Abstract
Chronic kidney disease (CKD) is a public health challenge worldwide. As CKD is associated with high rates of morbidity and mortality, identification of novel targets for effective therapy is urgently needed. Yan et al. provide evidence that the Src kinase plays a critical role in the pathogenesis of CKD by integrating multiple fibrogenic signal inputs. Therefore, targeted inhibition of Src kinase may hold promise as a new strategy in the fight against CKD.
CKD is increasingly becoming a public health problem on a global scale. Current therapeutic options for CKD in the clinical setting are scarce and often ineffective, and thus identifying and validating new therapeutic targets is paramount for developing effective strategies for the treatment of patients with CKD. Over the last 2 decades, extensive studies have characterized several key signal pathways and mediators in the pathogenesis of CKD.1,2 Among many factors identified thus far, transforming growth factor-β1 (TGF-β1) and angiotensin II are the most potent fibrogenic mediators. However, pharmacologic inhibition of these factors individually displays only limited therapeutic efficacy. In this context, it is desirable and important to identify a potential signal mediator that may integrate multiple fibrogenic signal inputs. In theory, targeting such a mediator might achieve maximal therapeutic efficacy by simultaneously blocking various fibrogenic signaling.
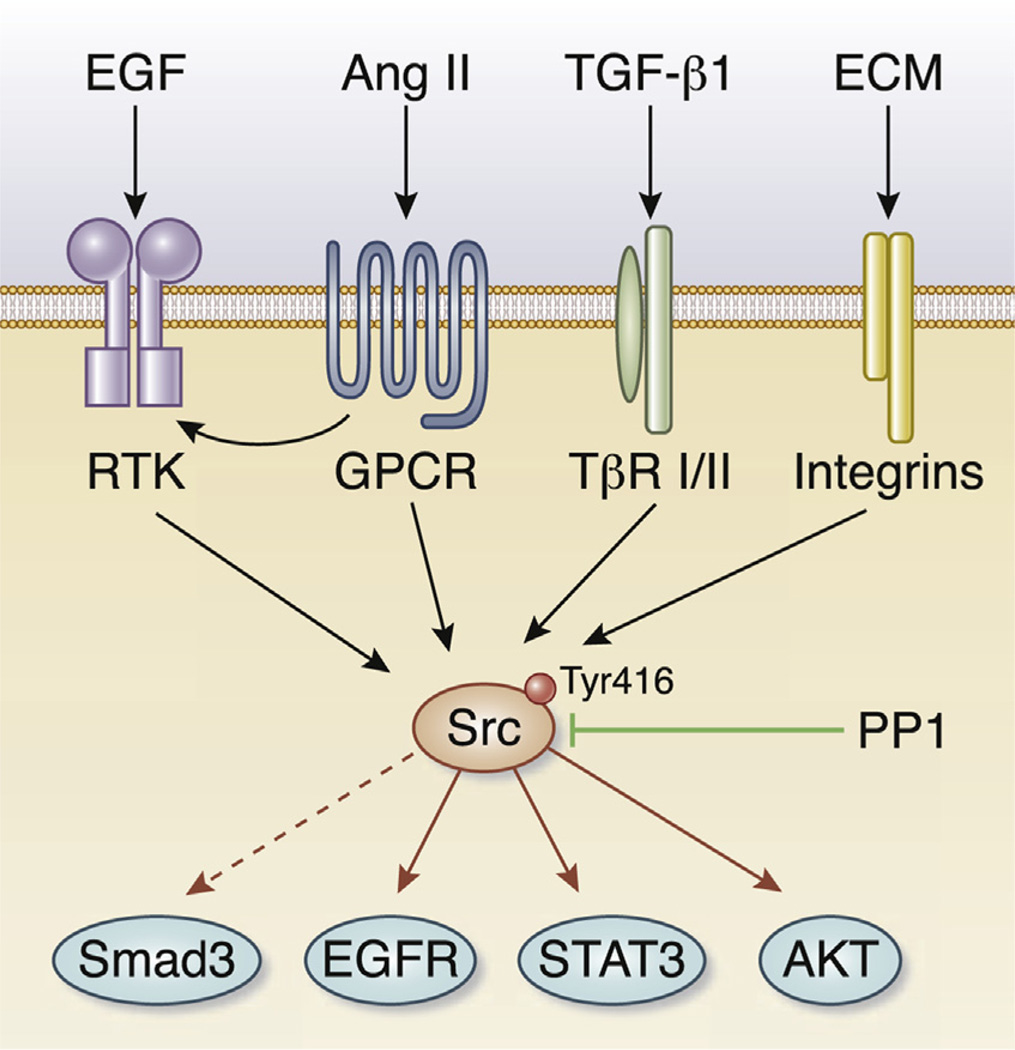
The Src kinase belongs to a family of non-receptor, intracellular protein tyrosine kinases, which includes Yes, Fyn, Lyn, and Lck, among others. Src is expressed ubiquitously in all cell types, whereas the other members of the family are often found in hematopoietic cells. Structurally, Src protein consists of several distinct regions, including Src homology 2 and 3 domains, a tyrosine kinase catalytic domain, and a short regulatory tail. Src can be activated by autophosphorylation at Tyr416, which is induced upon activation of a wide variety of transmembrane receptor proteins that include the receptor tyrosine kinases, G protein–coupled receptors, integrins, and cytokine receptors (Figure 1). When Src is activated, it triggers cascades of intracellular signal transduction by phosphorylating specific tyrosine residues in other substrate proteins such as STAT3 and AKT, and therefore regulates a multitude of biologic activities including cell survival, proliferation, and migration. In many aspects, Src is well positioned inside the cell as a platform to integrate multiple signal pathways in response to a wide array of stimuli, and is able to coordinate well-defined cellular responses (Figure 1).
Figure 1. The Src kinase promotes fibroblast activation and kidney fibrosis by integrating multiple fibrogenic signal inputs.
Upon stimulation by various extracellular cues such as transforming growth factor-β1 (TGF-β1), angiotensin II (Ang II), epidermal growth factor (EGF), or extracellular matrix (ECM), intracellular Src kinase is activated. Src activation leads to phosphorylation of the signaling proteins STAT3, AKT, and plasma membrane epidermal growth factor receptor (EGFR). Activation of Src also promotes TGF-β1–mediated Smad3 activation by multiple mechanisms (dashed line).
Src was originally identified as a protooncogene, and genetic mutations that result in increased activity or its overexpression are frequently found in human tumors from the colon, liver, lung, breast, and pancreas. Growing evidence, however, reveals that Src activation is also implicated in the pathogenesis of chronic, fibrotic disorders in diverse organs. For instance, in experimental models of dermal fibrosis and bleomycin-induced lung fibrosis, Src is activated, and inhibition of its activity is able to attenuate matrix expression and tissue fibrosis in these models.3,4 Src activation is also reported in animal models of diabetic nephropathy, HIV-associated nephropathy, and polycystic kidney disease.5,6 These observations strongly suggest that Src activation could be a common pathologic finding in the fibrotic diseases after chronic injury.
Yan et al.7 (2016) now demonstrate that Src kinase is activated in cultured kidney fibroblasts in response to TGF-β1 or serum and in the fibrotic kidney after unilateral ureteral obstruction.7 Using PP1 compound, a selective small molecule inhibitor of Src kinase, they have shown that pharmacologic inhibition of Src activation blocks myofibroblastic activation of fibroblasts in vitro and ameliorates renal fibrosis in vivo after unilateral ureteral obstruction. Mechanistically, inhibition of Src by PP1 appears to disrupt TGFβ1/Smad3 and epidermal growth factor receptor (EGFR) signaling, 2 key signal pathways that have been implicated in renal fibrogenesis. These studies establish that Src kinase acts as an integrator of multiple fibrogenic signals initiated by activation of diverse membrane receptors, and therefore could be a unique target for therapeutic intervention of fibrotic CKD.
Renal fibrogenesis is a dynamic and complex process in which multiple types of kidney cells and infiltrated inflammatory cells are involved.1,2 However, fibroblast activation and aberrant epithelial cell cycle progression are the most predominant events. As the major matrix-producing cells, activation of fibroblasts, characterized by α-smooth muscle actin expression and matrix production, plays a central role in the excessive production and deposition of extracellular matrix. Concurrently, chronic kidney injury induces growth arrest of tubular epithelial cells at the G2/M phase of the cell cycle, which enables the cells to produce and secrete profibrotic growth factors such as TGF-β1 and connective tissue growth factor.8 The most interesting finding of the study by Yan et al.7 is that Src kinase clearly elicits different, and somewhat opposing, biologic actions in renal interstitial fibroblasts and tubular epithelial cells. While Src activation induces cyclin D and cyclin E expression in fibroblasts and promotes their proliferation, this same signaling appears to inhibit cell proliferation in tubular epithelial cells, resulting in cell cycle arrest at the G2/M phase. Exactly how Src activation promotes fibroblast proliferation but inhibits the cell cycle progression of epithelial cells remains elusive at this stage and deserves further investigation in the future. Nevertheless, these findings underscore that Src kinase, as a single mediator, simultaneously regulates the survival, activation, and proliferation of interstitial fibroblasts and promotes cell cycle arrest at the G2/M phase of tubular epithelial cells. As a result, targeting this mediator would have the potential to precisely mitigate key pathologic events in renal fibrogenesis.
The signal routes and targets of Src activation have been widely studied, particularly in the oncology field. Activated Src can phosphorylate STAT3, AKT, and EGFR, and induces their activation. Indeed, Yan et al.7 demonstrate that inhibition of Src kinase by PP1 blocks the activation of EGFR and STAT3, both of which have been implicated in facilitating kidney fibrosis. Of particular interest, PP1 is able to block Smad3 phosphorylation and activation both in vitro and in vivo, suggesting a potential link of Src kinase to TGF-β1/Smad3 signaling. Therefore, Src inhibition by PP1 precisely targets multiple fibrogenic signaling in the pathogenesis of renal fibrosis (Figure 1).
One should interpret the results of the in vivo studies with caution, because the specificity of small molecule inhibitors in general, and PP1 in particular, is a potential concern. PP1 is characterized as a potent, adenosine triphosphate–competitive, and selective inhibitor of the Src family of protein tyrosine kinases. It is actually more potent in inhibiting other members of the Src family such as Fyn and Lck than Src itself. Therefore, one cannot completely rule out the possibility that other members of the Src family may also play a critical role in mediating the fibrogenic responses of the kidney after injury. Furthermore, there is a report that PP1 can block TGF-β1–mediated cellular activities by directly inhibiting type I and type II TGF-β receptors in a manner unrelated to Src signaling.9 Future studies using genetic approaches such as conditional knockout mice in which Src kinase is deleted in a cell type–specific fashion are warranted to fully address the role of Src in renal fibrogenesis.
The present study by Yan et al.7 also leaves many questions unanswered. For instance, because PP1 is given at the same time as when obstructive injury occurs, after unilateral ureteral obstruction, it remains a question whether inhibition of Src kinase at later time points, such as when kidney injury and fibrosis are already established, is therapeutically effective. Another clinically relevant question is whether the therapy with Src inhibition is truly superior to conventional anti–renin– angiotensin system remedies. A direct comparison of the therapeutic efficacy for CKD between Src inhibition and renin–angiotensin system blockers is needed, at least in the preclinical setting.
There is a growing recognition that dysregulated intracellular signaling plays a critical role in the evolution and progression of CKD. The present study of Yan et al.7 has shown that activation of Src, an intracellular, non-receptor tyrosine kinase that integrates multiple extracellular signal inputs, can trigger fibroblast activation and proliferation and enable tubular cells to acquire profibrotic phenotype by inducing cell cycle arrest, thereby leading to kidney fibrosis. As Src inhibitors are currently in clinical trials for cancer patients, these drugs can be readily translated into clinical care for patients with CKD. Hopefully, more studies will build on this line of investigation and eventually translate into effective remedies for those inflicted with CKD.
Acknowledgments
Work by the authors was supported by National Institutes of Health grants DK064005, DK091239, and DK106049.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 3.Hu M, Che P, Han X, et al. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351:87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skhirtladze C, Distler O, Dees C, et al. Src kinases in systemic sclerosis: central roles in fibroblast activation and in skin fibrosis. Arthritis Rheum. 2008;58:1475–1484. doi: 10.1002/art.23436. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi K, Xia L, Goldberg HJ, et al. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes. 2013;62:3874–3886. doi: 10.2337/db12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamzeh MT, Sridhara R, Alexander LD. Cyclic stretch-induced TGF-beta1 and fibronectin expression is mediated by beta1-integrin through c-Src- and STAT3-dependent pathways in renal epithelial cells. Am J Physiol Renal Physiol. 2015;308:F425–F436. doi: 10.1152/ajprenal.00589.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Y, Ma L, Zhou X, et al. Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int. 2016;89:68–81. doi: 10.1038/ki.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungefroren H, Sebens S, Groth S, et al. The Src family kinase inhibitors PP2 and PP1 block TGF-beta1-mediated cellular responses by direct and differential inhibition of type I and type II TGF-beta receptors. Curr Cancer Drug Targets. 2011;11:524–535. doi: 10.2174/156800911795538075. [DOI] [PubMed] [Google Scholar]