Abstract
Background
Debate remains over the role of surgical treatment in minimally displaced lateral compression (Young-Burgess, LC, OTA 61-B1/B2) pelvic ring injuries. Lateral compression type 1 (LC1) injuries are defined by an impaction fracture at the sacrum; type 2 (LC2) are defined by a fracture that extends through the posterior iliac wing at the level of the sacroiliac joint. Some believe that operative stabilization of these fractures limits pain and eases mobilization, but to our knowledge there are few controlled studies on the topic.
Questions/purposes
(1) Does operative stabilization of LC1 and LC2 pelvic fractures decrease patients’ narcotic use and lower their visual analog scale pain scores? (2) Does stabilization allow patients to mobilize earlier with physical therapy?
Methods
This retrospective study of LC1 and LC2 fractures evaluated patients treated definitively at one institution from 2007 to 2013. All patients treated surgically, all nonoperative LC2, and all nonoperative LC1 fractures with complete sacral injury were included. In general, LC1 or LC2 fractures with greater than 10 mm of displacement and/or sagittal/axial plane deformity on static radiographs were treated surgically. One hundred fifty-eight patients in the LC1 group (107 [of 697 screened] nonoperative, 51 surgical) and 123 patients in the LC2 group (78 nonoperative, 45 surgical) met inclusion criteria. The surgical and nonoperative groups were matched for fracture type. To account for differences between patients treated surgically and nonoperatively, we used propensity modeling techniques incorporating treatment predictors. Propensity scores demonstrated good overlap and were used as part of multiple variable regression models to account for selection bias between the surgically treated and nonoperative groups. Patient-reported pain scores and narcotic administration were tallied in 24-hour increments during the first 24 hours of hospitalization, at 48 hours after intervention, and in the 24 hours before discharge. Time from intervention to mobilization out of bed was recorded; intervention was defined as the date of definitive surgical intervention or the day the surgeon determined the patient would be treated without surgery.
Results
There was no difference in the narcotics distributed to any of the groups with the exception that the patients with surgically treated LC2 fractures used, on average (mean [95% confidence interval]) 40.2 (−72.9 to −7.6) mg morphine less at the 48-hour mark (p = 0.016). In general, there were no differences between the groups’ pain scores. The surgically treated patients with LC1 fractures mobilized 1.7 (−3.3 to −0.01) days earlier (p = 0.034) than their nonoperative counterparts. There was no difference in the LC2 cohort in terms of time to mobilization between those treated with and without surgery.
Conclusions
There were few differences in pain scores and morphine use between the surgical and nonoperative groups, and the differences observed likely were not clinically important. We found no evidence that surgical stabilization of certain LC1 and LC2 pelvic fractures improves patients’ pain, decreases their narcotic use, and improves time to mobilization. A randomized trial of patients with similar fractures and similar degrees initial displacement would help remove some of the confounders present in this study.
Level of Evidence
Level III, therapeutic study.
Introduction
Lateral compression-type pelvic ring injuries (Young Burgess LC, OTA B1, B2) remain the most common type of pelvic fractures encountered [9]. Lateral compression type 1 (LC1) are defined by an impaction fracture at the sacrum; type 2 (LC2) have a fracture that extends through the posterior iliac wing at the level of the sacroiliac joint [28]. Both patterns will have varying amounts of anterior pubic root/rami fractures. LC1 represents a broad spectrum of injury, including within the same category minimal “buckle” impaction fractures of the anterior sacrum to comminuted sacral fractures that extend to and through the posterior cortex [14]. There is general agreement that fractures displaced more than 1 cm benefit from surgical stabilization, but it is unclear if LC1 and LC2 fractures that are minimally displaced on radiographs obtained shortly after injury benefit from surgical intervention [7, 16, 20].
Surgeons might consider multiple factors in determining whether a fracture of this type would benefit from surgical stabilization. These include the surgeon’s assessment of fracture stability, the patient’s ability to mobilize, and the patient’s pain with the fracture. Fracture stability might be based on initial displacement of static radiographs, displacement on postmobilization radiographs [5], and/or displacement during an examination under anesthesia [24]. CT evaluation of LC fractures has shown that these represent a spectrum of injuries, and it remains unclear which fractures are covertly unstable [14]. Controversy remains regarding indications for surgical treatment of fractures displaced less than 1 cm on static radiographs. Few data correlate radiographic displacement with functional outcome and some exist demonstrating good outcomes with nonoperative treatment; this further clouds the decision process [10, 25]. We are interested in interventions that provide even short-term pain relief however; there is growing evidence linking acuity of acute pain to development of chronic pain and poor functional outcomes in trauma patients [8, 11].
We therefore asked whether if operative stabilization of LC1 and LC2 pelvic fractures impacted patients’ narcotic requirements, visual analog scale (VAS) pain scores, and time to mobilization.
Patients and Methods
This is a retrospectively matched cohort from a prospectively maintained database. Institutional review board approval was obtained.
Our institution’s prospectively collected pelvic trauma database was queried for all pelvic ring injuries treated from January 2007 to August 2013. Fracture classification was determined by the treating attending orthopaedic surgeon. Pain and ability to mobilize are not used in the decision to operate at our institution; initial displacement on static radiographs is our major criterion. As such, there is a large cohort of patients with complete LC1 and LC2 fractures who have been treated without patient pain or difficulty with mobilization being used in the treatment algorithm. During the study period at our institution there was no protocol in place for surgical versus nonoperative treatment so the decision for treatment was at the discretion of the attending orthopaedic trauma surgeon and at times with input from the patient and/or their family. In general, LC1 or LC2 fractures with greater than 10 mm of displacement and/or sagittal/axial plane deformity on static radiographs were treated surgically. Surgically treated patients were treated with a combination of posterior percutaneous fixation with or without anterior fixation. Anterior fixation included external fixation, percutaneous rami screws, and plating.
A total of 1546 patients existed in the pelvic database from 2007 to August 2013. Inclusion criteria for both LC1 and LC2 patterns were similar: treatment was definitively provided at our institution, injury radiographs and CT were reviewed by an attending orthopaedic surgeon at presentation, and all patients were skeletally mature. Combined ring/acetabular fractures were excluded from all groups. One hundred twenty-three LC2 fractures and 748 LC1 fractures were identified. All LC2 fractures (45 surgical, 78 nonoperative) were analyzed. All 51 surgically treated LC1 fractures were included as well. One hundred seven of the 697 nonoperative LC1 fractures were identified to have complete sacral fractures and constituted the nonoperative group analyzed in this article [10]. Polytraumatized patients and those with concomitant low extremity injury were included in both groups to make our results generalizable to the type of patients trauma surgeons treat in clinical practice. The patients were analyzed within their fracture group (surgical versus nonoperative LC1; surgical versus nonoperative LC2).
Both surgical and nonoperative groups were treated by the same physical therapy teams and were mobilized as soon as medically stable. Weightbearing was not standardized because many patients had other lower extremity injuries that impacted their therapy, but in general the patients were made toe-touch weightbearing on the side of their sacral injury. Pain regimens were the same and consisted of intravenous patient-controlled analgesia and oral narcotics administered by nursing staff in response to the patient’s VAS pain score.
Narcotic administration and patient-reported VAS scores were recorded from three specific time points. The narcotic figure represents the sum of all narcotic medications given in a 24-hour period converted to morphine equivalents. The VAS score is the average of all recorded scores in the same 24-hour period. These data were collected from electronic chart documentation of administration by nursing staff. The VAS scores of intubated or obtunded patients were excluded unless nursing documented a score based on their clinical assessment. There is no established minimum clinically important difference established for orthopaedic pelvic trauma, but a value of 2 on the 10-point scale is used in other specialties in orthopaedics [21].
The three time points are identified as the first 24 hours of hospitalization, 48 hours after intervention, and the last 24 hours before discharge. The first 24 hours of hospital stay represents time on the floor/intensive care unit; narcotics given in the resuscitation bay or operating room were not included. This was done to account for patients who arrived to our facility already intubated. The 48-hour time point is the 24-hour period beginning 48 hours after the initiation of treatment (completion of definitive pelvic surgery or decision to begin mobilizing with therapy). The last 24 hours in the hospital is as stated; inpatient rehabilitation values were not included. We were also interested in the pain and morphine requirements trends in the patient groups. The change in pain and morphine use from preoperative to postoperative (or premobilization to postmobilization) and the change in the 48 hours to the last day of hospitalization of each patient were calculated.
The time to mobilization represents the first date after intervention documented in the chart that a physical therapist was able to get a patient out of the hospital bed to a chair. Intervention was defined as the date of definitive surgical intervention or the day the attending surgeon determined the patient would be treated nonoperatively and mobilized with therapy. Using this time scheme, a patient who waited days for their surgery but then mobilized on postoperative day 1 had the same recorded time to mobilization as a nonsurgical patient who got up with physical therapy on the day after their injury. In general, patients were mobilized when they were medically stable to be out of bed, regardless of whether they had surgery or not. Transportation to a chair through a Hoyer or other lift was allowed in our recordkeeping in the case of head-injured or severely traumatized patients. In some cases, patients were mobilized before their definitive pelvic surgery; in this instance, the date recorded is the first postoperative mobilization date.
Statistical Analysis
Propensity score methods were used to account for the observational nature of the study. Propensity score matching is a statistical technique to account for known sources of bias (bias variables) in nonrandomized designs. Propensity scoring estimates the probability of treatment group membership based on all observed covariate data [22]. The propensity model was created incorporating documented risk factors for pain. Age, ethnicity, sex, presence of preinjury depression/anxiety/bipolar diagnosis, presence of preinjury narcotic use (recorded as binary data; amount not quantified), socioeconomic status (as estimated by 2000 census bureau data, calculated from listed zip code [18]), lower extremity Abbreviated Injury Score, and Charlson Comorbidity Index were gathered for each patient [1, 13]. Lower extremity bony injury was further subdivided into “low complexity/pain” and “high complexity/pain” injuries in anticipation of expected prolonged pain acuity. “High pain” injuries were those that required substantial joint reconstruction and have been documented to be at risk for prolonged pain (ie, pilon [19], plateau [3], calcaneus [6]).
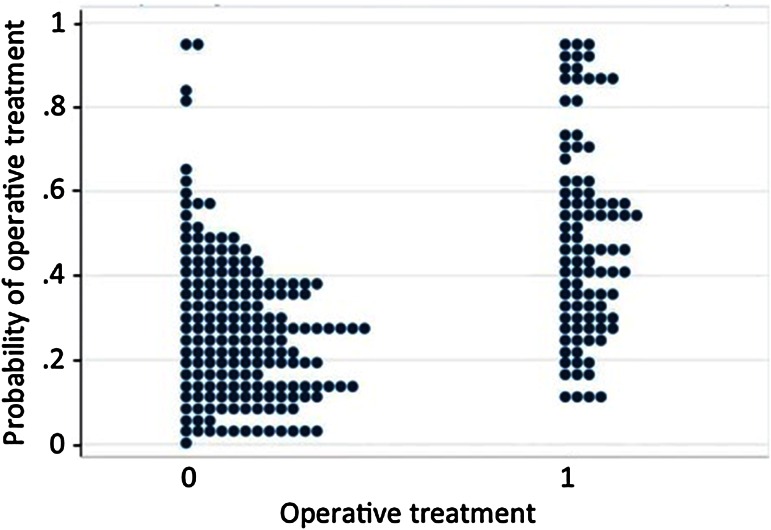
There were no significant differences in the comorbidities or predictors for pain between the groups (Table 1). The propensity model demonstrated moderate overlap (Fig. 1) and yielded McFadden’s R2 values of > 0.23 in all subsets and sensitivity analyses. This graphic representation demonstrates the probability of each patient being randomized to surgical treatment had this been a prospective trial. The more overlap of the data points on the horizon, the more strongly predictive the model.
Table 1.
Patient demographics
| LC1 | |||
|---|---|---|---|
| Demographic | Surgical (n = 51) | Nonoperative (n = 107) | p value* |
| Sex | M:21, F:30 | M:53, F:54 | 0.394 |
| Age (years) (mean ± SD) | 35 ± 13.4 | 41 ± 21 | 0.064 |
| Percent below poverty level | 12 | 10.0 | 0.788 |
| Preinjury narcotics (%) | 9 (17.6%) | 8 (7.5%) | 0.096 |
| Preinjury depression (%) | 10 (19.6%) | 20 (18.7%) | 0.99 |
| Ethnicity | 36 white, 15 nonwhite | 81 white, 26 nonwhite | 0.561 |
| CCI | 1 | 1 | 0.543 |
| LE AIS | 3 | 3 | 0.389 |
| LC2 | |||
|---|---|---|---|
| Demographic | Surgical (n = 45) | Nonoperative (n = 78) | p value* |
| Sex | M:22, F:23 | M:32, F:46 | 0.453 |
| Age (years) (mean ± SD) | 37 ± 15 | 45 ± 28 | 0.787 |
| Percent below poverty level | 9.8 | 9.8 | 0.99 |
| Ethnicity | 30 white, 15 nonwhite | 59 white, 19 nonwhite | 0.302 |
| Preinjury narcotics (%) | 5 (11.1%) | 7 (9%) | 0.757 |
| Preinjury depression (%) | 7 (15.6%) | 11 (14.1%) | 0.798 |
| CCI | 1 | 2 | 0.99 |
| LE AIS | 3 | 3 | 0.668 |
* Differences between means were evaluated using the Student’s t-test; differences in proportions for binary variables were evaluated using Fisher’s exact test; LC = lateral compression; CCI = Charlson Comorbidity Index; LE = lower extremity; AIS = Abbreviated Injury Score; M = male; F = female.
Fig. 1.
Propensity scores for treatment and control groups (0 = nonoperative, 1 = surgical); figure demonstrates moderate overlap of groups, making it a well-matched model.
Results
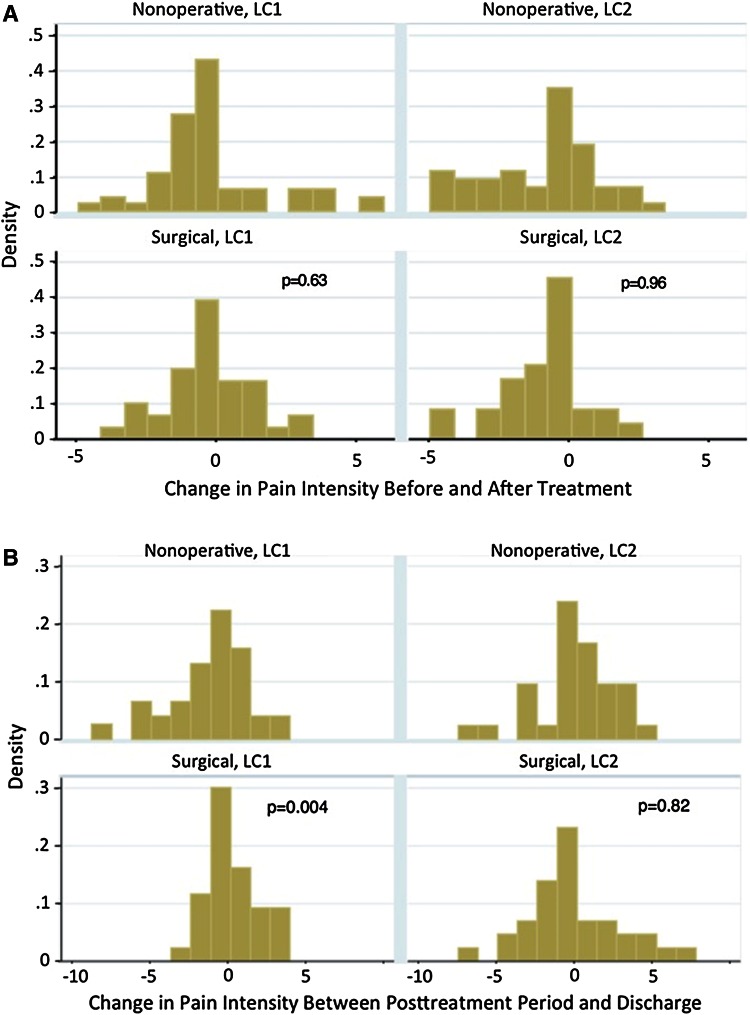
We found few differences in pain scores and narcotic use between the patients treated surgically and those treated nonoperatively, and the observed differences generally were small (Table 2). (mean [95% confidence interval]). The patient-reported pain in the last 24 hours of hospitalization was 1.2 (+0.2 to +2.2) points higher (p = 0.021) in the surgical LC1 group than the nonoperative group. In the LC2 cohort, surgical patients required 40.2 (−72.9 to −7.6) mg less morphine 48 hours after intervention (p = 0.016). When the change in narcotic administration and pain between the time periods was trended, again there was no difference between the LC1 surgically treated and nonoperative groups. The same decrease in morphine at the 48-hour mark was seen in the LC2 group (−31.9 [−65.2 to +1.4] mg, p = 0.06), but then there was a net increase in use between postoperative and time of discharge (+45.7 [+1.1 to 90.3] mg, p = 0.045). These results were graphed to ease interpretation (Fig. 2A–B). We calculated the change in use to account for patients who had preexisting narcotic use before the accident; in the case of chronic users, the absolute values of narcotic use might be erroneously elevated.
Table 2.
Results of surgical treatment effect
| Outcome | LC1 | LC2 | ||||
|---|---|---|---|---|---|---|
| Surgical treatment effect | Surgical treatment effect | |||||
| Mean (SD) | 95% CI | p value | Mean (SD) | 95% CI | p value | |
| Days to mobilization (days) | −1.7 (0.08) | −3.3 to −0.01 | 0.034 | +1.9 (−1.4) | −0.9 to +4.8 | 0.181 |
| Narcotic at 48 hours (mg) | +3.9 (16.1) | −28.1 to +35.9 | 0.811 | −40.2 (16.4) | −72.9 to −7.6 | 0.016 |
| Narcotic in last 24 hours (mg) | −0.49 (8.7) | −17.7 to +16.7 | 0.955 | +3.8 (9.4) | −15.0 to +22.6 | 0.689 |
| Pain at 48 hours | −.15 (0.5) | −1.1 to +0.8 | 0.764 | +0.35 (0.6) | −0.8 to +1.5 | 0.526 |
| Pain in last 24 hours | +1.2 (0.5) | +0.2 to +2.2 | 0.021 | +0.13 (0.6) | −1.1 to +1.3 | 0.837 |
| Change in morphine use from pretreatment to posttreatment | +3.2 (16.2) | −28.9 to +35.3 | 0.843 | −31.9 (16.8) | −65.2 to +1.4 | 0.06 |
| Change in morphine use from posttreatment to discharge | +0.2 (17.8) | −35.1 to +35.5 | 0.99 | +45.7 (22.4) | +1.1 to 90.3 | 0.045 |
| Change in pain from pretreatment to posttreatment | +0.02 (0.4) | −0.8 to +0.8 | 0.96 | +0.32 (0.4) | −0.6 to +1.2 | 0.47 |
| Change in pain from posttreatment to discharge | +1.85 (0.6) | +0.7 to +3.0 | 0.002 | −0.41 (0.7) | −1.8 to +1.0 | 0.56 |
LC = lateral compression; CI = confidence interval.
Fig. 2A–B.
(A) This graph demonstrates the change in pain intensity for the first 24 hours to 48 hours after treatment; the horizontal axis marks change in VAS score and the vertical axis is the percent distribution of the group. (B) This graph demonstrates the change in pain intensity between 48-hour mark and discharge.
In the LC1 groups, surgical stabilization shortened the time to mobilization by 1.7 days (−3.3 to −0.1; p = 0.034). There was no difference in the LC2 groups.
Discussion
Multiple authors cite pain and difficulty with mobilization as an indication for surgical intervention in LC-type pelvic ring fractures, although this has not been thoroughly investigated [15, 26]. Because pain and ability to mobilize are not included in the decision for surgery in our institution, we have the unique ability to have a nonoperative cohort of high-energy pelvic ring injuries. We found few differences between patients with complete but minimally displaced LC type 1 and 2 pelvic ring fractures who were treated without surgery and those with displaced fractures who were treated surgically in terms of their early pain, narcotic use, and time to mobilization. In general, the effect sizes of the differences we did find were small and may not have been clinically important.
There are several limitations of this study. There is a potential for selection bias in the groups with patients in the surgical group having more severe injuries and therefore perhaps greater pain. The decision to operate is almost exclusively made based on displacement of static radiographs; examinations under anesthesia are rarely done. The patients in both surgical groups therefore likely have more severe injuries than their nonoperative counterparts. The surgeons at our institution do not routinely record displacements less than 1 cm, which is why these data were not available for the model. The propensity model we used was meant to account for sources of variation and yielded McFadden’s R2 values of > 0.23 in all subsets and sensitivity analyses. McFadden regarded values in the 0.2 to 0.4 range as “extremely good model fits” [17]. Thus, we believe this was a strong propensity model and (as shown in the article) resulted in significant overlaps in scores between the two treatment groups. Although we would have preferred to have the initial displacement data, we believe the propensity score approach was still appropriate given the reasons stated. Furthermore, we believe it is important to recognize that propensity models seem to be somewhat robust to covariate set choices [2].
Our methodology worked to counter this using propensity modeling (sometimes called “pseudorandomization”), but this is not a randomized trial. This analysis (as is the case with most analyses) included multiple statistical tests, and we did consider adjustment for multiple comparisons. In this case, however, we decided to not use adjustments for three reasons. First, there is substantial controversy around the use of this type of adjustment, primarily centered around the concern that they reduce type I error at the expense of substantially increasing type II error [23]. Second, we felt that the both the outcomes and patient subgroups being examined here had a sound clinical basis, and we were by no means engaging in data mining. Finally, we felt that to the extent possible we have reported p values here rather than relying on arbitrary significance cutoffs and only reporting test results as greater than or less than a specific p value. We feel that this approach allows the readers to make their own choices regarding the true significance of the results. Another limitation in the study is the paucity of time points for data collection. The first 24- and 48-hour marks are very standardized between the groups. The majority of the surgical patients had their definitive surgery within the first few days of their hospitalization, so there is not a substantial time lapse between their 48-hour mark and the nonoperative patients’ 48-hour mark. What was harder to standardize was the last 24 hours. We did not exclude polytrauma patients so the length of stay of some patients was quite long, unrelated to their pelvic injury. We originally intended to follow the pain scores and narcotic requirements into the posthospital time course, but the data were abandoned as a result of inconsistency in followup. It would be interesting to see if the trends remain similar once the patients leave the hospital. Our institution has now moved to a fully electronic outpatient medical record, so prescription tracking will be more reliable in the future.
Although we used lower extremity Abbreviated Injury Score and complexity of lower extremity injury in the propensity model, we did not control for other organ system injury. There are many causes for pain in a polytraumatized patient. Interestingly, Hoffman et al. [12] found polytraumatized patients had lower pain scores at 6 months than their counterparts with isolated pelvic fracture. We anticipated difficulty delineating which effect the pelvic injury has relative to the whole picture, but we designed a pragmatic trial to include polytraumatized patients in our study because it is more reflective of our patient population and is part of our daily clinical decision-making, therefore making our findings more generalizable. A final but important limitation is the timeframe from which our data were gathered. We are unable to comment on the long-term effects of surgical stabilization on pain, healing, or functional outcome because we only analyzed in-hospital data. The purpose of the study is to evaluate the immediate impact of surgery on these patients, which it has, but we cannot extrapolate our findings beyond the time of discharge.
We did not find a substantial difference between the pain scores and narcotics administered to patients with surgical treatment of their LC-type pelvic ring fractures. This contradicts other findings in the literature. A study of 70 pelvic ring injuries of all severity (38 treated surgically and 32 nonoperatively) found a decrease in both VAS and narcotic requirement from pre- to postoperatively, although the VAS and narcotic requirements of the nonoperative group in the same time period were not reported [4]. A prospective but nonrandomized study of patients with LC1 fractures demonstrated a decreased VAS and occasions of narcotic administration at 72 hours postoperatively in the surgical group, but their numbers are quite small [27]. Our data are unique in that we have a large number of patients in each group, we are comparing similar fractures instead of simply the presence of a fracture, and we have reported the pain scores and narcotic quantity (not just frequency of administration) of both the surgical and nonoperative groups in detail. The reliability of our data therefore is high. The narcotic data were drawn from nursing documentation of narcotic administration, a metric closely monitored in our hospital. The physical therapy documentation was also very reliable because one of the criteria for their electronic consult report is the documentation of mobilization to a chair; these data were available in 100% of patients. Despite the limitations mentioned, we believe this study represents a strong step toward investigating the true impact of surgical stabilization of ring fractures on short-term pain.
There was a surgical benefit in the mobilization of LC1 fractures of almost 2 days, but not in the LC2 group. Although setting the mobilization goal to bed to chair is a minimal standard, it allowed us to include multiply injured patients in the analysis. It makes intuitive sense that improving the time to mobilization will help the patient’s overall recovery, pulmonary function, and perhaps other parameters, but that has yet to be shown in this patient population and is beyond the scope of this study.
We did not demonstrate a large difference in the pain scores and narcotic administration between patients with surgically and nonoperatively treated LC1 and LC2 fractures. Surgically treated patients with LC1 fractures mobilized more quickly than their nonoperative counterparts, but the same was not seen for patients with LC2 fractures. The indication to operate at our facility is based on fracture severity and initial displacement, biasing the more displaced fractures into the surgical groups. Because of this bias, we might expect the numbers to be clearly in favor of the nonoperative groups. This was not the case. Perhaps bringing the patients in the surgical group to the same pain and narcotic level as their nonoperative counterparts reflects some benefit. More work needs to be done investigating the theory that surgery helps pain, however, before we can speak with confidence on this topic. A randomized trial of patients with similar fractures and similar degrees of initial displacement would help remove some of the confounders present in this study. The trauma population represents a unique and difficult population to study, because these injuries seldom occur in isolation, but a carefully designed study could help guide our counseling of patients on their treatment.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the University of Maryland, Baltimore, MD, USA.
References
- 1.Agbbe B, Harrison J, Lyons R, Edwards E, Cameron P. Comparison of measure of comorbidity for predicting disability 12-months post-injury. BMC Health Serv Res. 2013;13:1–11. doi: 10.1186/1472-6963-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134:1128–1135. doi: 10.1016/j.jtcvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Barei D, Nork SE, Mills WJ, Coles CP, Henley MB, Benirschke SP. Functional outcomes of severe bicondylar tibial plateau fractures treated with dual incisions and medial and lateral plates. J Bone Joint Surg Am. 2006;88:1713–1721. doi: 10.2106/JBJS.E.00907. [DOI] [PubMed] [Google Scholar]
- 4.Barei D, Shafer B, Beingessner D, Gardner M, Nork S, Routt C. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68:949–953. doi: 10.1097/TA.0b013e3181af69be. [DOI] [PubMed] [Google Scholar]
- 5.Bruce B, Reilly M, Sims S. Predicting future displacement of nonoperatively managed lateral compression sacral fractures: can it be done? J Orthop Trauma. 2011;25:523–528. doi: 10.1097/BOT.0b013e3181f8be33. [DOI] [PubMed] [Google Scholar]
- 6.Buckley R, Tough S, McCormack R, Pate G, Leighton R, Petrie D, Galpin R. Operative compared with non-operative treatment of displaced intra-articular calcaneal fractures. J Bone Joint Surg Am. 2002;84:1733–1744. doi: 10.2106/00004623-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Burgess AR, Eastridge BJ, Young JW, Ellison TS, Ellison PS, Jr, Poka A, Bathon GH, Brumback RJ. Pelvic ring disruptions: effective classification system and treatment protocols. J Trauma. 1990;30:848–856. doi: 10.1097/00005373-199007000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Castillo R, Mackenzie E, Wegener S, Bosse M, the LEAP Study Group Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Dalal SA, Burgess AR, Siegel JH, Young JW, Brumback RJ, Poka A, Dunham CM, Gens D, Bathon H. Pelvic fracture in multiple trauma: classification by mechanism is key to pattern of organ injury, resuscitative requirements, and outcome. J Orthop Trauma. 1989;29:981–1002. [PubMed] [Google Scholar]
- 10.Gaski G, Manson T, Castillo R, Slobogean G, O’Toole R. Nonoperative treatment of lateral compression type 1 pelvic ring injuries with complete sacral fracture. J Orthop Trauma. 2014;28:674–680. doi: 10.1097/BOT.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 11.Harvey-Kelly K, Kanakaris N, Obakponovwe O, West R, Giannoudis P. Quality of life and sexual function after traumatic pelvic fracture. J Orthop Trauma. 2017;28:28–35. doi: 10.1097/BOT.0b013e31828fc063. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman M, Jones C, Sietsema D. Persistent impairment after surgically treated lateral compression pelvic injury. Clin Orthop Relat Res. 2012;470:2161–2172. doi: 10.1007/s11999-012-2247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes A, Williamson O, Hogg M, Arnold C, Prosser A, Clements J, Konstantatos A, O’Donnell M. Predictors of pain severity 3 months after serious injury. Pain Med. 2010;11:990–1000. doi: 10.1111/j.1526-4637.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 14.Khoury A, Kreder H, Skrinskas T, Hardisty M, Tile M, Whyne CM. Lateral compression fracture of the pelvis represents a heterogeneous group of complex 3D patterns of displacement. Injury Int J Care Injured. 2008;39:893–902. doi: 10.1016/j.injury.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Lefaivre K, Padalecki J, Starr A. What constitutes a young burgess lateral compression-I pelvic ring disruption? A description of computed tomography-based fracture anatomy and associated injuries. J Orthop Trauma. 2009;23:16–21. doi: 10.1097/BOT.0b013e31818f8a81. [DOI] [PubMed] [Google Scholar]
- 16.Lindahl J, Hirvensalo E. Outcome of operatively treated type-C injuries of the pelvic ring. Acta Orthop. 2005;76:667–678. doi: 10.1080/17453670510041754. [DOI] [PubMed] [Google Scholar]
- 17.Louviere JJ, Hensher AD, Swait DJ. Stated Choice Methods: Analysis and Applications. New York, NY, USA: Cambridge University Press; 2000. p. 55. [Google Scholar]
- 18.Marcin J, Schembri M, He J, Romano P. A population-based analysis of socioeconomic status and insurance status and their relationship with pediatric trauma hospitalization and mortality rates. Am J Public Health. 2003;93:461–466. doi: 10.2105/AJPH.93.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh JL, Weigel DP, Dirschi DR. Tibial plafond fractures: how do these ankle function over time? J Bone Joint Surg Am. 2003;85:287–295. [PubMed] [Google Scholar]
- 20.Oransky M, Tortora M. Nonunions and malunions after pelvic fractures: why they occur and what can be done? Injury. 2007;38:489–496. doi: 10.1016/j.injury.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Parker SL, Mendenhall SK, Shau DN, Adogwa O, Anderson WN, Devin CJ, McGirt MJ. Minimum clinically important difference in pain, disability, and quality of life after neural decompression and fusion for same-level recurrent lumbar stenosis: understanding clinical versus statistical significance. J Neurosurg Spine. 2012;16:471–478. doi: 10.3171/2012.1.SPINE11842. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 23.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Sagi HC, Coniglione F, Stanford J. Examination under anesthesia for occult pelvic ring instability. J Orthop Trauma. 2011;25:529–537. doi: 10.1097/BOT.0b013e31822b02ae. [DOI] [PubMed] [Google Scholar]
- 25.Sembler Soles G, Lien J, Tornetta P. Nonoperative immediate weightbearing of minimally displaced lateral compression sacral fractures does not result in displacement. J Orthop Trauma. 2012;26:563–567. doi: 10.1097/BOT.0b013e318251217b. [DOI] [PubMed] [Google Scholar]
- 26.Soles G, Lien J, Tornetta P., III Nonoperative immediate weightbearing of minimally displaced lateral compression sacral fractures does not result in displacement. J Orthop Trauma. 2012;26:563–567. doi: 10.1097/BOT.0b013e318251217b. [DOI] [PubMed] [Google Scholar]
- 27.Tosounidis T, Kanakaris N, Nikolaou V, Ta B, Giannoudis P. Assessment of lateral compression type I pelvic ring injuries by intraoperative manipulation: which fracture pattern is unstable? Int Orthop. 2012;36:2553–2558. doi: 10.1007/s00264-012-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young JWR, Burgess AR, Brumback RJ, Poka A. Lateral compression fractures of the pelvis: the importance of plain radiographs in the diagnosis and surgical management. Skeletal Radiol. 1986;15:103–109. doi: 10.1007/BF00350202. [DOI] [PubMed] [Google Scholar]