Abstract
Purpose
Inflammation associated with blood–retinal barrier (BRB) breakdown is a common feature of several retinal diseases. Therefore, the development of novel nonsteroidal anti-inflammatory approaches may provide important therapeutic options. Previous studies demonstrated that inhibition of dipeptidyl peptidase-IV, the enzyme responsible for the degradation of glucagon-like peptide-1 (GLP-1), led to insulin-independent prevention of diabetes-induced increases in BRB permeability, suggesting that incretin-based drugs may have beneficial pleiotropic effects in the retina. In the current study, the barrier protective and anti-inflammatory properties of exendin-4 (Ex-4), an analog of GLP-1, after ischemia-reperfusion (IR) injury were examined.
Methods
Ischemia-reperfusion injury was induced in rat retinas by increasing the intraocular pressure for 45 minutes followed by 48 hours of reperfusion. Rats were treated with Ex-4 prior to and following IR. Blood–retinal barrier permeability was assessed by Evans blue dye leakage. Retinal inflammatory gene expression and leukocytic infiltration were measured by qRT-PCR and immunofluorescence, respectively. A microglial cell line was used to determine the effects of Ex-4 on lipopolysaccharide (LPS)-induced inflammatory response.
Results
Exendin-4 dramatically reduced the BRB permeability induced by IR injury, which was associated with suppression of inflammatory gene expression. Moreover, in vitro studies showed that Ex-4 also reduced the inflammatory response to LPS and inhibited NF-κB activation.
Conclusions
The present work suggests that Ex-4 can prevent IR injury–induced BRB breakdown and inflammation through inhibition of inflammatory cytokine production by activated microglia and may provide a novel option for therapeutic intervention in diseases involving retinal inflammation.
Keywords: blood–brain barrier, inflammation, ischemia-reperfusion injury, microglia, vascular biology
A number of ocular diseases have been associated with retinal ischemia-reperfusion (IR) injury, including retinal vascular occlusion, acute glaucoma, diabetic retinopathy, and retinopathy of prematurity.1 The intraocular pressure IR model consists of transient ischemia followed by natural reperfusion, which causes neural cell damage, an inflammatory response,1 and blood–retinal barrier (BRB) alterations.2
Blood–retinal barrier breakdown associated with vascular hyperpermeability may lead to tissue edema with consequent vision loss.3 Sterile inflammation may contribute to BRB alterations in diabetic retinopathy, as a number of inflammatory cytokines are elevated in the vitreous of diabetic patients.4,5 In fact, inflammatory mediators have been shown to promote increased vascular permeability, junctional deregulation, leukocyte adhesion, and retinal cell death.6
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that enhances glucose-dependent insulin response via the G protein–coupled GLP-1 receptor (GLP-1R).7 Glucagon-like peptide-1 displays a very short half-life in circulation due to degradation by the enzyme dipeptidyl peptidase IV (DPP-IV); therefore, long-acting GLP-1 analogs like Ex-4 have been developed to promote insulin secretion in type 2 diabetic patients. Exendin-4 has been shown to have neuroprotective effects in retinas of streptozotocin-diabetic rats, preventing neuronal death, abnormalities in electroretinogram responses, and inner retinal layer thinning.8 Using the same animal model, we previously reported9 that a DPP-IV inhibitor (sitagliptin) prevents the diabetes-induced increase in BRB permeability, while exerting anti-inflammatory and antiapoptotic effects, independent of increased insulin secretion. A recent study10 also showed that intravitreal administration of Ex-4 was able to reduce retinal vascular permeability in type 2 diabetic Goto-Kakizaki rats and inhibit intercellular adhesion molecule-1 (ICAM-1) expression. These studies collectively suggest that GLP-1R activation may have benefits in treating diabetic retinopathy in addition to its role as an incretin. However, these studies provide little mechanistic understanding of the action of Ex-4 on retinal vascular permeability.
Previous studies demonstrated that retinal IR injury in the rat induces a VEGF-driven alteration in permeability and subsequent inflammatory response that maintains permeability at least 48 hours after ischemic injury.11–13 In the present work, we found that Ex-4 prevents the increase in BRB permeability and inhibits the expression of classical inflammation markers induced by IR injury at 48 hours. In vitro studies revealed that Ex-4 has anti-inflammatory effects that prevent the ability of microglia to produce factors that are known to cause vascular permeability, but fails to directly inhibit the permeability response of primary endothelial cell cultures treated with cytokines or VEGF. These data suggest that Ex-4 acts to suppress microglia activation in models of retinal inflammation reducing subsequent vascular permeability.
Materials and Methods
Retinal Ischemia-Reperfusion
Adult male Long-Evans rats (Charles River Laboratories, Wilmington, MA, USA) weighing 200 to 225 g were maintained in specific pathogenic-free conditions, monitored by quarterly sentinel testing and treated in accordance with the University of Michigan Committee on Use and Care of Animals (UCUCA) and consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research. All animal procedures were approved by the University of Michigan Committee on the Use and Care of Animals. Animals were anesthetized with intramuscular injection of ketamine and xylazine (66.7 mg/kg and 6.7 mg/kg body weight, respectively).
Ischemia was applied to the left eyes by increasing the intraocular pressure (IOP) to cut off the blood supply from the retinal artery as previously described.11–13 Increased IOP was achieved by infusing sterile saline to the anterior chamber through a 32-gauge needle connected to a syringe pump set at a constant flow rate of 40 μL/min. We monitored IOPs with a microtonometer designed for use on rodent eyes (TonoLab; Icare, Helsinki, Finland). Retinas were subjected to 45-minute ischemia followed by natural reperfusion of blood for a period of 48 hours. The contralateral eyes were subjected to needle puncture and served as sham controls. Exendin-4 (Tocris Bioscience, Bristol, UK), dissolved in 0.9% saline solution (pH 6), was administered twice daily as a subcutaneous injection of 10 μg/kg, with two initial administrations prior to ischemia and every 12 hours for the next 48 hours during the reperfusion period. Nontreated animals received saline vehicle injections. The last injection was given 1 hour prior to injection of Evans blue dye for the permeability assay or 1 hour prior to sacrificing the animals and collecting the retinas for the gene expression and whole-mount staining studies. A timeline for the drug administration protocol is provided in Supplementary Figure S1.
Evans Blue Assay
Retinal vascular permeability was measured by the accumulation of albumin-binding dye, Evans blue, according to the method described by Xu and colleagues.14
Isolation of Tissue and Cell RNA and Quantitative RT-PCR
Retinas and cell pellets were collected, flash-frozen in liquid nitrogen, and stored at −80°C until analysis. Total RNA was purified with an RNA preparation kit (RNeasy Plus Mini Kit; Qiagen, Venlo, Limburg, The Netherlands), and a homogenizer (QIAshredder; Qiagen) for dissociation of retinal tissues. Quantitative real-time (qRT)-PCR and duplex qPCRs were performed as previously described.13 Duplex qPCRs were performed using gene-specific primers and fluorescent dye-labeled probes (Applied Biosystems Life Technologies, Carlsbad, CA, USA). Primer-probe assay information and gene information are provided in Supplementary Table S1. Reactions were performed and monitored using a real-time PCR system (CFX384; Bio-Rad, Hercules, CA, USA). Relative normalized mRNA levels were calculated using the ΔΔCt method.
Retinal Whole-Mounts Immunostaining
Retina whole-mounts were prepared according to the procedure previously described.13 Retinas were stained with mouse anti-rat CD45 (1:50, BD Biosciences, Franklin Lakes, NJ, USA), AlexaFluor 488–labeled isolectin B4 (IB4) from Griffonia simplicifolia (1:75, Life Technologies) and 10 μg/mL Hoechst-33342 DNA stain (Life Technologies). Retinas were visualized under a confocal microscope (Leica TCS SP5 AOBS; Leica Microsystems, Wetzlar, Germany). Eight animals per group were used and from each retina eight fields were averaged and used to determine the number of CD45-positive cells in a masked fashion.
Measurement of Bovine Retinal Endothelial Cell Permeability
Bovine retinal endothelial cells (BREC) were isolated as previously described.15 We cultured BREC in flasks coated with 1 μg/cm2 fibronectin in MCDB-131 medium, supplemented with 10% fetal bovine serum (FBS), 22.5 μg/mL endothelial cell growth factor, 120 μg/mL heparin, 0.01 mL/mL antibiotic-antimycotic. For experimentation, BREC were used from passages 4 to 7. To measure cell monolayer permeability, BREC were grown to confluence on 0.4 μm pore transwell filters (Corning Costar, Acton, MA, USA) and then cell culture media was changed to MCDB-131 medium supplemented with 1% FBS, 0.01 mL/mL antibiotic/antimycotic, and 100 nmol/L hydrocortisone for 2 days before the experiment. Monolayer permeability of BREC to 70 kDa rhodamine isothiocyanate (RITC) dextran (Sigma-Aldrich Corp., St. Louis, MO, USA) was measured as previously described.15 We pretreated BREC with 10 nmol/L Ex-4, a concentration described to prevent increased permeability in endothelial cells.16 After 1 hour of incubation, BREC were then exposed to TNF (5 ng/mL for 1.5 hour), VEGF (50 ng/mL for 30 minutes), or the combination of both.
Murine Microglial Cell Line BV2 Culture and Treatment
The immortalized murine BV2 microglial cell line17 was cultured in Dulbecco's modified Eagle's medium (DMEM) containing L-alanyl-L-glutamine (Glutamax; Thermo Fisher Scientific, Inc., Rockville, MD, USA) and supplemented with 10% FBS, 10,000 U/mL penicillin, and 10 mg/mL streptomycin. The concentration of Ex-4 used for microglial cells was based on preliminary results showing a dose-dependent inhibition of IL-1β expression after treatment with LPS for 4 hours (data not shown). For the experiments, BV2 cells were plated at a density of 1 × 105 cells/cm2 in DMEM containing 1% FBS and then treated with 100 nmol/L Ex-4 1 hour prior to the addition of LPS (from Escherichia coli; Sigma-Aldrich Corp.) at a concentration of 100 ng/mL for the times specified in the respective figure legends.
Total Cell Lysates
Cell lysates were prepared as previously described.18 Protein concentration was determined by the BCA colorimetric assay (Pierce Biotechnology, Rockford, IL, USA). Samples were then denaturated with 6× Laemmli buffer, boiled for 5 minutes at 95°C, and stored at −20°C until use.
Measurement of Cyclic Adenosine Monophosphate (cAMP) Levels
BV2 cells were incubated with Ex-4 for the periods of time indicated in the respective figure legend. Cells were lysed in 0.1 mol/L HCl and the intracellular levels of cAMP were measured using a colorimetric competitive immunoassay kit according to the manufacturer instructions (Enzo Life Sciences, Farmingdale, NY, USA). Results were normalized to protein content.
Subcellular Fractionation Assay for Nuclear Extraction
Nuclear protein fractions were prepared according to the method previously described.18 Protein quantification and sample preparation were performed for the total extracts.
Immunoblot Analysis
For immunodetection of p65, p–cAMP-response element binding protein (CREB), CREB, and GLP-1R, 30 μg of protein from nuclear or total cell extracts were separated using SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Boehringer Mannheim, Mannheim, Germany). Western blot was performed as previously described.9 Membranes were probed with rabbit polyclonal anti-p65, anti-CREB, anti-phosphoCREB (1:1000, all from Cell Signaling Technology, Beverly, MA, USA) and anti-GLP-1R (1:300, Abcam, Cambridge, UK). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:10,000; Sicgen, Cantanhede, Portugal) and Lamin B (1:4000, Abcam) were used as loading controls for total and nuclear extracts, respectively. Immunoreactive bands were detected by enhanced chemiluminescence (ECL) substrate using an imaging system (VersaDoc 4000 MP; Bio-Rad) and quantification was performed using ImageJ software (version 1.47, http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).
Immunocytochemistry
V2 cells cultured on cover glass were washed with pre-warmed PBS and fixed with ice-cold 4% paraformaldehyde for 15 minutes at room temperature. Cells were then washed with PBS, blocked with 5% BSA in PBS containing 0.3% Triton X-100 (PBS-T) for 1 hour and incubated with the polyclonal primary antibody anti-p65 (Cell Signaling, 1:100 dilution in PBS-T containing 1% BSA) overnight at 4°C. After washing with PBS, cells were incubated at room temperature for 1 hour (kept dark) with the secondary antibody (1:1000 dilution in PBS-T) AlexaFluor 488 goat anti-rabbit (Life Technologies) and 10 μg/mL Hoechst-33342 DNA stain (Life Technologies) for nuclear staining. The coverslips were mounted upside down on glass slides and visualized under a confocal microscope (Leica Microsystems). Nuclear p65 immunostaining was measured using ImageJ software by averaging the fluorescence intensity of delimitated nuclei from four fields per slide from three experiments in a masked fashion.
Statistical Analysis
Results are expressed as mean ± SEM. One-way ANOVA followed by Bonferroni's post hoc test was employed to calculate the statistical difference between three or more groups. Statistical software (Prism 4.0; GraphPad Software, San Diego, CA, USA) was used and values of P < 0.05 were considered statistically significant.
Results
Ex-4 Prevents the Increase in BRB Permeability Induced by IR Injury
Vascular permeability was significantly increased by 3.8-fold (P < 0.001, vehicle-sham versus vehicle-IR, n = 16) in IR retinas at 48 hours after reperfusion when compared to nontreated sham retinas, as revealed by the increased retinal Evans Blue dye accumulation (Fig. 1). Treatment with Ex-4 by subcutaneous injection twice a day was able to significantly reduce the effect of IR on vascular permeability (P < 0.001, vehicle-IR versus Ex-4-IR, n = 16; Fig. 1).
Figure 1.
Ex-4 protects against the increase in retinal vascular leakage triggered by IR. Rats were treated twice daily with a subcutaneous injection of Ex-4 (10 μg/kg), with two initial administrations prior to ischemia and injections every 12 hours for the next 48 hours during the reperfusion period. Nontreated animals received saline vehicle injections. One eye of each animal was subjected to retinal ischemia for 45 minutes, followed by natural reperfusion. The contralateral eyes were subjected to needle puncture and served as sham controls. Evans Blue dye was injected in the tail vein and allowed to circulate 2 hours before flushing and determination of dye accumulation in retinal tissue. Data are expressed as mean ± SEM (n = 16 retinas per group). ***P < 0.001. ns, nonsignificant. One-way ANOVA followed by Bonferroni's post hoc test.
Ex-4 Treatment Alters the Inflammatory Response Following IR Injury Without Affecting the Number of CD45-Positive Cells
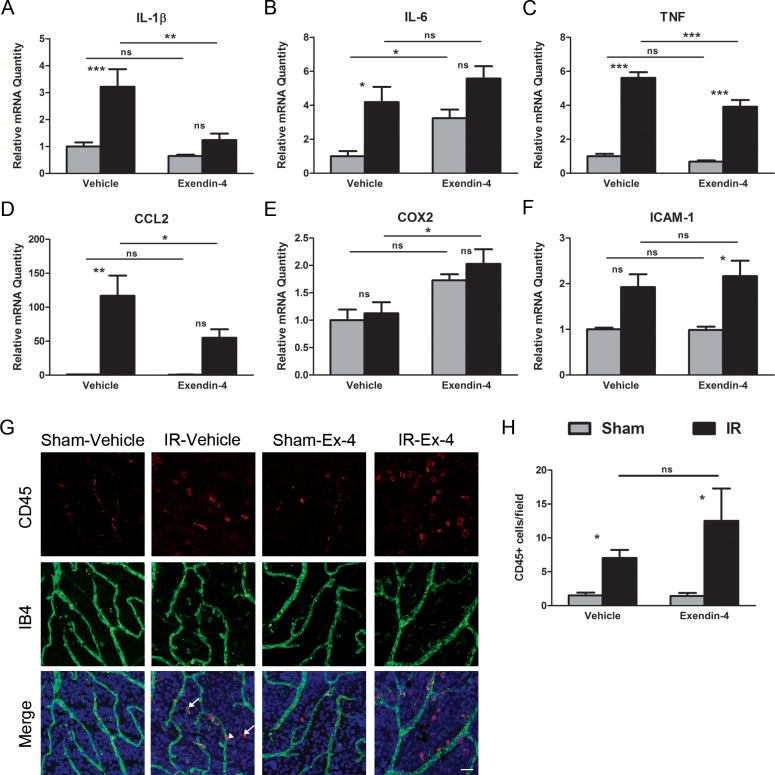
Ischemia-reperfusion injury induced a significant increase in retinal mRNA expression of several proinflammatory cytokines, including interleukin-1β (IL-1β, 3.2-fold, P < 0.001, vehicle-sham versus vehicle-IR, n = 8); interleukin-6 (IL-6, 4.2-fold, P < 0.05, vehicle-sham versus vehicle-IR, n = 8); TNF (5.6-fold, P < 0.001, vehicle-sham versus vehicle-IR, n = 8); and C-C motif chemokine ligand 2 (CCL2, 116.9-fold, P < 0.01, vehicle-sham versus vehicle-IR, n = 8; Figs. 2A–D). However, expression of mRNA for cyclooxygenase 2 (COX2) was not altered after 48 hours in IR retinas (Fig. 2E) and mRNA for ICAM-1 (1.9-fold) trended up (Fig. 2F).
Figure 2.
Exendin-4 inhibits the induction of the expression of IR-responsive genes associated with inflammation without affecting the number of CD45-positive cells. Rats were treated with Ex-4 (10 μg/kg) twice daily with two initial administrations prior to ischemia, and injections every 12 hours for the next 48 hours during the reperfusion period. Nontreated animals received saline vehicle injections. One eye of each animal was subjected to retinal ischemia for 45 minutes, followed by natural reperfusion. The contralateral eyes were subjected to needle puncture and served as sham controls. Total RNA was isolated from the retinas and the relative levels of mRNA of (A) IL-1β, (B) IL-6, (C) TNF, (D) CCL2, (E) COX2, and (F) ICAM-1 were determined by duplex qRT-PCR with β-actin serving as control. Data are expressed as mean ± SEM (n = 8 retinas per group). (G) Retinas were isolated and stained with antibodies to CD45 (red), isolectin B4 (IB4, green), and Hoechst nuclear stain (blue) and then flat mounted. The majority of CD45-positive cells are present in the perivascular region (arrows) with few cells positively staining inside the vessels (arrowhead). Scale bar: 25 μm. (H) We quantified CD45-positive cells in each retina from each group. Data are presented as CD45-positive cells per field and represent the mean ± SEM (n = 8 retinas per group). *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA followed by Bonferroni's post hoc test.
Comparison of mRNA expression levels in sham eyes of Ex-4-treated and nontreated rats revealed that the drug alone significantly affected the expression of IL-6 (increased 3.2-fold, P < 0.05, vehicle-sham versus Ex-4-sham, n = 8; Fig. 2B).
Treatment with Ex-4 significantly inhibited IR-induced upregulation of cytokines with known effects on vascular permeability; IL-1β (61% inhibition, P < 0.01, vehicle-IR versus Ex-4-IR, n = 8); TNF (30% inhibition, P < 0.001, vehicle-IR versus Ex-4-IR, n = 8); and CCL2 (53% inhibition, P < 0.05, vehicle-IR versus Ex-4-IR, n = 8) mRNAs (Figs. 2A, 2C, 2D, respectively). No effect on ICAM-1 mRNA expression levels was observed (Fig. 2F).
To determine whether Ex-4 treatment decreases leukocyte migration and infiltration into the retinal tissue following IR, retinal whole-mount retinas were stained with CD45, a marker of leukocytes, and with IB4, an isolectin binding to terminal alpha-D-galactose residues of glycoconjugates found on endothelial cells and some macrophages (Fig. 2G). The number of CD45-positive cells was significantly higher in IR retinas, relative to sham retinas. Most CD45-positive cells were present in the perivascular space after 48 hours of reperfusion (Fig. 2G, arrows). A few CD45-stained cells were also observed within vessels in IR-injured retinas (Fig. 2G, arrowhead). Exendin-4–treated IR retinas did not demonstrate any statistically significant decrease in CD45-positive cells when compared with vehicle-treated IR retinas (Fig. 2H), suggesting that the anti-inflammatory effects of Ex-4 are not due to a decrease in infiltrating leukocytes and are not sufficient to cause a decrease in leukocyte accumulation.
Ex-4 Has No Effect on TNF and/or VEGF-Induced Endothelial Cell Permeability
Given that Ex-4 was able to prevent the IR-induced BRB permeability in vivo, we hypothesized that it could have direct beneficial effects on endothelial barrier integrity. We therefore evaluated the effect of Ex-4 pretreatment on in vitro endothelial cell monolayer permeability to RITC-dextran following treatment with TNF and/or VEGF. As shown in Figure 3, treatment with TNF and/or VEGF resulted in a significant increase in 70 kDa RITC-dextran permeability across BREC monolayer (Fig. 3). Pretreatment of BREC with Ex-4 did not prevent or reduce the increase in permeability induced by TNF, VEGF, or both combined (Fig. 3).
Figure 3.
Effect of Ex-4 on TNF and/or VEGF-induced retinal endothelial cells permeability. We grew BREC to confluence on transwell filters and the monolayer permeability to RITC-70 kDa dextran was measured over the following 4 hours after the several treatments. Exendin-4 does not block TNF and/or VEGF-induced permeability in cultured BREC. We treated BREC with 10 nmol/L Ex-4 1 hour before TNF (5 ng/mL, 1.5 hours) and/or VEGF (50 ng/mL, 30 minutes) treatment. Average rate of Po for the control was 5.5 × 10−7 cm/s. The results represent the mean ± SEM (n ≥ 3). *P < 0.05, ***P < 0.001 versus control. One-way ANOVA followed by Bonferroni's post hoc test.
Ex-4 Modulates Inflammatory Response in BV2 Microglial Cells
Having established that Ex-4 reduced the production of proinflammatory cytokines without inhibition of induction of expression of ICAM-1 or accumulation of leukocytes occurring in IR retinas, we proceeded by assessing the impact of Ex-4 in microglia, the resident innate immune cells of the retina, and other neural tissues. Production of nitric oxide and proinflammatory cytokines by microglia may contribute to neuroinflammation in the IR injury model.19 In order to test the ability of Ex-4 to inhibit microglial activation, we used BV2, a microglia cell line, stimulated with LPS, which is a potent inducer of microglial activation and inflammatory gene expression.
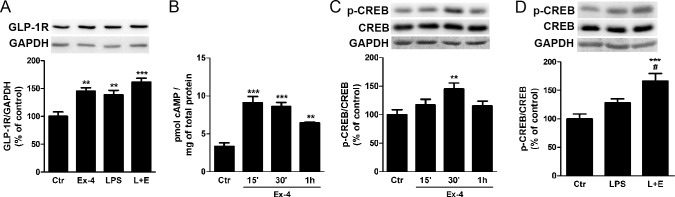
Exendin-4 is known to activate GLP-1R, a G-protein–coupled receptor, leading to activation of adenylyl cyclase, which results in generation of cAMP.7 We observed by Western blotting that Ex-4 and LPS each induced a significant increase in GLP-1R protein levels (P < 0.01, Control [Ctr] versus Ex-4, Ctr versus LPS, n = 5) that was not additive (P < 0.001, Ctr versus L + E, n = 5; Fig. 4A).
Figure 4.
Exendin-4 induces activation of GLP-1R and CREB in LPS-treated microglial BV2 cells. (A) We treated BV2 cells with Ex-4 (100 nmol/L) 1 hour prior to the LPS stimulus (100 ng/mL for 4 hours), and protein levels of GLP-1R were evaluated by Western blotting. We used GAPDH as a loading control. Quantification of GLP-1R protein levels was performed by densitometric analysis. Data represent the mean ± SEM (n = 5). (B) Intracellular cAMP levels were determined by ELISA after stimulating BV2 cells with Ex-4 (100 nmol/L) for 15 minutes, 30 minutes, and 1 hour. Data are presented as pmol of cAMP per mg of total protein and represent the mean ± SEM (n = 3). (C) We incubated BV2 cells with Ex-4 as described in (B) and protein levels of p-CREB and CREB were determined by Western blotting. (D) The effects of LPS on CREB phosphorylation were also assessed. Data represent the mean ± SEM (n = 5). **P < 0.01, ***P < 0.001 versus control. #P < 0.05 versus LPS; 1-way ANOVA followed by Bonferroni's post hoc test.
Treatment with 100 nmol/L of Ex-4 promoted a significant increase of intracellular cAMP levels by 15 minutes (P < 0.001, Ctr versus Ex-4 15′, Ctr versus Ex-4 30′, n = 3) that was maintained for at least 1 hour (P < 0.01, Ctr versus Ex-4 1h, n = 3) confirming GLP-1R activation (Fig. 4B).
Activation of GLP-1R was further confirmed by measuring the PKA-mediated phosphorylation of the transcription factor CREB. Treatment with Ex-4 promoted a peak in CREB phosphorylation after 30 minutes (P < 0.01, Ctr versus Ex-4 30′, n = 5; Fig. 4C). Further, Ex-4–induced CREB activation was maintained after incubation with LPS (Fig. 4D).
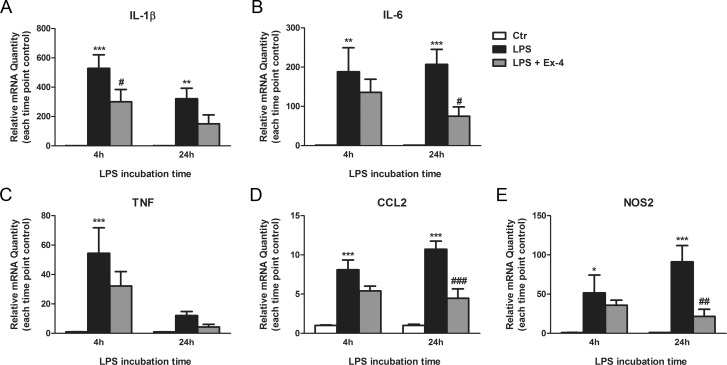
Treatment with Ex-4 was able to inhibit LPS-induced increase in mRNA expression of IL-1β (43% inhibition, P < 0.05, LPS versus LPS + Ex-4, n = 4) after 4 hours; and IL-6 (64% inhibition, P < 0.05, LPS versus LPS + Ex-4, n = 4), CCL2 (58% inhibition, P < 0.001, LPS versus LPS + Ex-4, n = 4), nitric oxide synthase 2 (NOS2; 76% inhibition, P < 0.01, LPS versus LPS + Ex-4, n = 4) after 24 hours (Figs. 5A–E). A nonsignificant trend toward a decrease in TNF mRNA levels after Ex-4 treatment was seen at both 4 hours (40% inhibition) and 24 hours (63% inhibition; Fig. 5A–E).
Figure 5.
Exendin-4 attenuates the inflammatory response of BV2 microglial cells to LPS. We treated BV2 cells with Ex-4 (100 nmol/L) 1 hour prior to the LPS stimulus (100 ng/mL for 4 hours or 24 hours). Total RNA was isolated from the cells and the relative levels of mRNA of (A) IL-1β, (B) IL-6, (C) TNF, (D) CCL2, and (E) NOS2 were determined by duplex qRT-PCR with β-actin serving as control. Data are presented as the relative mRNA quantity and represent the mean ± SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 versus control. #P < 0.05, ##P < 0.01, ###P < 0.001 versus LPS. Two-way ANOVA followed by Bonferroni's post hoc test.
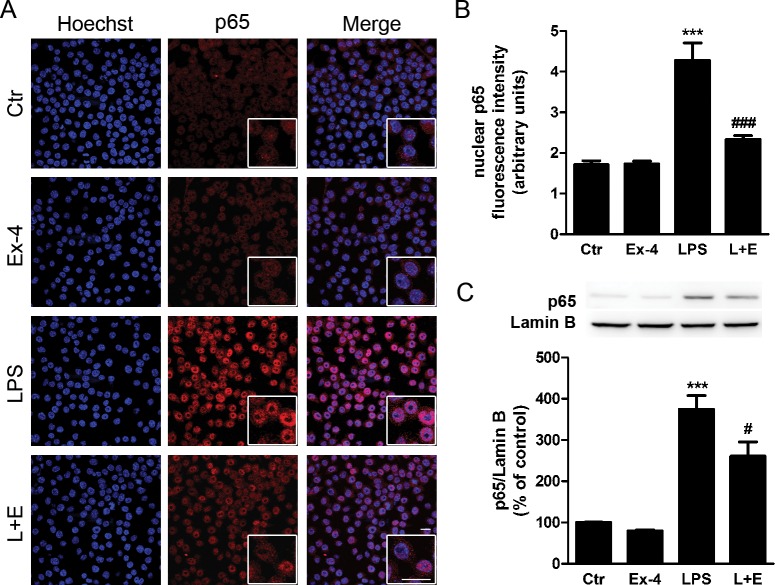
We next examined the effects of Ex-4 on nuclear factor-kappaB (NF-κB), which is an important transcription factor modulating cytokine gene expression in microglia. Immunocytochemical analysis shows that stimulation of BV2 cells with LPS for 30 minutes resulted in a strong nuclear expression of NF-κB p65 subunit, which was significantly inhibited by pretreatment with Ex-4 (Figs. 6A, 6B). Additionally, these results were confirmed by Western blotting using nuclear extracts from BV2 cells (Fig. 6C). These data suggest that modulation of NF-κB activation may be involved in the ability of Ex-4 to inhibit the expression of inflammatory mediators in microglial cells.
Figure 6.
Exendin-4 inhibits the nuclear accumulation of the NF-κB p65 subunit in LPS-stimulated BV2 microglial cells. (A) We treated BV2 cells with Ex-4 (100 nmol/L) 1 hour prior to the LPS stimulus (100 ng/mL for 30 minutes). Subcellular localization of p65 subunit (red) was evaluated by immunocytochemistry. Hoechst staining (blue) was used to visualize nuclei. Scale bar: 20 μm. (B) Quantification of nuclear fluorescence intensity for p65 immunoreactivity in BV2 cells. Data are presented as arbitrary fluorescence units and represent the mean ± SEM (n = 3). (C) We stimulated BV2 cells as described in (A). Subcellular fractionation was performed and nuclear extracts were separated by SDS-PAGE and immunoblotted with anti-p65 antibody. Lamin B was used as a loading control. Quantification of p65 protein levels was performed by densitometric analysis. Data are presented as mean ± SEM (n = 5). ***P < 0.001 versus control. #P < 0.05, ###P < 0.001 versus LPS. One-way ANOVA followed by Bonferroni's post hoc test.
Discussion
In the present study, we provide evidence that Ex-4, a GLP-1 analog, prevents the increase in BRB permeability and reduces the expression of several classical inflammatory markers induced by IR injury in rat retinas. Moreover, using a microglia cell line for in vitro studies, we demonstrate that Ex-4 also inhibits the inflammatory response to LPS activation, while reducing the amount of NF-κB p65 in the nucleus. Collectively, these results demonstrate, for the first time, that Ex-4 is able to modulate inflammation and prevent loss of the BRB in a retinal ischemia model.
Intraocular pressure–induced retinal IR injury provides a useful model of VEGF-driven vascular permeability followed by inflammatory response that maintains BRB loss, such as may be observed in a number of retinal eye diseases, including diabetic retinopathy and retinal vein occlusions.11 Evidence of retinal neurodegeneration, inflammation, including microglial activation, and BRB loss associated with tight junction alterations all may be observed after IR injury. Furthermore, this model does not include any systemic metabolic alterations, providing an opportunity to verify the direct effects of Ex-4 previously observed on diabetic retinas. Given its high lipophilicity, Ex-4 was shown to readily cross the blood–brain barrier, and therefore likely enters the retina as well.20 Thus, in contrast to previous studies8,10 where Ex-4 was delivered by intravitreal injection, we decided to administer Ex-4 by subcutaneous injection, which is the mode of administration indicated for GLP-1R agonists in the clinical practice. Furthermore, due to the high risk of infectious endophthalmitis associated with repeated intravitreal injections, any anti-inflammatory effects of Ex-4 could be masked in the case of this route of administration was used.
Exendin-4 has been reported to have multiple cellular protective effects, including the protection of endothelial cells and barrier function. A recent study showed that intravitreal injection of Ex-4 reduces retinal vascular leakage in type 2 diabetic Goto-Kakizaki rats.10 Moreover, Ex-4 was found to suppress LPS-mediated release of nuclear DNA-binding protein high-mobility group box 1 (HMGB1) and inhibits HMGB1-mediated hyperpermeability and leukocyte migration in septic mice.21 Previous studies16,22 have also shown that activation of GLP-1R in cultured endothelial cells could prevent the increase in monolayer permeability induced by LPS and thrombin. In the present study, systemic administration of Ex-4 was able to prevent increased retinal vascular permeability induced by IR injury at 48 hours of reperfusion. However, in primary cultures of retinal endothelial cells, Ex-4 could not prevent the increase in permeability induced by permeabilizing agents that are known to be increased in IR retinas, such as VEGF and TNF. These results point to indirect mechanisms accounting for the in vivo protective effects of Ex-4 in BRB, other than directly targeting the endothelial cells.
Inflammation triggered by reperfusion is a key mediator in retinal damage after ischemic injury and anti-inflammatory effects of Ex-4 have been proposed by recent studies.23–26 However, the current study is the first demonstration of the anti-inflammatory effects of Ex-4 on retinal IR injury. Exendin-4 inhibited the increase in the expression of classical inflammatory genes (IL-1β, TNF, and CCL2) that are responsive to IR injury. We also observed that the expression of another IR-responsive gene, IL-6, was further exacerbated by Ex-4. Interleukin 6 seems to have a dual role in inflammatory processes as increased IL-6 in microglia medium protects retinal ganglion cells (RGC) from pressure-induced death27 and intravitreal injection of IL-6 protects RGC layer neurons from IR injury.28 Thus, besides having anti-inflammatory effects, Ex-4 may also play an important role in neuroprotection, a question that was not addressed in the present study.
In response to neural tissue injury, such as in IR, microglial cells become activated, altering their morphology and gaining increased phagocytic ability. They migrate to the site of injury, proliferate and release a variety of factors, such as cytokines, chemokines, nitric oxide, reactive oxygen species, and matrix metalloproteinases, which are known to contribute to BRB dysfunction and breakdown.19 Weakening of the barrier and increased expression of adhesion molecules by endothelial cells promotes the infiltration of circulating leukocytes that may further exacerbate inflammation and retinal damage.19
In the present study, Ex-4 was neither able to prevent the increase in the number of CD45-positive cells nor inhibit the induction of expression of ICAM-1 in IR retinas, suggesting that the anti-inflammatory effects of Ex-4 are not due to inhibition of the accumulation of inflammatory cells, nor was it sufficient to block the accumulation of leukocytes in the injured retina. The current study cannot identify potential changes in subpopulations of circulating leukocytes and further studies are warranted to discern between the populations of CD45-positive cells and their activation state present in the ischemic retinas. Our analysis of CD45-positive cells in retinal whole-mounts cannot definitively differentiate activated resident microglia from invading leukocytes. Nevertheless, we observed a decrease in proinflammatory factors induced by Ex-4 in IR retinas and we clearly demonstrated that Ex-4 can modulate a microglia cell line's response to activation by inhibiting nuclear accumulation of NF-κB and decreasing the expression of classical inflammatory markers (IL-1β, IL-6, CCL2, and NOS2). Furthermore, Ex-4 is also known to suppress macrophage activation. Treatment of isolated mouse macrophages with Ex-4 suppresses LPS-induced gene expression of TNF and CCL2 by a mechanism dependent on adenylate cyclase and PKA activation, and prevents nuclear translocation of p65.26 Additionally, Ex-4 also attenuates high glucose–induced TNF and IL-1β expression and secretion by macrophages derived from the THP-1 human monocytic cell line in a GLP-1R–dependent manner.24 Thus, it is probable that both microglia and circulating monocytes were affected by systemic treatment with Ex-4. In accordance with our findings, other studies have pointed to microglia as a key mediator of GLP-1R–induced anti-inflammatory effects in animal models of neurodegenerative diseases.23,29–31
It is well known that GLP-1R activates the cAMP/PKA signaling pathway and a previous report32 has revealed that an increase in activity of this pathway suppresses NF-κB activity in monocytic THP-1 cells and in HUVEC. These findings support our observation that Ex-4 inhibited LPS-induced NF-κB activation, decreasing p65 nuclear accumulation, possibly via enhancement of cAMP following GLP-1R activation. Therefore, Ex-4 anti-inflammatory effects in microglia cells may be due to NF-κB inhibition and consequent decrease in proinflammatory cytokine gene transcription.
In conclusion, we report herein that Ex-4 confers significant protective effects in the ischemic retina, preventing BRB breakdown and inhibiting inflammation. Our in vitro studies suggest that the effects of Ex-4 on BRB are not mediated by direct effects on endothelial cells but by an inhibition of microglia activation that leads to a decrease in the inflammatory response and protects against barrier breakdown. These data identify Ex-4 as a potential therapeutic option for the treatment of retinal diseases characterized by increased vascular permeability and neuroinflammation, such as diabetic retinopathy.
Supplementary Material
Acknowledgments
The authors would like to thank Heather Lindner who aided in animal experimentation and Lijie Gong and Sumathi Shanmugam who helped with all facets of the BV2 culture and qRT-PCR assays.
Supported by NIH R01 EY012021 (DAA); Research to Prevent Blindness Jules and Doris Stein Professorship (DAA); NIH R01 EY007739 (SFA); the Core Center for Vision Research at the Kellogg Eye Center P30 EY007003; European Foundation for the Study of Diabetes (EFSD)/Glaxo Smith Kline Programme (RF); GIFT/Portuguese Society of Diabetes (RF); Foundation for Science and Technology (FCT Portugal, Strategic Project UID/NEU/04539/2013, Project PTDC/NEU-OSD/1113/2012-FCOMP-0124-FEDER-029665 (AFA); PhD fellowship SFRH/BD/103936/2014 (AG); COMPETE-FEDER; and a Fulbright Research Fellowship (AG).
Disclosure: A. Gonçalves, None; C.-M. Lin, None; A. Muthusamy, None; C. Fontes-Ribeiro, None; A.F. Ambrósio, None; S.F. Abcouwer, None; R. Fernandes, None; D.A. Antonetti, None
References
- 1. Osborne NN,, Casson RJ,, Wood JP,, Chidlow G,, Graham M,, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004. ; 23: 91–147. [DOI] [PubMed] [Google Scholar]
- 2. Zheng L,, Gong B,, Hatala DA,, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007. ; 48: 361–367. [DOI] [PubMed] [Google Scholar]
- 3. Cunha-Vaz J,, Faria de Abreu JR,, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975. ; 59: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adamiec-Mroczek J,, Oficjalska-Mlynczak J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes--role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008. ; 246: 1665–1670. [DOI] [PubMed] [Google Scholar]
- 5. Schwartzman ML,, Iserovich P,, Gotlinger K,, et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010. ; 59: 1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang J,, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011. ; 30: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell JE,, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013. ; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y,, Zhang J,, Wang Q,, et al. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci. 2011. ; 52: 278–285. [DOI] [PubMed] [Google Scholar]
- 9. Goncalves A,, Marques C,, Leal E,, et al. Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim Biophys Acta. 2014. ; 1842: 1454–1463. [DOI] [PubMed] [Google Scholar]
- 10. Fan Y,, Liu K,, Wang Q,, Ruan Y,, Ye W,, Zhang Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp Eye Res. 2014. ; 127: 104–116. [DOI] [PubMed] [Google Scholar]
- 11. Abcouwer SF,, Lin CM,, Wolpert EB,, et al. Effects of ischemic preconditioning and bevacizumab on apoptosis and vascular permeability following retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2010. ; 51: 5920–5933. [DOI] [PubMed] [Google Scholar]
- 12. Muthusamy A,, Lin CM,, Shanmugam S,, Lindner HM,, Abcouwer SF,, Antonetti DA. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J Cereb Blood Flow Metab. 2014. ; 34: 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abcouwer SF,, Lin CM,, Shanmugam S,, Muthusamy A,, Barber AJ,, Antonetti DA. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflammation. 2013. ; 10: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Q,, Qaum T,, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001. ; 42: 789–794. [PubMed] [Google Scholar]
- 15. Antonetti DA,, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods Mol Med. 2003. ; 89: 365–374. [DOI] [PubMed] [Google Scholar]
- 16. Li AQ,, Zhao L,, Zhou TF,, Zhang MQ,, Qin XM. Exendin-4 promotes endothelial barrier enhancement via PKA- and Epac1-dependent Rac1 activation. Am J Physiol Cell Physiol. 2015; 308: C164–C175. [DOI] [PubMed] [Google Scholar]
- 17. Blasi E,, Barluzzi R,, Bocchini V,, Mazzolla R,, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990; 27: 229–237. [DOI] [PubMed] [Google Scholar]
- 18. Aveleira C,, Castilho A,, Baptista F,, et al. High glucose and interleukin-1beta downregulate interleukin-1 type I receptor (IL-1RI) in retinal endothelial cells by enhancing its degradation by a lysosome-dependent mechanism. Cytokine. 2010. ; 49: 279–286. [DOI] [PubMed] [Google Scholar]
- 19. Madeira MH,, Boia R,, Santos PF,, Ambrosio AF,, Santiago AR. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015. ; 2015: 673090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kastin AJ,, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003. ; 27: 313–318. [DOI] [PubMed] [Google Scholar]
- 21. Lee W,, Ku SK,, Park EJ,, Na DH,, Kim KM,, Bae JS. Exendin-4 inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation. 2014. ; 37: 1876–1888. [DOI] [PubMed] [Google Scholar]
- 22. Dozier KC,, Cureton EL,, Kwan RO,, Curran B,, Sadjadi J,, Victorino GP. Glucagon-like peptide-1 protects mesenteric endothelium from injury during inflammation. Peptides. 2009. ; 30: 1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darsalia V,, Hua S,, Larsson M,, et al. Exendin-4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial M2 polarization. PLoS One. 2014. ; 9: e103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kodera R,, Shikata K,, Kataoka HU,, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011. ; 54: 965–978. [DOI] [PubMed] [Google Scholar]
- 25. Iwai T,, Ito S,, Tanimitsu K,, Udagawa S,, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006. ; 55: 352–360. [DOI] [PubMed] [Google Scholar]
- 26. Arakawa M,, Mita T,, Azuma K,, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010. ; 59: 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sappington RM,, Chan M,, Calkins DJ. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest Ophthalmol Vis Sci. 2006. ; 47: 2932–2942. [DOI] [PubMed] [Google Scholar]
- 28. Sanchez RN,, Chan CK,, Garg S,, et al. Interleukin-6 in retinal ischemia reperfusion injury in rats. Invest Ophthalmol Vis Sci. 2003. ; 44: 4006–4011. [DOI] [PubMed] [Google Scholar]
- 29. Lee CH,, Yan B,, Yoo KY,, et al. Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res. 2011. ; 89: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 30. Darsalia V,, Mansouri S,, Ortsater H,, et al. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond). 2012. ; 122: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim S,, Moon M,, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson's disease. J Endocrinol. 2009; 202: 431–439. [DOI] [PubMed] [Google Scholar]
- 32. Parry GC,, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol 1997. ; 159: 5450–5456. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.