Abstract
Objective
Prior research indicates off-label use is common in the intensive care unit (ICU); however the safety of off-label use has not been assessed. The study objective was to determine the incidence of adverse drug reactions (ADRs) associated with off-label use and evaluate off-label use as a risk factor for the development of ADRs in an adult ICU population.
Setting
Medical ICUs at three academic medical centers
Patients
Adult patients (age ≥ 18 years old) receiving medication therapy
Interventions
All administered medications were evaluated for Food and Drug Administration (FDA) approved or off-label use. Patients were assessed daily for the development of an ADR through active surveillance. Three ADR assessment instruments were used to determine the probability of an ADR resulting from drug therapy. Severity and harm of the ADR were also assessed. Cox proportional hazard regression was used to identify a set of covariates that influenced the rate of ADRs.
Measurements and Main Results
Overall, 1654 patient days (327 patients) and 16,391 medications were evaluated, with 43% of medications being used off-label. One hundred and sixteen ADRs were categorized dichotomously (FDA or off-label), with 56% and 44% being associated with FDA approved and off-label use, respectively. The number of ADRs for medications administered and number of harmful and severe ADRs did not differ for medications used for FDA approved or off-label use (0.74% vs 0.67%, p = 0.336; 33 vs. 31 events, p=0.567; 24 vs. 24 events, p = 0.276). Age, sex, number of high-risk medications, number of off-label medications, and severity of illness score were included in the Cox proportional hazard regression. It was found that the rate of ADRs increases by 8% for every one additional off-label medication (HR = 1.08; 95 % CI: 1.018–1.154).
Conclusion
While ADRs do not occur more frequently with off-label use, ADR risk increases with each additional off-label medication used.
Keywords: Adverse drug reaction, Off-label Drug Use, Off-label Prescribing, Intensive Care
Introduction
The off-label use of medications poses several clinical, safety, ethical, and legal issues. Off-label prescribing refers to the use of medications in a manner different from the structured product label.1 Pharmaceutical manufacturers must demonstrate the safety and efficacy of a medication for indications listed in a proposed package labeling before obtaining approval for sale from the Food and Drug Administration (FDA).2 However, the FDA does not regulate medical practice or how these medications are prescribed following approval.3,4 Physicians are allowed to prescribe FDA-approved medications for indications, doses, routes of administration, and patient populations (e.g, pediatrics, pregnant women, etc.) beyond manufacturer recommendations provided in the package insert.3 Off-label use may be warranted based upon published clinical evidence supporting its use or in clinical scenarios lacking robust data where the theoretical benefit outweighs the potential risks.1 Unfortunately, federal regulating agencies do not provide guidance for appropriate off-label prescribing practices and this has created patient safety concerns.5
Off-label prescribing is common in several patient populations. About 21% of medications prescribed in an outpatient setting were used for non-FDA-approved indications with the vast majority (73%) having little or no evidence supporting their use.6 Several published reports have found the off-label rate of medication use in neonatal and pediatric patient populations in the ICU or emergency department to range from 26% to 59%.7–9 The incidence of off-label use for the five most commonly prescribed chemotherapeutic agents has been reported to exceed 50%.10 Furthermore, off-label prescribing patterns are common in adult critically ill patients.11
The intensive care unit (ICU) population is of particular interest for medication safety concerns due to the severity of illness and the numerous medications that patients receive.12–14 Up to 36% of medications ordered in the ICU are used off-label.11,15 Gastrointestinal, antibiotic, antiepileptic, and immunologic agents are the most common therapeutic drug classes administered in the ICU that are not supported by information provided in the product label.11 More concerning is the high incidence of off-label drug use that has minimal or no published evidence supporting the unapproved use. The rates of off-label drug use in the critically ill population with a low level of evidence and weak recommendation for use is 48.3% and 43.5%, respectively.11 The lack of evidence supporting off-label prescribing practices raises significant concerns about patient safety and the additional risk of adverse drug reactions (ADR).14
Nevertheless, the association between off-label use of medications and the risk of ADRs in adult ICUs has not been evaluated. Due to the high frequency of off-label medication use in an ICU setting, it is important to examine the impact this practice has on patient safety outcomes.11 The purpose of this study was to determine the incidence of ADRs associated with off-label drug use and to evaluate off-label drug use as a risk factor for the development of ADRs in an adult ICU patient population.
Materials and Methods
This investigation was a prospective evaluation of all patients admitted to the medical intensive care unit (MICU) at three academic medical centers: University of Pittsburgh Medical Center Presbyterian Hospital, Pittsburgh, PA, Banner Good Samaritan Medical Center, Phoenix, AZ, and the University of Chicago Medical Center, Chicago, IL. The MICU at Presbyterian Hospital is a 24-bed unit, divided into two medical services, with each service consisting of twelve beds and a clinical pharmacist participates in daily work rounds on all beds. One medical service (12 beds) was included from UPMC Presbyterian hospital. Banner Good Samaritan Hospital is a 32-bed MICU where a clinical pharmacist participates in daily work rounds on 16-beds. These 16-beds were included in this study. Partial units were selected because these patients were in the care of the investigators (PS, MB). The University of Chicago is a 16-bed MICU with a usual census of approximately 20 patients on the MICU service that accounts for overflow in other units. The clinical pharmacist cares for all patients on the service. Institutional Review Board approval was obtained at each study site prior to initiation of this study.
Off-label Drug Use Evaluation
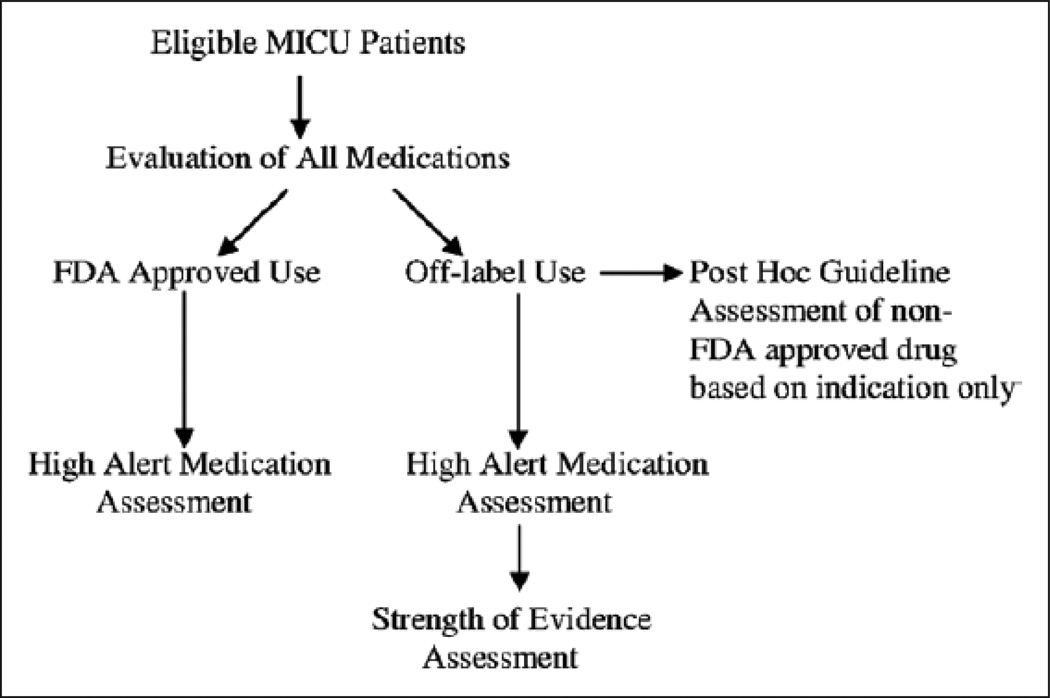
This evaluation occurred over a three-month time period at each institution. Adult patients (age ≥ 18 years old) receiving medication therapy on the MICU service where a clinical pharmacist was present were included in the study. Upon admission of the patient to the MICU, and each subsequent weekday morning (Monday through Friday) throughout the subject’s entire ICU stay, any new medications prescribed in the previous 24 hours were evaluated. The new medications that were added to the medication profile over the weekend were included in Monday’s analysis. All medications that were administered, regardless of the route, including scheduled and “as needed” medications, were included and assessed. Medication-specific information collected included: dose, duration, route of administration, frequency, indication, as well as the location of origin for when the medication was started (before hospital admission, before admission to the ICU, after admission to the ICU). If details regarding the medication order were not easily available, such as the indication for use, the site investigator questioned and confirmed the medication-related information with the prescriber and this information was recorded. All inpatient medications were then evaluated to determine if they were being utilized for FDA-approved or off-label use.16 All of the off-label medications were subsequently classified into one of the following categories based upon why the medication was considered to be off-label: 1) patient population; 2) indication of use; 3) dose; 4) route of administration.8 Medications were also assessed to determine if they were considered to be a high alert medication as described by the Institute for Safe Medication Practice (ISMP).16 The strength of evidence supporting each off-label indication was evaluated through the use of a nationally recognized drug information database, DRUGDEX (DRUGDEX, MICROMEDEX Healthcare Series, Thomson Reuter, Greenwood,CO) .17 We dichotomized the DRUGDEX strength of evidence categories and defined DRUGDEX categories “A” or “B” as “high evidence” and DRUGDEX strength of evidence categories “C” or “none” as “low evidence”. Patient age, sex, and ICU length of stay were also collected. Severity of illness was determined by calculating the Acute Physiology Assessment and Chronic Health Assessment Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) score.18, 19Figure 1 displays the steps taken to evaluate the medications.
Figure 1.
Medication assessment. FDA = Food and Drug Administration, MICU = medical ICU.
Adverse drug reaction Evaluation
All patients were evaluated daily for the development of an ADR by the clinical pharmacist through communication during patient care rounds and chart review. To minimize variability in assessment, only one clinical pharmacist at each site evaluated medications for the development of an ADR. An ADR was defined as “an undesirable clinical manifestation that is consequent to and caused by the administration of a particular drug”.20 The clinical pharmacist assessing the suspected ADR was not part of the evaluation of the off-label or FDA-approved use status of the medications and therefore was blinded to those determinants outside of their personal knowledge. When the clinical pharmacist identified a suspected ADR, the ADR was assessed using three objective, previously published ADR assessment determination instruments.20–22 These tools have been utilized to determine whether the signs, symptoms, or laboratory abnormalities are the result of drug-induced injury or from non-drug-related causes. These three tools contain assessment categories such as previous documentation of a reaction in the literature, alternative causes of the reaction, timing of the reaction, availability of objective evidence, symptom resolution following discontinuation of suspected drug, and similar reaction after rechallenged with suspected medication.20–22 An event was considered to be an ADR when two of the three instruments had an agreement of possible, probable or definite. Possible is a commonly used categorization for a suspected ADR occurred and the 2 out of 3 agreement method has been used previously to increase the rigor of the ADR evaluation.24–26 The severity of the ADR was then classified based upon the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE).27 The ADR was graded on a scale of 1 to 5 with death as a result of the ADR represented as a level 5.27 For this investigation, a Grade of 1–2 was considered “mild/moderate”, whereas a Grade of 3–5 was considered “severe/life threatening”. A modified version of the MEDMARX® form was used as an assessment of harm to the patient as a result of the ADR.28 Categories A through I were included in the evaluation. Category A events were defined as those that had the capacity to cause patient harm (least severe), while Category I events represented events that were the most severe and contributed to or resulted in a patient’s death.28 For this investigation, we defined MEDMARX categories A-D as “no harm” and categories E-I as “harm” which is consistent with the National Coordinating Council for Medication Error Reporting and Prevention interpretation.29,30 Events associated with harm from drugs are considered adverse drug events (ADEs).29
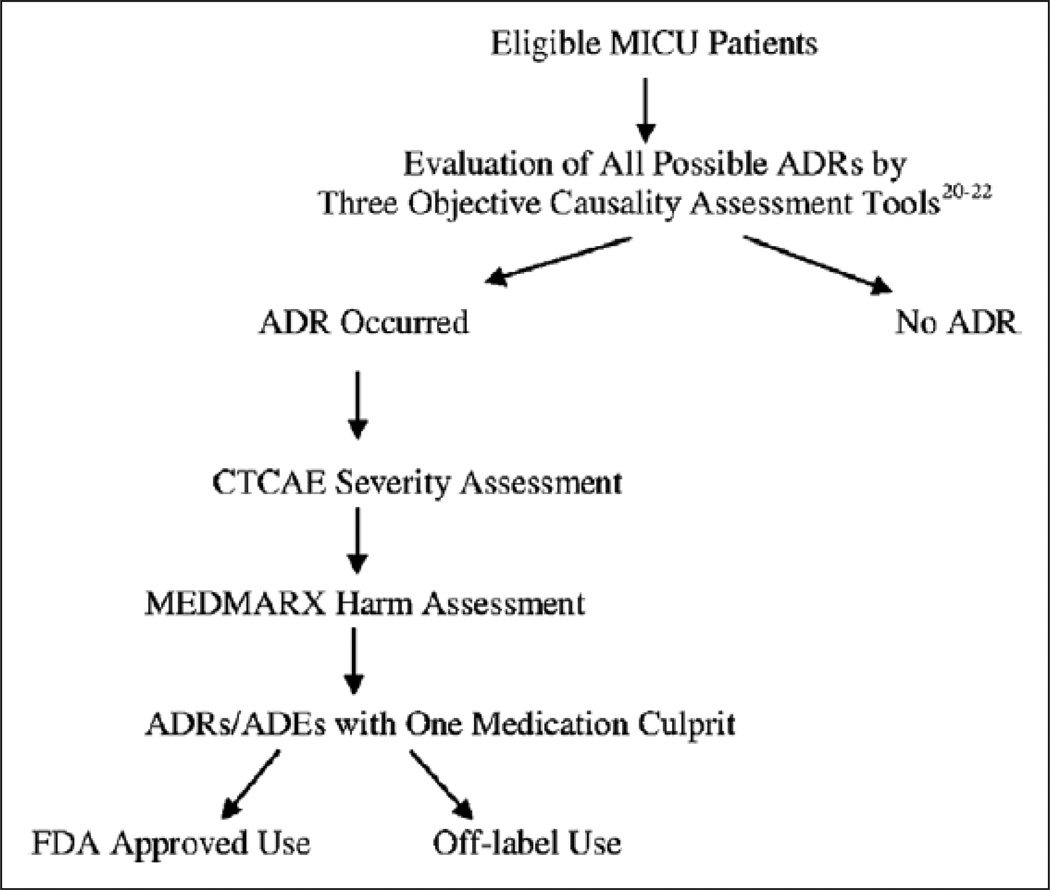
The type of reaction, its level of severity, and related harm were recorded. After the ADR was identified, assessed, and recorded, the medication suspected of causing the ADR was then mapped to the FDA-approved or off-label use data. Based upon the clinical pharmacist’s patient care responsibilities, when an ADR was identified, the ADR was reported to the attending physician caring for the patient and submitted to the institution’s ADR reporting system. Figure 2 displays the steps taken to evaluate the ADRs.
Figure 2.
Evaluation of adverse drug reactions (ADRs). ADEs = adverse drug events, CTCAE = Common Terminology Criteria for Adverse Events, FDA = Food and Drug Administration.
Guideline Comparison
A review of published guidelines was conducted to further clarify the data supporting off-label drug use. A post-hoc assessment of the 25 most common non-FDA-approved indications identified in this study was conducted to determine if the non-FDA-approved indication was supported by recommendations in published guidelines. The term “guideline” plus drug class and the indication was searched for the past 10 years in Medline using Pubmed. A second strategy was also employed that consisted of a search for guidelines for each of the top 25 non-indicated uses in guidelines.gov.
Risk Factor Evaluation
Based upon the literature, risk factors for the development of an ADR are numerous, and specific risk factors have been identified to contribute to the risk of ADRs in critically ill patients.13 These include patient and drug-related risk factors.13 Specific risk factors, such as patient age, severity of illness, total medications received, total number of high alert medications received, as well as the number of off-label medications received, were evaluated.
Statistical Analysis
Descriptive statistics and student t-test, chi-square, and Mann-Whitney-U tests were used to summarize and compare the patient population that developed one or more ADRs to patients that did not develop an ADR. Chi-square was used to compare the ADRs resulting from medications with high or low levels of evidence as well as the number of harmful and severe ADRs resulting from FDA-approved or off-label medications. The ADRs that occurred were classified and analyzed according to their assignment in the off-label or FDA approved use category and the categories for severity and harm. Only ADRs attributed to one causal medication that could be dichotomized to either FDA approved use or off-label use were considered. A p-value of ≤ 0.05 was considered to be statistically significant. For patients that developed an ADR, only medications received before the ADR were included in the analysis, as medication received after would not impact the ADR that already occurred. For patients that did not develop an ADR, all medications were included as an ADR could have occurred with even the last medication received prior to the patient leaving the MICU.
To evaluate the strength of the linear association between the number of medications received and the number of ADRs a Spearman correlation was used. To assess the linear association between the day of ADR occurrence in the total patient’s length of stay and the total length of stay, a Pearson correlation was used.
A Cox proportional hazard regression was used to test the relationship between the drug status (off-label vs. FDA-approved) and the ADR outcome. We hypothesized that patients that received more off-label medications had a greater risk of developing an ADR. The method of variable selection used was the Forward stepwise likelihood ratio criterion. The variables initially included were age, sex, length of stay, SOFA score, number of medications before the ADR, number of high-risk medications before the ADR, and the number of off-label medications before the ADR. In addition, interactions were created between SEX and LOS, SOFA, # of meds before ADR, # HIGH risk meds before ADR, # of off-label meds before ADR. The APACHE II score was not included due to multicollinearity with SOFA score.
We also built two additional models. One model tested if a low level of evidence for an off-label indication was a risk factor for the development of an ADR. A second model tested if the different type of off-label use (patient population, indication, dose, route of administration) was a risk factor for the development of an ADR. Forward stepwise likelihood ratio criterion, which is more robust than forward conditional and forward Walds methods, was used for both models.31 In addition to main effects, interaction effects of sex with other predictors were entered into the models. The variables included initially were age, sex, SOFA score, high-risk medication status, and the level of evidence (high/low) for the first model, and age, sex, SOFA score, high-risk medication status, and the type of off-label use for the second model. Statistical analyses were completed using STATA software, version 12, College Station, Texas.
Results
A total of 1654 patient days (327 patients) and 16,391 medications were evaluated. Demographics of all patients are displayed in Table 1. Of the medications prescribed, 7056 (43.0%) were considered to be off-label. Upon evaluation of the categories of off-label use, 5756 (81.6%) was attributed to a non-indicated use and 895 (12.7 %) was attributed to a non-indicated dose (Table 2). Of the medications used for an off-label indication, 51.7 % (2976 /5756) had a low strength of evidence.
table 1.
Comparison of Patient Characteristics Between Those With and Without an Adverse Drug Reaction
| Characteristic | Total (n=327) | No ADR (n = 237) | ADR (n = 90) |
p (Between No ADR and ADR) |
|---|---|---|---|---|
| Patient age, median (IQR)a | 60 (48–70) | 60 (47–70) | 61 (50–73) | 0.687 |
| Patient sex, % male | 51.07 | 48.94 | 56.66 | 0.212 |
| Acute Physiology and Chronic Health Evaluation score, mean ± SD |
19.3 ±7.6 | 18.6 ±7.7 | 21.1 ±7.2 | 0.004 |
| Sequential Organ Failure Assessment score, mean + SD |
6.3 + 0.2 | 5.6±0.2 | 8 ±0.5 | < 0.001 |
| Patient ICU length of stay, median (IQR)a | 3 (2–7) | 3 (2–4) | 6 (4–11) | < 0.001 |
| No. of medications received before ADR, median (IQR)a |
13(9–18) | 13 (8–18)b | 14.5 (11 −22) | 0.008 |
| No. of high-alert medications received before ADR, median (IQR)a |
4 (2–6) | 4 (2–6)b | 4 (3–7) | < 0.001 |
| No. of off-label medications before ADR, median (IQR)a |
5 (3–8) | 5 (3–7)b | 6 (4–9) | 0.031 |
| No. of Food and Drug Administration- approved medications before ADR, median (IQR)a |
8(5–11) | 8 (5–11)b | 9 (5–11) | 0.028 |
ADR = adverse drug reaction. IQR = interquartile range.
Mann-Whitney U test.
For patients that did not experience an ADR, the “number of medications received before ADR,” the “number of high-alert medications received before ADR,” the “number of off label medications before ADR,” and the “number of Food and Drug Administration-approved medications before ADR” are the total medications received.
TABLE 2.
Medications, Off-Label Use, and Adverse Drug Reaction: By Site
| Description | Total | Site 1 | Site 2 | Site 3 |
|---|---|---|---|---|
| No. of medications evaluated | 16,391 | 8,821 | 4,386 | 3,184 |
| Patient days | 1,654 | 931 | 367 | 356 |
| Medications off-label | 7,056 | 3,846 | 1,791 | 1,419 |
| Nonindicated population | 159 | 0 | 4 | 14 |
| Nonindicated use | 5,756 | 3,182 | 1,540 | 1,119 |
| Nonindicated dose | 895 | 544 | 187 | 220 |
| Nonindicated route | 246 | 120 | 60 | 66 |
| Total ADRs | 126 | 49 | 58 | 19 |
| ADRs with one drug culprit | 116 | 47 | 50 | 19 |
| Off-label | 51 | 16 | 24 | 18 |
| Off-label due to indication | 45 | 12 | 17 | 16 |
ADR = adverse drug reaction.
There were 126 ADRs identified, resulting in 76.2 ADRs per 1000 patient days. One hundred and sixteen ADRs resulting from either approved use or an off-label use, were included in further analysis as they were attributed to one causal medication that could be dichotomized to either FDA approved use or off-label use. Of these 116 ADRs, corticosteroids and opioids were the most commonly implicated classes of medications. Corticosteroids and opioids accounted for 12% and 11.2% of the ADRs, respectively (Table 3). Upon review of the types of ADRs, hypotension was the most frequently occurring outcome (12.2%) followed by delirium (11.2%). Table 4 displays the types of ADRs that were identified and the frequency of occurrence. Upon comparison of patients that developed an ADR to those that did not, patients that developed an ADR had a higher mean APACHE II score (p = 0.004) and mean SOFA score (p < 0.001). Patients that developed an ADR had a longer median ICU length of stay (p<0.001), a higher mean number of total medications (p < 0.001) and more high alert medications received (p<0.001), as well as a higher median number of the total number of off-label medications received (p<0.001). In patients that developed an ADR, the number of medications, number of high alert medications, and number of off-label medications that were received prior to the ADR were also identified and displayed in Table 1. There was a statistically significant difference between the number of medications (p = 0.008), number of high alert medications (p<0.001), and number of off-label medications (p <0.001) that were received prior to the ADR to the total number of medications that patient received that did not develop an ADR (Table 1).
table 3.
Description of Adverse Drug Reactions: By Causal Medication Class
| Medication Class | No. of ADRs (%) | No. of Serious ADR (n = 48; Common Terminology Criteria for Adverse Events severity rating 3–5) |
No. of Medications (n = 16,391) |
No. of Off-Label Medications (n = 7,056) |
Percent Off-Label |
|---|---|---|---|---|---|
| Corticosteroids | 14 (1 2.07) | 6 | 493 | 202 | 40.97 |
| Opioids | 13 (11.21) | 5 | 1,108 | 197 | 17.78 |
| Sedatives | 12 (10.34) | 3 | 343 | 21 | 6.12 |
| Anti-infective | 12 (10.34) | 5 | 2,368 | 859 | 36.28 |
| Other | 11 (9.48) | 9 | 6,818 | 4,409 | 76.78 |
| Anticoagulants | 9 (7.76) | 3 | 800 | 150 | 18.75 |
| Antihypertensives | 9 (7.76) | 3 | 861 | 174 | 20.21 |
| Beta agonists | 9 (6.18) | 1 | 319 | 139 | 43.57 |
| Benzodiazepines | 7 (6.03) | 6 | 629 | 196 | 31.16 |
| Vasopressors | 6 (6.18) | 2 | 474 | 170 | 35.86 |
| Insulin | 5 (4.31) | 3 | 823 | 302 | 36.70 |
| Antiarrhythmic | 3 (3.54) | 1 | 245 | 96 | 39.18 |
| Diuretics | 3 (2.57) | 1 | 226 | 33 | 14.60 |
| Laxatives | 3 (2.57) | 0 | 884 | 108 | 12.22 |
ADR = adverse drug reaction.
table 4.
Description of Adverse Drug Reactions
| Description | Number (%) (n = 116) |
|---|---|
| Hypotension | 14(12.07) |
| Delirium | 13(11.21) |
| Tachycardia | 12(10.34) |
| Hyperglycemia | 10(8.62) |
| Other (e.g., tremor, ototoxicity, and seizure) |
11 (9.46) |
| Bleeding | 8 (690) |
| Thrombocytopenia | 7 (6.03) |
| Constipation | 5 (5.17) |
| Elevated triglycerides | 6 (5.17) |
| Hypoglycemia | 5 (431) |
| Electrolyte abnormalities (e,g, hyperkalemia and hypokalemia) |
5 (431) |
| Acute kidney injury | 4 (3.45) |
| Diarrhea | 4 (3.45) |
| Impaired oxygenation/shortness of breath |
4 (3.45) |
| Rash | 4 (3.45) |
| Nausea | 3 (257) |
Adverse drug reaction Definitions/Descriptions: hypotension = blood pressure < 90/60mm Hg; delirium = per standardized scale or physician judgment; tachycardia = heart rata > 100 beats/min; hyperpylcemia = blood sugar > 1 80; bleeding = loss of blood or visible signs of bleeding; constipation = < bowel movements in 1 week; elevated triglycerides = triglycerides > 200; hypoglycemia = blood sugar < 70 mg/dL; electrolyte abnormalities = electrolyte readmg outside of the normal range; acute kidney injury = an increase in the serum creatinine by two-fold or a decrease in the glomerular filtration rate 50%, a urine output of < 0.5 mL/kg/hr for 12 hr; diarrhea = three or more watery stools per day; impaired oxygenation/shortness of breath = increase in oxygen requirements, mechanical ventilation support, or patient subjective complaint; rash = a skin eruption that may be accompanied by itching; nausea = a sensation often associated, but not limited to vomiting.
Of the 116 ADRs that could be categorized dichotomously, 56 % (65/116) of the ADRs were associated with FDA-approved use, whereas 44 % (51/116) of the ADRs were associated with off-label use. The frequency of ADRs, normalized for the number of medications in each group, did not differ irrespective of the medication’s off-label or FDA-approved use (p = 0.336) as displayed in Table 5. For the ADRs that were dichotomized, overall, there were 70.1 ADRs per 1000 patient-days, with 39 ADRs per 1000 patient days that were due to an FDA-approved use and 30.8 ADRs per 1000 patient days that were due to an off-label medication. There were 45 ADRs that resulted from off-label medications that were due to non-indicated use. Of these ADRs, 25 were attributed to medications with a low strength of evidence, and 20 ADRs being attributed to a medication with a high strength of evidence (p = 0.33).
table 5.
Adverse Drug Reaction Rate, Severity, and Harm: By Food and Drug Administration-Approved or Off-Label Status
| Description | n | FDA-Approved Use | Off-Label Use |
p(Between FDA-Ap proved and Off-Label Use) |
|---|---|---|---|---|
| No. of ADRs attributed to one drug |
116 | 63 | 53 | 0.336 |
| Harmful ADRs (MEDMARX categories E-l) |
64 | 33 | 31 | 0.576 |
| Severe ADRs (Common Terminology Criteria for Adverse Events severity rating 3–5) |
48 | 24 | 24 | 0.276 |
FDA = Food and Drug Administration. ADR = adverse drug reaction.
The resultant harm and severity of the ADR caused by FDA-approved or off-label use was also assessed. Harmful ADRs, defined as MEDMARX categories E-I, were identified and compared and 64 ADRs were considered to result in patient harm (Table 5). The number of harmful ADRs did not differ for medications used for FDA-approved or off-label use (33 vs. 31 events, respectively; p = 0.567). Severe ADRs, defined as a CTCAE severity rating of 3–5 (severe/life threatening), were identified and compared and 48 ADRs were considered to result in a severe outcome (Table 5). The number of severe ADRs did not differ for medications used for FDA-approved or off-label use (24 vs. 24; p = 0.276). It was also found that there was a moderate linear association between the number of medications received and the number of ADRs (r = 0.408; p < 0.001) and that there was a strong strength linear association between the day of ADR occurrence in the total patient’s length of stay and the total length of stay (r = 0.754; p < 0.001).
For the Cox proportional hazard regression, four variables (length of stay, number of medications received before the ADR, number of high-risk medications received, number of off-label medications received), were selected at the final step and were included in the final model. All four variables were significant (Table 6). For the primary variable of interest, number of off-label medications received, it was found that the rate of all ADRs increases by 8% for every one additional off-label medication received (Hazard Ratio = 1.08; 95 % CI: 1.018 – 1.154).
table 6.
Cox Proportional Hazard Regression of Potential Relationships Between Drug Status and Adverse Drug Reactions
| Covariate | Hazard Ratio (95% CI) |
p |
|---|---|---|
| Length of stay | 0.911 (0.856–0.969) | 0003 |
| No. of medications before ADR |
0.897 (0.862–0.934) | < 0.001 |
| No, of high-alert medications received before ADR |
1.259(1.127–1.407) | < 0.001 |
| No. of off-label medications before ADR |
1.084(1.018–1.154) | 0.012 |
ADR = adverse drug reaction.
The number of high-risk medications was the only variable included in the final model used to test the risk of developing an ADR with consideration to the level evidence supporting the off-label drug use. Medications with a low strength of evidence did not significantly predict the occurrence of an ADR as compared to medications with a high strength of evidence. However, the odds of developing an ADR was 2.76 times higher with each additional high-risk medication received (OR 2.763; 95% CI: 1.531–4.987).
The results for the second model that was tested to determine if the different type of off-label use (patient population, indication, dose, route of administration) was a risk factor for the development of an ADR, produced similar findings. The different type of off-label use (patient population, indication, dose, route of administration) was not a predictor for the development of an ADR, as the number of high-risk medications was the only variable selected in the final model. (Odds Ratio (OR) = 2.749; 95% CI: 1.523–4.962) .
Lastly, 97 medications were considered in the 25 most common, off-label, non-indicated uses evaluation. Of these 97 medications, 57 medications (58.8 %) were highlighted as possible therapeutic options in at least one guideline. The drug class, non-indicated use, and guideline are displayed in Supplemental Digital Content - Table 7.32–50
Discussion
This is the first multicenter investigation in the ICU to assess the impact of off-label medication use on the frequency, severity, and harm of ADRs. Upon evaluation of the medication use in our population, we found an incidence of off-label use to be 48 % of the medications prescribed. Our rate is higher than the 36% of off-label use that was identified in a recent ICU snapshot evaluation.11 The difference could be explained by the different study designs between the evaluations. The incidence of off-label use has also been described in the hospitalized pediatric and neonatal population and ranges from 26% to 59% in the ICU and emergency department settings.7–9 The high incidence of off-label use in the pediatric population was primarily due to the fact that many medications do not receive regulatory approval in the pediatric population.57,58 Also, the lack of clinical trial data supporting the drug’s indication and doses higher than recommended in package inserts were other common reasons contributing to high off-label use in pediatrics.57,58
The ordering of an off-label medication does not necessarily imply a lack of evidence to support its use. However, there is also a dearth of supporting evidence for use of many off-label uses.11 The lack of regulation and evidence suggest a medication safety concern. Therefore, our investigation aimed to evaluate the incidence and association of ADRs with off-label medication use in the ICU.
We specifically aimed to evaluate the difference in frequency, severity, and harm of the ADRs caused by medications being used for off-label use as compared to their FDA-approved indication. Our results indicate there is no difference between the frequency, severity, or harm of the ADRs between medications used for FDA-approved or off-label use. The evaluation of ADRs associated with off-label use was also conducted in a pediatric emergency department.8 This investigation found a rate of ADRs to be 0.6% (40/6675) with 12.5 % of those ADRs resulting from an off-label medication, which is lower than what we observed in our investigation.8 They concluded that the frequency of ADRs from off-label medications was less frequent than ADRs that resulted from FDA-approved use.8 This investigation has several significant differences as compared to our study. First of all, pediatric emergency department patients are very different than an adult ICU population. Besides age of the patient, the adult ICU patient’s length of stay is considerably longer than the short period of time a patient spends in the emergency department. This would allow for increased numbers of medications to be given and for changes in organ function to occur, which results in fluctuations in drug metabolism and elimination.59 Also, the method of ADR detection differed. We used an active surveillance method by having a clinical pharmacist concurrently evaluate patients for ADRs and conduct medical chart reviews, whereas the study conducted by Phan and colleagues retrospectively reviewed the medical chart to identify the ADRs. This may explain the increase in the frequency of ADRs we identified as compared to the study by Phan and colleagues.8, 60 Furthermore, Phan et al, did not assess severity or harm to the patient, which is an important aspect of our investigation.8
Overall, we found 70.1 ADRs per 1000 patient days for all medication use in this multicenter study. This rate was similar to a previously published report, which observed 86.5 total ADRs per 1000 patient days in a MICU at a single center.61 Previous investigations have identified rates of ADRs ranging between 13.8 to 116.8 events per 1000 patient days in the ICU.13, 61–64 The wide variability in the incidence of ADRs could be explained by the mode of detection. Voluntary reporting of ADRs underestimates the actual adverse event rate and has been shown to be an unreliable method of detection.59 However, the use of multiple modes and active surveillance strategies improve the detection of ADRs, which was utilized in our investigation.65
The five most common classes of medications implicated in the development of an ADR in our study, corticosteroids, opioids, sedatives, anti-infectives, and anticoagulants, also mirrored those identified in previous investigations.3,62 We also found a strong correlation between the day of ADR occurrence in the total patient’s length of stay and the total length of stay and a moderate correlation between the number of total medications received and the number of ADRs a patient develop. These results are consistent with known patient- and medication-related risk factors associated with the development of ADRs.13,14 Additional investigations need to be conducted to further explore this relationship.
We found that the number of off-label medications received increased the rate of all ADRs by 8% for every one additional off-label medication, but off-label use does not appear to result in ADRs that are of higher severity or harm. A focus on the safe use and monitoring of high alert medications is paramount in the prevention of ADRs. The use of daily patient care rounds as a time to enhance communication between patient care providers is also key in the prevention of ADRs.14 The inclusion of a pharmacist as a member of the care team is another strategy to aid in the prevention of ADRs. A clinical pharmacist’s presence on daily patient care rounds has been shown to decrease the incidence of ADRs and their associated costs.66 The rates of ADRs have also been shown to be higher in units with high patient:nurse ratio as well as greater nursing workloads.12 Therefore, Adequately staffing ICUs to ensure optimal patient:nurse ratios is another important aspect of patient safety and the prevention of ADRs. The implementation and utilization of a computerized physician order entry process and clinical decision support software is another powerful tool in reducing errors and ADRs and should be considered when possible.63
The results of this investigation do not only add to the repertoire of tools clinicians possess to improve patient safety in the ICU, but is may assist pharmacy and therapeutics committees during their formulary decisions. Clinicians should be aware as the number of off-label medications used increase, the rates of ADRs increase. Clinicians also need to be aware of the strength of evidence supporting the off-label use of medications in their patient population. Pharmacy and therapeutics committees should also be concerned about the strength of evidence surrounding off-label use when making formulary and guideline decisions. In our investigation, we identified that the majority of medications involved with the top 25 of-label indications were mentioned in guidelines as possible therapeutic options which may have been a driving factor of why the level of evidence was not identified as a predictor of an ADR. When specifically evaluating a medication to be added to a hospital formulary, a similar investigation should be conducted.
Our study provides valuable information in regards to the role of off-label medication use and the development, severity, and harm of ADRs. However, several limitations should be noted. First of all, these results may not be generalizable to community or rural hospital settings; institutions without a clinical pharmacist’s presence on daily ICU patient care rounds; non-adult ICU populations; or facilities without patient safety technology. This study was conducted at three academic medical centers with a dedicated clinical pharmacist in each MICU and computerized physician order entry as well as clinical decision support software. These resources may affect the prevention, identification, and reporting of ADRs. A second limitation is the inclusion and analysis of only ADRs that could be dichotomized into off-label or FDA-approved use categories. While ten ADRs were not included in the analysis, the authors chose this method of analysis to achieve the primary objective of the study, to determine the incidence of ADRs associated with off-label drug use and evaluate off-label drug use as a risk factor for the development of ADRs in an adult ICU patient population. The inclusion of ADRs with two or more potentially causal medications, representing both the off-label and FD- approved category, would prevent dichotomization of the outcome. Another limitation, the clinical pharmacists performing the ADR assessments were blinded to the results of the FDA-approved and off-label uses. This was completed to prevent assessment bias. Even while blinded, the clinical pharmacists they may have known some of the approved indications due to experience. However, since off-label use occurs frequently it is highly possible that this was not the case. In addition, a combination of prospective ADR identification and chart review was utilized to as a more inclusive approach to identify ADRs.65 Our ADR rate is consistent with the literature, however, there is always the possibility that the detection of a few ADRs did not occur or that the experience of the clinical pharmacist impacted the identification of ADRs. A final limitation is that an assessment of medication errors was not conducted with the ADRs, therefore, the dichotomization of preventable vs. non-preventable adverse reactions could not be conducted. It should also be noted that identifying ADRs in the midst of critical illness related events may be difficult. However, through the use of validated causality assessment tools, the identification of ADRs and the causal medication is made more objective. Lastly, a post-hoc assessment of off-label use and discussion in guidelines was only conducted for non-indicated use and not dose, population, or route of administration as this type of detail is atypical in most guidelines.
Conclusion
Off-label use of medications frequently occurs in the ICU. While the incidence of ADRs does not differ between medications used for FDA-approved versus off-label uses, clinicians should be aware that the risk of developing an ADR increases with the increasing number of off-label medications received, irrespective of the level of evidence to support the use of the off-label medication. The prevention of ADRs is important to patient safety and should be at the forefront of safe medication practices.
Acknowledgments
Financial Support: This work was supported by a grant through the American Society of Health-System Pharmacists Research and Education Foundation
Contributor Information
Pamela L. Smithburger, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy.
Mitchell S. Buckley, Department of Pharmacy, Banner Good Samaritan Medical Center.
Mark A. Culver, Department of Pharmacy, Banner Good Samaritan Medical Center.
Sarah Sokol, Department of Pharmaceutical Services, University of Chicago Medical Center.
Ishaq Lat, Department of Pharmaceutical Services, University of Chicago Medical Center.
Steven M. Handler, Departments of Geriatric Medicine and Biomedical Informatics, University of Pittsburgh School of Medicine.
Levent Kirisci, Department of Pharmaceutical Sciences, University of Pittsburgh School of Pharmacy.
Sandra L. Kane-Gill, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy.
References
- 1.Gazarian M, Kelly M, McPhee JR, Graudins LV, Ward RL, Campbell TJ. Off-label use of medicines: consensus recommendations for evaluating appropriateness. MJA. 2006;185:544–548. doi: 10.5694/j.1326-5377.2006.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed January 20, 2014]; http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/
- 3.Blum RS. Legal considerations in off-label medication prescribing. Arch Intern Med. 2002;162:1777–1778. doi: 10.1001/archinte.162.15.1777. [DOI] [PubMed] [Google Scholar]
- 4.Gillick MR. Controlling off-label medication use. Ann Intern Med. 2009;150:344–347. doi: 10.7326/0003-4819-150-5-200903030-00108. [DOI] [PubMed] [Google Scholar]
- 5.Stafford RS. Regulating off-label drug use - rethinking the role of the FDA. N Engl J Med. 2008;35:1427–1429. doi: 10.1056/NEJMp0802107. [DOI] [PubMed] [Google Scholar]
- 6.Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006:1021–1026. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 7.Barr J, Brenner-Zada G, Heiman E, et al. Unlicensed and off-label medication use in a neonatal intensive care unit: a prospective study. Am J Perinatol. 2002;19:67–72. doi: 10.1055/s-2002-23557. [DOI] [PubMed] [Google Scholar]
- 8.Phan H, Leder M, Fishley M, Moeller M, Nahata M. Off-label and unlicensed medication use and associated adverse drug reactions in a pediatric emergency department. Pediatr Emer Care. 2010;26:424–430. doi: 10.1097/PEC.0b013e3181e057e1. [DOI] [PubMed] [Google Scholar]
- 9.Jong GW, Vulto AG, de Hoog M, et al. Unapproved and off-label use of drugs in a children’s hospital. N Engl J Med. 2000;343:1125–1126. doi: 10.1056/NEJM200010123431515. [DOI] [PubMed] [Google Scholar]
- 10.Eastman P. Reimbursement policies discourage off-label drug use. Oncology Times. 2005;27:8–10. [Google Scholar]
- 11.Lat I, Micek S, Janzen J, et al. Off-label medication use in adult critical care patients. Journal of Critical Care. 2011;26:89–94. doi: 10.1016/j.jcrc.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Seynaeve S, Verbrugghe W, Claes B, Vandenplas D, et al. Adverse drug reactions in intensive care units: a cross-sectional study of prevalence and risk factors. Am J Crit Care. 2011;20:e131–e140. doi: 10.4037/ajcc2011818. [DOI] [PubMed] [Google Scholar]
- 13.Kane-Gill SL, Kirisci L, Verrico MM, Rothschild JM. Identification of risk factors for adverse drug reactions in critically ill patients. Crit Care Med. 2012;40:823–828. doi: 10.1097/CCM.0b013e318236f473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane-Gill SL, Jacobi J, Rothschild JM. Adverse drug reactions in intensive care units: risk factors, impact, and the role of the team care. Crit Care Med. 2010;38(Suppl):S83–S89. doi: 10.1097/CCM.0b013e3181dd8364. [DOI] [PubMed] [Google Scholar]
- 15.AlbalADRjo P, Caillet B, Moine P, et al. Off-label prescriptions in an adult surgical intensive care unit. Presse Med. 2001;30:1484–1488. [PubMed] [Google Scholar]
- 16. [Accessed August 5, 2012];Institute for Safe Medication Practices: ISMP’S list of high alert medications. Available at www.ismp.org/tools/highalertmedications.pdf.
- 17.DRUGDEX, MICROMEDEX Healthcare Series, Thomson Reuter, Greenwood CO [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1995;13:818–829. [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA. 1979;242:623–632. [PubMed] [Google Scholar]
- 21.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 22.Jones JK. Definition of events associated with drugs: regulatory perspectives. J Rheumatol. 1998;17(suppl):14–19. [PubMed] [Google Scholar]
- 23.Goh CL. An approach to the evaluation and documentation of adverse drug reaction. Sing Med J. 1989;30:285–289. [PubMed] [Google Scholar]
- 24.Kane-Gill SL, Visweswaran S, Saul MI, et al. Computerized detection of adverse drug reactions in the medical ICU. Int J Med Inform. 2011;80:570–578. doi: 10.1016/j.ijmedinf.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harinstein LM, Kane-Gill SL, Smithburger PL, et al. Use of a laboratory-drug combination alert to detect drug-induced thrombocytopenia in critically ill patients. J Crit Care. 2012;27(3):242–249. doi: 10.1016/j.jcrc.2012.02.014. PMID:22520497. [DOI] [PubMed] [Google Scholar]
- 26.Rivkin A. Admissions to a medical intensive care unit related to adverse drug reactions. Am J Health-Syst Pharm. 2007;64:1840–1843. doi: 10.2146/ajhp060641. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. Publish Date: June 14th, 2010. [Google Scholar]
- 28.MEDMARX® Medication Error Data Entry Form. The United States Pharmacopeia Convention, Inc. 2005 [Google Scholar]
- 29.Kane-Gill SL, Kowiatek JG, Weber RJ. A comparison of voluntarily reported medication errors in intensive care and general care units. Qual Saf Health Care. 2010;19:55–59. doi: 10.1136/qshc.2008.027961. [DOI] [PubMed] [Google Scholar]
- 30.National Coordinating Council on Medication Error Reporting and Prevention. [accessed 27 May 2008];NCC MERP Index for categorizing medication errors. doi: 10.1002/pds.1423. http://www.nccmerp.org/pdf/indexColor2001-06-12.pdf. [DOI] [PubMed]
- 31.Hosmer DW, Lemeshow A. Applied Logistic Regression. second. New York, NY: John Wiley and Sons; 2000. [Google Scholar]
- 32.University of Michigan Health System. Management of acute atrial fibrillation and atrial flutter in non-pregnant hospitalized adults. Ann Arbor MI: University of Michigan Health System; 2014. May, p. 26p. [PubMed] [Google Scholar]
- 33.January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.03.022. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 34.National Clinical Guideline Venter. Atrial fibrillation: the management of atrial fibrillation. London (UK: National Institute for Health and Care Excellence (NICU); 2014. Jun, p. 49p. (Clinical guideline; no. 180) [Google Scholar]
- 35.Bushnell C, McCullough L. Stroke prevention in women: synopsis of the 2014 American Heart Association/ American Stroke Association Guidelines. An Intern Med. 2014;160:853–857. doi: 10.7326/M14-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Culebras A, Messe SR. Summary of evidence-based guideline update: Prevention of stroke in nonvalvular atrial fibrillation: Report of the guideline development subcommittee of the American AcADRmy of Neurology. Neurology. 2014;83(13):716–724. doi: 10.1212/WNL.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 37.Barr, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 38.National Clinical Guideline Centre for Acute and Chronic Conditions. Alcohol-use disorders. Diagnosis and clinical management of alcohol-related physical complications. London UK: National Institute for Health and Clinical Excellence (NICE); 2010. p. 30p. Hun. (Clinical guideline; no. 100) [Google Scholar]
- 39.Sveum R, Bergstrom J, Brottman G, Hanson M, Heiman M, Johns K, Malkiewicz J, Manney S, Moyer L, Myers C, Myers N, O’Brien M, Rethwill M, Schaefer K, Uden D. Institute for Clinical Systems Improvement. Diagnoais and Management of Asthma. Updated. 2012 Jul [Google Scholar]
- 40.Lalloo UG, Ainslie GM, Abdool-Gaffar MS, et al. Guideline for the management of acute asthma in adults: 2013 update. S Afr Med J. 2012;103(3 Pt 2):189–198. doi: 10.7196/samj.6526. [DOI] [PubMed] [Google Scholar]
- 41.Umpierrez GE, Hellman R, Korytkpwski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: An Endocrine Society Clinical Practice Guideline. JCEM. 2012;97(1):16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 42.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 43.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficule infection in adults:2010 update by the Scoeity of Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of American (IDSA) Infection Control and Hospital Epidemiology. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 44.McKay SL, Fravel M, Scanlon C. Management of constipation. Iowa City IA: University of Iowa Gerontological Nursing Interventions Research Center, Research Translation and Dissemination Core; 2009. Oct, p. 51p. [Google Scholar]
- 45.Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2014), executive summary. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014 Available from: http://www.goldcopd.org/
- 46.Dellinger PR, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 47.Bodmann KF. Current guidelines for the treatment of severe pneumonia and sepsis. Chemotherapy. 2005;51(5):227–233. doi: 10.1159/000087452. [DOI] [PubMed] [Google Scholar]
- 48.National Institute for Health and Clinical Excellence (NICE) Acute upper gastrointestinal bleeding: management. London UK: National Institute for Health and Clinical Excellence NICE; 2012. Jun, p. 23p. (Clinical guideline; no 141) [Google Scholar]
- 49.University of Texas at Austin School of Nursing. Family Nurse Practitioner Program. Clinical guideline for the treatment of primary insomnia in middle-aged and older adults. Austin (TX): University OF Texas at Austin, School of Nursing; 2014. May, p. 28p. [Google Scholar]
- 50.Guideline development group for the management of patients with insomnia in primary care. Clinical practice guidelines for the management of patients with insomnia in primary care. MADRid (Spain): Health Technology Assessment Unit, Lain Entralfo Agency, Ministry of Health, Social Services and Equality (Spain); 2009. p. 159p. [Google Scholar]
- 51.Baughman RP, Meyer KC, Nathanson I, et al. Monitoring of nonsteroidal immunosuppressive drugs in patients with lung disease and lung transplant recipients: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;142(5):e1S–E111S. doi: 10.1378/chest.12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Thoracic Society Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 53.Rosendorff C. Hypertension and coronary artery disease: a summary of the American Heart Association scientific statement. J Clin Hypertens. 2007;9(10):790–795. doi: 10.1111/j.1751-7176.2007.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 54.Luehr D, Woolley T, Burke R, et al. Hypertension diagnosis and treatment. Bloomington (MN): Institution for Clinical Systems Improvement (ICSI) 2012 Nov;:67. [Google Scholar]
- 55.National Institute for Health and Care Excellence (NICE) The pharmacological management of neuropathic pain in adults in non-specialist settings. London (UK): National Institute for Health and Care Excellence (NICE); 2013. Nov, Neuropathic pain - pharmacological management; p. 41p. (Clinical guideline; no. 173) [PubMed] [Google Scholar]
- 56.Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T. European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010 Sep;17(9) doi: 10.1111/j.1468-1331.2010.02999.x. 1113-e88. [DOI] [PubMed] [Google Scholar]
- 57.Pandolfini C, Impicciatore P, Provasi D, et al. Off-label use of drugs in Italy: a prospective, observational and multicenter study. Acta Paediatr. 2002;91:339–347. doi: 10.1080/08035250252834030. [DOI] [PubMed] [Google Scholar]
- 58.Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164:552–558. doi: 10.1007/s00431-005-1698-8. [DOI] [PubMed] [Google Scholar]
- 59.Kane-Gill SL, Kowiatek JG, Weber RJ. A comparison of voluntarily reported medication errors in intensive care and general care units. Qual Saf Health Care. 2010;19:55–59. doi: 10.1136/qshc.2008.027961. [DOI] [PubMed] [Google Scholar]
- 60.Kane-Gill SL, Van Den Bos J, Handler SM. Adverse drug reactions in hospital and ambulatory care settings identified using a large administrative database. Ann Pharmacother. 2010;44:983–993. doi: 10.1345/aph.1M726. [DOI] [PubMed] [Google Scholar]
- 61.Rothschild JM, Landrigan CP, Cronin JW, et al. The critical care safety study: the incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33:1694–1700. doi: 10.1097/01.ccm.0000171609.91035.bd. [DOI] [PubMed] [Google Scholar]
- 62.Cullen DJ, Sweitzer BJ, Bates DW, et al. Preventable adverse drug reactions in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med. 1997;25:1289–1297. doi: 10.1097/00003246-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 63.Benkirane RR, R-Abouqal R, Haimeur CC, et al. Incidence of adverse drug reactions and medication errors in intensive care units: a prospective multicenter study. J Patient Saf. 2009;5:16–22. doi: 10.1097/PTS.0b013e3181990d51. [DOI] [PubMed] [Google Scholar]
- 64.Anthes AM, Harinstein LM, Smithburger PL, et al. Improving adverse drug reaction detection in critically ill patients through screening intensive care unit transfer summaries. Pharmacoepidemiol Drug Saf. 2013;22(5):510–516. doi: 10.1002/pds.3422. [DOI] [PubMed] [Google Scholar]
- 65.Stockwell DC, Kane-Gill SL. Developing a patient safety surveillance system to identify adverse events in the intensive care unit. Crit Care Med. 2010;38(6 Suppl):S117–S125. doi: 10.1097/CCM.0b013e3181dde2d9. [DOI] [PubMed] [Google Scholar]
- 66.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug reactions in the intensive care unit. JAMA. 1999;282:267–270. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]