Abstract
Vestibular stimulation has been reported to alleviate central pain. Clinical and physiological studies confirm pervasive interactions between vestibular signals and somatosensory circuits, including nociception. However, the neural mechanisms underlying vestibular-induced analgesia remain unclear, and previous clinical studies cannot rule out explanations based on alternative, non-specific effects such as distraction or placebo. To investigate how vestibular inputs influence nociception, we combined caloric vestibular stimulation (CVS) with psychophysical and electrocortical responses elicited by nociceptive-specific laser stimulation in humans (laser-evoked potentials, LEPs). Cold-water CVS applied to the left ear resulted in significantly lower subjective pain intensity for experimental laser pain to the left hand immediately after CVS, relative both to before CVS, and to 1 hour after CVS. This transient reduction in pain perception was associated with reduced amplitude of all LEP components, including the early N1 wave reflecting the first arrival of nociceptive input to primary somatosensory cortex. We conclude that cold left ear CVS elicits a modulation of both nociceptive processing and pain perception. The analgesic effect induced by CVS could be mediated by either subcortical gating of the ascending nociceptive input, or by direct modulation of the primary somatosensory cortex.
Keywords: Vestibular system, Nociception, Caloric Vestibular Stimulation, Laser-evoked potentials, Analgesia, Multisensory modulation
1. Introduction
Vestibular input contributes to perception in a very wide sense, through its integration with input from other sensory modalities. Consistent with this view, primate studies revealed that vestibular input does not project to a “primary vestibular cortex”, but to a network of multimodal areas. In primates, the dominant pole in this network is the parieto-insular vestibular cortex (PIVC), an area comprising the posterior insula/retroinsular cortex in the bank of the lateral sulcus (Guldin and Grüsser 1998). The human homologue of primate PIVC may not be a single area, but a distributed set of regions. Recent functional neuroimaging studies in humans have shown that artificial galvanic, caloric, or acoustic vestibular stimulation elicits responses in a wide range of multimodal cortical areas, including the posterior and anterior insula, temporoparietal junction, superior temporal gyrus, inferior parietal lobule, and somatosensory cortices (Lopez et al. 2012a; zu Eulenburg et al. 2012). The parietal operculum (area OP2) of the right hemisphere has been identified as the core region of this network (zu Eulenburg et al. 2012).
Interestingly, several classical somatosensory areas also receive vestibular inputs. The primary and the secondary somatosensory cortex respond to both vestibular and somatosensory inputs (Bottini et al. 1995), and are thus good candidates to mediate the powerful interactions between vestibular and somatosensory systems. For example, caloric vestibular stimulation (CVS) modulated psychophysical thresholds for both touch and pain (Ferrè et al. 2013), and enhanced the N80 wave of somatosensory-evoked potentials (SEPs) elicited by median nerve stimulation (Ferrè et al. 2012). Interestingly, the N80 wave may be generated in the parietal operculum (Jung et al. 2009; Eickhoff et al. 2010), a region receiving strong vestibular projections. Also clinical observations give support to the notion of powerful crossmodal interactions between vestibular and somatosensory systems (Vallar et al. 1990; Vallar et al. 1993).
CVS has been reported to reduce both experimental pain (Ferrè et al. 2013) and clinical central pain (Ramachandran et al. 2007; McGeoch et al. 2008a; McGeoch et al. 2008b). At least two possible mechanisms could underlie these vestibular-nociceptive interactions. Most accounts suggest that vestibular stimulation influences somatosensory perception indirectly, for example via a high-level supramodal attentional mechanism (Vallar et al. 1990; Vallar et al. 1993). Alternatively, vestibular projections might modulate somatosensory processing directly, for example by a gain-control or gating mechanism within ascending somatosensory pathways (Ferrè et al. 2011; Ferrè et al. 2012).
In an attempt to resolve this issue we recorded psychophysical and electrophysiological responses elicited by selective stimulation of skin nociceptors using laser pulses, before and after CVS. We have used the classical left cold CVS paradigm since previous studies indicated that this has stronger somatosensory effects than other CVS paradigms. For example, the inverse paradigm, cold right CVS, did not reliably affect somatosensory perception (Vallar et al. 1993; Bottini et al. 2005). Critically, several studies confirm a functional specialisation of the right hemisphere in vestibular processing, identified by combining functional neuroimaging with quantified stimulation of both vestibular organs. For example, vestibular cortical projections are more extensive in the right than in the left hemisphere in right-handed subjects (Bense et al. 2001; Suzuki et al. 2001; Dieterich et al. 2003; Janzen et al. 2008).
Nociceptive stimuli trigger a series of time-specific cortical processes, reflected in distinct waves of the laser-evoked potential (LEP). The N1 wave represents early processing of nociceptive input in the primary somatosensory cortex, while N2 and P2 waves represent later processing stages attributed to multimodal cortical areas (Mouraux and Iannetti 2009). Thus, if CVS interferes with the ascending nociceptive input in its early processing, for example by a subcortical modulation, we should observe modulation of all LEP waves. In contrast, if CVS influences the late nociceptive processing, for example by altering arousal or attention, we should observe the early N1 wave to be unaffected, and a possible selective modulation of later N2 and P2 waves, thought to be related to attentional and cognitive processing.
2. Materials and Methods
2.1. Participants
Ten naïve paid right-handed healthy volunteers (three females and seven males), aged between 20 and 33 years (26.7 ± 4.4 years; mean ± SD), participated in the experiment. Participants were recruited from the University College London's subject pool. The gender balance of the sample reflects those volunteering for the experiment. Exclusion criteria were any history of neurological or psychiatric disorders. All participants gave their written informed consent and were paid for their participation. The sample size was set in advance of testing, and was also used as data-collection stopping rule. No participants withdrew from the study. The study conformed to the Declaration of Helsinki and was approved by the local ethics committee.
2.2. Nociceptive stimulation
Pulses of noxious radiant heat were generated by an infrared neodymium yttrium aluminium perovskite (Nd:YAP) laser with a wavelength of 1.34 μm (Electronical Engineering, Florence, Italy). These laser pulses directly activate nociceptive terminals in superficial skin layers (Baumgärtner et al. 2005). The laser beam was transmitted via an optic fiber, and its diameter was set at approximately 8 mm (~50 mm2) by focusing lenses. Laser pulses were administered within a 5 × 5 cm area on the dorsum of the left hand. The duration of the laser pulses was 4 ms, and the energy level was set at 3.5 J. Laser pulses were confirmed to elicit a clear “pricking pain” sensation in all participants, consistent with activation of Aδ nociceptors (Treede et al. 1995). After each stimulus, the laser beam target was shifted by approximately 1 cm in a random direction, to avoid nociceptor fatigue or sensitization.
2.3. Caloric Vestibular Stimulation (CVS)
CVS was performed by slowly pouring 30 ml of cold (~0.4°C temperature) water close to the tympanic membrane into the external left auditory canal, using a syringe. The participant’s head was positioned 30° backward from the horizontal plane, thus orienting the lateral semicircular canal vertically, and 30° away from the irrigated side (Coats and Smith 1967). Irrigation lasted approximately 30 s. Participants were asked to close their eyes during the stimulation, to reduce discomfort. Immediately after CVS, the head was positioned in the upright position.
Since we were focussing on short-term after effects induced by the stimulation, LEP recording was administered just after CVS irrigation, but never during it. CVS activates the vestibular organs by creating convection currents within the semicircular canal fluid, but the effects of the stimulation considerably outlast the convention currents, and also outlast the thermotactile and noxious sensation caused by the water. In other words, CVS has specific physiological effects on brain function and processing, that outlast the peripheral stimulation, as shown by several perceptual, neuroimaging and neurophysiological studies (Bottini et al. 1994; Bottini et al. 1995; Fasold et al. 2002, Vallar et al. 1990; Vallar et al. 1993; Bisiach et al. 1991; Bottini et al. 2005; Ferrè et al. 2011; Ferrè et al., 2012).
After the irrigation, the water remaining in the auditory canal was carefully removed from the participant’s ear by the experimenter using absorbent material. CVS effectiveness was verified by visually checking for presence of ipsilateral slow-phase nystagmus. The experimenter then verbally checked whether participants felt any CVS-related symptoms, such as vertigo, or somatosensory sensations (i.e. cold) in the ear. These symptoms subsided within 3 minutes from the end of irrigation in all participants, at which point laser stimulation resumed.
Previous findings using cold CVS found strongest somatosensory effects following vestibular stimulation designed to activate the vestibular network in the right hemisphere (i.e. left cold CVS). This presumably reflects the right-hemisphere specialisation for somatosensory representation (Vallar et al. 1993; Bottini et al. 1995), rather than differences between the left and right vestibular organs themselves. Left cold CVS inhibits signals from the respective vestibular organ by reducing the spontaneous discharge rate of the horizontal semicircular canal thereby creating a relative right-sided excitation at the level of the vestibular nuclei and consequentially an activation of the right hemisphere, contralateral to the side of stimulation. Further, functional imaging studies have shown that cortical projections of the vestibular system are asymmetrically organised. The cortical vestibular network has been located primarily in the non-dominant right hemisphere in right-handed subjects (Bense et al. 2001; Suzuki et al. 2001; Dieterich et al. 2003; Janzen et al. 2008). We therefore hypothesized that delivering the nociceptive stimulation to the left hand, i.e., ipsilateral to the ear to which CVS was applied, and thus contralateral to the cerebral hemisphere predominantly activated by left-cold CVS, would provide the strongest modulatory effects.
2.4. Experimental design
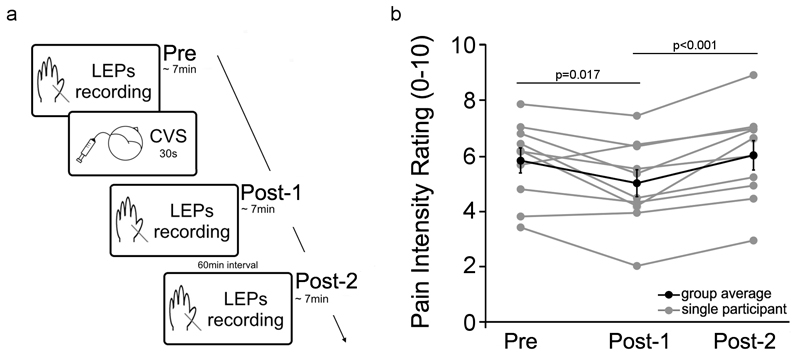
Participants were tested in a single session, consisting of three recording blocks: one block before CVS (‘Pre’), one block shortly after CVS (‘Post-1’), and a further block one hour after CVS (‘Post-2’) (Figure 1a). In each block we delivered two series of 30 laser pulses, using an inter-stimulus interval ranging between 6 s and 8 s with a uniform random distribution. After each laser stimulus participants were asked to rate verbally the intensity of the pinprick sensation elicited by the laser stimulus, using a numerical rating scale ranging from 0 (not painful) to 10 (extremely painful). The two series in each block were separated by approximately two minutes. After the first block, the CVS was delivered. The interval between the end of the first block (‘Pre’) and the beginning of the second block (‘Post-1’) was approximately 20 minutes. The third and final block (‘Post-2’) occurred 1 hour after the end of CVS. The ‘Post-2’ block was included to check whether acute effects of vestibular stimulation between ‘Pre’ and ‘Post-1’ blocks might be confounded with effects of time-dependent pain habituation. In particular, habituation should produce monotonic changes in pain perception across the three successive blocks, while acute vestibular activation should produce changes between ‘Pre’ and ‘Post-1’, followed by return to the ‘Pre’ baseline by ‘Post-2’. The ‘Post-1’ electrophysiological recording began only few minutes after the CVS, but not before CVS-induced symptoms of vertigo and dizziness had ceased. The Post-1 session was completed within 15 minutes (i.e. within the time window during which CVS effects on the vestibular system are known to persist; Bottini et al. 1995).
Figure 1. Experimental procedure and pain rating results.
(a) Participants were tested in a single session, consisting of three recording blocks: one block before CVS (‘Pre’), one block shortly after CVS (‘Post-1’), and a further block one hour after CVS (‘Post-2’). LEPs were recorded in each block. After the ‘Pre’ block, CVS was delivered. The interval between the end of the ‘Pre’ block and the beginning of the ‘Post-1’ block was approximately 20 minutes. The ‘Post-2’ block occurred 1 hour after the end of CVS.
(b) Subjective ratings of pain intensity in the three experimental blocks: before left-ear CVS (‘Pre’), shortly after left-ear CVS (‘Post-1’) and one hour after left-ear CVS (‘Post-2’). Note the CVS-induced reduction of pain intensity in the ‘Post-1’ block. Note also the similar pain ratings in the ‘Pre’ and the ‘Post-2’ blocks. Error bars show SE across participants.
2.5. EEG recording and data pre-processing
Participants were seated on a comfortable chair in a silent, temperature-controlled room. They were asked to place their left hand on a desk, and to keep their eyes open and gaze slightly downwards on a fixation point. A screen blocked vision of both the laser and the stimulated hand. The EEG was recorded using 32 Ag–AgCl electrodes, placed on the scalp according to the International 10–20 system and referenced to the nose. The electro-oculogram (EOG) was recorded using two surface electrodes, one placed over the right lower eyelid, the other placed lateral to the outer canthus of the right eye. White noise was presented over headphones during the experiment, to mask the sounds made by the laser. Signals were amplified and digitized at a sampling rate of 1,024 Hz.
EEG data were preprocessed and analysed using Letswave (http://amouraux.webnode.com) (Mouraux and Iannetti, 2008) and EEGLAB (http://sccn.ucsd.edu). EEG epochs were extracted from 500 ms prior to each laser pulse to 1000 ms after, and baseline corrected using the mean pre-stimulus value. Trials contaminated by eye-blinks and movements were corrected using an Independent Component Analysis (ICA) algorithm (Jung et al. 2000). In all datasets, ICs related to eye movements had a large EOG channel contribution and a frontal scalp distribution. Since filtering and ICA changed the EEG signals, a second baseline correction was performed after the ICA, again using the pre-stimulus interval as baseline.
Average waveforms for ‘Pre’, ‘Post-1’ and ‘Post-2’ blocks were calculated for each subject. Grand average scalp topographies of LEPs were plotted at the peak latency of the N1, N2, and P2 waves. The N2 and P2 waves were identified at Cz, as the most negative and positive deflection after stimulus onset, respectively. The N1 wave was identified using the C4–Fz montage for left hand stimulation (Hu et al. 2011).
3. Results
3.1. CVS
Although CVS is mildly unpleasant, no participant reported any particular discomfort, nor withdrew from the study. Immediately after CVS, the experimenter noted that all participants showed clear horizontal nystagmus, with a slow phase ipsilateral to the stimulated ear. Most participants also experienced typical CVS symptoms of dizziness and vertigo. The experimenter waited for approximately three minutes, until these CVS-induced symptoms disappeared. The ‘Post-1’ recording block was then started.
3.2. Pain psychophysics
In all participants, laser stimuli elicited a clear pinprick sensation, consistent with activation of Aδ fibers (Bromm and Treede 1984). We performed a repeated-measures, one-way ANOVA on pain intensity ratings, with ‘block’ as experimental factor (three levels: ‘Pre’, ‘Post-1’, and ‘Post-2’). The ANOVA revealed that pain ratings were significantly different across the three blocks (F(2,18)=9.914; p=0.001). Post-hoc t tests revealed a clear analgesic effect of left-ear CVS. Indeed, pain ratings in the ‘Post-1’ block were significantly lower than pain ratings in ‘Pre’ block (t(9)=2.914, p=0.017) and ‘Post-2’ block (t(9)=4.987, p<0.001). There were no differences in pain ratings between the ‘Pre’ and the ‘Post-2’ blocks (t(9)=-0.81, p=0.439) (Figure 1b).
3.3. LEP waveforms and topographies
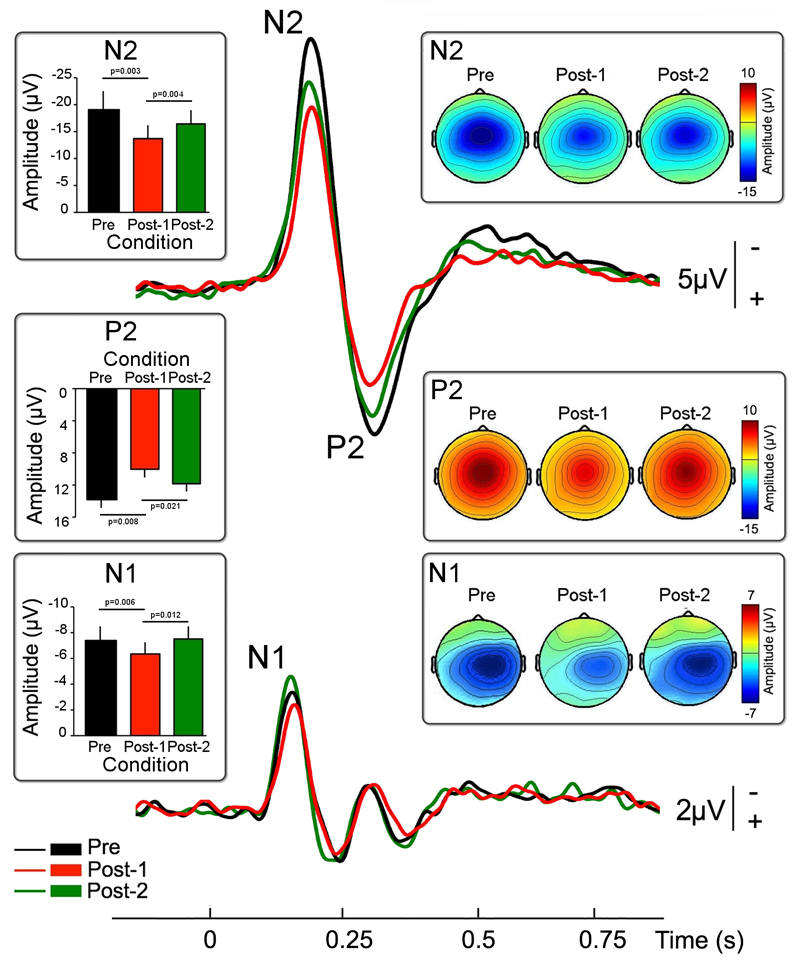
Nd:YAP laser stimulation evoked clear time-locked LEPs, consistent with the conduction velocity of Aδ afferents, in all participants. Figure 2 shows the grand average LEP waveform at Cz, with the scalp maps at the peak latencies of the N2 and P2 waves. As expected, LEPs consisted of a large negative-positive biphasic complex (N2 and P2 waves) that was maximal over the scalp vertex (electrode Cz) (Mouraux and Iannetti 2009). The scalp topography of both the N2 and P2 was centrally distributed. The scalp topography of the N2 wave extended bilaterally towards the temporal regions (electrodes T3 and T4). Figure 2 also shows the grand average of the N1 wave, with the scalp maps at the N1 peak latency. The scalp topography of the N1 activity showed a maximum at the central-parietal electrodes contralateral to the laser stimulation.
Figure 2. CVS induced modulation of LEPs.
Group averaged LEP waveforms elicited by nociceptive stimulation of the left hand dorsum in the three experimental blocks: before left-ear CVS (‘Pre’, in black), shortly after left-ear CVS (‘Post-1’, in red) and one hour after left-ear CVS (‘Post-2’, in green). Note the significant reduction of peak amplitude in all the main LEP waves (N1, N2, and P2) selectively induced by vestibular stimulation.
Error bars show SE across participants.
We performed a repeated-measures, one-way ANOVA with ‘block’ as experimental factor (three levels: ‘Pre’, ‘Post-1’, and ‘Post-2’). This analysis showed that left-ear CVS reduced the amplitude of all main LEP waves in the ‘Post-1’ block.
The amplitude of the N1 wave was significantly different across the three blocks (F(2,18)= 6.409; p=0.008). Post-hoc t tests showed that the N1 amplitude in the ‘Post-1’ block was significantly lower than the N1 amplitude in the ‘Pre’ block (t(9)=-3.535, p=0.006) and the ‘Post-2’ block (t(9)=-3.157, p=0.012). In contrast, the amplitudes of the N1 wave in the ‘Pre’ and ‘Post-2’ blocks were not different (t(9)=-0.235, p=0.819).
A similar analysis applied to the amplitude of the N2 and P2 waves revealed again a significant main effect of block (N2: F(2,18)=11.473; p=0.001; P2: F(2,18)=7.495; p=0.004). Post-hoc t tests showed that the amplitudes of the N2 and P2 waves in the ‘Post-1’ block were significantly lower than the amplitude of the N2 and P2 waves in the ‘Pre’ block (N2: t(9)=-4.13, p=0.003; P2: t(9)=3.377, p=0.008) and in the ‘Post-2’ block (N2: t(9)=-3.892, p=0.004; P2: t(9)=2.78, p=0.021). In contrast to what was observed for the N1 wave, there were trends for habituation of the N2 and P2 amplitudes between the ‘Pre’ and ‘Post-2’ blocks (N2: t(9)=-2.09, p=0.066; P2: t(9)=1.815, p=0.103).
4. Discussion
We have explored the effect of cold left ear CVS on the psychophysical and cortical responses elicited by nociceptive-specific laser stimuli. We observed that CVS induces a significant analgesic effect (Figure 1b) and inhibits the earliest cortical responses elicited by nociceptive laser stimulation (Figure 2). This finding indicates that the analgesic effect induced by CVS involves modulation of the primary somatosensory processing. Such modulation could either reflect a reduced afferent input to the primary somatosensory cortex as a result of some gating mechanism, or a reduced processing within primary somatosensory cortex itself, or both. Critically, the concentration of the CVS-induced analgesic effect on the earlier, rather than the later, cortical potentials evoked by laser stimulation, appears to rule out the possibility that CVS-induced analgesia merely reflects non-specific changes in arousal or spatial attention.
Vestibular information is essential for virtually all everyday behaviours. Through integration with other sensory modalities, the vestibular system can orient the body to the environment (Berthoz et al. 1996), detect self-motion (Berthoz et al. 1995) and even provide a foundation for bodily self-consciousness (Blanke et al. 2002). The latter function implies a direct interaction between the vestibular and somatosensory cortical systems. Consistent with this hypothesis, we recently demonstrated that CVS decreases detection thresholds for touch, but also increases detection thresholds for pain (Ferrè et al. 2013). Importantly, since in those studies we employed nociceptive-specific laser stimulation, the inhibitory effect of CVS on pain is independent of any effect of CVS on touch (Ferrè et al. 2013; Ferrè et al. 2011).
A nociceptive stimulus elicits a series of temporally distinct cortical processes. The N1 wave of the LEPs represents an early stage of sensory processing related to ascending nociceptive input. N2 and P2 waves represent a later processing stage related to the activity of multimodal cortical areas, and may primarily reflect a general factor of stimulus saliency (Mouraux and Iannetti 2009; Ronga et al. 2013). Identifying specific LEP components that are modified by CVS could potentially clarify the neural processing stage at which the modulation takes place.
Modulation of the N1 wave suggests a direct multisensory interaction between vestibular and ascending nociceptive input at an early stage of processing, localisable before the input reaches the primary somatosensory cortex, or at its level (Tarkka and Treede 1993; Valentini et al. 2012). Therefore, our results indicate for the first time that even early cortical processing of nociceptive input is strongly influenced by CVS. In fact, it is also possible that CVS inhibits the incoming nociceptive input subcortically, through a thalamic gating of nociceptive afferent input (Odkvist et al. 1974; Grüsser et al. 1990; Guldin et al. 1992; Guldin and Grüsser 1998).
Our N1 results are not easily reconciled with the view that CVS only influences later processing of painful stimuli, via non-specific mechanisms such as spatial attention (Vallar et al. 1990; Vallar et al. 1993). We did additionally find reduction of later N2 and P2 waves. These modulations of later components could indeed be explained either by inhibition of somatosensory-specific cortical responses, or by an independent modulation of late, multimodal cortical areas (Mouraux and Iannetti 2009). Our data cannot distinguish between these two possibilities.
We stimulated the left ear with cold CVS, and the left hand with nociceptive stimulation. We chose this combination because the strongest vestibular-somatosensory interactions were reported following left cold CVS (Vallar et al. 1990; Vallar et al. 1993). For instance, the irrigation of the left ear canal with cold water temporarily ameliorated tactile hemianaesthesia on the left arm (Vallar et al. 1990). Importantly, the mirror-reversed paradigm, i.e. right ear cold CVS in right hemianaesthesia showed no modulatory effect (Vallar et al. 1993; Bottini et al. 2005). These results have been interpreted as a modulation of somatosensory perception induced by CVS and mediated by a specific right hemispheric neural network involved in somatosensory processing. Cerebral lateralisation for nociceptive processing appears to be minimal or absent (but see Schlereth et al. 2003 for a study using equivalent source dipole currents). Therefore, we chose to follow the tradition of other somatosensory modalities, by focussing on right hemisphere processing. Importantly, lateralisation of CVS effects does not reflect differences in the vestibular periphery or innervation per se, but rather reflects differences in the hemispheric lateralization of neurocognitive functions modulated by vestibular stimulation. For instance, left cold CVS improves deficits in bodily awareness in right-brain damage patients (Bisiach et al. 1991), while right cold CVS influences aphasia in left-brain damage patients (Wilkinson et al. 2013). These contrasting effects do not reflect differences between left and right vestibular organs. Rather, they occur because bodily awareness is predominantly lateralised in the right hemisphere and language in the left hemisphere. Interestingly, several neuroimaging studies also confirm a functional specialisation of the right hemisphere in vestibular processing, in addition to the right-hemisphere specialisation for somatosensory function. For example, vestibular cortical projections are more extensive in the right than in the left hemisphere (Bense et al. 2001; Suzuki et al. 2001; Dieterich et al. 2003; Janzen et al. 2008). These studies lend additional support to our choice of investigating CVS-induced analgesia using left-ear cold CVS. Future studies might interestingly compare these effects with those of right-ear cold CVS.
LEPs, like other sensory ERPs, show time-dependent habituation at various time scales (Iannetti et al. 2008; Valeriani et al. 2003). Our design avoided short-term habituation (Iannetti et al. 2008) by using a long and variable interstimulus interval. However, longer-term habituation to nociceptive stimulation across successive blocks might have occurred (Valeriani et al. 2003). Indeed, N2 and P2 waves showed lower amplitudes in both the ‘Post-1’ and ‘Post-2’ blocks, compared to the ‘Pre’ block. However, non-specific mechanisms such as long-term habituation cannot readily explain the full pattern of our results – as both N2 and P2 amplitudes were significantly smaller in the ‘Post-1’ than in the ‘Post-2’ block (Figure 2), suggesting that CVS had an additional acute effect over and above general habituation mechanisms. Even more strikingly, the N1 wave did not show any habituation, when comparing ‘Pre’ to ‘Post-2’. Thus, we can rule out the possibility that the observed reduction of amplitude of the N1 wave was due to habituation. Instead, it must reflect a direct consequence of CVS on nociceptive processing. For the N2 and P2 waves, some contribution of habituation over the course of the experiment cannot be excluded. However, the significant difference between Pre and Post-1 blocks suggest an additional effect of CVS.
CVS effects on somatosensory perception have previously been explained as shifts of spatial attention towards the stimulated side (Vallar et al. 1990; Vallar et al. 1993). This hypothesis cannot readily explain our data. Indeed, directing spatial attention to noxious stimulation produces hyperalgesia (Scharein and Bromm 1998; Liu et al. 2011), and also an increase of amplitude of all LEP waves, including the N1 (Legrain et al. 2002). We found an effect of CVS in the opposite direction: CVS exerted a clear analgesic effect (Figure 1; see also Ferrè et al., 2013) and reduced the amplitude of all LEP waves (Figure 2).
Could our effects be due to some aspect of the CVS procedure, other than vestibular-nociceptive interactions? Evoked potentials were recorded not during CVS itself, but a few minutes after irrigation. By this time, nystagmus and vertigo have subsided (Miller et al., 2000; Ngo et al., 2007; Ngo et al. 2008). However, it has been demonstrated the activation of vestibular projections lasts over the oculo-motor reflex for few minutes. These effects have been extensively tested in cognitive neuroimaging (Bottini et al. 1994; Bottini et al. 1995; Fasold et al. 2002), clinical (Vallar et al. 1990; Vallar et al. 1993) and experimental studies (Lenggenhager et al. 2014; Lopez et al. 2012b; Ferrè et al. 2011; Ferrè et al. 2013). In most experimental paradigms, the effects induced by CVS are measured immediately after irrigation, but not during it, to avoid the side effects discussed previously. Our experimental procedure followed this established protocol. We can therefore exclude explanations based on effects of vestibular-induced gaze modulation and also acute vestibular symptoms such as vertigo.
However, CVS does not only affect the vestibular system. Several additional neural systems receive corollary stimulation when the ear is irrigated, triggering autonomic, thermal and nociceptive responses. For instance, caloric irrigation produces changes in heart rate variability, blood pressure variability and respiratory frequency – since these changes were identical in healthy controls and in a labyrinthine-defective patient, they were attributed to autonomic rather than vestibular effects (Jauregui-Renaud et al. 2000). Anatomically, the external ear and the tympanic membrane are innervated by the trigeminal, glossopharyngeal and vagal cranial nerves, which are known to contribute to autonomic responses (Alvord and Farmer, 1997; Drake et al. 2008; Truex et al. 1969). Arnold’s reflex (coughing when the wall of the ear canal is touched) is attributed to sensory vagal innervation of this area (Ryan et al. 2014). Although reflex coughing was not observed in our study, these afferents were presumably stimulated by CVS. Physiological studies demonstrated that nerve endings in the tympanic membrane are also involved in inflammatory and nociceptive responses (Uddman et al. 1988). One of the most important afferent inputs from the tympanic membrane is constituted by the nociceptive trigeminal afferents, which disruption results in anaesthesia of the ear drum (Saunders and Weider, 1985). Thus, these thermal, autonomic and nociceptive responses can be triggered and modulated by the water stimulation that is an inevitable part of caloric irrigation. In our case, we cannot exclude the possibility that the analgesic effects of CVS partly reflect these accessory stimulations, in addition to stimulation of the vestibular organs themselves. Although CVS is an established procedure, it is hard to have a good control for these accessory stimulations. For instance, applying body temperature water to the auditory canal would not trigger the same vagal, thermal and somatosensory sensations. Thus, it would not control for the non-specific changes occurring during CVS. Similarly, it would not cause the typical vestibular induced side effects (dizziness and vertigo).
5. Conclusion
Vestibular inputs have widespread effects within multisensory cortical networks. Previous studies focussed on anatomical (Bottini et al. 1995), clinical (Vallar et al. 1990) and perceptual (Ferrè et al. 2011) interactions between vestibular stimulation and tactile somatosensation. Here we show, for the first time, that cold left-ear CVS reduces the earliest responses to purely nociceptive stimulation. These effects are already present at the level of primary somatosensory cortex, or may even occur at earlier levels, such as in the thalamus.
Acknowledgements
This research was supported by a Wellcome Trust Project Grant 094863/Z/10/Z to GDI and PH. EF was supported by a BIAL Foundation bursary. PH was additionally supported by a Major Research Fellowship from Leverhulme Trust, by EU project VERE (WP1), by ERC Advanced Grant HUMVOL, and by an ESRC Professorial Research Fellowship. GDI is University Research Fellow of The Royal Society and acknowledges the support of The Wellcome Trust.
Abbreviations
- CVS
caloric vestibular stimulation
- LEPs
laser-evoked potentials
- PIVC
parieto insular vestibular cortex
- SEPs
somatosensory-evoked potentials
Footnotes
Conflict of interest:
The authors declare no competing financial interests.
References
- Alvord LS, Farmer BL. Anatomy and orientation of the human external ear. J Am Acad Audiol. 1997;8:383–390. [PubMed] [Google Scholar]
- Baumgärtner U, Cruccu G, Iannetti GD, Treede RD. Laser guns and hot plates. Pain. 2005;116:1–3. doi: 10.1016/j.pain.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI) J Neurophysiol. 2001;85(2):886–899. doi: 10.1152/jn.2001.85.2.886. [DOI] [PubMed] [Google Scholar]
- Berthoz A. How does the cerebral cortex process and utilize vestibular signals? In: Baloh RW, Halmagyi GM, editors. Disorders of the vestibular system. New York: Oxford University Press; 1996. pp. 113–125. [Google Scholar]
- Berthoz A, Israel I, Georges-Francois P, Grasso R, Tsuzuku T. Spatial memory of body linear displacement: what is being stored? Science. 1995;269:95–98. doi: 10.1126/science.7604286. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Rusconi ML, Vallar G. Remission of somatoparaphrenic delusion through vestibular stimulation. Neuropsychologia. 1991;29(10):1029–1031. doi: 10.1016/0028-3932(91)90066-h. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Bottini G, Paulesu E, Gandola M, Loffredo S, Scarpa P, Sterzi R, Vallar G. Left caloric vestibular stimulation ameliorates right hemianesthesia. Neurology. 2005;65(8):1278–1283. doi: 10.1212/01.wnl.0000182398.14088.e8. [DOI] [PubMed] [Google Scholar]
- Bottini G, Paulesu E, Sterzi R, Warburton E, Wise RJ, Vallar G, Frackowiak RS, Frith CD. Modulation of conscious experience by peripheral sensory stimuli. Nature. 1995;376(6543):778–781. doi: 10.1038/376778a0. [DOI] [PubMed] [Google Scholar]
- Bottini G, Sterzi R, Paulesu E, Vallar G, Cappa SF, Erminio F, Frackowiak RS. Identification of the central vestibular projections in man: a positron emission tomography activation study. Experimental Brain Research. 1994;99(1):164–169. doi: 10.1007/BF00241421. [DOI] [PubMed] [Google Scholar]
- Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol. 1984;3(1):33–40. [PubMed] [Google Scholar]
- Coats AC, Smith SY. Body position and the intensity of caloric nystagmus. Acta Otolaryngol. 1967;63:515–532. doi: 10.3109/00016486709128785. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13(9):994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Drake RL, Drake RL, Gray H. Gray's atlas of anatomy. Churchill Livingstone/Elsevier; Philadelphia: 2008. [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30(18):6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17(3):1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Ferrè ER, Bottini G, Haggard P. Vestibular modulation of somatosensory perception. Eur J Neurosci. 2011;34(8):1337–1344. doi: 10.1111/j.1460-9568.2011.07859.x. [DOI] [PubMed] [Google Scholar]
- Ferrè ER, Bottini G, Haggard P. Vestibular inputs modulate somatosensory cortical processing. Brain Struct Funct. 2012;217(4):859–864. doi: 10.1007/s00429-012-0404-7. [DOI] [PubMed] [Google Scholar]
- Ferrè ER, Bottini G, Iannetti GD, Haggard P. The balance of feelings: Vestibular modulation of bodily sensations. Cortex. 2013;49(3):748–758. doi: 10.1016/j.cortex.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Grüsser OJ, Pause M, Schreiter U. Localization and responses of neurones in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis) J Physiol. 1990;430:537–557. doi: 10.1113/jphysiol.1990.sp018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldin WO, Akbarian S, Grüsser OJ. Cortico-cortical connections and cytoarchitectonics of the primate vestibular cortex: A study in squirrel monkeys (saimiri sciureus) J Comp Neurol. 1992;326(3):375–401. doi: 10.1002/cne.903260306. [DOI] [PubMed] [Google Scholar]
- Guldin WO, Grüsser OJ. Is there a vestibular cortex? Trends Neurosci. 1998;21:256–259. doi: 10.1016/s0166-2236(97)01211-3. [DOI] [PubMed] [Google Scholar]
- Hu L, Liang M, Mouraux A, Wise RG, Hu Y, Iannetti GD. Taking into account latency, amplitude, and morphology: improved estimation of single-trial ERPs by wavelet filtering and multiple linear regression. J Neurophysiol. 2011;106(6):3216–3229. doi: 10.1152/jn.00220.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol. 2008;100(2):815–828. doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen J, Schlindwein P, Bense S, Bauermann T, Vucurevic G, Stoeter P, Dieterich M. Neural correlates of hemispheric dominance and ipsilaterality within the vestibularsystem. Neuroimage. 2008;42(4):1508–1518. doi: 10.1016/j.neuroimage.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Jauregui-Renaud K, Yarrow K, Oliver R, Gresty MA, Bronstein AM. Effects of caloric stimulation on respiratory frequency and heart rate and blood pressure variability. Brain Res Bull. 2000;53(1):17–23. doi: 10.1016/s0361-9230(00)00304-x. [DOI] [PubMed] [Google Scholar]
- Jung P, Baumgärtner U, Stoeter P, Treede RD. Structural and functional asymmetry in the human parietal opercular cortex. J Neurophysiol. 2009;101(6):3246–57. doi: 10.1152/jn.91264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol. 2000;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Hilti L, Palla A, Macauda G, Brugger P. Vestibular stimulation does not diminish the desire for amputation. Cortex. 2014;54:210–212. doi: 10.1016/j.cortex.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Legrain V, Guérit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99:21–39. doi: 10.1016/s0304-3959(02)00051-9. [DOI] [PubMed] [Google Scholar]
- Liu CC, Veldhuijzen DS, Ohara S, Winberry J, Greenspan JD, Lenz FA. Spatial attention to thermal pain stimuli in subjects with visual spatial hemi-neglect: extinction, mislocalization and misidentification of stimulus modality. Pain. 2011;152(3):498–506. doi: 10.1016/j.pain.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Blanke O, Mast FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience. 2012a;212:159–179. doi: 10.1016/j.neuroscience.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Lopez C, Schreyer HM, Preuss N, Mast FW. Vestibular stimulation modifies the body schema. Neuropsychologia. 2012b;50(8):1830–1837. doi: 10.1016/j.neuropsychologia.2012.04.008. [DOI] [PubMed] [Google Scholar]
- McGeoch PD, Ramachandran VS. Vestibular stimulation can relieve central pain of spinal origin. Spinal Cord. 2008a;46(11):756–757. doi: 10.1038/sc.2008.47. [DOI] [PubMed] [Google Scholar]
- McGeoch PD, Williams LE, Lee RR, Ramachandran VS. Behavioural evidence for vestibular stimulation as a treatment for central post-stroke pain. J Neurol Neurosurg Psychiatry. 2008b doi: 10.1136/jnnp.2008.146738. [DOI] [PubMed] [Google Scholar]
- Miller SM, Liu GB, Ngo TT, Hooper G, Riek S, Carson RG, Pettigrew JD. Interhemispheric switching mediates perceptual rivalry. Curr Biol. 2000;10:383–392. doi: 10.1016/s0960-9822(00)00416-4. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging. 2008;26:1041–1054. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- Ngo TT, Liu GB, Tilley AJ, Pettigrew JD, Miller SM. Caloric vestibular stimulation reveals discrete neural mechanisms for coherence rivalry and eye rivalry: a meta-rivalry model. Vision Res. 2007;47(21):2685–99. doi: 10.1016/j.visres.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Ngo TT, Liu GB, Tilley AJ, Pettigrew JD, Miller SM. The changing face of perceptual rivalry. Brain Res Bull. 2008;75(5):610–8. doi: 10.1016/j.brainresbull.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Odkvist LM, Schwarz DW, Fredrickson JM, Hassler R. Projection of the vestibular nerve to the area 3a arm field in the squirrel monkey (saimiri sciureus) Exp Brain Res. 1974;21(1):97–105. doi: 10.1007/BF00234260. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, McGeoch PD, Williams L, Arcilla G. Rapid relief of thalamic pain syndrome induced by vestibular caloric stimulation. Neurocase 2007. 2007;13(3):185–188. doi: 10.1080/13554790701450446. [DOI] [PubMed] [Google Scholar]
- Ronga I, Valentini E, Mouraux A, Iannetti GD. Novelty is not enough: laser-evoked potentials are determined by stimulus saliency, not absolute novelty. J Neurophysiol. 2013;109(3):692–701. doi: 10.1152/jn.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MN, Gibson PG, Birring SS. Arnold's nerve cough reflex: evidence for chronic cough as a sensory vagal neuropathy. J Thorac Dis. 2014;6:S748–752. doi: 10.3978/j.issn.2072-1439.2014.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RL, Weider D. Tympanic membrane sensation. Brain. 1985;108:387–404. doi: 10.1093/brain/108.2.387. [DOI] [PubMed] [Google Scholar]
- Scharein E, Bromm B. The intracutaneous pain model in the assessment of analgesic efficacy. Pain Rev. 1998;5(4):216–246. [Google Scholar]
- Schlereth T, Baumgärtner U, Magerl W, Stoeter P, Treede RD. Left-hemisphere dominance in early nociceptive processing in the human parasylvian cortex. Neuroimage. 2003;20(1):441–454. doi: 10.1016/s1053-8119(03)00345-8. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, Shiino A, Kitajima K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Cognitive Brain Research. 2001;12:441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- Tarkka IM, Treede RD. Equivalent electrical source analysis of pain-related somatosensory evoked potentials elicited by a CO2 laser. J Clin Neurophysiol. 1993;10:513–519. doi: 10.1097/00004691-199310000-00009. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483(3):747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truex RC, Carpenter MB, Strong OS. Human neuroanatomy. Williams & Wilkins; Baltimore: 1969. [Google Scholar]
- Uddman R, Grunditz T, Larsson A, Sundler F. Sensory innervation of the ear drum and middle-ear mucosa: retrograde tracing and immunocytochemistry. Cell Tissue Res. 1988;252:141–146. doi: 10.1007/BF00213835. [DOI] [PubMed] [Google Scholar]
- Valentini E, Hu L, Chakrabarti B, Hu Y, Aglioti SM, Iannetti GD. The primary somatosensory cortex largely contributes to the early part of the cortical response elicited by nociceptive stimuli. Neuroimage. 2012;59(2):1571–1581. doi: 10.1016/j.neuroimage.2011.08.069. [DOI] [PubMed] [Google Scholar]
- Valeriani M, De Tommaso M, Restuccia D, Le Pera D, Guido M, Iannetti GD, Cruccu G. Reduced habituation to experimental pain in migraine patients: a CO(2) laser evoked potential study. Pain. 2003;105(1):57–64. doi: 10.1016/s0304-3959(03)00137-4. [DOI] [PubMed] [Google Scholar]
- Vallar G, Bottini G, Rusconi ML, Sterzi R. Exploring somatosensory hemineglect by vestibular stimulation. Brain. 1993;116(1):71–86. doi: 10.1093/brain/116.1.71. [DOI] [PubMed] [Google Scholar]
- Vallar G, Sterzi R, Bottini G, Cappa S, Rusconi ML. Temporary remission of left hemianesthesia after vestibular stimulation. A sensory neglect phenomenon. Cortex. 1990;26:123–131. doi: 10.1016/s0010-9452(13)80078-0. [DOI] [PubMed] [Google Scholar]
- Zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012;60(1):162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Morris R, Milberg W, Sakel M. Caloric vestibular stimulation in aphasic syndrome. Frontiers in integrative neuroscience. 2013;7 doi: 10.3389/fnint.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]