Abstract
Radiation therapy is not only a mainstay in the treatment of many malignancies but also results in collateral obliteration of microvasculature and dermal/subcutaneous fibrosis. Soft tissue reconstruction of hypovascular, irradiated recipient sites through fat grafting remains challenging; however, a coincident improvement in surrounding skin quality has been noted. Cell-assisted lipotransfer (CAL), the enrichment of fat with additional adipose-derived stem cells (ASCs) from the stromal vascular fraction, has been shown to improve fat volume retention, and enhanced outcomes may also be achieved with CAL at irradiated sites. Supplementing fat grafts with additional ASCs may also augment the regenerative effect on radiation-damaged skin. In this study, we demonstrate the ability for CAL to enhance fat graft volume retention when placed beneath the irradiated scalps of immunocompromised mice. Histologic metrics of fat graft survival were also appreciated, with improved structural qualities and vascularity. Finally, rehabilitation of radiation-induced soft tissue changes were also noted, as enhanced amelioration of dermal thickness, collagen content, skin vascularity, and biomechanical measures were all observed with CAL compared to unsupplemented fat grafts. Supplementation of fat grafts with ASCs therefore shows promise for reconstruction of complex soft tissue defects following adjuvant radiotherapy.
Keywords: Cell-assisted lipotransfer, Irradiation, Radiation fibrosis, Fat grafting, Adipose-derived stromal cells, Adipose-derived stem cells
Introduction
Adjuvant radiation therapy is highly effective in reducing local recurrence risk following resection of various tumors [1, 2]. However, detrimental collateral changes frequently occur to the surrounding tissue, with macroscopic discoloration and loss of skin elasticity as well as microscopic obliteration of small vessels and dermal thickening, making reconstruction of irradiated wounds and soft-tissue defects challenging [3–5]. Rehabilitation may be addressed through autologous fat grafting, but transfer of avascular fat to hypovascular postradiation recipient beds has been associated with poor fat graft survival [6, 7]. Nonetheless, clinical fat grafting has been recognized to possess a regenerative effect, with improved skin quality and restoration of vascular networks and reduction in collagen disorganization [6]. Several lines of evidence support the notion that adipose-derived stem/stromal cells (ASCs) found within grafted fat confer much of this capacity. This includes reduction of fibrosis following mesenchymal cell injection and the known ability for ASCs to secrete both angiogenic and antiapoptotic growth factors [8–10]. Furthermore, enrichment of lipoaspirated fat with supplemental ASCs, known as cell-assisted lipotransfer (CAL), has been shown to enhance fat graft retention at healthy recipient sites [11, 12]. In this study, we determined the ability for CAL to promote fat graft survival in irradiated soft tissue and evaluated the capacity for supplemental ASCs to augment the regenerative effects of fat on the overlying fibrotic skin.
Materials and Methods
Animal Irradiation and Fat Grafting
All studies were performed in accordance with Stanford University animal guidelines. A total of 30 Gy external beam radiation was delivered to the scalp of adult 60-day-old male Crl:NU-Foxn1nu immunocompromised mice in six fractionated doses of 5 Gy each over 12 days, followed by 5 weeks of recovery [7]. Lipoaspirate was obtained from two healthy female donors, ages 45 and 49 years. ASCs were harvested from half of the lipoaspirate while the other half was processed for grafting by centrifugation [13, 14]. For CAL, 250,000 supplemental cells were added in a small volume (20 μl) to a larger volume of fat (5 ml). Fat (200 μl), with or without supplemental cells (10,000 cells per graft), was then injected beneath the scalp of age-matched irradiated and non-irradiated mice [7, 14–16]. This well-established mouse model facilitates in vivo study of human grafts with minimal immunological reaction.
Fat Graft Analysis
Fat graft volume retention was measured over 8 weeks using microcomputed tomography (n = 6 animals per group), and images were reconstructed as three-dimensional surfaces through cubic-spline interpolation [15]. Fat grafts were removed after 8 weeks, and eight hematoxylin and eosin (H&E) stained sections from each group were evaluated for graft integrity, cysts/vacuoles, inflammation, and fibrosis by three blinded, independent reviewers [17]. CD31 fluorescent immunohistochemical staining (Ab28364; Abcam, Cambridge, MA, http://www.abcam.com) was performed to assess vascularity and then quantified by ImageJ analysis [16].
Skin Analysis
Scalp skin was harvested prior to and 8 weeks following grafting. Sections were stained with H&E and Masson’s Trichrome to analyze dermal thickness and collagen density/structure. Vascularity was determined with CD31 immunohistochemical staining. Mechanical analysis of harvested skin was performed on a MTS Bionix 200 (MTS Systems, Eden Prairie, MN) with an Interface SM-10 force transducer. Stress-strain curves were generated to calculate Young’s modulus.
Statistical Analysis
Data are presented as means ± SE. Analyses of variance were used for multiple group comparisons, and two-tailed Student’s t tests were used for comparisons between two groups. All analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA). A p value <.05 was considered statistically significant.
Results
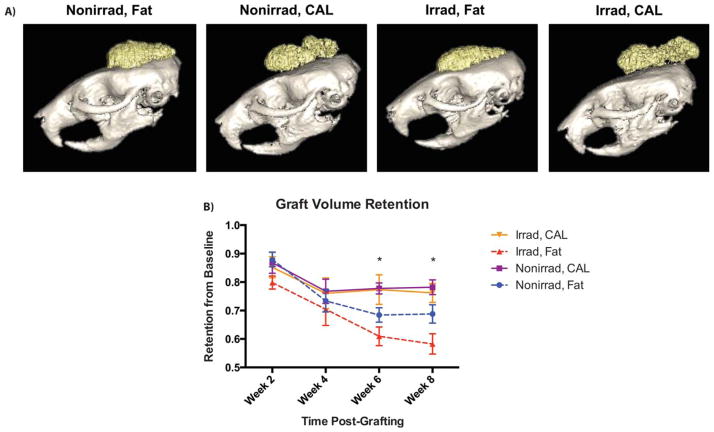
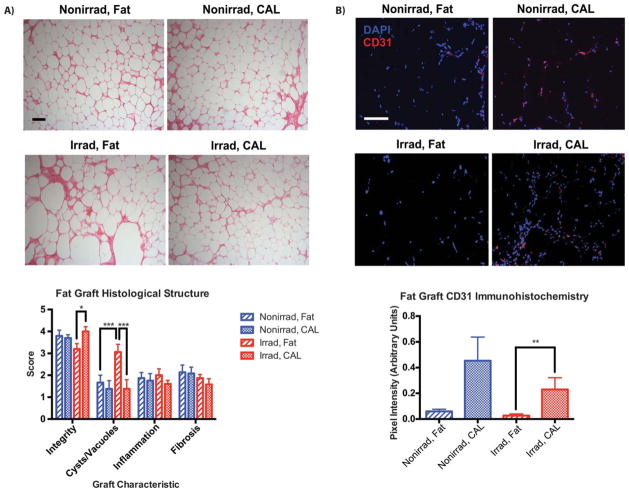
Volumetric analysis of fat grafts demonstrated significantly improved retention with CAL in irradiated scalps (Fig. 1A). Fat graft survival for CAL in irradiated animals (76.2% ± 3.3% at week 8) was similar to non-irradiated animals receiving CAL (78.2% ± 2.6%) (Fig. 1B). Both groups outperformed fat alone in non-irradiated scalps (68.8% ± 3.2%), and the worst volume retention was observed with unsupplemented fat grafts placed at irradiated recipient sites (58.2% ± 3.6%). These findings are congruent with histological analyses which found the worst graft integrity and the greatest presence of cysts/vacuoles when fat alone was grafted into irradiated scalp (Fig. 2A). Both of these measures were significantly improved with CAL in irradiated animals. Increased graft vascularity, as shown by CD31 immunofluorescent staining, may have contributed to these histological findings. Not surprisingly, fat grafts in irradiated scalps had the lowest vascularity, and this was significantly enhanced by CAL (Fig. 2B). The ability for supplemental ASCs to secrete angiogenic cytokines when grafted with fat further support this observation [16].
Figure 1.
Volume retention of grafts over time. (A): Representative reconstructed microcomputed tomography images of fat-only and CAL grafts at week 8 postgrafting. (B): Average graft volume retention (n = 6 animals per group), expressed as a percentage with respect to baseline volume, measured over 8 weeks. *, p <.05. Abbreviation: CAL, cell-assisted lipotransfer.
Figure 2.
Histological and immunohistochemical assessment of graft structure and vascularity at 8 weeks (n = 4 animals per group). (A): Representative H&E staining of sections from fat grafts and CAL grafts with or without prior irradiation of the recipient site, × 10 magnification (top). Scoring of graft architectural characteristics, based on H&E stained sections (bottom). (B): Representative CD31 immunofluorescent staining of fat and CAL grafts, × 20 magnification (top). Quantification of CD31 staining, demonstrating vascularity of grafts (bottom). Scale bar = 100 μm. *, p <.05; **p <.01; ***p <.001. Abbreviations: CAL, cell-assisted lipotransfer; DAPI, 4′,6-diamidino-2-phenylindole.
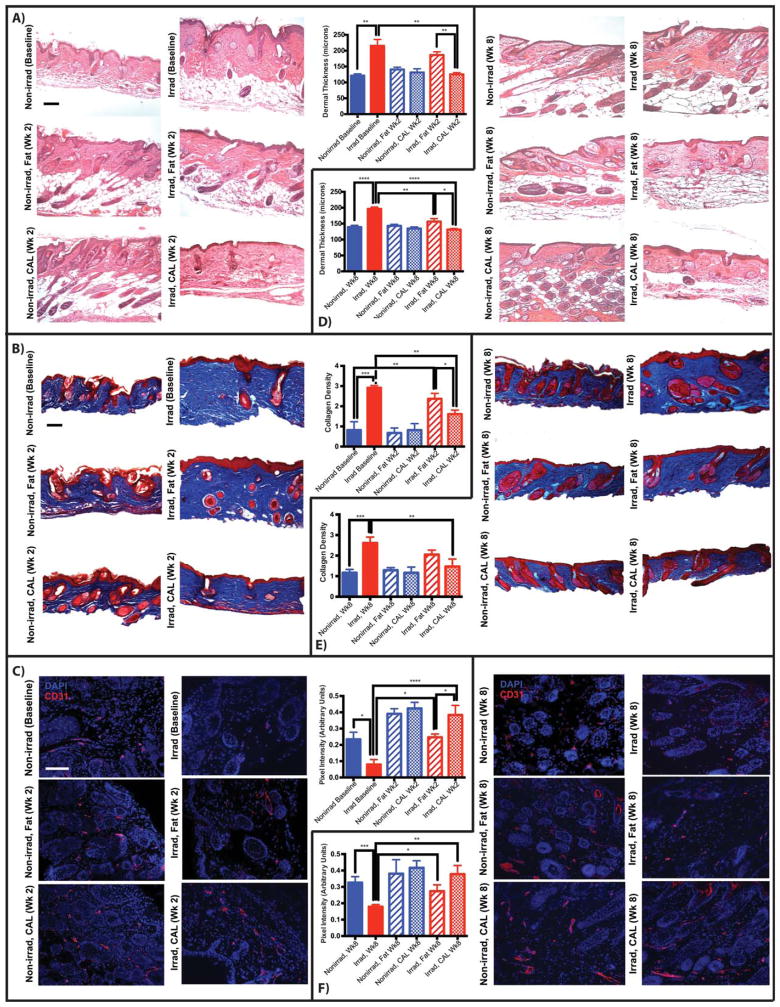
The regenerative effect of fat on the overlying fibrotic skin was also enhanced by CAL. Radiation-induced dermal fibrosis was reflected in a thicker dermis (215 ± 20 μm) and increased collagen content at baseline, and while fat grafts alone attenuated some of this pathologic change (186 ± 10 μm), CAL was found to significantly reduce dermal thickness (125 ± 6 μm) and collagen content, with amounts similar to non-irradiated skin (121 ± 6 μm) 2 weeks after graft placement (Fig. 3A, 3B). Skin hypovascularity following radiation was also partially ameliorated by unsupplemented fat, as previously demonstrated, but was significantly improved with CAL (Fig. 3C) [7]. Parallel observations were made at 8 weeks, with notable improvement in dermal thickness, collagen content, and hypovascularity of irradiated skin overlying fat grafts without ASC supplementation. These observations, however, were still greater with CAL (Fig. 3D–3F). From our results and others in which multiple points were sampled [7], these measurements did not vary significantly across skin overlying graft sites. Studies have shown persistent dysregulation of fibroinflammatory cytokines following radiation exposure, and fat grafting may reverse some of pathologic changes through downregulation of profibrotic factors such as transforming growth factor-beta [7, 18–20]. Further inhibition of Smad3 signaling in concert with potential immunomodulatory effects of supplemental ASCs in CAL may limit dermal collagen production, as observed in other settings [21–24].
Figure 3.
Histological and immunohistochemical assessment of skin (n = 4 animals per group). (A): Representative images (× 10 magnification) and dermal thickness derived from two H&E stained sections from the scalp of each animal, each with three measurements at regular intervals, week 2. (B): Representative sections (× 10 magnification) and quantified collagen density of skin stained with Masson’s Trichrome, week 2. (C): Representative images (× 20 magnification) and quantification of vascular density of sections stained for CD31, week 2. (D): Dermal thickness, week 8. (E): Collagen content, week 8. (F): Vascular density, week 8. Scale bar = 100 μm. *, p <.05; **, p <.01; ***, p <.001; ****, p <.0001. Abbreviation: CAL, cell-assisted lipotransfer.
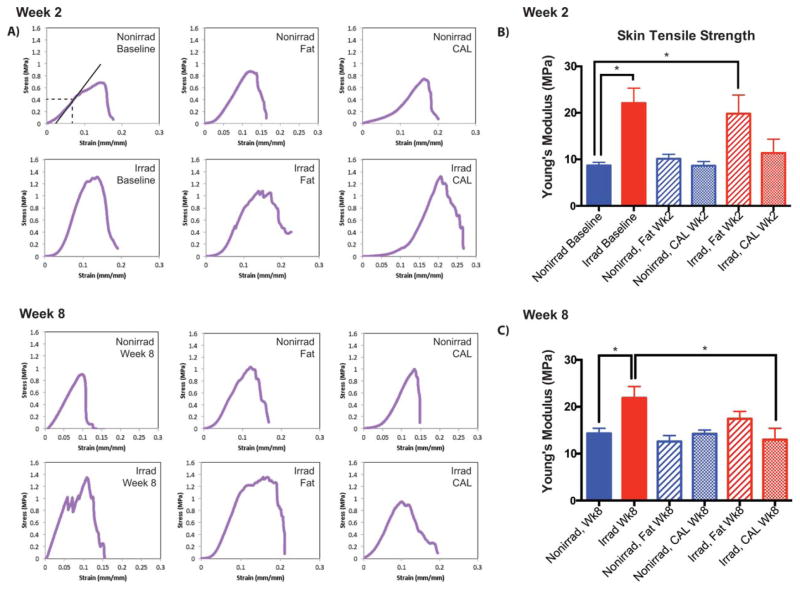
These improved histologic findings in irradiated skin were also reflected in enhanced biomechanical metrics, as demonstrated by measures of material stiffness. Tensile testing generated stress-strain curves to determine the Young’s modulus (Fig. 4A). Stiffness of irradiated skin 2 weeks after grafting decreased with unsupplemented fat, but this was further improved with CAL (Fig. 4B). By 8 weeks, CAL significantly reduced irradiated skin stiffness (13.0 ± 2.4 MPa, from 21.9 ± 2.4 MPa), returning measured levels to non-irradiated skin controls (14.3 ± 1.1 MPa) (Fig. 4C). These findings are congruent with previous studies showing improved elasticity in irradiated wounds injected with ASCs [25]. The advantages of CAL over fat grafting alone thus extend beyond histopathological improvements to provide a functional regenerative benefit, with supplemental ASCs augmenting the ability for fat to reverse many chronic radiation-induced cutaneous changes.
Figure 4.
Tensile testing of skin overlying grafts (n = 4 animals per group). Samples were trimmed to 1.8 cm × 0.5 cm and positioned into the machine with 8 mm gauge length and crosshead speed moving at a constant rate of 5.1 mm/minute along the sample axis. (A): Representative stress-strain curves of samples, demonstrating measurement of Young’s modulus. (B): Young’s modulus of irradiated and non-irradiated skin, and either without grafting, 2 weeks after fat grafting, or after CAL grafting. (C): Young’s modulus, week 8. *, p <.05. Abbreviation: CAL, cell assisted lipotransfer.
Discussion
Radiation therapy results in deleterious soft tissue changes which complicate soft tissue reconstruction. Fat graft outcomes, while worse at irradiated sites, can be significantly improved with ASC supplementation. Furthermore, CAL augments the regenerative effects of fat on the overlying skin, resulting in histolopathologic and functional improvements of radiation-injured skin. Importantly, concern has been raised regarding use of stromal cells in the setting of prior malignancy, and while clinical studies such as RESTORE II have not demonstrated increased rates of cancer recurrence, need for long-term follow-up has thus far limited widespread clinical adoption. Nonetheless, CAL shows promise for reconstruction of soft tissue deficits following adjuvant radiotherapy, functioning both as an autologous filler and as a regenerative therapeutic.
Significance Statement.
Radiation therapy results in deleterious soft tissue changes which complicate soft tissue reconstruction. Fat graft outcomes, while worse at irradiated sites, can be significantly improved with adipose-derived stem cell supplementation, also known as cell-assisted lipotransfer. Furthermore, cell-assisted lipotransfer augments the regenerative effects of fat on the overlying skin, resulting in histolopathologic and functional improvements of radiation-injured skin. Cell-assisted lipotransfer therefore shows promise for reconstruction of soft tissue deficits following adjuvant radiotherapy, functioning both as an autologous filler and as a regenerative therapeutic.
Acknowledgments
We thank the surgeons and medical staff at Plastic Surgery Center Palo Alto for their assistance providing biological samples for these experiments and the Stanford Small Animal Imaging Facility for allowing use of their imaging instruments; Krysta Biniek, Daisy Yuen, and the laboratory of Dr. Reinhold H. Dauskardt for the gracious use of their biomechanical testing equipment and software. M.T.L. was supported by NIH grants U01 HL099776, R01 DE021683-03, R21 DE024230-02, the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Gunn/Olivier Fund. D.C.W. was supported by NIH grant K08 DE024269, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award.
Footnotes
Data in this manuscript have been previously presented at the 65th Annual Meeting of the California Society of Plastic Surgeons in May 2015.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author Contributions
A.L.: study design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; D.D. and A.J.W.: collection and/or assembly of data, data analysis and interpretation, and final approval of manuscript; K.J.P., E.R.Z., E.A.B., D.A.A., and M.S.H.: collection and/or assembly of data and final approval of manuscript; G.K.L. and G.C.G.: financial support, administrative support, and final approval of manuscript; M.T.L.: financial support, administrative support, provision of study material, and final approval of manuscript; D.C.W.: conception and design of study, financial support, administrative support, provision of study material, data analysis and interpretation, manuscript writing, and final approval of manuscript
References
- 1.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 2.Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25:1043–1047. doi: 10.1200/JCO.2006.07.9319. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Kolozsvary AJ, Jenrow KA, et al. Mechanisms of radiation-induced skin injury and implications for future clinical trials. Int J Radiat Biol. 2013;89:311–318. doi: 10.3109/09553002.2013.765055. [DOI] [PubMed] [Google Scholar]
- 4.Archambeau JO, Ines A, Fajardo LF. Response of swine skin microvasculature to acute single exposures of X rays: Quantification of endothelial changes. Radiat Res. 1984;98:37–51. [PubMed] [Google Scholar]
- 5.Zawaski JA, Yates CR, Miller DD, et al. Radiation combined injury models to study the effects of interventions and wound biomechanics. Radiat Res. 2014;182:640–652. doi: 10.1667/RR13751.1. [DOI] [PubMed] [Google Scholar]
- 6.Phulpin B, Gangloff P, Tran N, et al. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast Reconstr Surg. 2009;123:1187–1197. doi: 10.1097/PRS.0b013e31819f2928. [DOI] [PubMed] [Google Scholar]
- 7.Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plast Reconstr Surg. 2014;134:249–257. doi: 10.1097/PRS.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 10.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. discussion 56–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet. 2013;382:1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 13.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PloS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paik KJ, Zielins ER, Atashroo D, et al. Studies in fat grafting: Part V. Cell-assisted lipotransfer to enhance fat graft retention is dose dependent. Plast Reconstr Surg. 2015;136:67–75. doi: 10.1097/PRS.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung MT, Hyun JS, Lo DD, et al. Microcomputed tomography evaluation of human fat grafts in nude mice. Tissue Eng Part C, Methods. 2013;19:227–232. doi: 10.1089/ten.tec.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garza RM, Rennert RC, Paik KJ, et al. Studies in fat grafting: Part IV. Adipose-derived stromal cell gene expression in cell-assisted lipotransfer. Plast Reconstr Surg. 2015;135:1045–1055. doi: 10.1097/PRS.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Kirkham JC, McCormack MC, et al. The effect of pressure and shear on autologous fat grafting. Plast Reconstr Surg. 2013;131:1125–1136. doi: 10.1097/PRS.0b013e3182879f4a. [DOI] [PubMed] [Google Scholar]
- 18.Martin M, Lefaix J, Delanian S. TGF-β1 and radiation fibrosis: A master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 19.Martin M, Lefaix JL, Pinton P, et al. Temporal modulation of TGF-β1 and β-actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res. 1993;134:63–70. [PubMed] [Google Scholar]
- 20.Sultan SM, Stern CS, Allen RJ, Jr, et al. Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg. 2011;128:363–372. doi: 10.1097/PRS.0b013e31821e6e90. [DOI] [PubMed] [Google Scholar]
- 21.Yun IS, Jeon YR, Lee WJ, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: A pilot study. Dermatol Surg. 2012;38:1678–1688. doi: 10.1111/j.1524-4725.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 22.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Pinheiro CH, de Queiroz JC, Guimaraes-Ferreira L, et al. Local injections of adipose-derived mesenchymal stem cells modulate inflammation and increase angiogenesis ameliorating the dystrophic phenotype in dystrophin-deficient skeletal muscle. Stem Cell Rev. 2012;8:363–374. doi: 10.1007/s12015-011-9304-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Iyyanki TS, Branch-Brooks CD, et al. Abstract P39: Adipose-derived stem cell therapy to improve musculofascial healing in the irradiated abdominal wall. Plast Reconstr Surg. 2013;131:183. [Google Scholar]