Abstract
Homeostasis is a central pillar of modern Physiology. The term homeostasis was invented by Walter Bradford Cannon in an attempt to extend and codify the principle of ‘milieu intérieur,’ or a constant interior bodily environment, that had previously been postulated by Claude Bernard. Clearly, ‘milieu intérieur’ and homeostasis have served us well for over a century. Nevertheless, research on signal transduction systems that regulate gene expression, or that cause biochemical alterations to existing enzymes, in response to external and internal stimuli makes it clear that biological systems are continuously making short-term adaptations both to set-points, and to the range of ‘normal’ capacity. These transient adaptations typically occur in response to relatively mild changes in conditions, to programs of exercise training, or to sub-toxic, non-damaging levels of chemical agents; thus the terms hormesis, heterostasis, and allostasis are not accurate descriptors. Therefore, an operational adjustment to our understanding of homeostasis suggests that the modified term, Adaptive Homeostasis may be useful especially in studies of stress, toxicology, disease, and aging. Adaptive Homeostasis may be defined as follows: ‘The transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, non-damaging, signaling molecules or events, or the removal or cessation of such molecules or events.”
Keywords: Homeostasis, Adaptation, Stress, Hormesis, Nrf2, Aging
Introduction to Homeostasis
The concept of milieu intérieur, or a constant interior bodily environment, was developed by the celebrated French physiologist Claude Bernard in 1865 (1). The word ‘homeostasis’ was coined by the Harvard Physiologist, Walter Bradford Cannon in 1926 to describe and extend Bernard’s milieu intérieur concept (2), and popularized (in 1932) in his highly successful and persuasive book, The Wisdom of the Body (3). Cannon combined two words from from Ancient Greek ὅμoς (hómos, “similar”) + ιστημι (histēmi, “standing still”)/stasis (from στάσις) into a Modern Latin form to invent his term homeostasis.
Cannon wrote, “The constant conditions which are maintained in the body might be termed equilibria. That word, however, has come to have fairly exact meaning as applied to relatively simple physico-chemical states, in closed systems, where known forces are balanced. The coordinated physiological processes which maintain most of the steady states in the organism are so complex and so peculiar to living beings - involving, as they may, the brain and nerves, the heart, lungs, kidneys and spleen, all working cooperatively - that I have suggested a special designation for these states, homeostasis. The word does not imply something set and immobile, a stagnation. It means a condition - a condition which may vary, but which is relatively constant.”
Claude Bernard (July 12, 1813 – February 10, 1878) who is considered by many to have been the “father” of modern experimental physiology is quoted as having said that, “The laboratory is the temple of the science of medicine.” (4). Working at a time when cells were just beginning to be thought of as the basic structural unit of tissue and organ anatomy, Bernard was able to add an entirely new level of functional integration. Bernard concluded that, “The constancy of the internal environment is the condition for free and independent life: the mechanism that makes it possible is that which assured the maintenance, within the internal environment, of all the conditions necessary for the life of the elements.” (5–7). An important biography of Claude Bernard has been written by Charles Gross (7).
Prior to gaining his medical degree in 1900, Walter Cannon (October 19, 1871 – October 1, 1945) was a student of Physiologist Henry Pickering Bowditch, who became Dean of Harvard’s Medical School. Bowdich, in turn, had studied in Paris with Claude Bernard in the late 1860’s, after graduating from Harvard College. It is clearly no accident that Cannon, who in 1906 became Higginson Professor and chair of the department of physiology at Harvard Medical School, went on to further clarify and classify Claude Bernard’s concept of milieu intérieur, presumably passed on via Bowdich, into his own terminology of Homeostasis. No stranger to the concept of fluid metabolic states, Cannon had previously (in 1915), coined the term Fight or Flight to describe an animal’s response to threats (9).
In the The Wisdom of the Body (3), Cannon listed four core concepts that defined his idea of homeostasis:
Constancy in an open system, such as our bodies represent, requires mechanisms that act to maintain this constancy.
Steady-state conditions require that any tendency toward change automatically meets with factors that resist change.
The regulating system that determines the homeostatic state consists of a number of cooperating mechanisms acting simultaneously or successively.
Homeostasis does not occur by chance, but is the result of organized self-government.
According to Arthur C. Guyton’s immensely influential Textbook of Medical Physiology (10), “The term homeostasis is used by physiologists to mean, maintenance of nearly constant conditions in the internal environment.”
The basic idea of homeostasis is shown in Fig. 1 below. The Y axis of Fig 1 can be any biological/physiological function, such as blood pressure, heart rate, core temperature, blood glucose, NAD+/NADH and NADP+/NADPH ratios, superoxide dismutase and glutathione peroxidase levels and activities, Proteasome and Lon levels and activities, or the capacity and effectiveness of DNA repair systems. The X axis is calibrated by time, whose units can be seconds, minutes, hours, days, weeks, or even years (if one considers aging). The classical homeostasis graph of Fig. 1 reveals that while there is a mean value for any physiological attribute, we actually spend most of our time away from that mean, oscillating between a minimum ‘normal’ and a maximum ‘normal’ value. The span from low normal to high normal is then considered the normal physiological or homeostatic range.
Fig 1.

Claude Bernard (1813–1878)
The celebrated French physiologist who developed concept of milieu intérieur, or a constant interior bodily environment in 1865 (1).
Image attribution: https://upload.wikimedia.org/wikipedia/commons/e/e7/Bernard_Claude.jpg
The Importance of Stress
It is, of course, many years since Bernard and Cannon made their important contributions, and a great deal has changed in our basic appreciation of how living organisms function. When one considers, for example, that Watson & Crick (11) published their three-dimensional structure of the DNA double helix some 27 years after Cannon proposed homeostasis, it is not difficult to imagine that certain aspects of the homeostatic principle might need reconsideration or even revision. Indeed, looked at in that light, it is quite remarkable that a 90 year-old theorem based on 150 year-old observations has survived as a central dogma of physiology.
Nevertheless, it could be argued that Hans Selye began a reassessment in the 1950’s, based on his observations of behavioral responses to stress. Selye was born in Vienna, then part of the Austro-Hungarian Empire, on 26 January 1907. He grew up in Komárom, Hungary and studied medicine and chemistry in Prague. In 1931 Selye moved to Johns Hopkins University and then took a position at McGill University in Montreal. In 1945, he was recruited by the Université de Montréal, where he became professor and director of the Institute of Experimental Medicine and Surgery. In 1975 he created the International Institute of Stress and, in 1979, Selye and Arthur Antille started the Hans Selye Foundation. Behavioral Physiologists define stress as how the body reacts to a stressor, real or imagined, a stimulus that causes stress. Acute stressors affect an organism in the short term while chronic stressors exert their effects over the longer term. General Adaptation Syndrome (GAS), developed by Hans Selye (12), is a profile of how organisms respond to stress; GAS is characterized by three phases: a nonspecific mobilization phase, which promotes sympathetic nervous system activity; a resistance phase, during which the organism makes efforts to cope with the threat; and an exhaustion phase, which occurs if the organism fails to overcome the threat and depletes its physiological resources.
Clearly, Selye was focusing on how the nervous system coordinates many behavioral and physiological responses to stress, often through the use of hormones (13). In fact, this nervous system and hormonal approach to the coordination of homeostasis was already part of Walter Cannon’s thinking when he proposed adrenaline as the common mediator for the regulation of both temperature and blood sugar (of course, this was subsequently found to be incorrect). Similarly, Cannon was also juggling both homeostasis and stress responses when he developed his Fight or Flight theories (9).
Heterostasis & Allostasis
Responding to a growing awareness of the body’s ability to cope with toxic xenobiotics, Hans Selye proposed the new term heterostasis (‘heteros’ meaning other, and ‘stasis’ meaning fixity) as a counterpart of homeostasis to describe a new state induced by excessive amounts of a toxin. Selye wrote (14), “I propose to speak of heterostasis (heteros=other; stasis=fixity) as the establishment of a new steady state by exogenous (pharmacologic) stimulation of adaptive mechanisms through the development and maintenance of dormant tissue reactions.” Selye’s vision of heterostasis was of an entirely nonspecific reaction to toxic chemical stressors. He wrote, “By chemical treatment this process induces the body to raise production of its own natural nonspecific (multipurpose) remedies.” (15).
Psychologists and behavioral physiologists have often preferred the term Allostasis, also approximately meaning ‘other-fixity’ or ‘other-sameness.’ Allostasis envisions responses to a challenge and typically involves cephalic control or involvement in anticipating changing needs and, ultimately, restoring the homeostatic state (16–18). Allostasis also adds the extra dimension of energy expenditure and Allostatic Load, which is the concept that responding to stress costs energy and that repeated or, especially, chronic incursions into the allostatic mode predispose individuals to chronic degenerative diseases (16–18).
Day (19) has seriously criticized the entire idea of allostasis and allostatic load as unnecessary and, even, incorrect usages of homeostasis. Day argues that, “Indeed, rather than clarifying the concept of stress, the primary effort seems to be directed at subsuming the concept of stress with the concept of allostasis, which has the inadvertent effect of collapsing the study of homeostatic responses and stress responses together.” (19). Day goes even further to conclude that, “The attempt to subsume the concept of stress within the concept of allostasis is also counterproductive in that it distracts stress researchers from the important task of developing conceptual frameworks that allow us to tackle fundamental issues such as how the organism differentiates stressful from non-stressful challenges.” (19).
Basically, there appears to be very little difference between the terms heterostasis and allostasis, except for the introduction of allostatic load by the latter. To this investigator, allostatic load is really just an hypothetical outcome of repeated or lengthy deviations from homeostasis, rather than an actual physiological principle. Similarly, heterostasis fails to account for specific, transient, and reversible adaptive changes in homeostasis.
Hormesis
The term hormesis was suggested in 1943 by Southam & Erlich (20) to describe the process by which sub-lethal damage caused by small doses of a toxin or poison would produce an exaggerated repair response in which the organism actually becomes stronger than it was previously. The first recorded use of the term hormesis actually occurred in 1941 in Chester M. Southam’s University of Idaho undergraduate thesis. In many ways, hormesis appears to be a biological corollary of the famous pronouncement by German philosopher Friedrich Wilhelm Nietzsche (1844 –1900): “That which does not kill us makes us stronger.”
Hormesis has more recently been championed by University of Massachusetts at Amherst physiologist and toxicologist, Edward J. Calabrese. Biphasic dose-response curves have formed the basis for much of Calabrese’s impressive contributions to the toxicology literature. Calabrese has led a major effort to completely rethink the scientific foundations of our risk assessment and environmental regulation processes, and is also trying to gain acceptance for hormetic principles in drug discovery and clinical treatments (21–23). Despite the apparent ubiquity of biphasic dose-response relationships, both the concept of hormesis and Calabrese’s attempts to revolutionize environmental and medical regulations have not been without their detractors (24,25). Nevertheless, Calabrese’s work (21–23) has effected a major change in our understanding of biphasic dose-responses, and the importance of hormetic reactions to damaging levels of various environmental and industrial toxins.
For the current discussion, however, the problem with hormesis is its association with repair or restoration of damage, to produce a stronger organism. Instead, we now have numerous examples of situations in which the homeostatic range for multiple functions is transiently expanded or contracted, without any damaging initiating stimulus and, therefore, with no repair process, as will be explained in the next section.
Adaptive Homeostasis
Despite the somewhat idyllic picture of internal consistency, painted by the concept of homeostasis, successful survival in the real world actually involves dealing with fluctuating levels of both internal and environmental stresses; these threats include heat stress, cold stress, exercise, oxidative stress, food deprivation, hypoxic or anoxic stress, chemical toxins, heavy metals, mechanical stress, salt, alcohol, osmolarity, and even emotional and psychological stresses, as well as many more (26–29). A sauna or spa can certainly be considered a heat shock or stress, when one considers that temperatures of up to 104°F (40°C) are routinely encountered. Conversely, in the United States Midwest, or North East Europe, or Northern China, winter temperatures of −31°F (−35°C) must certainly be considered a cold shock or stress. Anyone who is involved in arc welding is exposed to significant oxidative stress in the form of the ozone (O3) generated, and various oxides that are partial combustion products (30). Similarly, just driving on a busy freeway, expressway, or motorway (or living next to one) exposes people to chronic damage from thousands of free radical, partial combustion products of carbon, oxygen, and nitrogen as well as oxidizing ultrafine particulates (31,32). Millions of people every year suffer ischemia/reperfusion injuries from cerebral strokes or heart attacks that involve the transient stresses of hypoxia or anoxia and reoxygenation (33). Hunger (nutrient deprivation shock) is, unfortunately, still hardly a stranger to much of the third world, and all human beings suffer from transient emotional or psychological stresses at some point in life.
If we consider the ability to cope with these various cellular or organismal stressors, it is immediately clear that they can all rise, or fall, to levels that are not accommodated by the ‘normal’ homeostatic range of stress resistance. How then do cells and whole organisms (including people) deal with fluctuating levels of stress?
In the last several years, we have discovered that resistance to multiple forms of stress is not a static property of cells, tissues, or organisms. Indeed, multiple protective systems demonstrate great transient plasticity in response to very small changes in, oxygen, oxidants, temperature, acid, alkali, salt, exercise, etc. In numerous well-documented examples, these are such small changes that they cause no damage at all (e.g. 34–42). Since these transient modifications of the homeostatic range are not examples of repair or restoration of damage to produce a stronger organism, they do not qualify as examples of hormesis. Similarly, neither hereostasis nor allostasis, with their psychological overtones and requirements for overall nervous system control, seem adequate to describe transient variations in the homeostatic range that occur as discrete responses to (non-damaging) changes in the levels of internal or environmental factors.
It is now clear that cells and whole organisms make transient and reversible adjustments to their stress resistance or resilience, in response to fluctuating metabolic and environmental conditions, and to exercise. These adjustments in stress resistance can either have a biochemical, post-translational basis, or can depend on alterations in gene expression. Based on the principle that one should largely discuss the subjects one knows best, and about which one has most expertise, I will use protein turnover and oxidative stress as my exemplars for the following discourse. Thus, using the need to control and regulate protein turnover as an example, and using signaling by oxidants as a model, we have found that the proteolytic enzymes Proteasome and Lon can undergo biochemical alteration to differentially modify the cellular proteome. The nuclear form of Proteasome, for example, undergoes post-translational ADP-ribosylation by poly ADP-ribose polymerase in response to signaling by oxidants such as H2O2, and this modification increases nuclear Proteasome’s ability to degrade histone proteins (43). Mitochondria have no Proteasome, but they do contain the Lon protease which is involved in both protein quality control in the matrix, and mitochondrial DNA maintenance and mitochondrial proliferation. Normally, Lon is bound in the D-loop of the mitochondrial genome, where it is required for DNA maintenance and mitochondrial proliferation (44,45). Following signaling by (non-damaging) nanomolar to low micromolar amounts of H2O2, Lon is actually released from the mitochondrial DNA (attached to the inner surface of the inner mitochondrial membrane) and migrates to the matrix where it can selectively degrade soluble mitochondrial proteins (46).
Although post-translational homeostatic adaptations can produce extremely rapid responses to changing environments, transient adaptive responses in gene expression profiles can allow cells and organisms to cope with a far greater range of conditions. Many such adaptive alterations to the homeostatic range are mediated by discrete signal transduction pathways that transiently alter transcription/translation (47–53). A good example of such pathways is the Keep1-Nrf2 system (35–37, 54–56). Nrf2 (nuclear factor erythroid 2-related factor 2) is a basic leucine zipper protein, with a Cap “n” Collar (CCC) structure. Nrf2 is normally found in the cytoplasm of mammalian cells, where it is bound to the Keep 1 (Kelch ECH associating protein1) an E3 ubiquitin ligase that actually polyubiquitinylates Nrf2 and targets it for proteolytic degradation by the 26S Proteasome. This process keeps cellular Nrf2 levels low, and prevents Nrf2 translocation to the nucleus where it would have signaling effects. In response to a wide variety of electrophiles and oxidants, Nrf2 avoids proteolytic digestion, undergoes phosphorylation, and translocates to the nucleus where it binds to Electrophile Response Elements (EPRE’s) which are also called Antioxidant Response Elements (ARE’s) within target gene sequences. Binding of Nrf2 to a gene’s EPRE or ARE (along with other proteins, such as MafG) causes increased expression of that gene, for a limited period: a transient increase in homeostatic levels.
Most of the Nrf2 target genes are involved in providing cellular protection against damage by electrophilic or oxidizing toxicants. Naturally, early studies of the Keep1-Nrf2 signal transduction pathway focused on its role in response to toxic exposures to electrophiles and oxidants (54–56). Subsequently, it has become abundantly clear that entirely sub-toxic, non-damaging, levels of the same electrophiles or oxidants is sufficient to activate the pathway (35–37). Essentially, sub-toxic, non-damaging, levels of Nrf2-inducing agents or conditions act as priming doses to activate pathways and enzymes that would provide protection, should it subsequently become necessary. Thus, the Keep1-Nrf2 signal transduction pathway can effect transient but powerful changes in the homeostatic range of cellular defenses against electrophiles and oxidants, yet it is not an example of heterostasis, allostasis, or hormesis.
I propose that the term Adaptive Homeostasis more adequately and appropriately describes this important cellular capability. How then could we define the term Adaptive Homeostasis? I suggest that Adaptive Homeostasis be defined as follows: ‘The transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, non-damaging, signaling molecules or events, or the removal or cessation of such molecules or events.’ This definition allows us to predict and explain the transient expansion of the homeostatic range that occurs upon exposure to nanomolar, even picomolar, levels of agents that would only be damaging or toxic in the millimolar range: Positive Adaptive Homeostasis. Similarly, the contraction of the homeostatic range that occurs many hours after initial expansion is understandable as Negative Adaptive Homeostasis, occurring as a result of removal or metabolism of the initiating agent. Negative Adaptive Homeostasis can also describe a transient contraction of the homeostatic range in response to a negative signaling molecule or event. For example, production of amino acid synthetases is turned off transiently if organisms consume a diet very rich in all amino acids (Negative Adaptive Homeostasis) and the homeostatic range of amino acid synthesis capacity is transiently decreased.
Of course, transient adaptation is also seen in conditions where cells or organisms are exposed to toxic levels of agents, or to damaging conditions, unless the toxicity or damage is so great that irreparable harm or even death occurs (57). Such conditions may actually include genetic or metabolic responses to actual damage, i.e. hormesis. A few examples of such truly hormetic responses are well-characterized in the literature, such as the induction of DNA repair capacities in response to frank DNA damage (58). In most cases, however, transient changes in protection or repair capacities can clearly be seen as the outcome of signal transduction pathways that are activated by very low, even trace, levels of initiating agents, or conditions: i.e. they are true examples of Adaptive Homeostasis.
Summary & Conclusions
Our understanding of transient adaptive changes in homeostatic capacities has been irrevocably altered by the discovery of multiple signal transduction pathways that respond not to damage, toxicity, or random events, but rather to highly predictable reactions of agents or environmental conditions with key protein sensors that activate, modulate, or disable the transcription/translation machinery for vital responsive genes. As our knowledge of such pathways and their molecular mechanisms increases, it has become increasingly clear that such terms as hormesis, heterostasis, and allostasis are simply inadequate or even inaccurate descriptors. Therefore, I propose that the term Adaptive Homeostasis be used to describe transient changes in the homeostatic range that occur in response to sub-toxic, non damaging, conditions or agents that act via pre-existing signal transduction pathways to biochemically modify existing enzymes, or to regulate transcription/translation.
Positive Adaptive Homeostasis, thus describes the transient increase in protective homeostatic capacity that occurs in response to signaling by an agent or condition. Negative Adaptive Homeostasis describes a return to the basal homeostatic range that occurs following metabolism of a signaling molecule, or removal of/from the initiating condition. Negative Adaptive Homeostasis could also describe a transient decrease in the homeostatic range, selectively induced by specific signaling agents or conditions. Adaptive Homeostasis may, thus be defined as follows: ‘The transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, non-damaging, signaling molecules or events, or the removal or cessation of such molecules or events.”
Fig. 2.

Walter Bradford Cannon (1871–1945)
The Harvard Physiologist who coined the term, Homeostasis in 1926 to describe and extend Bernard’s milieu intérieur concept (2). Homeostasis was subsequently popularized (in 1932) in Cannon’s highly influential book, The Wisdom of the Body (3).
Image attribution: https://upload.wikimedia.org/wikipedia/commons/7/78/Walter_Bradford_Cannon_1934.jp
Fig. 3.
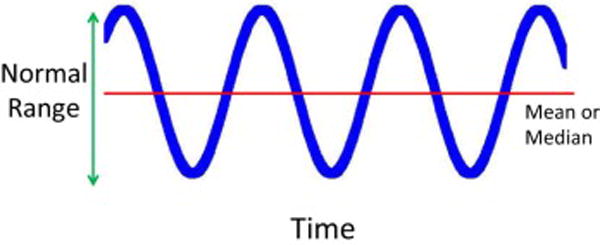
A Graphic Depiction of the Principle of Homeostasis. According to Arthur C. Guyton’s Textbook of Medical Physiology (10), “The term homeostasis is used by physiologists to mean, maintenance of nearly constant conditions in the internal environment.” Any biological function or measurement, therefore, will oscilate around a mean or median, within a range that is considered a ‘normal’ or physiological.
Fig. 4.
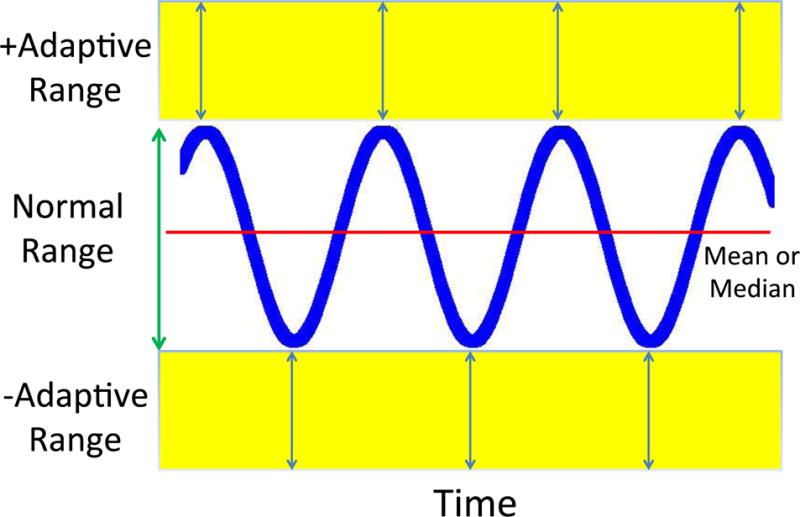
A Graphic Representation of Adaptive Homeostasis. Here, in addition to the normal or physiological range, are added both positive and negative adaptive ranges that can be transiently induced via signal transduction pathways in response to sub-toxic, non-damaging, stimuli. Thus, for example, a signal given by nanomolar levels of H2O2 can act via the Keep1-Nrf2 system to increase synthesis of protective levels of proteasome, immunoproteasome, and Pa28 (or 11S) proteasome regulator for a period of several hours (34–36): an example of positive Adaptive Homeostasis. Loss of the H2O2 stimulus then returns the system to the basal homeostatic range. Similarly, when organisms are exposed to a diet rich in amino acids, they turn off production of amino acid synthetases, thus decreasing capacity to synthesize amino acids: an example of Negative Homeostasis. Restoration of a ‘normal’ diet would then reverse the transient decrease in capacity back within the normal homeostatic range.
Acknowledgments
KJAD was supported by grant #ES003598 from the National Institute of Environmental Health Sciences of the US National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernard Claude. An Introduction to the Study of Experimental Medicine. 1865. originally published in 1865; first English translation by Henry Copley Greene, published by Macmillan & Co., Ltd. 1927; Dover edition, 1957. [Google Scholar]
- 2.Cannon WB. Physiological regulation of normal states: some tentative postulates concerning biological homeostatics. In: Pettit A, editor. A Charles Richet: ses amis, ses collègues, ses élèves (in French) Paris: Les Éditions Médicales; 1926. p. 91. [Google Scholar]
- 3.Cannon WB. The Wisdom of the Body. New York: W. W. Norton & Company; 1932. pp. 177–201. [Google Scholar]
- 4.Schafer AI. The Vanishing Physician-scientist. Cornell University Press; 2009. p. 29. [Google Scholar]
- 5.Bernard C. In: Lectures on the phenomena common to animals and plants. Trans Hoff HE, Guillemin R, Guillemin L, editors. Springfield (IL): Charles C Thomas; 1974. [Google Scholar]
- 6.Bernard Claude. In: Lectures on the Phenomena of Life Common to Animals and Plants. Hoff Hebbel E, Guillemin Roger, Guillemin Lucienne., translators. Springfield, Illinois USA: Charles C Thomas; 1974. p. 84. [Google Scholar]
- 7.Gross Charles G. Claude Bernard and the constancy of the internal environment. The Neuroscientist. 1998;4:380–385. [Google Scholar]
- 8.Cooper SJ. From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite. 2008;51:419–427. doi: 10.1016/j.appet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Cannon Walter Bradford. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement. Appleton and company; New York: 1915. pp. 1–334. [Google Scholar]
- 10.Guyton AC. Textbook of Medical Physiology. 8th. Philadelphia: W.B. Saunders; 1991. [Google Scholar]
- 11.Watson JD, Crick FHC. A structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 12.Selye H. The Stress of Life. New York: McGraw-Hill Book Company; 1956. [Google Scholar]
- 13.Timiras PS. Stress, Adaptation, Longévité. Economica Press; Paris, France: 2004. [Google Scholar]
- 14.Selye H. Homeostssis and heterostasis. In: Day Stacey B., editor. Trauma: Clinical and Biological Aspects. Springer-Verlag; New York: 1975. pp. 25–29. [Google Scholar]
- 15.Selye H. In: The Nature of Stress and its Relation to Cardiovascular Disease In Hearts and Heart-like Organs. Bourne GH, editor. Academic Press; London: 1980. p. 302. [Google Scholar]
- 16.Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason JT, editors. Handbook of life stress, cognition, and health. Chicester, NY: Wiley; 1988. pp. 629–649. [Google Scholar]
- 17.McEwen Bruce S. “Protective and Damaging Effects of Stress Mediators”. Seminars in Medicine of the Beth Israel Deaconess Medical Center. N Engl J Med. 1998a;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 18.Wingfield John C. “Control of behavioural strategies for capricious environments”. Anniversary Essays. Anim Behav. 2003;66(5):807–16. doi: 10.1006/anbe.2003.2298. [DOI] [Google Scholar]
- 19.Day Trevor A. Defining stress as a prelude to mapping its neurocircuitry: No help from allostasis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1195–1200. doi: 10.1016/j.pnpbp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Southam CM, Ehrlich J. Effects of Extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- 21.Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief - Hormesis demands a reappraisal of the way risks are assessed. Nature. 2003;421(6924):691–692. doi: 10.1038/421691a. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese EJ. Hormesis: A revolution in toxicology, risk assessment and medicine. EMBO Report Mattson, MP, and Calabrese, EJ (2010) 2004;5(Suppl 1):S37–40. doi: 10.1038/sj.embor.7400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattson MP, Calabrese EJ, editors. Hormesis: A revolution in biology, toxicology and medicine. Humana Press Inc; Hormesis: What it is and why it matters; pp. 1–13. [Google Scholar]
- 24.Kaiser J. HORMESIS Sipping From a Poisoned Chalice. Science. 2003;302:376–379. doi: 10.1126/science.302.5644.376. [DOI] [PubMed] [Google Scholar]
- 25.Axelrod D, Burns K, Davis D, von Larebeke N. Hormesis–an inappropriate extrapolation from the specific to the universal. Int J Occup Environ Health. 2004;10:335–339. doi: 10.1179/oeh.2004.10.3.335. [DOI] [PubMed] [Google Scholar]
- 26.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 27.Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–57. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 28.Saunders LR, Verdin E. Stress Response and Aging. Science. 2009;323:1021–1022. doi: 10.1126/science.1170007. [DOI] [PubMed] [Google Scholar]
- 29.de Nadal E, Ammerer G, Posas F. Controlling gene expression in response to stress. Nature Reviews Genetics. 2011;12:833–845. doi: 10.1038/nrg3055. [DOI] [PubMed] [Google Scholar]
- 30.Liu HH, Wu YC, Chen HL. Production of Ozone and Reactive Oxygen Species After Welding. Arch Environ Contam Toxicol. 2007;53:513–518. doi: 10.1007/s00244-007-0030-1. [DOI] [PubMed] [Google Scholar]
- 31.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Liu H, Davies KJA, Sioutas C, Morgan T, Finch CE, Forman HJ. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic Biol Med. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasoulis Marios-Konstantinos, Douzinas EE. Hypoxemic reperfusion of ischemic states: an alternative approach for the attenuation of oxidative stress mediated reperfusion injury. J Biomed Sci. 2016;23:1–8. doi: 10.1186/s12929-016-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJA. The immunoproteasome, the 20S proteasome and the PA28 proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochemical Journal. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJA. Nrf2 dependent induction of proteasome and Pa28αβ regulator is required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJA. A Conserved Role for the 20S Proteasome and Nrf2 Transcription Factor in Oxidative-Stress Adaptation in Mammals, C elegans and D melanogaster. J Exptl Biol. 2013;216:543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88(Part B):314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demirovic D, Rattan SIS. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Experimental Gerontol. 2013;48:94–98. doi: 10.1016/j.exger.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Hohmann S. Osmotic Stress Signaling and Osmoadaptation in Yeasts. Microbiol Mol Biol Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins TJ, Swain PS. (Strategies for cellular decision-making. Mol Systems Biol. 5:1–15. doi: 10.1038/msb.2009.83. Article #326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monge C, Leon-Velarde F. Physiological Adaptation to High Altitude: Oxygen Transport in Mammals and Birds. Physiol Rev. 1991;71:1135–1172. doi: 10.1152/physrev.1991.71.4.1135. [DOI] [PubMed] [Google Scholar]
- 42.Cecia M, Ross J, Jr, Condorellia G. Molecular determinants of the physiological adaptation to stress in the cardiomyocyte: a focus on AKT. J Mol Cell Cardiol. 2004;37:905–912. doi: 10.1016/j.yjmcc.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Ullrich O, Reinheckel T, Sitte N, Haass G, Grune T, Davies KJA. Poly-ADP-ribose-polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc Natl Acad Sci (USA) 1999;96:6223–6228. doi: 10.1073/pnas.96.11.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu B, Yadav S, Shah PG, Liu T, Tian B, Pukszta S, Villaluna N, Kutejová E, Newlo CS, Santos JH, Suzuki CK. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J Biol Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- 45.Matsushimaa Y, Gotob Y, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proc Natl Acad Sci USA. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bota D, Davies KJA. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nature Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 47.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 48.Shadel GS, Horvath T. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 50.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Guo-Tao, Ma Shi-Liang, Bai Li-Ping, Zhang Li, Ma Hui, Jia Ping, Liu Jun, Zhong Ming, Guo Zhi-Fu. Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Reports. 2012;39:969–987. doi: 10.1007/s11033-011-0823-1. [DOI] [PubMed] [Google Scholar]
- 52.Fossett N. Signal transduction pathways, intrinsic regulators, and the control of cell fate choice. Biochim Biophys Acta - Gen Sub. 2013;1830:2375–2384. doi: 10.1016/j.bbagen.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lloyd Alison C. The Regulation of Cell Size. Cell. 2013;154:1194–1205. doi: 10.1016/j.cell.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 54.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 55.Ma Q. Role of Nrf2 in Oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang DD. mechanistic studies of the nrf2-keap1 signaling pathway. Drug Metabol Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 57.Wiese AG, Pacifici RE, Davies KJA. Transient adaptation to oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318:231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Bin-Bing S, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]


