ABSTRACT
Acute-onset arthritis is a common clinical problem facing both the general clinician and the rheumatologist. A viral aetiology is though to be responsible for approximately 1% of all cases of acute arthritis with a wide range of causal agents recognised. The epidemiology of acute viral arthritis continues to evolve, with some aetiologies, such as rubella, becoming less common due to vaccination, while some vector-borne viruses have become more widespread. A travel history therefore forms an important part of the assessment of patients presenting with an acute arthritis. Worldwide, parvovirus B19, hepatitis B and C, HIV and the alphaviruses are among the most important causes of virally mediated arthritis. Targeted serological testing may be of value in establishing a diagnosis, and clinicians must also be aware that low-titre autoantibodies, such as rheumatoid factor and antinuclear antibody, can occur in the context of acute viral arthritis. A careful consideration of epidemiological, clinical and serological features is therefore required to guide clinicians in making diagnostic and treatment decisions. While most virally mediated arthritides are self-limiting some warrant the initiation of specific antiviral therapy.
KEYWORDS: Arthritis, arthralgia, alphavirus, parvovirus B19, hepatitis
Introduction
Viral infections are a well-recognised cause of acute arthralgia and arthritis with a large number of causative agents reported. The diagnosis of virally induced arthritis can be difficult to confirm but should be considered in all patients presenting with acute-onset polyarticular symptoms. In addition to serological testing for the causative agent there may be associated clinical features that point clinicians to a specific virus such as the typical ‘slapped cheek’ rash seen in parvovirus-associated arthritis or jaundice associated with acute hepatitis B (HBV) infection. In many cases however, these features may be subtle, absent or temporally distant from the joint symptoms making the diagnostic process difficult. Therefore when a virally mediated arthritis is suspected, serological testing should be based on both clinical and epidemiological data.
Epidemiology
Accurate data on the incidence and prevalence of virally induced arthritis are lacking. Most studies have investigated a limited number of aetiologies and there is likely to be significant geographic variation in the frequency and causes of virally mediated arthritis. Studies that have screened patients presenting with acute arthritis have suggested a viral aetiology in about 1% of cases1–3 and in the largest study, the cost per individual tested was €85.05.
Worldwide, epidemics of arbovirus-related arthritis are increasingly recognised. In Europe there have been recent outbreaks of sindbis virus4 and chikungunya (CHIKV).5 While there is increasing recognition, and possibly incidence, of vector-borne causes of arthritis, other causes of viral-induced arthritis may be becoming less common. Routine vaccination against mumps and rubella for example, is likely to have reduced the frequency with which arthritis associated with these two viruses is seen.6
Causative organisms
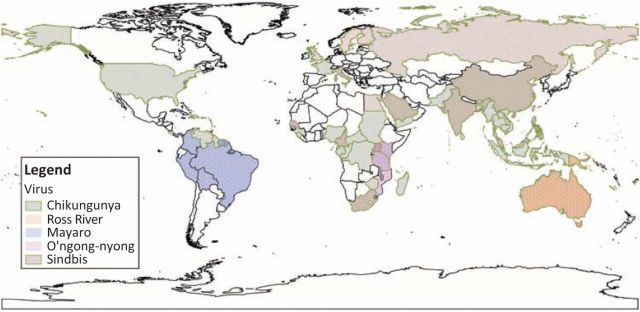
A broad range of viruses, many of which have specific geographic niches (Table 1 and Fig 1), can cause arthritis, highlighting the importance of a travel history. In patients where an imported aetiology is being considered, consultation with a specialist in infectious disease is recommended to guide investigations.
Table 1.
Key features of major viral arthritides.
| Virus | Epidemiology | Common clinical features | Diagnostic test |
|---|---|---|---|
| Parvovirus B19 | Exposure to young children | Large joint oligoarticular in children, RA-like pattern more common in adults. | B19 IgM |
| Hepatitis B | Congenital transmission in high-risk countries MSM, IDU | RA-like pattern of arthritis lasting days to months with rash, malaise or myalgia. Joint symptoms often resolve with onset of jaundice. | HBsAg, anti-HBc, HBV DNA |
| HCV | Worldwide MSM, IDU, blood transfusions | Mild RA-like pattern of arthritis/ arthralgia. | Anti-HCV, HCV-RNA |
| HCV-associated cryoglobulinaemia | As above | Large joint oligoarticular non-erosive arthritis (often involving ankles). | Anti-HCV, HCV-RNA, cryoglobulins, low C4 |
| HIV | Worldwide – especially sub-Saharan Africa, multiple sexual partners, MSM, IDU, blood transfusions | Variety of presentations reported. Joint symptoms as part of an immune-reconstitution syndrome also described with initiation of retroviral therapy. | Anti-HIV1/2, HIV-RNA |
| Alphaviruses | Specific geographical range for each virus – See Fig 1 | Chronic arthritis/arthralgia associated with chikungunya infection, as well as tenosynovitis, carpal tunnel syndrome and new-onset Raynaud phenomenon. Associated thrombocytopenia common. | Specific-viral IgM, specific-viral RNA |
| HTLV-1 | Japan, Caribbean | Chronic large and medium joint oligoarthritis. Fever myalgia and skin lesions common at onset of joint symptoms. Associated inflammatory eye, skin and muscle disorders reported. | Anti-HTLV-1, HTLV-1-DNA |
CHIKV = chikungunya; HBV = hepatitis B virus; HCV = hepatitis C virus; HTLV-1 = human T-lymphotrophic virus type-1; IDU = injecting drug user; MSM = men who have sex with men; RA = rheumatoid artritis.
Parvovirus B19
Parvovirus B19 is a single-stranded DNA virus. The virus is globally distributed and most healthy adults are seropositive. While outbreaks of parvovirus are uncommon they have been reported.7 Many cases of parvovirus B19 are asymptomatic but the virus is also responsible for a wide spectrum of clinical manifestations, including erythema infectiosum, aplastic anaemia and an acute arthritis.
Acute symptomatic parvovirus B19 infection is associated with elevated levels of a wide range of pro-inflammatory cytokines.8 Parvovirus-B19 DNA has been found in the synovial fluid of inflamed joints, though it is just as commonly found in synovial tissue from controls.9 The development of arthritis is associated with the development of parvovirus B19 specific antibodies, suggesting that immune complexes may be involved in the development of parvovirus arthritis.10,11
The reported frequency of arthritis varies with age of infection, from 8% in children to as high as 50–80% in adults.10,12 In children an asymmetric large-joint oligoarthritis, usually involving the knee, is most common, whereas adults tend to present with a rheumatoid arthritis (RA)-like symmetrical small joint pattern involving the wrists, metacarpophalangeals and proximal interphalengeals.13 A number of autoantibodies have been reported to occur transiently with parvovirus-arthritis (usually at low titres) including rheumatoid factor (RF), antinuclear antibody (ANA) and a variety of extractable nuclear antigens (ENAs). Arthritis symptoms are usually short-lived but can persist for a number of months and can be managed with non-steroidal anti-inflammatory drugs (NSAIDs). There are case reports of severe parvovirus arthritis associated with persistent viraemia responding favourably to intravenous immunoglobulin.14
Fig 1.
Geographic range of alphaviruses.
HIV
Arthritis can be seen at any stage of HIV infection but the spectrum of manifestations is changing following the roll-out of combination antiretroviral therapy (cARV).11
Arthralgia is a well-described feature of primary HIV infection illnesses,15 but true arthritis is relatively uncommon. In the setting of untreated HIV, joint symptoms are common with around 11% developing a mono or polyarticular arthritis.16 Joint symptoms, including reactive arthritis, have been reported to respond well to the initiation of cARV.17
The spectrum of rheumatic complications of HIV has changed following uptake of cARV. A longitudinal study conducted between 1989 and 2000 revealed a marked decline in the occurrence of classical rheumatic complications of HIV such as reactive arthritis and psoriatic arthritis.18 Autoimmune rheumatic diseases such as RA are relatively uncommon in the setting of untreated HIV but initiation of cARV can result in an immune reconstitution syndrome which may unmask underlying autoimmune rheumatological disease.11,18
Clinicians must bear in mind that joint symptoms in patients with HIV may represent manifestations of co-infection with other blood-borne viruses such as HBV and hepatitis C (HCV), a reactive arthritis following an alternative infection or an arthropathy related to a sexual transmitted infection such as gonorrhoea or syphilis.19
Hepatitis B
HBV, a dsDNA virus of the Hepadnaviridae family, is estimated to affect around 400 million people worldwide. Transmitted vertically, sexually or through blood-borne contact (transfusion or intravenous drug use), around 95% of adults exposed to the virus will mount an appropriate immune response leading to eventual viral clearance.
Arthritis in patients with HBV is mediated through the formation and deposition of immune complexes containing viral antigens and their respective antibodies in the synovial tissues.20 Arthritis occurs in both the prodromal phase of acute infection and during chronic HBV infection.
Arthritis can be the only presenting feature of acute HBV infection,21 and in the prodromal phase of infection often resembles RA, with a symmetrical polyarticular distribution involving proximal interphalengeal joints, ankles and knees.22 The presence of rash, fever, malaise or myalgia may provide clues to the underlying diagnosis. Arthritis symptoms typically last days to months23 and often resolve with the onset of jaundice. RF can be elevated in around 25% of cases whereas C3 and C4 are found to be low in around 40%, indicative of an immune-complex mediated process.11
Up to 25% of patients with chronic HBV infection report joint symptoms, though overt synovitis is uncommon and joint damage is rare. The presence of chronic arthritis or arthralgia should prompt consideration of associated immune complex deposition syndromes, such as polyarteritis nodosa, or cryoglobulinaemia.
Hepatitis C
Approximately 3–4 million individuals are infected with HCV annually. The World Health Organization estimate that around 3% of the world's population have chronic HCV infection.24
Between 40 and 70% of patients with acute HCV infection are reported to experience at least one extra-hepatic manifestation during their illness.25,26 Arthritis symptoms are common and have been reported in more than 70% of patients27 with two distinct clinical patterns described: a more common polyarticular small joint arthritis resembling RA which usually runs a milder disease course; and an oligoarticular medium and large joint arthritis (often affecting the ankles) that tends to be non-erosive, and is frequently associated with mixed cryoglobulinaemic vasculitis (MCV).28 An RA-like polyarthritis has also been reported as a complication of interferon treatment for HCV patients.29
As with HBV, RF is positive in HCV-related arthritis (in up to 80% of cases),30 and anti-citrullinated protein antibodies, while highly specific for RA, are also reported to occur in 4.5% of HCV-related arthritis cases. 31
Symptomatic treatment with analgesics and NSAIDs is recommended for the management of HCV-related arthritis in the absence of MCV. For patients with MCV, antiviral treatment is recommended to achieve a sustained virological response. There is a strong correlation between clinical improvement of MCV-associated arthritis and the disappearance of HCV RNA and cryoglobulins in the serum.32
Hepatitis E
Until recently, hepatitis E virus (HEV) was considered solely as the cause of an acute self-limited hepatitis, similar to hepatitis A. It is now clear that infection with HEV serotypes 3 or 4 may be associated with chronic infection33 and a range of extra-hepatic manifestations including joint symptoms and cryoglobulinaemia.34
HTLV
Human T-lymphotrophic virus type-1 (HTLV-1) was the first identified retrovirus and it is estimated that 20 million individuals are infected35 predominantly in Japan and the Caribbean. HTLV-1 is the cause of HTLV-associated myelopathy and adult T-cell leukaemia/lymphoma (ATLL). There is increasing evidence that infection with both HTLV-1 and -2 is associated with acute arthritis, as well as inflammatory eye, muscle and skin disorders.
Clinically HTLV-associated arthropathy has been reported as a chronic oligoarthritis preferentially affecting the shoulders, wrists and knees. Fever, myalgia and skin lesions commonly occur at the onset of polyarthritis36 and both RF and ANA positivity are reported. There is no established treatment for HTLV-associated arthropathy though corticosteroid therapy is often used. Despite concerns regarding an increased risk of ATLL in the context of immunosuppression, safe use of anti-tumour necrosis factor agents for RA in patients with HTLV infection has been reported.37 Treatment with interferon-α has been reported to improve joint symptoms in some patients with chronic HTLV-associated arthropathy.38
Arboviruses
Arthropod-borne-viruses (arboviruses) are a broad group of viruses transmitted predominantly by mosquitoes. Two main genera of arboviruses are associated with acute arthritis; alphaviruses and flaviruses.
Alphaviruses
Alphaviruses are a genus of RNA virus, usually transmitted by mosquitoes. ‘New World’ alphaviruses are commonly associated with encephalitis while ‘Old World’ alphaviruses, of which CHIKV is the most well known, are commonly associated with a syndrome of fever and arthralgia.39,40 The incubation period for all the alphaviruses is approximately 3–15 days41,42 and epidemics are well recognised.42–44 Macrophages are thought to be responsible for much of the pathology associated with alphavirus infection through the release of pro-inflammatory cytokines and matrix metalloproteinases.41,45
Chikungunya
CHIKV is responsible for disease throughout Africa and Asia, and more recently the Caribbean.46 Smaller outbreaks have also occurred in Europe.5Aedes mosquitoes are the vector of CHIKV.42 In Africa the virus is maintained in a sylvatic transmission cycle involving small non-human primates, small mammals and mosquitoes, while in Asia CHIKV is predominantly urban, involving a human–mosquito transmission cycle.
The incubation period of CHIKV is normally 2–4 days followed by the onset of an acute febrile illness, characterised by fever, arthralgia, myalgia, headache and rash,42 and accompanying viraemia.39 The joint symptoms of acute CHIKV can involve both large and small joints (often 10 or more joints) and may be accompanied by effusions. Clinically acute CHIKV can be difficult to differentiate from other arboviruses, especially dengue. One small study suggested that a platelet count <100x109/L was the most useful factor in differentiating dengue from CHIKV.47
While acute CHIKV normally lasts for about a week, the disease is notable for the occurrence of long-lasting arthritis that may persist up to 36 months.5,48 Tenosynovitis, carpal tunnel syndrome (secondary to synovial hypertrophy) and new-onset Raynaud's phenomena are also reported in patients with persistent symptoms.49 The arthritis of CHIKV is typically symmetrical and most commonly involves the fingers, wrists, knees and ankles. Chronic joint symptoms have been reported to be relapsing and remitting in 60–80% of patients and unremitting in 20–40%. In patients with chronic joint symptoms they were relapsing and remitting in 60–80% of patients and unremitting in 20–40%. Age, female gender and pre-existing rheumatic disease have been associated with an increased risk of prolonged arthralgia,5,48 and cryoglobulinaemia has been reported in more than 90% of patients with persistent symptoms in one case series.50
Ross River virus
The Ross River virus (RRV) is the most common mosquito-transmitted infection in Australia with around 8,000 cases reported annually.43 RRV was first identified in Queensland in 195951 and is recognised as the cause of a debilitating disease, characterised by headache, fever, rash and joint symptoms. Females are affected slightly more frequently than males and there are seasonal variations in transmission, likely reflecting the impact of rainfall on the mosquito vector.51
The incubation period for RRV is 5–15 days43 followed by an acute-onset febrile illness that is similar to other alphavirus infections. In a prospective study of patients with RRV, knees, wrists and fingers were the joints most frequently affected. On follow-up, symptoms improved significantly over a 3–6 month period. Persistent joint symptoms were frequently associated with the presence of other comorbidities such as depression or underlying rheumatological disease. The extent to which RRV itself causes persistent joint symptoms in the absence of comorbidities remains unclear; it is possible that in patients diagnosed with Ross River disease clinicians may underdiagnose alternative causes of persistent joint symptoms.41
Other alphaviruses
A number of other alphaviruses cause clinically similar syndromes to those seen with CHIKV and Ross River.
Sindbis is one of the most widely distributed of the alphaviruses and is reported in Europe, Africa, Asia and Australia.52 Wild birds are the major reservoir and a range of mosquito species serve as vectors. Seroprevalence surveys in Europe have shown that exposure to sindbis virus is common but the frequency of associated arthritis is unknown.
The O'nyong-nyong virus has been responsible for several epidemics in East Africa. The name means ‘joint breaker’ a description given to it by the Acholi tribe.40 The disease was first described following an epidemic involving almost two million people between 1959–1961. The disease subsequently re-emerged following a major epidemic in Uganda in the 1990s.44,53
Outbreaks of Mayaro virus have been reported across the northern region of South America and the Amazon basin.54 Forest-dwelling mosquitoes are thought to be the major vector, with monkeys serving as the major reservoir of infection.40
Flavirviruses
Dengue is an acute viral infection caused by one of five serotypes of the genus Flavivirus. The infection is transmitted by Aedes mosquitoes and causes an acute illness characterised by fever, rash, myalgia and headache.55 The disease is widely distributed and clinically it can be difficult to distinguish dengue from other arboviral infections such as CHIKV. However unlike CHIKV, while arthralgia is a common feature of dengue, true arthritis and synovitis are rare in dengue.
Other viruses
A number of other viruses have rarely been associated with an acute arthritis, including the herpes viruses, coxsackie viruses, measles, mumps and rubella. Immunisation with the latter three viruses has also been reported to occasionally trigger an acute arthritis.6,56–9
Conclusions
Acute-onset polyarticular arthritis is a common clinical problem facing both the general clinician and the rheumatologist. A wide spectrum of both acute and chronic viral infections can manifest with arthritis emphasising the importance of a thorough history, in particular of travel, when assessing patients presenting with acute arthritis. Our understanding of the epidemiology of virally mediated arthritis continues to evolve and increasing travel abroad is likely to result in more individuals presenting with arthritis secondary to ‘tropical’ viral infections. As well as increasing travel, the geographic distribution of many viruses continues to expand. The recent outbreak in the Caribbean of CHIKV is likely to result in a significant increase in the number of cases of this potentially disabling arthritis being seen in returning travellers. Although viruses cause only a small proportion of all cases of acute arthritis, differentiation of virally mediated arthritis from primary rheumatological disease is important for several reasons. First, unlike immune-mediated rheumatological disease, most virally mediated arthritis is self-limiting and does not require initiation of any specific disease-modifying agents. Conversely, certain viral infections may require initiation of specific antiviral therapy. Finally, the finding of low-titre autoantibodies, such as RF and ANA, in the context of acute viral arthritis has the potential to mislead clinicians when making diagnostic and treatment decisions.
Funding
MM is supported by a Wellcome Trust Clinical Research Fellowship (102807).
References
- 1.Ansemant T, Ornetti P, Garrot J-F, et al. Usefulness of routine hepatitis C and hepatitis B serology in the diagnosis of recent-onset arthritis. Systematic prospective screening in all patients seen by the rheumatologists of a defined area – brief report. Jt Bone Spine Rev Rhum 2012;79:268–70. [DOI] [PubMed] [Google Scholar]
- 2.Zerrak A, Bour JB, Tavernier C, Dougados M, Maillefert JF. Usefulness of routine hepatitis C virus, hepatitis B virus, and parvovirus B19 serology in the diagnosis of recent-onset inflammatory arthritides. Arthritis Rheum 2005;53:477–8. [DOI] [PubMed] [Google Scholar]
- 3.Varache S, Narbonne V, Jousse-Joulin S, et al. Is routine viral screening useful in patients with recent-onset polyarthritis of a duration of at least 6 weeks? Results from a nationwide longitudinal prospective cohort study. Arthritis Care Res 2011;63:1565–70. [DOI] [PubMed] [Google Scholar]
- 4.Kurkela S, Manni T, Myllynen J, Vaheri A, Vapalahti O. Clinical and laboratory manifestations of sindbis virus infection: prospective study, Finland, 2002–2003. J Infect Dis 2005;191:1820–9.15871114 [Google Scholar]
- 5.Moro ML, Grilli E, Corvetta A, et al. Long-term chikungunya infection clinical manifestations after an outbreak in Italy: A prognostic cohort study. J Infect 2012;65:165–72. [DOI] [PubMed] [Google Scholar]
- 6.Tingle AJ, Allen M, Petty RE, Kettyls GD, Chantler JK. Rubella-associated arthritis. I. Comparative study of joint manifestations associated with natural rubella infection and RA 27/3 rubella immunisation. Ann Rheum Dis 1986;45:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tello-Winniczuk N, Díaz-Jouanen E, Díaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. JCR J Clin Rheumatol 2011;17:449–50. [DOI] [PubMed] [Google Scholar]
- 8.Kerr JR, Cunniffe VS, Kelleher P, Coats AJS, Mattey DL. Circulating cytokines and chemokines in acute symptomatic parvovirus B19 infection: negative association between levels of pro-inflammatory cytokines and development of B19-associated arthritis. J Med Virol 2004;74:147–55. [DOI] [PubMed] [Google Scholar]
- 9.Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004;350:586–97. [DOI] [PubMed] [Google Scholar]
- 10.Kerr JR. Pathogenesis of human parvovirus B19 in rheumatic disease. Ann Rheum Dis 2000;59:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vassilopoulos D, Calabrese LH. Virally associated arthritis 2008: clinical, epidemiologic, and pathophysiologic considerations. Arthritis Res Ther 2008;10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corcoran A, Doyle S. Advances in the biology, diagnosis and host–pathogen interactions of parvovirus B19. J Med Microbiol 2004;53:459–75. [DOI] [PubMed] [Google Scholar]
- 13.Moore TL. Parvovirus-associated arthritis. Curr Opin Rheumatol 2000;12:289–94. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa E, Otaguro S, Murata M, et al. Intravenous immunoglobulin therapy for severe arthritis associated with human parvovirus B19 infection. J Infect Chemother 2008;14:377–82. [DOI] [PubMed] [Google Scholar]
- 15.Lavreys L, Thompson ML, Martin HL, et al. Primary human immunodeficiency virus type 1 infection: clinical manifestations among women in Mombasa, Kenya: Clin Infect Dis 2000;30:486–90. [DOI] [PubMed] [Google Scholar]
- 16.Calabrese LH, Kelley DM, Myers A, O'Connell M, Easley K. Rheumatic symptoms and human immunodeficiency virus infection. The influence of clinical and laboratory variables in a longitudinal cohort study. Arthritis Rheum 1991;34:257–63. [DOI] [PubMed] [Google Scholar]
- 17.Scott C, Brand A, Natha M. Reactive arthritis responding to antiretroviral therapy in an HIV-1-infected individual. Int J STD AIDS 2012;23:373–4. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese LH, Kirchner E, Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: emergence of a new syndrome of immune reconstitution and changing patterns of disease. Semin Arthritis Rheum 2005;35:166–74. [DOI] [PubMed] [Google Scholar]
- 19.Phillips C, Reno H, Atkinson JP, Ranganathan P. Human immunodeficiency virus infection presenting as an unusual arthropathy. Arthritis Care Res 2011;63:450–3. [DOI] [PubMed] [Google Scholar]
- 20.Wands JR, Mann E, Alpert E, Isselbacher KJ. The pathogenesis of arthritis associated with acute hepatitis-B surface antigen-positive hepatitis. Complement activation and characterization of circulating immune complexes. J Clin Invest 1975;55:930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pease C, Keat A. Arthritis as the main or only symptom of hepatitis B infection. Postgrad Med J 1985;61:545–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inman RD. Rheumatic manifestations of hepatitis B virus infection. Semin Arthritis Rheum 1982;11:406–20. [DOI] [PubMed] [Google Scholar]
- 23.Mirise RT, Kitridou RC. Arthritis and hepatitis. West J Med 1979;130:12–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Houghton M. The long and winding road leading to the identification of the hepatitis C virus. J Hepatol 2009;51:939–48. [DOI] [PubMed] [Google Scholar]
- 25.Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d'Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l'Hepatite C. Medicine (Baltimore) 2000;79:47–56. [DOI] [PubMed] [Google Scholar]
- 26.Cacoub P, Poynard T, Ghillani P, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum 1999;42:2204–12. [DOI] [PubMed] [Google Scholar]
- 27.Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic manifestations of chronic HCV infection. J Gastrointest Liver Dis JGLD 2007;16:65–73. [PubMed] [Google Scholar]
- 28.Rosner I, Rozenbaum M, Toubi E, et al. The case for hepatitis C arthritis. Semin Arthritis Rheum 2004;33:375–87. [DOI] [PubMed] [Google Scholar]
- 29.Mazzaro C, Zorat F, Caizzi M, et al. Treatment with peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study. J Hepatol 2005;42:632–8. [DOI] [PubMed] [Google Scholar]
- 30.Sayiner ZA, Haque U, Malik MU, Gurakar A. Hepatitis C virus infection and its rheumatologic implications. Gastroenterol Hepatol 2014;10:287–93. [PMC free article] [PubMed] [Google Scholar]
- 31.Antonelli A, Ferri C, Galeazzi M, et al. HCV infection: pathogenesis, clinical manifestations and therapy. Clin Exp Rheumatol 2008;26:S39–47. [PubMed] [Google Scholar]
- 32.Kemmer NM, Sherman KE. Hepatitis C-related arthropathy: Diagnostic and treatment considerations. J Musculoskelet Med 2010;27:351–4. [PMC free article] [PubMed] [Google Scholar]
- 33.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med 2012;367:1237–44. [DOI] [PubMed] [Google Scholar]
- 34.Pischke S, Behrendt P, Manns MP, Wedemeyer H. HEV-associated cryoglobulinaemia and extrahepatic manifestations of hepatitis E. Lancet Infect Dis 2014;14:678–9. [DOI] [PubMed] [Google Scholar]
- 35.Caskey MF, Morgan D, Porto AF, et al. Clinical manifestations associated with HTLV type i infection: a cross-sectional study. AIDS Res Hum Retroviruses 2007;23:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guérin B, Arfi S, Numéric P, et al. Polyarthritis in HTLV-1-infected patients. A review of 17 cases. Rev Rhum Engl Ed 1995;62:21–8. [PubMed] [Google Scholar]
- 37.Umekita K, Umeki K, Miyauchi S, et al. Use of anti-tumor necrosis factor biologics in the treatment of rheumatoid arthritis does not change human T-lymphotropic virus type 1 markers: a case series. Mod Rheumatol 2015;25:794–7. [DOI] [PubMed] [Google Scholar]
- 38.Aoyagi T, Maeda K, Furuichi I, et al. Treatment of patients with polyarthritis and anti-HTLV-I antibodies with interferon-alpha. Ann Rheum Dis 1994;53:80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suhrbier A, Jaffar-Bandjee M-C, Gasque P. Arthritogenic alphaviruses – an overview. Nat Rev Rheumatol 2012;8:420–9. [DOI] [PubMed] [Google Scholar]
- 40.Tesh RB. Arthritides caused by mosquito-borne viruses. Annu Rev Med 1982;33:31–40. [DOI] [PubMed] [Google Scholar]
- 41.Suhrbier A, La Linn M. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr Opin Rheumatol 2004;16:374–9. [DOI] [PubMed] [Google Scholar]
- 42.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet 18;379:662–671. [DOI] [PubMed] [Google Scholar]
- 43.Mylonas AD, Brown AM, Carthew TL, et al. Natural history of Ross River virus-induced epidemic polyarthritis. Med J Aust 2002;177:356–60. [DOI] [PubMed] [Google Scholar]
- 44.Rwaguma EB, Lutwama JJ, Sempala SD, et al. Emergence of epidemic O'nyong-nyong fever in southwestern Uganda, after an absence of 35 years. Emerg Infect Dis 1997;3:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assunção-Miranda I, Bozza MT, Da Poian AT. Pro-inflammatory response resulting from sindbis virus infection of human macrophages: Implications for the pathogenesis of viral arthritis. J Med Virol 2010;82:164–74. [DOI] [PubMed] [Google Scholar]
- 46.Van Bortel W, Dorleans F, Rosine J, et al. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill 2014;19:20759. [DOI] [PubMed] [Google Scholar]
- 47.Lee VJ, Chow A, Zheng X, et al. Simple clinical and laboratory predictors of chikungunya versus dengue infections in adults. PLoS Negl Trop Dis 2012;6:e1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schilte C, Staikovsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis 2013;7:e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parola P, Simon F, Oliver M. Tenosynovitis and vascular disorders associated with Chikungunya virus-related rheumatism. Clin Infect Dis Off Publ Infect Dis Soc Am 2007;45:801–2. [DOI] [PubMed] [Google Scholar]
- 50.Oliver M, Grandadam M, Marimoutou C, et al. Persisting mixed cryoglobulinemia in chikungunya infection. PLoS Negl Trop Dis 2009;3:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu W, Mengersen K, Dale P, et al. Epidemiologic patterns of Ross River virus disease in Queensland, Australia, 2001–2011. Am J Trop Med Hyg 2014;91:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laine M, Luukkainen R, Toivanen A. Sindbis viruses and other alphaviruses as cause of human arthritic disease. J Intern Med 2004;256:457–1. [DOI] [PubMed] [Google Scholar]
- 53.Kiwanuka N, Sanders EJ, Rwaguma EB, et al. O'nyong-nyong fever in South-Central Uganda, 1996–1997: clinical features and validation of a clinical case definition for surveillance purposes. Clin Infect Dis 1999;29:1243–50 [DOI] [PubMed] [Google Scholar]
- 54.Tesh RB, Watts DM, Russell KL, et al. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis 1999;28:67–73. [DOI] [PubMed] [Google Scholar]
- 55.Simmons CP, Farrar JJ, Nguyen van VC, Wills B. Dengue. N Engl J Med 2012;366:1423–32. [DOI] [PubMed] [Google Scholar]
- 56.Nussinovitch M, Harel L, Varsano I. Arthritis after mumps and measles vaccination. Arch Dis Child 1995;72:348–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fontebasso M. Mumps polyarthritis. J R Coll Gen Pract 1985;35:152. [PMC free article] [PubMed] [Google Scholar]
- 58.Di Loreto S, Fabiano C, Nigro G. High prevalence of streptococcal or Epstein-Barr virus infections in children with acute non-septic monoarthritis. New Microbiol 2014;37:81–6. [PubMed] [Google Scholar]
- 59.Contamin C, Brion JP, Bayle F, Morand P, Peo’ch M, Vialtel P. A case of arthritis caused by cytomegalovirus after kidney transplantation. Transpl Infect Dis 2004;6:87–9. [DOI] [PubMed] [Google Scholar]