Abstract
Purpose
The purpose of this study was to investigate 13C hyperpolarization of α-ketobutyrate (αKB), an endogenous molecular analog of pyruvate, and its in vivo enzymatic conversion via lactate dehydrogenase (LDH), using localized MR spectroscopy.
Methods
Hyperpolarized (HP) 13C MR experiments were conducted using [13C]αKB with rats in vivo and with isolated LDH enzyme in vitro, along with comparative experiments using [13C]pyruvate. Based on differences in the kinetics of its reaction with individual LDH isoforms, HP [13C]αKB was investigated as a novel MR probe with added specificity for activity of LDHB expressed H (“heart”-type) subunits of LDH (e.g. constituents of LDH-1 isoform).
Results
Comparable T1 and polarization values to pyruvate were attained (T1= 52s at 3T, polarization= 10%, at C1). MR experiments showed rapid enzymatic conversion with substantially increased specificity. Formation of product HP [13C]α-hydroxybutyrate (αHB) from αKB in vivo was increased 2.7-fold in cardiac slabs relative to liver and kidney slabs. In vitro studies resulted in 5.0-fold higher product production from αKB with bovine heart LDH-1, as compared with pyruvate.
Conclusions
HP [13C]αKB may be a useful MR probe of cardiac metabolism and other applications where the role of H subunits of LDH is significant (e.g. renal cortex and brain).
Keywords: 13C, DNP, LDHB, LDHA, isoenzymes, hydroxybutyrate
Introduction
Dissolution dynamic nuclear polarization (DNP) for hyperpolarized (HP) 13C MRI has enabled localized, non-invasive monitoring of real-time enzymatic conversions through key biochemical pathways in vivo (1-3). The observable conversion of HP substrate [1-13C]pyruvate to HP [1-13C]lactate catalyzed by lactate dehydrogenase (LDH) has generated particular interest as a glycolytic marker, with promising applications in cancer (4), cardiovascular disease (5,6), and diabetes (7), including a recent “first-in-man” HP 13C MRI clinical Phase 1 safety trial in prostate cancer patients (8).
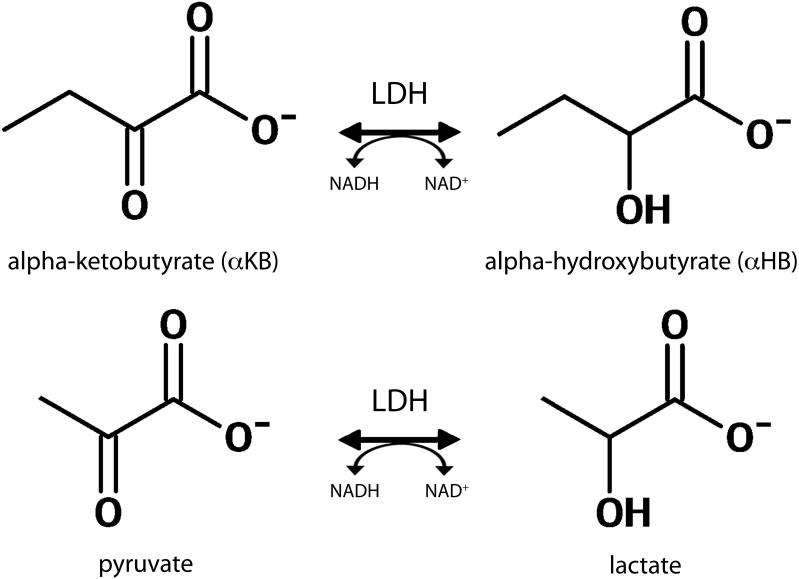
In contrast to many other important enzymes, for instance phosphoenolpyruvate carboxykinase (PEPCK) (9), LDH is characterized by relatively broad substrate specificity, exhibiting substantial activity with several α-keto acids (10). α-Ketobutyrate (αKB) is one such, endogenous structural analog of pyruvate that is also readily reduced via LDH as shown in Figure 1, to α-hydroxybutyrate (αHB). In this work we investigated HP [13C]αKB as a potentially valuable [13C]pyruvate analog with added specificity for measuring the activity of LDHB expressed H (“heart”-type) subunits of LDH, in cardiac and other tissues where H subunits are important (e.g. brain and renal cortex).
Figure 1.
Redox interconversion of αKB and αHB also catalyzed by LDH (top row), as compared with the standard interconversion of pyruvate and lactate (bottom row).
αKB actually differs biochemically from pyruvate in multiple potentially interesting ways, including 1) the aforementioned differential activity with individual LDH isoforms (10) as well as other enzymes that act on pyruvate (11), 2) possible differences in vascular permeability and/or cellular transport (12), and 3) different endogenous pool sizes (~0.1mM for pyruvate as compared with low μM range for αKB (13)). In this work we focused on the reported variable activity of individual LDH isoforms with αKB as compared to pyruvate (10). While the reported activity of LDHB expressed H subunits is similar between αKB and pyruvate, the activity of LDHA expressed M (“muscle”) subunits with αKB is greatly attenuated, indicating that HP αKB could provide useful added specificity for activity with H (“heart”) subunits. Moreover, the activity of pyruvate with H subunits in particular is inhibited by high concentrations of pyruvate, an effect which appears to be diminished in the case of αKB (14). The known differential activity of pyruvate and αKB was previously widely exploited clinically to measure serum isoenzyme fractions in the diagnosis of myocardial infarction (15), prior to supplantation by the current standard method of measuring serum cardiac troponin levels (16). Specific localized measures of cardiac LDH activity could be useful in the diagnosis and management of cardiovascular diseases.
Interestingly, a recent metabolomics discovery study singled out αHB among 485 possible biochemicals as the single best predictor of early stage insulin resistance (13), and αHB appears to be the most promising existing plasma biomarker in early diabetes research (17,18). This endogenous αHB is normally generated via LDH from αKB according to the reaction in Figure 1. Under normal conditions αKB is a dilute product of amino acid catabolism (threonine and methionine) and glutathione anabolism (cysteine formation pathway), and is ultimately recycled through the Krebs cycle via succinyl CoA. The modulation of αHB levels in early diabetes thus may be related to changes in LDH and/or glutathione metabolism. The potential ability to obtain tissue-specific measurements of αHB metabolism using HP 13C MRI may thus represent a useful new molecular imaging tool for diabetes research.
The purpose of this initial study was to investigate the feasibility of hyperpolarizing αKB via dissolution DNP and detecting its rapid enzymatic conversion to αHB catalyzed by LDH in vivo. Localized MR experiments demonstrating this conversion and comparing HP [13C]αKB and [13C]pyruvate metabolic imaging were conducted both in vivo in rats and in vitro with isolated LDH in solution. These experiments also explored the specificity of HP [13C]αKB for measuring the activity of LDHB expressed H subunits of LDH.
Methods
Modeling Reaction Rates of LDH Isoforms
LDH catalyzes the interconversion of pyruvate and lactate, permitting organisms to sustain a “temporary oxygen debt” in the form of accumulated lactate to be later reoxidized as oxygen becomes available (19). In higher vertebrates, functional LDH in vivo exists as a tetramer consisting of assembled combinations of any four individual M (“muscle”) and/or H (“heart”) subunits expressed by the genes LDHA and LDHB, respectively, giving five isoforms LDH1-5. Skeletal muscle and notably, rodent and human hepatocytes as well as most cancers, contain almost exclusively the homotetramer of M subunits (known as LDH-5 isoform), while the heart contains almost exclusively the H homotetramer (LDH-1 isoform). Most other tissues contain varying combinations of M and H subunits. The testicular isoform LDH-X is coded by a third gene whose expression is restricted to the testis. The individual isoforms differ greatly in their kinetic properties and substrate specificity.
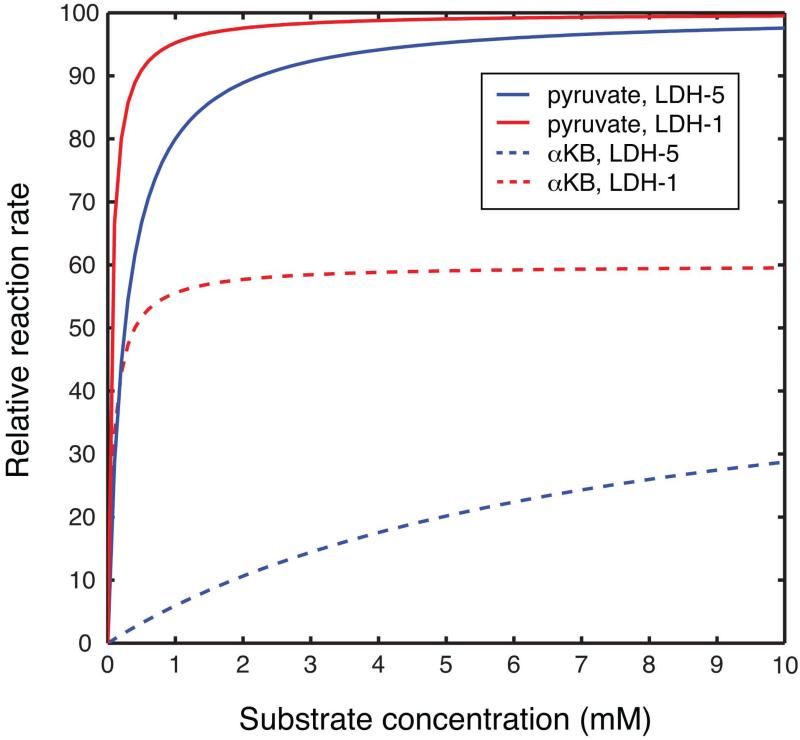
Under Michaelis-Menten (MM) enzyme kinetics, the ratio of Kcat (the catalytic constant) to Km (the Michaelis constant) is known as the specificity constant, indexing the specificity of an enzyme for alternative substrates. The specificity constant of rabbit muscle LDH-5 with αKB is 50x lower than pyruvate (for reduction), while the specificity constant of bovine heart LDH-1 with αKB is only 2.5x lower than pyruvate (10). αKB is thus 25x more specific for LDH-1 than LDH-5, relative to pyruvate. Calculated MM reaction rates based on published kinetic constants (10) and relevant substrate concentrations illustrate the potential specificity of HP αKB for LDH-1. Given the appropriate assumptions, the MM kinetic model predicts the initial reaction rate
where [E] and [S] denote the concentrations of enzyme and substrate, respectively. LDH-1 has lower Km values (Km<0.1mM) and absolute Kcat values for reduction than LDH-5 (Km≥0.25mM), features which are thought to contribute to its functional role in the oxidation of lactate as an energy source (19). Plots of predicted relative reaction rate versus substrate concentration are shown in Figure 2. Taking for example 10mM substrate concentration, the MM model predicts a relative reaction rate of 3.4x times lower for αKB with equal amounts of LDH-5 (isolated from rabbit muscle) as compared with pyruvate, but αKB retains 60% of activity with LDH-1 (bovine heart) relative to pyruvate. As the concentration of αKB is reduced below its Km value for LDH-5 (7.4mM), such as by further dilution of the HP infusion and metabolic conversion, the specificity for LDH-1 may be further increased.
Figure 2.
Relative reaction rates for the reduction of pyruvate (solid lines) and αKB (dotted lines) via LDH-5 (blue) and LDH-1 (red) as a function of substrate concentration, as predicted by Michaelis-Menten kinetics. Curves for each isoform were normalized to the maximum rate with pyruvate.
Hyperpolarization
The sodium salt of [U-13C]αKB (Cambridge Isotopes Laboratories, Andover, MA) was dissolved to a concentration of 3.4M in 50/50 glycerol/water (vol/vol), and mixed with trityl radical OX063 (15mM) (Oxford Instruments, Abingdon, UK) as well as Gd-DOTA (1.0mM) (Guerbet, Roissy, France). For each experiment, ~60mg of this mixture was polarized using a HyperSense dissolution DNP system (Oxford Instruments) and rapidly dissolved in 1X phosphate buffered saline (PBS) to yield 40mM solutions of HP [U-13C]αKB. For comparison, [1,2-13C]pyruvic acid was also polarized using similar methods to also yield 40mM solutions of HP [1,2-13C]pyruvate (with similar pH, close to neutral). Although only the C1 resonances of both molecules were directly relevant to this study, doubly labeled pyruvate was used to produce a similar appearance of the NMR spectra as [U-13C]αKB, which is the form of 13C-labeled αKB that was readily available commercially. Hyperpolarized C3 and C4 resonances of αKB were not observable due to their very short T1 relaxation times due to directly attached protons.
MR experiments
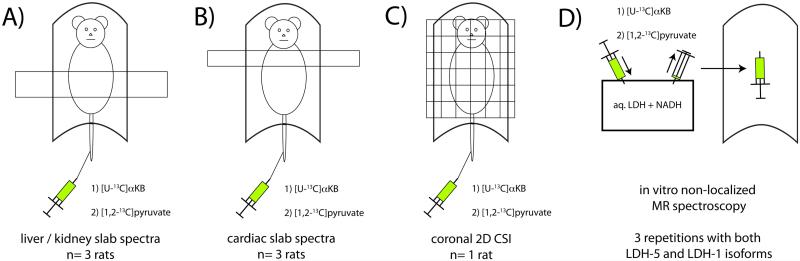
Liquid state polarizations and aqueous T1 relaxation times at 3T were measured for both the HP [U-13C]αKB and HP [1,2-13C]pyruvate samples. To demonstrate the feasibility of detecting localized conversion of HP αKB to αHB in vivo, and perform a paired comparison with HP pyruvate, three rats were scanned by sequential slab-localized MRS acquisitions of HP [U-13C]αKB and HP [1,2-13C]pyruvate (2.5mL 40mM injections over 12s via tail vein). In all cases, the αKB experiment preceded the corresponding pyruvate experiment by approximately one hour. All MR experiments were conducted in a 3T clinical scanner (GE Healthcare, Waukesha, WI) equipped with multinuclear 13C capability, using a dual tuned 1H / 13C volume transceiver radiofrequency coil designed for imaging rats. Rats were anesthetized using inhalational isoflurane delivered via nose cone (1.5%, 1L/min flow rate) and placed on a heated water pad inside the coil for the duration of the experiments. Typical physiologic parameters measured for rats handled in this manner in our lab are: body temperature= 36°C, respiration rate= 1 breath/s, heart rate= 300 beats/minute, blood glucose concentration= 100mg/dL. All animal studies were conducted in accordance with a protocol approved by the Institutional Review Board (IRB). A 5.5cm axial slab covering liver and kidneys was repeatedly excited (20°) every 3s over 30s (pulse bandwidth=2.8kHz, slice center frequency= ~178ppm), starting at the end of injection. MR signals were refocused using a pair of adiabatic spin echo pulses (TE=120ms), resulting in B1-insensitive narrowing of the spectral linewidths (20). To investigate the conversion of HP αKB in the heart (which contains only LDH-1 isoform), three additional rats were again scanned by sequential slab-localized MRS experiments with both HP [U-13C]αKB and HP [1,2-13C]pyruvate, using identical study parameters as above, except in this case a 15mm axial slab through the heart was excited instead, every 5s from 25-40s from the start of injection, with increasing flip angle over time to fully utilize all of the HP magnetization. The experiments performed in this study are diagrammed in Figure 3.
Figure 3.
Schematic representation of in vivo (A-C) and in vitro (D) MR experiments conducted using both HP [U-13C]αKB and HP [1,2-13C]pyruvate.
To demonstrate the basic feasibility of MR spectroscopic imaging (MRSI) of HP αKB, one additional rat was imaged by 2D chemical shift imaging (CSI), again sequentially with HP [U-13C]αKB and then HP [1,2-13C]pyruvate approximately one hour later. For 2D CSI, the concentration of each injection was raised to 80mM. The acquisition was exactly identical for both compounds. The injection procedure was identical to the other experiments but in this case the scan was initiated 30s after the start of injection. The 2D CSI acquisition was encoded as a coronal projection image (matrix= 12x8) but excited with axial slice selection (12cm slab) in order to avoid signal wrap around from the tail and brain. The other acquisition parameters were: spatial resolution= 1cm × 1cm, spectral resolution= 12.2Hz, spectral bandwidth= 25kHz, slice center frequency= ~178ppm, TR= 108ms, total scan time= 10s, centric view order, and increasing flip angle over phase encoding steps. Despite the potential benefit of narrower spectral linewidths, double spin echo refocusing was not employed in the 2D CSI acquisition for two reasons. First, extending the acquisition time well beyond 10s as required would introduce increased blurring due to T1 decay, motion, and metabolic conversion. Second, the SNR could be significantly degraded at the longer TE, a potentially important effect given the higher degree of localization in this experiment as compared with the slab spectra.
In vitro studies were conducted to evaluate the potential specificity of αKB for activity of H subunits, by comparing the in vitro activity of both compounds with isolated LDH-5 and LDH-1 isoforms. For each compound, 30μmol aqueous HP substrate (0.5mL) was introduced into a previously mixed solution of ~100 “units” of either isolated LDH-5 or LDH-1 enzyme and a slight excess (~33μmol) of reduced β-nicotinamide adenine dinucleotide (NADH) coenzyme (Sigma Aldrich, St. Louis, MO), in 2.5mL 1X phosphate buffered saline (PBS) maintained at ~37°C. This solution was freshly prepared just prior to each HP experiment and then mixed rapidly with the HP substrate and a portion was quickly transferred to the RF coil for MR measurements. In this case, spectra with a 5° flip angle were acquired every 3s over 42s. This experiment was repeated using LDH derived from rabbit muscle (LDH-5) and bovine heart (LDH-1) (Sigma Aldrich). In each case, the concentration of HP substrate was ~10mM and the concentration of LDH was in the low nM range. Each experiment was repeated three times.
Results
For both HP [U-13C]αKB and HP [1,2-13C]pyruvate, doublets were observed for C1 resonances due to J coupling with co-labeled C2. Estimated liquid state polarizations of [U-13C]αKB were 10% for C1 and 9% for C2, back-calculated to time of dissolution. Aqueous T1’s at 3T were 52s for C1 and 38s for C2. For [1,2-13C]pyruvate, the estimated liquid state polarizations were 17% for C1 and 16% for C2 (back-calculated), and the T1’s were 50s for C1 and 37s for C2. Thus the liquid state polarizations and T1 relaxation times of both molecules are comparable, with the neat pyruvic acid preparation attaining somewhat higher polarization.
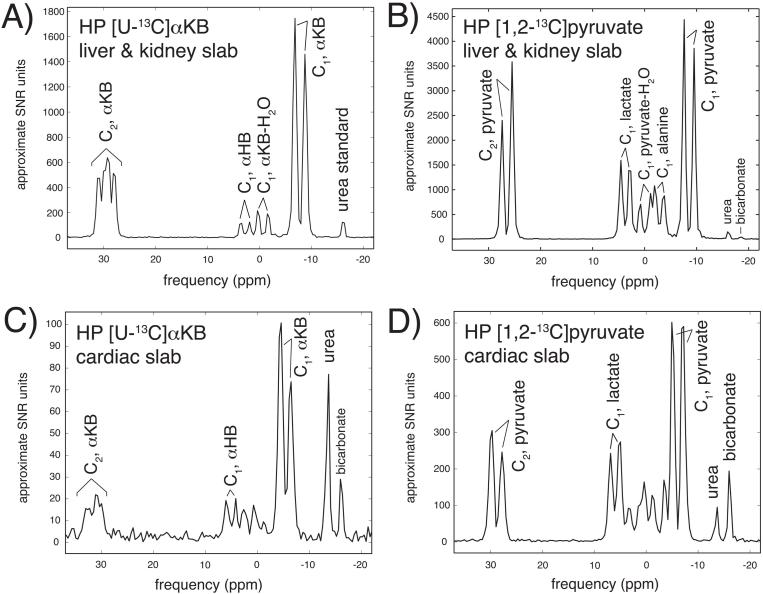
Rapid in vivo conversion of HP αKB to HP αHB via LDH was detected in all in vivo spectra. In the liver and kidney slab spectra of the first three rats scanned with both αKB and pyruvate, the mean summed HP αKB-to-αHB ratio was 5.2 ± 0.6 (mean ± s.d.) times the HP pyruvate-to-lactate ratio observed after HP pyruvate infusion in the same animals (Figure 4A,B). Unlike HP pyruvate spectra which show a significant alanine peak, no transamination product (i.e. α-aminobutyrate) was observed after HP αKB infusions, and only a very small degree of decarboxylation by PDH was detected in these spectra (11), by the appearance of a small HP [13C]bicarbonate peak in some of the data. By contrast, the cardiac slab yielded much more αHB signal than the liver and kidney slab, relative to lactate produced in the corresponding pyruvate experiment (Figure 4C,D). In scans from the second group of three rats scanned with both αKB and pyruvate, the cardiac mean summed αKB-to-αHB ratio was only 1.9 ± 0.1 times the HP pyruvate-to-lactate ratio, representing a 2.7-fold increase in product production over the liver and kidney slab, relative to pyruvate. Interestingly, some cardiac αKB spectra (e.g. Figure 4C) also revealed a large bicarbonate peak, despite the much slower predicted decarboxylation rate of αKB (11).
Figure 4.
Slab-localized 13C spectra (summed over acquisition) obtained after infusion of HP [U-13C]αKB (A,C) and HP [1,2-13C]pyruvate (B,D), in liver / kidney slab (A,B) and cardiac slab (C,D). NMR signal is scaled to approximate SNR level, and frequency is referenced to slab center frequency (i.e. ~178ppm in absolute units).
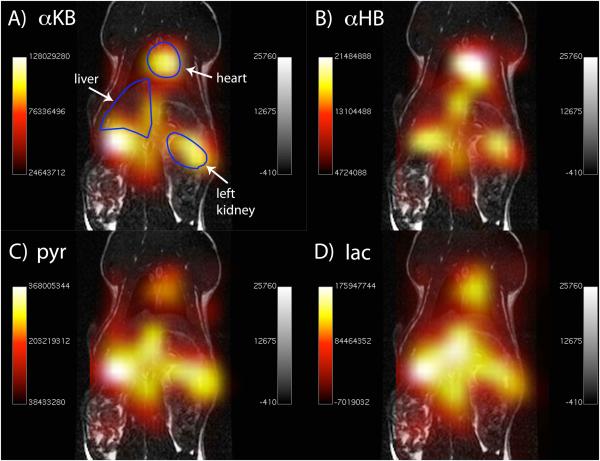
Spectroscopic images derived from the 2D CSI experiments (obtained by integrating the localized HP metabolite peaks) in one additional rat are shown in Figure 5. Injections of both HP compounds resulted in substantial HP product formation (i.e. αHB or lactate) in multiple anatomic regions. In the case of αKB, the largest product formation was in the region of the heart (Fig. 5B), while in the case of pyruvate the largest product formation was in the kidneys and liver. Since these images were acquired at relatively low true spatial resolution (1cm × 1cm), the localization of HP signals to these tissues was not extremely precise, but it appears that the signals largely originate from these tissues as described.
Figure 5.
Spectroscopic images (coronal projection) of injected HP [13C]αKB (A) and product [13C]αHB (B), in comparison with images of injected HP [13C]pyruvate (C) and product [13C]lactate (D). 13C metabolite images are overlaid in color on anatomic 1H images shown in grayscale.
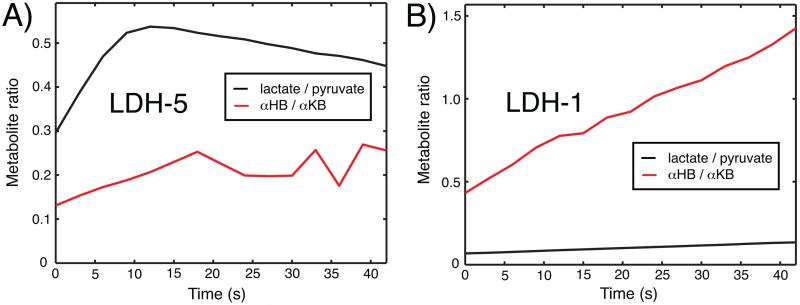
The in vitro HP experiments evidenced high specificity of αKB for LDH-1, even higher than predicted by the MM model with published kinetic constants. Dynamic metabolic curves for the LDH-5 and LDH-1 reactions from one set of experiments are plotted in Figure 6. In the LDH-1 reactions, the integrated area of the HP αHB-to-αKB ratio was actually higher than the integrated HP lactate-to-pyruvate ratio by a factor of 5.0 ± 2.9 across the three sets of experiments. Meanwhile, the LDH-5 experiments yielded lower activity with αKB by a factor of 3.1 ± 1.6, by the corresponding calculation for that experiment.
Figure 6.
In vitro activity of HP αKB (red lines) and HP pyruvate (black lines) with LDH-5 (A) and LDH-1 (B) isoforms, measured by dynamic 13C MR with experimental conditions described in text.
Discussion
We have demonstrated the hyperpolarization and rapid in vivo enzymatic conversion via LDH of [13C]αKB, a molecular analog of [13C]pyruvate which attains comparable polarization levels and has similar T1 relaxation times, but exhibits varying specificity for LDH isoforms. Prior in vitro studies as well as our own in vitro experiments indicate relatively high specificity of this molecule for activity with LHDB expressed H subunits, in comparison with pyruvate. The results of our in vivo rat studies are consistent with such specificity, since HP αKB yielded much higher fractional conversion in the heart, a site where only LDHB has significant expression, as compared with the liver and kidney. Furthermore, the 2D CSI experiments with αKB resulted in greatest HP product αHB formation in the heart, in contrast with experiments with pyruvate which resulted in the most lactate being formed in the kidneys and liver. Results observed in vivo are of course complicated by many factors including NADH availability, fractional blood volume, and vascular permeability and cellular transport. However, the high numerical consistency of these results is persuasive. The potential influence of any other potential differences from pyruvate, such as differences in vascular permeability and cellular transport of αKB and its metabolites, requires further study.
While the in vitro results showed that the activity of αKB with LDH-5 was greatly reduced in comparison to pyruvate, as expected, they also showed greatly increased activity with LDH-1. While unproven, this could be due to less inhibition by the formation of an abortive ternary complex (e.g. LDH-NAD+-substrate) involving αKB, such as is known to occur at high pyruvate concentration (19). LDHB expressed H subunits are known to be much more sensitive to such inhibition than M units. A relative absence of inhibition of chicken LDH-1 by αKB, as compared to pyruvate (despite both being effective substrates), has been noted previously (14). This apparent reduced potential for inhibition could prove valuable if H subunits of LDH are in fact inhibited by pyruvate administered during HP experiments. Substantial conversion of pyruvate to lactate was however observed in the cardiac slab in vivo. This could be due to differences between the in vitro and in vivo situations as alluded to above, or the lactate could be generated outside the heart.
Universally labeled αKB was used in this study due to practical reasons of availability as noted above, although co-labeling at the C2 position does result in some moderate shortening of the T1 relaxation time of the C1 carbon in both αKB and pyruvate (21). For comparison, we polarized natural abundance αKB (where co-labeling is essentially negligible) and measured an aqueous T1 of 65s for the C1 carbon at 3T, in comparison with ~52s for [U-13C]αKB and [1,2-13C]pyruvate. Therefore, we anticipate somewhat improved performance with singly labeled αKB due to increased polarization as well as improved discrimination of individual spectral resonances due to elimination of carbon-carbon coupling. The sodium salt form of αKB, as opposed to the neat acid, was also selected based on availability. As in the case of pyruvate, the increased concentration of a neat acid form of αKB should greatly benefit its polarization, even though unlike pyruvate αKB is also a solid at room temperature.
Notably, one prior related HP MR study sought to exploit the low substrate specificity of LDH (specifically the testicular LDH-X isoform, which has especially broad specificity) in the design of a reporter protein system based on a HP [13C]pyruvate analog that was desired to react specifically with LDH-X but not LDH1-5 (22). The selected molecule (“pyruvic acid derivative Y”) is apparently identical to keto-isocaproate (KIC), which was previously proposed by another group as a novel HP 13C probe of branched chain amino acid metabolism (23). αKB was not among the candidate molecules for the reporter system, presumably because it reacts much too greatly with LDH1-5, as shown in this study.
Based on limited published data and our own initial experience with HP 13C MRI of αKB in rats, the acute toxicity of αKB appears to be low. Published mouse LD50 values of αKB (2960 mg/kg, subcutaneous route) (24) and pyruvate (3533 mg/kg, also subcutaneous) (25) are similar. Accumulation of αKB is thought to be toxic to certain bacteria (26). Further study is required to investigate any potential acute toxicity of αKB in the context of HP 13C MRI. Should αKB lack the potential inotropic effects of pyruvate (27), it may be an attractive alternative for monitoring localized LDH activity in cardiovascular patients.
Potential value of the described approach might be seen by analogy with positron emission tomography (PET). In that modality, [18F]fluorodeoxyglucose (FDG) is an ideal probe by virtue of its difference from the endogenous substance (glucose), which greatly increases its specificity. It remains to be seen whether molecular analogs of key biomolecules could be similarly useful for HP 13C MRI, even though much higher concentrations are of course required. In this case, both pyruvate and αKB are endogenous substances, although αKB is normally far less abundant. It also remains to be seen what the most useful applications of HP αKB could be, but they could extend beyond cardiovascular disease and diabetes as described above. A recent study showed that LDHB is an essential gene in triple negative breast cancer, and high LDHB expression correlated with poor clinical outcome, indicating a localized non-invasive assay of LDHB expression could provide value in that context as well. In the near term, future technical developments will include improved localization of αKB signals, which would be facilitated by a C1-labeled version and obtaining a neat acid form with potentially improved polarization.
Acknowledgements
We gratefully acknowledge the critical assistance of Hsin-Yu Chen, Hong Shang, Eugene Milshteyn, and Mark Van Criekinge with experiments described in this manuscript. We also gratefully acknowledge grant support from NIH K01DK099451 and P41EB013598.
References
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golman K, Zandt R, Thaning M. Real-time metabolic imaging. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. in 't. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurd RE, Yen Y-F, Chen A, Ardenkjaer-Larsen JH. Hyperpolarized 13C metabolic imaging using dissolution dynamic nuclear polarization. Journal of Magnetic Resonance Imaging. 2012;36:1314–1328. doi: 10.1002/jmri.23753. doi: 10.1002/jmri.23753. [DOI] [PubMed] [Google Scholar]
- 4.Kurhanewicz J, Vigneron DB, Brindle K. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia. 2011;13(2):81–97. doi: 10.1593/neo.101102. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rider OJ, Tyler DJ. Clinical implications of cardiac hyperpolarized magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance. 2013;15:93. doi: 10.1186/1532-429X-15-93. doi: 10.1186/1532-429X-15-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malloy CR, Merritt ME, Dean Sherry A. Rizi R, editor. Could 13C MRI assist clinical decision-making for patients with heart disease? NMR in Biomedicine. 2011;24:973–979. doi: 10.1002/nbm.1718. doi: 10.1002/nbm.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laustsen C, Østergaard JA, Lauritzen MH, Nørregaard R, Bowen S, Søgaard LV, Flyvbjerg A, Pedersen M, Ardenkjaer-Larsen JH. Assessment of early diabetic renal changes with hyperpolarized [1-13C]pyruvate. Diabetes/Metabolism Research and Reviews. 2013;29:125–129. doi: 10.1002/dmrr.2370. doi: 10.1002/dmrr.2370. [DOI] [PubMed] [Google Scholar]
- 8.Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med. 2013;5:198ra108–198ra108. doi: 10.1126/scitranslmed.3006070. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidinger PF, Nowak T. Analogs of oxalacetate as potential substrates for phosphoenolpyruvate carboxykinase. Archives of Biochemistry and Biophysics. 1990;278:131–141. doi: 10.1016/0003-9861(90)90241-p. doi: 10.1016/0003-9861(90)90241-P. [DOI] [PubMed] [Google Scholar]
- 10.Kim MJ, Whitesides GM. L-Lactate Dehydrogenase - Substrate-Specificity and Use as a Catalyst in the Synthesis of Homochiral 2-Hydroxy Acids. Journal of the American Chemical Society. 1988;110:2959–2964. doi: 10.1007/BF02921743. [DOI] [PubMed] [Google Scholar]
- 11.Lapointe DS, Olson MS. Alpha-Ketobutyrate Metabolism in Perfused Rat-Liver - Regulation of Alpha-Ketobutyrate Decarboxylation and Effects of Alpha-Ketobutyrate on Pyruvate-Dehydrogenase. Archives of Biochemistry and Biophysics. 1985;242:417–429. doi: 10.1016/0003-9861(85)90226-7. doi: 10.1016/0003-9861(85)90226-7. [DOI] [PubMed] [Google Scholar]
- 12.Halestrap AP. The monocarboxylate transporter familyuStructure and functional characterization. Iubmb Life. 2012;64:1–9. doi: 10.1002/iub.573. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 13.Gall WE, Beebe K, Lawton KA, et al. Federici M, editor. α-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE. 2010;5:e10883. doi: 10.1371/journal.pone.0010883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everse J, Berger RL, Kaplan NO, Ehrenberg A. Complexes of pyridine nucleotides and their function, in Structure and Function of Oxidation and Reduction Enzymes. In: Akeson A, editor. Pergamon Press; Oxford: 1972. [Google Scholar]
- 15.Rosalki SB, Wilkinson JH. Serum alpha-hydroxybutryate dehydrogenase in diagnosis. JAMA. 1964;189:61–63. doi: 10.1001/jama.1964.03070010067019. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe AS, Landt Y, Parvin CA, Abendschein DR, Geltman EM, Ladenson JH. Comparative sensitivity of cardiac troponin I and lactate dehydrogenase isoenzymes for diagnosing acute myocardial infarction. Clinical Chemistry. 1996;42:1770–1776. [PubMed] [Google Scholar]
- 17.Lowe WLJ, Bain JR. “Prediction Is Very Hard, Especially About the Future”: New Biomarkers for Type 2 Diabetes? Diabetes. 2013;62:1384–1385. doi: 10.2337/db13-0057. doi: 10.2337/db13-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730–1737. doi: 10.2337/db12-0707. doi: 10.2337/db12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markert CL. Lactate dehydrogenase. Biochemistry and function of lactate dehydrogenase. Cell Biochemistry and Function. 1984;2:131–134. doi: 10.1002/cbf.290020302. doi: 10.1002/cbf.290020302. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham CH, Chen AP, Albers MJ, Kurhanewicz J, Hurd RE, Yen Y-F, Pauly JM, Nelson SJ, Vigneron DB. Double spin-echo sequence for rapid spectroscopic imaging of hyperpolarized 13C. J. Magn. Reson. 2007;187:357–362. doi: 10.1016/j.jmr.2007.05.014. doi: 10.1016/j.jmr.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Chen AP, Hurd RE, Schroeder MA, Lau AZ, Gu Y-P, Lam WW, Barry J, Tropp J, Cunningham CH. Simultaneous investigation of cardiac pyruvate dehydrogenase flux, Krebs cycle metabolism and pH, using hyperpolarized [1,2-C-13(2)]pyruvate in vivo. NMR in Biomedicine. 2012;25:305–311. doi: 10.1002/nbm.1749. doi: 10.1002/nbm.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishihara T, Nonaka H, Naganuma T, Ichikawa K, Sando S. Mouse lactate dehydrogenase X: A promising magnetic resonance reporter protein using hyperpolarized pyruvic acid derivative Y. Chemical Science. 2012;3:800–806. doi: 10.1039/C1SC00919B. [Google Scholar]
- 23.Karlsson M, Jensen PR, Zandt R, Gisselsson A, Hansson G, Duus JØ, Meier S, Lerche MH. Imaging of branched chain amino acid metabolism in tumors with hyperpolarized 13C ketoisocaproate. International Journal of Cancer. 2010;127:729–736. doi: 10.1002/ijc.25072. in 't. doi: 10.1002/ijc.25072. [DOI] [PubMed] [Google Scholar]
- 24.2-Ketobutyric acid, sodium salt (MSDS) Santa Cruz Biotechnology. Url: http://datasheets.scbt.com/sc-206463.pdf. Published 4-21-2010. Accessed 1-19-2015.
- 25.Pyruvic acid (MSDS) TCI America. Url: https://www.spectrumchemical.com/MSDS/TCI-P0579.pdf. Published 5-2-2007. Accessed 1-19-2015.
- 26.LaRossa RA, Van Dyk TK, Smulski DR. Toxic accumulation of alpha-ketobutyrate caused by inhibition of the branched-chain amino acid biosynthetic enzyme acetolactate synthase in Salmonella typhimurium. J. Bacteriol. 1987;169:1372–1378. doi: 10.1128/jb.169.4.1372-1378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermann H-P, Pieske B, Schwarzmöller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. The Lancet. 1999;353:1321–1323. doi: 10.1016/s0140-6736(98)06423-x. doi: 10.1016/S0140-6736(98)06423-X. [DOI] [PubMed] [Google Scholar]