal contraception: Physicians’ and residents’ knowledge, attitudes, and practices. Canadian Family Physician. 2012;58:e677–e683. [PMC free article] [PubMed] [Google Scholar]
- Witting K., Santtila P., Alanko K., Harlaar N., Jern P., Johansson A., Sandnabba N. K. Female sexual function and its associations with number of children, pregnancy, and relationship satisfaction. Journal of Sex and Marital Therapy. 2008;34:89–106. doi: 10.1080/00926230701636163. [DOI] [PubMed] [Google Scholar]
- Witting K., Santtila P., Jern P., Varjonen M., Wager I., Höglund M., Sandnabba N. K. Evaluation of the Female Sexual Function Index in a population-based sample from Finland. Archives of Sexual Behavior. 2008;37:912–924. doi: 10.1007/s10508-007-9287-8. [DOI] [PubMed] [Google Scholar]
- Wonglikhitpanya N., Taneepanichskul S. Effects of biphasic oral contraceptives containing desogestrel (Oilezz) on cycle control facial acne and seborrhea in healthy Thai women. Journal of the Medical Association of Thailand. 2006;89:755–760. [PubMed] [Google Scholar]
- Woodsong C., Alleman P. Sexual pleasure, gender power, and microbicide acceptability in Zimbabwe and Malawi. AIDS Education and Prevention. 2008;20:171–187. doi: 10.1521/aeap.2008.20.2.171. [DOI] [PubMed] [Google Scholar]
- Xu F., Sternberg M. R., Markowitz L. E. Women who have sex with women in the United States: Prevalence, sexual behavior, and prevalence of herpes simplex virus type 2 infection: Results from National Health and Nutrition Examination Survey 2001–2006. Sexually Transmitted Diseases. 2010;37:407–413. doi: 10.1097/OLQ.0b013e3181db2e18. [DOI] [PubMed] [Google Scholar]
- Zimmerman Y., Eijkemans M. J., Coelingh Bennink H. J., Blankenstein M. A., Fauser B. C. The effect of combined oral contraception on testosterone levels in healthy women: A systematic review and meta-analysis. Human Reproduction Update. 2014;20:76–105. doi: 10.1093/humupd/dmt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman Y., Foidart J.-M., Pintiaux A., Minon J.-M., Fauser B., Cobey K., Bennink H. Restoring testosterone levels by adding dehydroepiandrosterone to a drospirenone containing combined oral contraceptive: II. Clinical effects. Contraception. 2015;91:134–142. doi: 10.1016/j.contraception.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Zubowicz E. A., Oakes J. K., Short M. B., Perfect M. M., Succop P. S., Rosenthal S. L. Adolescents’ descriptions of the physical characteristics of microbicide surrogates and experiences of use. Journal of Women’s Health. 2006;15:952–961. doi: 10.1089/jwh.2006.15.952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


