Abstract
Purpose
The impact of adjuvant radiotherapy for pancreatic adenocarcinoma (PAC) remains controversial. We examined effects of adjuvant therapy on overall survival (OS) in PAC, using the National Cancer Data Base (NCDB).
Methods
Patients with resected PAC from 1998 to 2002 were queried from the NCDB. Factors associated with receipt of adjuvant chemotherapy (ChemoOnly) versus adjuvant chemoradiotherapy (ChemoRad) versus no adjuvant treatment (NoAdjuvant) were assessed. Cox proportional hazard modeling was used to examine effect of adjuvant therapy type on OS. Propensity scores (PS) were developed for each treatment arm and used to produce matched samples for analysis to minimize selection bias.
Results
From 1998 to 2002, a total of 11,526 patients underwent resection of PAC. Of these, 1,029 (8.9 %) received ChemoOnly, 5,292 (45.9 %) received ChemoRad, and 5,205 (45.2 %) received NoAdjuvant. On univariate analysis, factors associated with improved OS included: younger age, higher income, higher facility volume, lower tumor stage and grade, negative margins and nodes, and absence of adjuvant therapy. On multivariate analysis with matched PS, factors independently associated with improved OS included: younger age, higher income, higher facility volume, later year of diagnosis, smaller tumor size, lower tumor stage, and negative tumor margins and nodes. ChemoRad had the best OS (hazard ratio 0.70, 95 % confidence interval 0.61–0.80) in a PS matched comparison with ChemoOnly (hazard ratio 1.04, 95 % confidence interval 0.93–1.18) and NoAdjuvant (index).
Conclusions
Adjuvant chemotherapy with radiotherapy is associated with improved OS after PAC resection in a large population from the NCDB. On the basis of these analyses, radiotherapy should be a part of adjuvant therapy for PAC.
Pancreatic adenocarcinoma (PAC) remains an aggressive malignancy, with a 5-year disease-specific survival of ∼5 %.1 Surgical therapy offers the best chance of meaningful survival for those patients with tumors amenable to resection, but even then, survival rates improve to only 15–20 %, demonstrating the need for effective multimodal therapy.1,2 Adjuvant therapy is now standard practice after resection of PAC on the basis of results of several clinical trials, but the ideal regimen remains controversial, especially with regard to use of adjuvant radiotherapy (ART).
In the United States, adjuvant chemoradiotherapy (ChemoRad) is typically administered after PAC resection on the basis of the results of the 1985 Gastrointestinal Tumor Study Group (GITSG) randomized, prospective, multicenter clinical trial.3 Despite limited accrual of only 43 patients in 11 years, interim analysis demonstrated a significant median survival advantage for the treatment arm over the observation arm (20 vs. 11 months; p = 0.035), and this approach became common practice in the United States. In Europe, the European Study Group of Pancreatic Cancer (ESPAC)-1 trial, published in 2001 and updated in 2004, supported the use of adjuvant chemotherapy only (ChemoOnly), while identifying a deleterious effect of ChemoRad on overall survival (OS) after resection of PAC.4,5 Patients who received ChemoOnly had improved overall 5-year survival compared with those who did not (21 vs. 8 %, p = 0.009), and those who received ChemoRad actually did worse than those who did not (10 vs. 20 %, p = 0.05). This led the investigators to conclude that ChemoOnly was beneficial, while ChemoRad was detrimental for patients who underwent resection for PAC, thus establishing the standard in Europe.
The purpose of this analysis was to compare OS rates for a large, representative U.S. cohort of resected PAC patients stratified by adjuvant therapy type and adjusted for patient and provider factors affecting outcomes, using records from the National Cancer Data Base (NCDB). Our main objective was to examine the differential impact of no adjuvant therapy (NoAdjuvant) versus ChemoRad versus ChemoOnly in this NCDB cohort who underwent surgical resection for PAC. To mitigate the impact of any treatment selection biases in this observational study, we used a propensity score (PS) matching method. The PS enables us to balance observed patient characteristics across treatment groups and hence simulate the key feature of successful randomization.6 Typically, PS matching methods are applied for two-treatment comparisons. In the current study, we extend this approach to accommodate the comparison for more than two interventions using an innovative matching algorithm.
Methods
Data Source
Our sample was obtained from the NCDB's Participant Use Data File (PUF) for pancreatic neoplasms. The NCDB, the largest disease-specific clinical registry in the United States, is jointly supported and managed by the American College of Surgeons' Commission on Cancer (CoC) and the American Cancer Society. Created in 1988, the NCDB contains detailed clinical, pathological, and demographic data on ∼70 % of all U.S. incident cancer cases, as reported on an ongoing basis by the roughly 1,500 CoC-approved cancer programs.7
The Pancreatic PUF is a Health Insurance Portability and Accountability Act (HIPAA)-compliant data file containing deidentified patient-level data that thus do not identify hospitals, providers, or patients. These tumor site–specific PUFs are designed to provide CoC-approved cancer programs with a resource to review and advance the quality of care delivered to cancer patients.
Study Population
Our sample consisted of all NCDB incident cases of PAC diagnosed between 1998 and 2002, based on availability of survival data at the time of analysis. We included cases for which a primary tumor site in the pancreas could be identified. From this group, we selected cases with adequate survival data, excluding patients with missing information. We included only patients who underwent surgical resection of the primary PAC and selected patients with information available on ART and adjuvant chemotherapy. We excluded patients who had radiotherapy as their only form of adjuvant therapy, and those who died within 60 days of surgical resection.
Study Variables
Variables selected for impact on OS are listed in Table 1. Footnotes are provided to clarify those variable in need of explanation. The measures for education level, income, and urban/rural status were determined for each patient's area of residence by matching the zip code of the patient at the time of diagnosis to 2000 U.S. Census data.
Table 1. Univariate analysis of factors according to type of adjuvant therapy received in patients with pancreatic adenocarcinoma from the National Cancer Data Base, 1998–2002 (N = 11,526).
| Adjuvant therapy N (Row %) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Covariate | Level | Total | NoAdjuvant | ChemoOnly | ChemoRad | p* |
| Age at diagnosis | ≤50 years | 2,690 | 1,075 (39.96) | 254 (9.44) | 1,361 (50.59) | <0.001 |
| 50–65 years | 3,039 | 1,142 (37.58) | 289 (9.51) | 1,608 (52.91) | ||
| 65–75 years | 3,911 | 1,776 (45.41) | 367 (9.38) | 1,768 (45.21) | ||
| >75 years | 1,886 | 1,212 (64.26) | 119 (6.31) | 555 (29.43) | ||
| Gender | Male | 5,854 | 2,517 (43) | 527 (9) | 2,810 (48) | <0.001 |
| Female | 5,670 | 2,686 (47.37) | 502 (8.85) | 2,482 (43.77) | ||
| White race | No | 1,398 | 658 (47.07) | 129 (9.23) | 611 (43.71) | 0.209 |
| Yes | 10,128 | 4,547 (44.9) | 900 (8.89) | 4,681 (46.22) | ||
| Median income, 2000a | <$30,000 | 1,372 | 666 (48.54) | 139 (10.13) | 567 (41.33) | <0.001 |
| $30,000–$34,999 | 1,892 | 913 (48.26) | 179 (9.46) | 800 (42.28) | ||
| $35,000–$45,999 | 3,117 | 1,406 (45.11) | 273 (8.76) | 1,438 (46.13) | ||
| >$46,000 | 4,575 | 1,983 (43.34) | 385 (8.42) | 2,207 (48.24) | ||
| Insurance type | Uninsured | 294 | 151 (51.36) | 25 (8.5) | 118 (40.14) | <0.001 |
| Medicaid | 424 | 172 (40.57) | 44 (10.38) | 208 (49.06) | ||
| Private/other | 8,619 | 3,763 (43.66) | 769 (8.92) | 4,087 (47.42) | ||
| Medicare | 1,619 | 850 (52.5) | 145 (8.96) | 624 (38.54) | ||
| Facility typeb | CCP | 1,049 | 411 (39.18) | 128 (12.2) | 510 (48.62) | <0.001 |
| CCCP | 4,288 | 1,673 (39.02) | 406 (9.47) | 2,209 (51.52) | ||
| ARCP | 6,189 | 3,121 (50.43) | 495 (8) | 2,573 (41.57) | ||
| Facility volumec | n | 11,526 | 5,205 | 1,029 | 5,292 | <0.001 |
| Mean | 45.24 | 52.11 | 38.68 | 39.76 | ||
| Median | 18.5 | 21 | 15 | 17 | ||
| Year of diagnosis | 1998 | 1991 | 929 (46.66) | 180 (9.04) | 882 (44.3) | 0.117 |
| 1999 | 2086 | 928 (44.49) | 178 (8.53) | 980 (46.98) | ||
| 2000 | 2255 | 1,009 (44.75) | 178 (7.89) | 1,068 (47.36) | ||
| 2001 | 2463 | 1,119 (45.43) | 214 (8.69) | 1,130 (45.88) | ||
| 2002 | 2731 | 1,220 (44.67) | 279 (10.22) | 1,232 (45.11) | ||
| Primary site | Head | 9224 | 3,918 (42.48) | 769 (8.34) | 4,537 (49.19) | <0.001 |
| Body | 649 | 307 (47.3) | 70 (10.79) | 272 (41.91) | ||
| Tail | 1,389 | 828 (59.61) | 176 (12.67) | 385 (27.72) | ||
| Body/tail | 264 | 152 (57.58) | 14 (5.3) | 98 (37.12) | ||
| Tumor size | ≤25 mm | 3,616 | 1,667 (46.1) | 242 (6.69) | 1,707 (47.21) | <0.001 |
| 25–35 mm | 2,730 | 1,114 (40.81) | 232 (8.5) | 1,384 (50.7) | ||
| 35–45 mm | 1,783 | 750 (42.06) | 168 (9.42) | 865 (48.51) | ||
| >45 mm | 2,061 | 1,052 (51.04) | 224 (10.87) | 785 (38.09) | ||
| Staged | I | 2,274 | 1,431 (62.93) | 97 (4.27) | 746 (32.81) | <0.001 |
| II | 2,046 | 938 (45.85) | 145 (7.09) | 963 (47.07) | ||
| III | 5,044 | 1,724 (34.18) | 444 (8.8) | 2,876 (57.02) | ||
| IV | 1,493 | 586 (39.25) | 285 (19.09) | 622 (41.66) | ||
| Grade | Unspecified | 1,739 | 1,074 (61.76) | 128 (7.36) | 537 (30.88) | <0.001 |
| I | 1,374 | 750 (54.59) | 92 (6.7) | 532 (38.72) | ||
| II | 4,809 | 1,900 (39.51) | 431 (8.96) | 2,478 (51.53) | ||
| III/IV | 3,604 | 1,481 (41.09) | 378 (10.49) | 1,745 (48.42) | ||
| Surgical margin positive | No | 7,861 | 3,781 (48.1) | 606 (7.71) | 3,474 (44.19) | <0.001 |
| Yes | 2,631 | 943 (35.84) | 286 (10.87) | 1,402 (53.29) | ||
| LN positive | No | 4,494 | 2,459 (54.72) | 305 (6.79) | 1,730 (38.5) | <0.001 |
| Yes | 5,931 | 2,165 (36.5) | 603 (10.17) | 3,163 (53.33) | ||
| No. of examined LNs > 12 | No | 7,615 | 3,657 (48.02) | 701 (9.21) | 3,257 (42.77) | <0.001 |
| Yes | 3,050 | 1,191 (39.05) | 239 (7.84) | 1,620 (53.11) | ||
Data are presented as n (%) unless otherwise indicated
NoAdjuvant no adjuvant chemotherapy, ChemoOnly chemotherapy only, ChemoRad adjuvant chemoradiotherapy, LN lymph node, CoC Commission on Cancer
Bold values (p < 0.05) are statistically significant
The parametric p value is calculated by ANOVA for numerical covariates and Chi-square test for categorical covariates
The measures for urban/rural status, income, and education level were determined for each patient's area of residence by matching the zip code of the patient at the time of diagnosis to 2000 U.S. Census data
CCP Community Cancer Program, CCCP Comprehensive Community Cancer Programs, ARCP Academic/Research Cancer Program, all as defined by the CoC
Defined as total number of pancreatic adenocarcinoma analytical cases in a given facility (regardless of facility type) over the 1998–2002 observation period within a given CoC-designated facility type (e.g., CCP), a cancer program was high volume if its number of analytical cases over the 1998–2002 period was at or above the median for all programs nationwide; similarly, a cancer program was considered low volume if its case load was below the median of all programs within that facility type
Stage reflects the AJCC 5th ed.
Statistical Methods
Descriptive Analysis
Categorical variables were summarized as frequencies, and the median and range were reported for each continuous variable. The univariate survival analyses were conducted by associating OS with each variable individually, using both the Kaplan–Meier method (with log rank test p values) and a Cox proportional hazard model (yielding hazard ratios [HR], with 95 % confidence intervals [CI]). The association between the adjuvant therapy type (NoAdjuvant, ChemoOnly, and ChemoRad) and each of potential confounder variables was examined by the chi-square test for categorical covariates or ANOVA for continuous covariates.
Multivariable Analyses
Cox proportional hazard modeling was used to estimate the relative impacts of the three adjuvant therapy strategies on 5-year OS. Two Cox modeling strategies were used to adjust for patient-level differences potentially influencing survival outcomes in this study design. The first model uses traditional multivariable regression modeling (in a nonstratified Cox model) and assumes that systematic differences across patients in the three intervention groups were adequately captured and controlled via inclusion of the potentially confounding covariates shown in Table 2. The second model was built by backward elimination with a p value of <0.05, balancing between goodness of fit and simplicity. The start-up variable list was created from those factors in Table 2 that were statistically significant in the univariate survival analysis.
Table 2. Univariate analysis of factors influencing overall survival in resected patients with pancreatic adenocarcinoma from the National Cancer Data Base, 1998 to 2002 (N = 11,526).
| Covariate | Level | HR | 95 % CI Low | 95 % CI High | HR p value |
|---|---|---|---|---|---|
| Adjuvant therapy | ChemoRad | 1.170 | 1.120 | 1.224 | <0.001 |
| ChemoOnly | 1.592 | 1.479 | 1.714 | <0.001 | |
| NoAdjuvant | – | – | – | – | |
| Age at diagnosis | ≤50 years | 0.606 | 0.566 | 0.648 | <0.001 |
| 50–65 years | 0.780 | 0.732 | 0.832 | <0.001 | |
| 65–75 years | 0.856 | 0.806 | 0.910 | <0.001 | |
| >75 years | – | – | – | – | |
| Gender | Male | 1.049 | 1.006 | 1.094 | 0.025 |
| Female | – | – | – | – | |
| White race | No | 0.963 | 0.902 | 1.028 | 0.259 |
| Yes | – | – | – | – | |
| Median income, 2000 | <$30,000 | 1.166 | 1.089 | 1.248 | <0.001 |
| $30,000-$34,999 | 1.121 | 1.054 | 1.191 | <0.001 | |
| $35,000-$45,999 | 1.067 | 1.013 | 1.124 | 0.015 | |
| >$46,000 | – | – | – | – | |
| Insurance type | Uninsured | 0.900 | 0.782 | 1.037 | 0.146 |
| Medicaid | 0.768 | 0.678 | 0.869 | <0.001 | |
| Private/other | 0.821 | 0.774 | 0.871 | <0.001 | |
| Medicare | – | – | – | – | |
| Facility type | CCP | 1.231 | 1.144 | 1.325 | <0.001 |
| CCCP | 1.171 | 1.120 | 1.224 | <0.001 | |
| ARCP | – | – | – | – | |
| Facility volume (unit = 50) | 0.943 | 0.927 | 0.959 | <0.001 | |
| Year of diagnosis | 1998 | 1.106 | 1.036 | 1.182 | 0.003 |
| 1999 | 1.071 | 1.003 | 1.143 | 0.041 | |
| 2000 | 1.038 | 0.974 | 1.107 | 0.252 | |
| 2001 | 1.016 | 0.954 | 1.081 | 0.626 | |
| 2002 | – | – | – | – | |
| Primary site | Head | 1.297 | 1.122 | 1.499 | <0.001 |
| Body | 1.120 | 0.946 | 1.326 | 0.189 | |
| Tail | 0.844 | 0.720 | 0.988 | 0.035 | |
| Body/tail | – | – | – | – | |
| Tumor size | ≤25 mm | 0.913 | 0.857 | 0.973 | 0.005 |
| 25–35 mm | 1.143 | 1.070 | 1.221 | <0.001 | |
| 35–45 mm | 1.228 | 1.142 | 1.321 | <0.001 | |
| >45 mm | – | – | – | – | |
| Stage | I | 0.365 | 0.338 | 0.394 | <0.001 |
| II | 0.606 | 0.563 | 0.652 | <0.001 | |
| III | 0.822 | 0.773 | 0.874 | <0.001 | |
| IV | – | – | – | – | |
| Grade | Unspecified | 0.365 | 0.339 | 0.393 | <0.001 |
| I | 0.410 | 0.380 | 0.443 | <0.001 | |
| II | 0.764 | 0.729 | 0.801 | <0.001 | |
| III/IV | – | – | – | – | |
| Surgical margin positive | No | 0.654 | 0.623 | 0.687 | <0.001 |
| Yes | – | – | – | – | |
| LN positive | No | 0.579 | 0.553 | 0.606 | <0.001 |
| Yes | – | – | – | – | |
| No. of examined LNs > 12 | No | 0.958 | 0.913 | 1.005 | 0.076 |
| Yes | – | – | – | – |
To further reduce patient selection bias in our second model, a PS method was used that was based on matching.7,8 Under this particular PS approach, each patient is assigned, ex post facto, a predicted probability (i.e., a PS) of having received each of the competing adjuvant therapy strategies (NoAdjuvant, ChemoRad, ChemoOnly) on the basis of a multivariable regression analysis. Each patient receiving a given intervention, e.g., ChemoRad, is matched with a patient who received ChemoOnly and a patient who received NoAdjuvant on the basis of the approximate equality of their PS across all three interventions. Under PS matching, the three patients may be regarded post hoc as having roughly the same probability distribution of having received any of the three therapeutic options (Supplementary materials). After forming the matched sample, the Cox proportional hazard model was performed, stratified by matched groups to estimate treatment effect.
This matching process reduces the effective sample size as unmatched patient data are eliminated from the analysis. For comparison purposes, the multivariable regression findings regarding the impact of these interventions on survival are presented both without and with PS adjustment. All Cox proportional hazard analyses were performed by SAS 9.2, and the PS matching algorithm was implemented by R, with the significance level set at 0.05.
Results
Study Population
The original NCDB pancreatic PUF (1998–2007) contained 208,930 patients diagnosed with PAC. The selected time interval provided 94,385 patients meeting histologic criteria for PAC. Subsequent inclusion criteria were: primary anatomic tumor site in the pancreas could be identified (n = 69,268); adequate survival data (n = 69,240); surgical resection of the primary PAC (n = 13,385); information on ART (n = 13,385); and adjuvant chemotherapy (n = 12,881). We excluded patients who had received radiotherapy as their only form of adjuvant therapy (n = 438), and those who died within 60 days of resection (n = 917), leaving a final sample of 11,526 patients.
Patient and Disease Variables
Patient-related and disease-specific variables are summarized in Table 1, which also summarizes information on adjuvant therapy grouping (NoAdjuvant 45.2, ChemoOnly 8.9, and ChemoRad 45.9 %).
Univariate Analysis for Receipt of Adjuvant Treatment and OS
The univariate results for associations between patient and disease variables and type of adjuvant treatment received are provided in Table 1. Factors associated with receiving adjuvant therapy included younger patient age, male gender, having either private insurance or Medicaid, and having positive surgical margins and positive lymph nodes.
Univariate OS results by patient and disease variable are shown in Table 2. Factors associated with better OS included younger age, being female, having private insurance or Medicaid, having a higher median income, having negative surgical margins, and having negative lymph nodes. Although the proportionate distribution of patients across treatment types did not change significantly over time (Table 1), OS improved progressively (Table 2), with patients treated in 1998 experiencing the worst OS (HR 1.11, p = 0.003), compared with patients treated in 2002 (the final diagnosis year of our study population). Patients who received ChemoRad had better OS than the ChemoOnly patients, but worse survival than those receiving NoAdjuvant therapy (HR 1.17, p < 0.001).
Multivariable Analysis for Factors Associated with OS
Table 3 shows the Cox model analysis for OS without PS adjustment. Although no patient level variable had more than 12 % of observations missing and most had fewer than 5 % missing, the resulting available sample for analysis was 7,288. The impact of individual factors on survival is summarized by HR and 95 % CI. In this non-PS-adjusted analysis, use of ChemoRad was associated with a survival advantage compared with NoAdjuvant (HR 0.79, p < 0.001). By contrast, patients receiving ChemoOnly experienced the similar OS as NoAdjuvant patients (HR 1.08, p = 0.108).
Table 3. Multivariable proportional hazard model without propensity score adjustment (N = 7,288).
| Covariate | Level | HR | 95 % CI Low | 95 % CI High | HR p value |
|---|---|---|---|---|---|
| Age at diagnosis | ≤50 y | 0.689 | 0.632 | 0.751 | <0.001 |
| 50–65 y | 0.791 | 0.731 | 0.857 | <0.001 | |
| 65–75 y | 0.886 | 0.821 | 0.955 | 0.002 | |
| >75 y | – | – | – | – | |
| Median income, 2000 | <$30,000 | 1.170 | 1.077 | 1.272 | <0.001 |
| $30,000-$34,999 | 1.089 | 1.010 | 1.174 | 0.027 | |
| $35,000-$45,999 | 1.025 | 0.963 | 1.092 | 0.441 | |
| >$46,000 | – | – | – | – | |
| Year of diagnosis | 1998 | 1.078 | 0.993 | 1.171 | 0.073 |
| 1999 | 1.026 | 0.947 | 1.112 | 0.524 | |
| 2000 | 1.020 | 0.942 | 1.104 | 0.628 | |
| 2001 | 0.935 | 0.867 | 1.009 | 0.085 | |
| 2002 | – | – | – | – | |
| Facility volume (unit = 50) | 0.962 | 0.941 | 0.983 | <0.001 | |
| Facility typea | CCP | 1.104 | 1.002 | 1.217 | 0.047 |
| CCCP | 1.064 | 1.002 | 1.131 | 0.044 | |
| ARCP | – | – | – | – | |
| Primary site | Head | 1.160 | 0.958 | 1.405 | 0.128 |
| Body | 1.172 | 0.938 | 1.465 | 0.162 | |
| Tail | 0.930 | 0.751 | 1.151 | 0.505 | |
| Body/tail | – | – | – | – | |
| Tumor size | ≤25 mm | 0.733 | 0.677 | 0.794 | <0.001 |
| 25–35 mm | 0.884 | 0.815 | 0.959 | 0.003 | |
| 35–45 mm | 0.942 | 0.863 | 1.029 | 0.187 | |
| >45 mm | – | – | – | – | |
| Stage | I | 0.592 | 0.520 | 0.674 | <0.001 |
| II | 0.809 | 0.715 | 0.916 | <0.001 | |
| III | 0.806 | 0.732 | 0.886 | <0.001 | |
| IV | – | – | – | – | |
| Grade | Unspecified | 0.441 | 0.395 | 0.492 | <0.001 |
| I | 0.455 | 0.413 | 0.501 | <0.001 | |
| II | 0.768 | 0.725 | 0.813 | <0.001 | |
| III/IV | – | – | – | – | |
| Surgical margin positive | No | 0.709 | 0.668 | 0.753 | <0.001 |
| Yes | – | – | – | – | |
| LN positive | No | 0.724 | 0.641 | 0.818 | <0.001 |
| Yes | – | – | – | – | |
| No. of examined LNs > 12 | No | 1.109 | 1.046 | 1.175 | <0.001 |
| Yes | – | – | – | – | |
| Adjuvant therapy | ChemoRad | 0.784 | 0.739 | 0.831 | <0.001 |
| ChemoOnly | 1.076 | 0.977 | 1.186 | 0.139 | |
| No adjuvant therapy | – | – | – | – |
The model was built by backward elimination with a staying p value of <0.05. The starting list contains all variables that demonstrated significance at the 0.05 level in <T2>Table 2
HR hazard ratio, CI confidence interval, LN lymph node, ChemoOnly chemotherapy only, ChemoRad adjuvant chemoradiotherapy
CCP Community Cancer Program, CCCP Comprehensive Community Cancer Programs, ARCP Academic/Research Cancer Program, all as defined by the Commission on Cancer
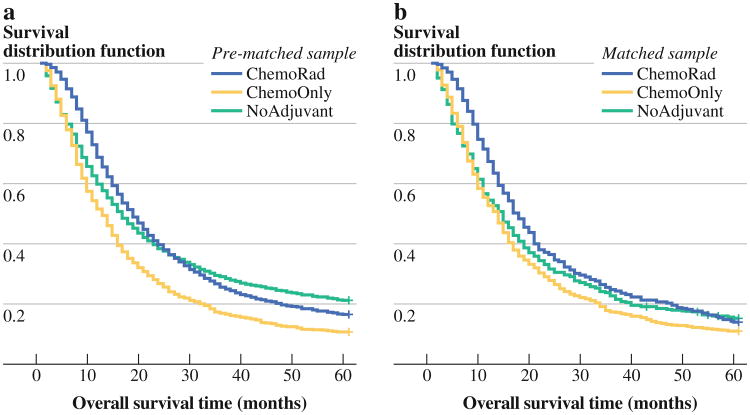
In the Cox model stratified by propensity-score matched groups (N = 1,650), the resulting multivariable estimates were similar to those for the non-PS-adjusted Cox model shown in Table 3, but the survival benefit for ChemoRad patients was greater when compared with that of NoAdjuvant patients (HR 0.70, p < 0.001). Figure 1 illustrates the change of Kaplan–Meier curves before and after selection bias has been removed. There was still no significant survival difference between ChemoOnly and NoAdjuvant (HR 1.04, p = 0.77). Further discussion of these PS matched-sample analyses is provided in the Supplementary materials.
Fig. 1.
Kaplan–Meier survival curves for the three therapy groups (heavy line NoAdjuvant, light line ChemoOnly, hatched line ChemoRad) for both the prematched sample (n = 7,288, left) and one of matched samples (n = 1,650, right). Note the overlap between the NoAdjuvant and ChemoRad groups before propensity score matching, which disappears after matching
Discussion
The purpose of this study was to assess the value of adjuvant therapy on OS in a large cohort of patients with PAC who underwent surgical resection. The sample consisted of all incident cases of PAC over the 1998–2002 period reported to the NCDB meeting study inclusion criteria. In multivariable survival analyses that also attempted to adjust for possible treatment selection bias through PS matching methods, we demonstrated that patients who received ChemoRad experienced better OS compared with those who had ChemoOnly or NoAdjuvant; ChemoOnly OS was similar to NoAdjuvant. Novel aspects of this study include the use of the NCDB to address this question of adjuvant therapy used in resected PAC, and the novel PS matching method, which allowed us to assess three treatment arms simultaneously.
Adjuvant therapy for resected pancreatic cancer provides patients with a survival benefit based on results of several randomized trials.3,8,9 The GITSG study, while small and flawed, set the standard for adjuvant therapy practices for PAC in the United States.3 The European ESPAC-1 demonstrated a survival advantage associated with adjuvant ChemoOnly and reduced survival attributable to ChemoRad.5 Review of other randomized trials for adjuvant therapy of resected PAC does not provide any further clarification on the role of ChemoRad versus ChemoOnly. The 2007 Charite Onkologie (CONKO-001) trial compared adjuvant gemcitabine with observation.8 The European Organization for Research and Treatment of Cancer (EORTC) 40891 trial did not support the benefit of ChemoRad over observation.10 The Radiation Therapy Oncology Group (RTOG) 9704 trial demonstrated no difference in OS between gemcitabine chemotherapy over 5-fluorouracil (5-FU) when combined with ART.11 The most recent ESPAC-3 trial dropped the ChemoRad arm and also demonstrated no difference between 5-FU and gemcitabine ChemoOnly approaches.9 In summary, no existing randomized trial can definitively answer the question of the optimal adjuvant therapy approach for resected PAC.
Although at risk for selection bias, retrospective data can provide useful perspectives on questions such at this. Retrospective data may include larger samples than randomized clinical trials, and indeed it is unlikely that further, large-scale randomized trials will be launched anytime soon to study the issues addressed here. Hence, large cohort studies of the type undertaken here likely represent the best practical approach going forward to enrich the evidence base on treatment effectiveness.
Capitalizing on the NCDB PAC data set, we carried out a PS matching procedure to minimize potential bias in the sorting of patients across three treatment groups. An innovative three-way matching of these PS was performed, lowering the number of evaluable patients but improving within-group homogeneity. The sample that emerged with PS matching was still significantly larger than any previous randomized trials examining adjuvant therapy for PAC. Our matched PS multivariable results reveal an OS advantage for those patients who received ChemoRad compared with other adjuvant therapies.
Other large retrospective studies have looked at the question of ChemoRad for PAC. Several studies have assessed data from the Surveillance, Epidemiology and End Results (SEER) registry, which includes information on incident cancer cases representing about 26 % of the U.S. population.12–15 These studies have identified a benefit of ART when combined with adjuvant chemotherapy for PAC. A substantial limitation of these analyses is that SEER does not include specific patient-level information on the use of adjuvant chemotherapy or detail on radiation dosing. The only available option for obtaining chemotherapy-specific data on SEER patients is through analysis of the linked SEER–Medicare data base, but the latter includes only individuals who were age 65 or over and enrolled in Medicare fee for service at the time of cancer diagnosis. Although SEER data are high quality and population based, the NCDB data are also highly representative of U.S. cancer patients and include about 70 % of all incident cases nationwide.
Among analyses of the impact of adjuvant therapy on pancreatic cancer survival, only two reports (those of McDade et al. and Hsu et al.) used PS adjustments.15,16 McDade et al. examined 5,676 patients from SEER between 1988 and 2005 and found an OS benefit for patients receiving ART compared with those who did not (HR 0.773; 95 % CI 0.714–0.836, p < 0.05)—a result notably consistent with those reported here.15 However, McDade et al. were not able to report on the impact of adjuvant chemotherapy. In contrast, we were able to assess three treatment groups (NoAdjuvant, ChemoOnly, and ChemoRad) and demonstrated superior results for ChemoRad, providing stronger support for a radiation-inclusive adjuvant strategy. The findings in our paper support those of McDade et al. and those demonstrated by GITSG, demonstrating the benefit of ChemoRad in the adjuvant therapy of PAC.
Hsu et al.16 performed Cox survival and PS analyses assessed associations with OS for 1,272 patients combined from 2 centers, using PS analysis and matched-pair analysis by treatment group (1:1) on the basis of institution, age, sex, tumor size/stage, differentiation, margin, and node positivity. They found that patients who received chemoradiotherapy had significantly better median survival than that of patients who did not receive adjuvant therapy. Again, chemotherapy alone was assessed.
The strengths of the current study include its data source (NCDB), large sample, use of propensity adjustment, and the further matching of the PS to achieve more homogeneous comparison groups. Limitations of our work include the retrospective nature of our data and the selection of data from an earlier time period (1998 to 2002), as these included the cases for which survival data were available. We did not have comorbidity data available because this information was added to NCDB only in more recent years. We cannot comment on the exact nature of the chemotherapy or on comorbid conditions of the patients examined; thus, as with any population study analysis, selection bias may play a substantial role in our defining our findings. Importantly, only 8.9 % of the patients received ChemoOnly. The time period was a transitional period for the use of the three treatment arms assessed. Perhaps the ChemoOnly patients had more comorbid conditions precluding them from receiving concomitant radiotherapy and worsening their survival. It is also important to note that both 5-FU and gemcitabine were used during this time period in the adjuvant setting for PAC, but NCDB data do not provide detail on specific agents. However, the ESPAC-3 trial demonstrated that there was no difference in survival between groups receiving ChemoOnly with 5-FU versus gemcitabine.
This study is the first to demonstrate, using a large national database, an OS advantage for resected PAC patients who received adjuvant therapy consisting of both chemotherapy and radiotherapy over those who received no adjuvant therapy or adjuvant chemotherapy alone. It is also the first to use matched PS to examine the value of these specific approaches, and the first (in the pancreatic cancer domain) to make this assessment between three treatment groups as opposed to two groups (adjuvant therapy vs. no adjuvant therapy). Given the shifting paradigm toward increasing preoperative therapy strategies, this question of optimal adjuvant treatment continues to evolve, especially with new response data to FOFIRINOX, a more aggressive chemotherapy regimen.17–21 In light of the conflicting conclusions from existing randomized trials, our findings support the use of combination radiotherapy and chemotherapy as adjuvant therapy for patients who undergo surgical resection of pancreatic ductal adenocarcinoma.
Supplementary Material
Acknowledgments
Supported in part by grant 5P30CA138292 from the National Cancer Institute.
Footnotes
Podium presentation at the 65th annual meeting of the Society of Surgical Oncology, March 24, 2012, Orlando, FL.
Electronic supplementary material: The online version of this article (doi:10.1245/s10434-013-3047-x) contains supplementary material, which is available to authorized users.
Disclosure: None.
References
- 1.Simons JP, Ng SC, McDade TP, Zhou Z, Earle CC, Tseng JF. Progress for resectable pancreatic [corrected] cancer? A population-based assessment of US practices. Cancer. 2010;116:1681–90. doi: 10.1002/cncr.24918. [DOI] [PubMed] [Google Scholar]
- 2.Crane CH, Varadhachary GR, Wolff RA, Fleming JB. Challenges in the study of adjuvant chemoradiation after pancreaticoduodenectomy. Ann Surg Oncol. 2010;17:950–2. doi: 10.1245/s10434-009-0859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–85. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 7.Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3. doi: 10.1002/jso.10320. [DOI] [PubMed] [Google Scholar]
- 8.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Stocken DD, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 2009;100:246–50. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776–82. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–26. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazard L, Tward JD, Szabo A, Shrieve DC. Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: results of a study from the Surveillance, Epidemiology, and End Results (SEER) registry data. Cancer. 2007;110:2191–201. doi: 10.1002/cncr.23047. [DOI] [PubMed] [Google Scholar]
- 14.Artinyan A, Hellan M, Mojica-Manosa P, et al. Improved survival with adjuvant external-beam radiation therapy in lymph node-negative pancreatic cancer: a United States population-based assessment. Cancer. 2008;112:34–42. doi: 10.1002/cncr.23134. [DOI] [PubMed] [Google Scholar]
- 15.McDade TP, Hill JS, Simons JP, et al. A national propensity-adjusted analysis of adjuvant radiotherapy in the treatment of resected pancreatic adenocarcinoma. Cancer. 2010;116:3257–66. doi: 10.1002/cncr.25069. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital–Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–90. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214:33–45. doi: 10.1016/j.jamcollsurg.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101:587–92. doi: 10.1002/jso.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Scodan R, Mornex F, Girard N, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol. 2009;20:1387–96. doi: 10.1093/annonc/mdp015. [DOI] [PubMed] [Google Scholar]
- 20.Bickenbach KA, Gonen M, Tang LH, et al. Downstaging in pancreatic cancer: a matched analysis of patients resected following systemic treatment of initially locally unresectable disease. Ann Surg Oncol. 2012;19:1663–69. doi: 10.1245/s10434-011-2156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.