Abstract
[Purpose] This study systematically reviewed the antalgic effects of non-invasive physical modalities (NIPMs) on central post-stroke pain (CPSP). [Subjects and Methods] Clinical studies were sought on September 2015 in 10 electronic databases, including Medline and Scopus. The searching strings were “central pain and stroke” and “treatment, and physical or non-pharmacological”. The inclusion and exclusion criteria were set for screening the clinical articles by two reviewers. Pain scores on visual analog scale in an article were used as the outcome measure for resulting judgment. The NIPMs intervention summarized from the eligible articles was rated from Levels A to C according to Evidence Classification Scheme for Therapeutic Interventions. [Results] Over 1200 articles were identified in the initial searches and 85 studies were retrieved. Sixteen studies were eligible and judged. Caloric vestibular stimulation (n=3), heterotopic noxious conditioning stimulation (n=1), and transcutaneous electrical stimulation (n=1) were rated below Level C. Transcranial direct current stimulation (TDCS; n=2) and transcranial magnetic stimulation (TMS; n=9) were rated as Level B. [Conclusion] The findings suggest that TMS and TDCS were better than other treatments for CPSP relief but the studies were of insufficient quality.
Key words: Non-invasive physical modality, Central post-stroke pain, Analgesia
INTRODUCTION
Central post-stroke pain (CPSP) is generally referred as pain originating from damage to the central nervous system after stroke. It was first documented as occurring in the limbs after a contra-lateral thalamic stroke1). The pain was then described as a spontaneous pain, and a painful over-reaction to external stimulation resulting from lesions confined to the central nervous system2). Although central pain after stroke was originally described as ‘thalamic pain’, it is currently recognized that strokes involving the sensory tracts in the various brain regions can produce pain similar to central pain3, 4).
Because of a growing elderly population, treatments for CPSP have been gaining more attention5,6,7,8). Pain often has a negative impact on patient’s quality of life9). CPSP is mainly caused by cerebrovascular diseases, which are common among the elderly10). The current mainstream treatment for CPSP control is pharmacology11). However, CPSP is frequently persistent and pharmacological treatments have unpleasant side effects. This drives researchers to search for other techniques to control the pain of CPSP patients.
In recent decades, non-invasive physical modalities (NIPMs) have been extensively utilized in physical therapy12, 13). They induce neuro-modulatory effects for either motor improvement or pain control8, 14, 15). The term, NIPMs, refers to the techniques involving the application of physical modalities across the intact surface of the skin for treatment purposes such as pain relief16, 17). NIPMs are mainly used by physicians, health professionals, or are self-administered by patient. Many NIPMs are safe, inexpensive, easy to operate, and have no profile of toxicity in long term use, e.g. transcutaneous electrical nerve stimulation (TENS)17,18,19,20).
NIPMs have been used for decades, little is known about their effects on the reduction of CPSP. This was our motives for exploring the antalgic effects of NIPMs on CPSP in a systematic review of clinical reports in order to summarize the efficacies of various NIPMs. To date, no study has systematically reviewed the clinical evidences to determine the antalgic effects of NIPMs on CPSP. This review should provide the most up-to-date profile of the effect of NIPMs used to control CPSP.
SUBJECTS AND METHODS
Clinical studies were searched in September 2015 using the following electronic databases: 1) Academic Search Premier (1975–present) ; 2) CINAHL Plus with full text (1937–present); 3) Medline Proquest (1966–present); 4) Medline Ovid SP(1946–present); 5) ProQuest Health and Medical Complete (1986–present); 6) Pubmed (1946–present); 7) Science Direct online (SODL; 1997–present); 8) Scopus (1823–present); 9) The Cochrane Library (1993–present); and 10) Web of Science (1955–present). The initial search was done using the search string, “central pain and stroke”, for potential articles, and a second search was sequentially done using the other searching strings “treatment”, “physical” or “non-pharmacological”. The articles were screened by two independent reviewers. A third reviewer exercised when there were disagreements over the searched articles between the aforementioned two reviewers. The inclusion criteria were studies that had: 1) been published as a full clinical study in English; 2) investigated the antalgic effects of the treatments using the physical modalities after a stroke; 3) recorded outcomes as changes in the pain intensity. Initially, study title and abstract were used to search for potential studies which were further checked to identify the full published reports. The reference lists of retrieved or relevant articles were also screened for reports that were not identified through the electronic searches. Studies and/or comparisons were excluded if a treatment was invasive or a physical modality was not used for treatment purpose.
Information was extracted and trial outcome judgment was made. A statistically significant reduction on a visual analogue scale of pain at any time throughout a study was regarded as an effective outcome. A selected study was classified into one of four classes (Classes I to IV) with declining study quality according to the table of Evidence Classification Scheme for Therapeutic Interventions21). All classified studies with various study quality in each NIPM were accumulated and rated using one of the three recommendation levels (Levels A to C) with declining evidence strength in alphabetic order21).
RESULTS
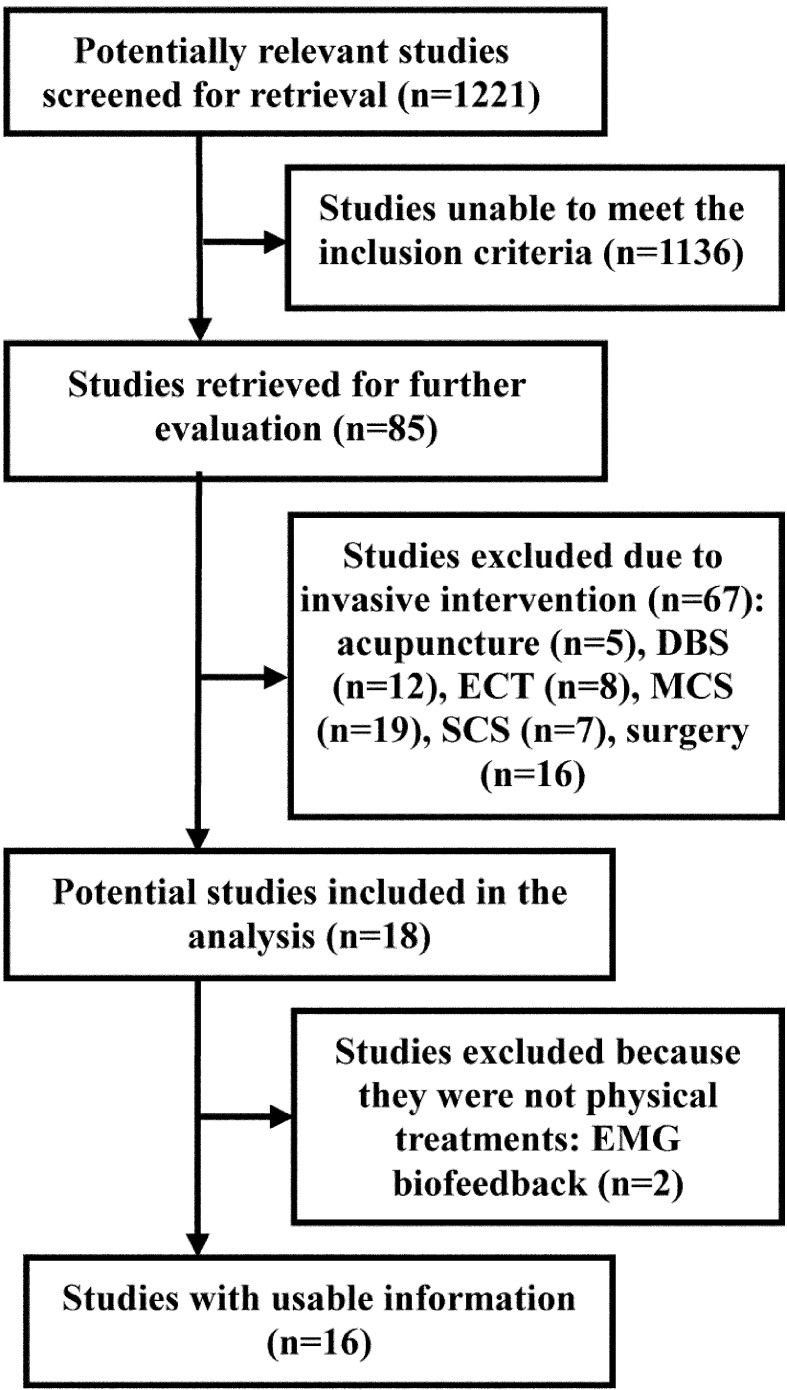
Over 1,200 articles were identified by the first and second searches of the electronic databases (Fig. 1). Among them, 1,136 articles did not to meet the inclusion criteria. Only 85 full-published reports were potentially relevant for retrieval (Fig. 1). Of the 85 reports, 67 were excluded due to invasive interventions including acupuncture (n=5), deep brain stimulation (DBS, n=12), spinal cord stimulation (SCS, n=7), electroconvulsive therapy (ECT, n=8), motor cortex stimulation (MCS, n=19), and surgery (n=16). Of the remaining 18 reports, EMG biofeedback (n=2) were excluded due to the intervention not as a physical treatment.
Fig. 1.
Procedure of search and selection
Only sixteen studies were eligible for the review. Five types of non-invasive modality intervention were found in the review: 1) caloric vestibular stimulation22,23,24) (CVS; n=3), 2) heterotopic noxious conditioning stimulation25) (HNCS; n=1), 3) transcutaneous electrical stimulation26) (TENS; n=1), 4) transcranial direct current stimulation27, 28) (TDCS; n=2), and 5) transcranial magnetic stimulation29,30,31,32,33,34,35,36,37) (TMS, n=9). The CVS studies reported that the mean VAS scores was reduced 2.6 cm by the intervention23), 4.0 cm in the 24 hours following the intervention22), or 4.4 cm after 4 to 7 weeks of the intervention24). The HNCS study reported that the mean VAS score was significantly increased 0.2 cm by the intervention25). The TENS studies reported that three of the fifteen CPSP patients were reduced more than 20% of VAS scores after 23 to 30 months of the intervention26). The TDCS studies reported that the mean VAS score was reduced 3.3 cm by the intervention27) and significantly 1.2 cm after 3 weeks of the intervention28). The TMS studies reported that the mean VAS scores were reduced 1.6 cm by the intervention36), non-significantly 1.0 cm by 8 days of intervention37), and significantly 3.2 cm by 2 weeks of the duration34); and the other TMS studies reported that the VAS scores were significantly reduced 10.8% to 32.6% by the intervention30,31,32,33, 35). However, the most recent study of TMS reported no significant difference between the intervention and sham groups. The effects of CVS, HNCS, and TENS were rated as below Level C. TDCS and TMS were rated as Level B. These studies are summarized in Table 1.
Table 1. Pain relieving effects of NIPMs intervention in CPSP.
| Study | Size* | Age* (y/o) | Duration* (years) | Baseline* (VAS; cm) | Results* (VAS; cm) | Class** | Rating*** | |
|---|---|---|---|---|---|---|---|---|
| I. Caloric vestibular stimulation | ||||||||
| 1. McGeoch et al., 200922) | 1 | 58.0 | 0.25 | 9.0 | −4.0 in 24 hours | IV | Below C | |
| 2. McGeoch et al., 200823) | 9 | 59.9 | 9.2 | 5.8 | −2.6 | III | ||
| 3. Ramachandran et al., 200724) | 2 | 78.0 | 6.0 | 7.8 | −4.4 in 4 to 7 weeks | IV | ||
| II. Heterotopic noxious conditioning stimulation | ||||||||
| 1. Tuveson et al., 200925) | 10 | 60.2 | 5.0 | 6.7 | +0.2 (SD) | III | Below C | |
| III. Transcutaneous electrical nerve stimulation | ||||||||
| 1. Leijon et al., 198926) | 15 | 67.0 | 4.1 | NA | >−20%† in 3/15 patients in 23 to 30 months | III | Below C | |
| IV. Transcranial direct current stimulation | ||||||||
| 1. Morishita et al., 201527) | 1 | 72.0 | 0.75 | 6.0 | −3.3 | IV | B | |
| 1. Bae et al., 201428) | 14 | 51.1 | 12.2 | 4.3 | −1.2 in 3 weeks (SD; Sham: NSD) [RCT] | II | ||
| V. Transcranial magnetic stimulation | ||||||||
| 1. De Oliveira et al., 201429) | 11 | 55.0 | 5.3 | 6.8 | NSD from Sham in 1 to 10 days [RCT] | II | B | |
| 2. Hosomi et al., 201330) | 21 | 59.6 | 4.0 | 7.8 | −32.6%† (SD) | III | B | |
| 3. Ohn et al., 201231) | 22 | 54.9 | 1.8 | 6.6 | −10.8%† (SD) | III | ||
| 4. Goto et al., 200832) | 17 | 63.1 | 5.1 | 8.1 | −24.5%† (SD) | III | ||
| 5.Hirayama et al., 200633) | 12 | 59.4 | 4.7 | NA | −22.8%† (SD) | III | ||
| 6. Khedr et al., 200534) | 24 | 52.3 | 1.5 | 8.6 | −3.2 in 2 weeks (SD; Sham: NSD) [RCr] | III | ||
| 7. Lefaucheur et al., 200435) | 12†† | 54.6 | NA | NA | −20.3%† compared to Sham (SD) [RCr] | III | ||
| 8. Lefaucheur et al., 2001a36) | 6†† | 54.7 | NA | NA | −1.6 (Sham: NSD) [RCr] | III | ||
| 9. Lefaucheur et al., 2001b37) | 7†† | 57.2 | NA | NA | −1.0 in 8 days (NSD; Sham: NSD) [RCr] | III | ||
i) *Data presented as sample mean; **Classification of study with declining evidence strength by number: I>II>III>IV; ***Rating levels. ii) †Percentage from bassline VAS score; ††Thalamic pain. iii) NA: not available; NSD: no significant difference; SD: significant difference; RCr: randomized crossover design; RCT: randomized controlled trial; VAS: visual analogue scale
DISCUSSION
Among the searched studies, TMS and TDCS were rated as Level B. Most of the studies investigated the effect of TMS on CPSP. The 5 Hz TMS reduced the VAS pain score by 22.8%33) and 32.6%30) from the baseline, and the 20 Hz34) did the score by 3.2 cm. In contrast, The 10 Hz TMS reduced the VAS score by 10.8%31) and 24.5%32) from the baseline, or by 1.037) and 1.636) cm of VAS score. These results indicated that 5 Hz or 20 Hz TMS might be helpful for reducing CPSP more than 10 Hz TMS. However, the most recent study used 10 Hz TMS29) and it reported there was no significant difference in pre-post CPSP. The inconsistency of the results among the 10 Hz TMS29, 31, 32, 36, 37) may arise from the location of brain lesions, the areas of TMS, and the baseline measures of cortical activity. Study quality was also an issue as some29, 36, 37) of the studies had small sizes.
TDCS was rated as B level. It reduced VAS scores by 1.2 (n=14)28) and 3.3 (n=1)27) cm at intensities of 2.0–2.5mA applied for 20 minutes. Among the CPSP patients with various brain lesions, TMS was found to be more effective than TDCS in 2 to 3 weeks of intervention [3.234) vs. 1.228) VAS scores]. In contrast, TDCS was found to be more effective than TMS just after the intervention [3.327) vs. 1.636, 37) VAS scores] when the lesion was only in the thalamus. This indicated that damage in various brain areas may influence the antalgic effects due to the introceptive circuits of pain control involved. Further to this evidence, the utilization of TMS and TDCS at different times may be an effective clinical strategy for eliciting an antalgic effect. However, the issue of poor study quality still exists, because of the small sample size (n=1) of the TDCS study27). Clinically, TDCS might be appropriate for home care due to its portability for CPSP patients, and a guideline for remotely-supervised TDCS application in clinical trial has been announced38).
HNCS, TENS and CVS were rated below C level. Poor study quality due to small sample size, various brain lesions, or no randomized control design was an issue with these studies. HNCS26) with a modified submaximal effort tourniquet to induce ischemic discomfort induced a slight increase in CPSP in 15 minutes post-intervention, but the long-term effects were not investigated. TENS27) of ether high (70 Hz) or low frequency (2 Hz) induced reductions in VAS pain scores of more than 20% from baseline in around 20% of the patients with short and long-term effects. Compared to TMS or TDCS, a TENS effect was only seen in some of the patients, but it is suggested for some patients with financial limits, because of its low-cost advantage. CVS with cold water (4° C) irrigated into external auditory canal for vestibular stimulation in one minute, realized VAS pain score reductions in the range of 2.6 to 4.4 cm23,24,25), which observably better than those of TMS, 1.0 to 3.235, 37, 38) cm, and TDCS, 1.2 to 3.328, 29) cm. Although the CVS effects were promising, the temporary discomfort after the CVS might restrict its clinical application due to side-effects of vertigo and nystagmus. Due to low cost and simple application, CVS is still a valuable NIPM intervention for CPSP. However, CVS was only effective when the relay stations of introceptive circuits of pain control, such as the brain stem, were spared from stroke damage. These studies also had quality issues, such as small sample size (n=123), 225) or 924)).
In summary, TMS and TDCS had higher levels of CPSP control efficacy than the other interventions. They showed better reduction of CPSP than TENS and HNCS. Compared to TMS and TDCS, the antalgic effect of low-cost CVS was promising but its clinical application may be restricted by post-application symptoms. TENS is a low-cost intervention, but the strength for its efficacy was poor and it was only effective for some of CPSP patients. The selected NIPMs with central approaches, such as CVS, TMS, or TDCS, might be beneficial for CPSP control. Due to inconsistent results and the relatively low quality of the current evidence for the efficacy of NIPMs, more evidence backed by higher quality studies is needed to establish the effects of NIPMs, especially their long-term effects on CPSP control.
Acknowledgments
This project was funded by Chang Gung University (CMPRD1D0171 and EMRPD1E1711). All of the authors have no potential conflicts of interest. No commercial party has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
REFERENCES
- 1.Greiff: Zur Localisation der Hemichorea. Archiv Fur Psychologie und Nervenkrankheiten, 1883.
- 2.Riddoch G: The clinical features of central pain. Lancet, 1938, 231: 1093–1098. [Google Scholar]
- 3.Hong JH, Choi BY, Chang CH, et al. : The prevalence of central poststroke pain according to the integrity of the spino-thalamo-cortical pathway. Eur Neurol, 2012, 67: 12–17. [DOI] [PubMed] [Google Scholar]
- 4.Lee IH, Kim YN, Son CS, et al. : Clinical aspects of screening test tools for central neuropathic pain in patients with thalamic stroke. J Phys Ther Sci, 2011, 23: 749–752. [Google Scholar]
- 5.Tao W, Fu Y, Hai-Xin S, et al. : The application of sonography in shoulder pain evaluation and injection treatment after stroke: a systematic review. J Phys Ther Sci, 2015, 27: 3007–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim TG, Bae SH, Kim GY, et al. : The effects of extracorporeal shock wave therapy on stroke patients with plantar fasciitis. J Phys Ther Sci, 2015, 27: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva FC, da Silva DF, Mesquita-Ferrari RA, et al. : Correlation between upper limb function and oral health impact in stroke survivors. J Phys Ther Sci, 2015, 27: 2065–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HS, Cha HG: Effect of mirror therapy with tDCS on functional recovery of the upper extremity of stroke patients. J Phys Ther Sci, 2015, 27: 1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiliç Z, Erhan B, Gündüz B, et al. : Central post-stroke pain in stroke patients: incidence and the effect on quality of life. Turk Fiz Tip Rehab Derg, 2015, 61: 142–147. [Google Scholar]
- 10.Harrison RA, Field TS: Post stroke pain: identification, assessment, and therapy. Cerebrovasc Dis, 2015, 39: 190–201. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS: Pharmacological management of central post-stroke pain: a practical guide. CNS Drugs, 2014, 28: 787–797. [DOI] [PubMed] [Google Scholar]
- 12.Lim KO, Lee DY, Shin WS: The effects of a warm whirlpool bath on pain and stiffness of patients with chronic stroke induced knee osteoarthritis. J Phys Ther Sci, 2013, 25: 873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HS, Kim JU: The effect of self-directed exercise using a task board on pain and function in the upper extremities of stroke patients. J Phys Ther Sci, 2013, 25: 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade DC, Borges I, Bravo GL, et al. : Therapeutic time window of noninvasive brain stimulation for pain treatment: inhibition of maladaptive plasticity with early intervention. Expert Rev Med Devices, 2013, 10: 339–352. [DOI] [PubMed] [Google Scholar]
- 15.Bi X, Lv H, Chen BL, et al. : Effects of transcutaneous electrical nerve stimulation on pain in patients with spinal cord injury: a randomized controlled trial. J Phys Ther Sci, 2015, 27: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CC, Huang WB, Chuang YF, et al. : Effects of transcutaneous electrical nerve stimulation (TENS) on experimental blunt pressure pain in healthy participants in randomized controlled trial: pulse frequency and pad size. J Med Biol Eng, 2015, 35: 500–509. [Google Scholar]
- 17.Chen CC, Chuang YF, Yang HC, et al. : Neuromuscular electrical stimulation of the median nerve facilitates low motor cortex excitability in patients with spinocerebellar ataxia. J Electromyogr Kinesiol, 2015, 25: 143–150. [DOI] [PubMed] [Google Scholar]
- 18.Chen CC, Johnson MI: A comparison of transcutaneous electrical nerve stimulation (TENS) at 3 and 80 pulses per second on cold-pressor pain in healthy human participants. Clin Physiol Funct Imaging, 2010, 30: 260–268. [DOI] [PubMed] [Google Scholar]
- 19.Chen CC, Johnson MI: An investigation into the hypoalgesic effects of high- and low-frequency transcutaneous electrical nerve stimulation (TENS) on experimentally-induced blunt pressure pain in healthy human participants. J Pain, 2010, 11: 53–61. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Johnson MI: Differential frequency effects of strong nonpainful transcutaneous electrical nerve stimulation on experimentally induced ischemic pain in healthy human participants. Clin J Pain, 2011, 27: 434–441. [DOI] [PubMed] [Google Scholar]
- 21.Leone MA, Brainin M, Boon P, et al. European Federation of Neurological Societies: Guidance for the preparation of neurological management guidelines by EFNS scientific task forces—revised recommendations 2012. Eur J Neurol, 2013, 20: 410–419. [DOI] [PubMed] [Google Scholar]
- 22.McGeoch PD, Williams LE, Song T, et al. : Post-stroke tactile allodynia and its modulation by vestibular stimulation: a MEG case study. Acta Neurol Scand, 2009, 119: 404–409. [DOI] [PubMed] [Google Scholar]
- 23.McGeoch PD, Williams LE, Lee RR, et al. : Behavioural evidence for vestibular stimulation as a treatment for central post-stroke pain. J Neurol Neurosurg Psychiatry, 2008, 79: 1298–1301. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran VS, McGeoch PD, Williams L, et al. : Rapid relief of thalamic pain syndrome induced by vestibular caloric stimulation. Neurocase, 2007, 13: 185–188. [DOI] [PubMed] [Google Scholar]
- 25.Tuveson B, Leffler AS, Hansson P: Influence of heterotopic noxious conditioning stimulation on spontaneous pain and dynamic mechanical allodynia in central post-stroke pain patients. Pain, 2009, 143: 84–91. [DOI] [PubMed] [Google Scholar]
- 26.Leijon G, Boivie J: Central post-stroke pain—the effect of high and low frequency TENS. Pain, 1989, 38: 187–191. [DOI] [PubMed] [Google Scholar]
- 27.Morishita T, Hyakutake K, Saita K, et al. : Pain reduction associated with improved functional interhemispheric balance following transcranial direct current stimulation for post-stroke central pain: a case study. J Neurol Sci, 2015, 358: 484–485. [DOI] [PubMed] [Google Scholar]
- 28.Bae SH, Kim GD, Kim KY: Analgesic effect of transcranial direct current stimulation on central post-stroke pain. Tohoku J Exp Med, 2014, 234: 189–195. [DOI] [PubMed] [Google Scholar]
- 29.de Oliveira RA, de Andrade DC, Mendonça M, et al. : Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J Pain, 2014, 15: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 30.Hosomi K, Kishima H, Oshino S, et al. : Cortical excitability changes after high-frequency repetitive transcranial magnetic stimulation for central poststroke pain. Pain, 2013, 154: 1352–1357. [DOI] [PubMed] [Google Scholar]
- 31.Ohn SH, Chang WH, Park CH, et al. : Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair, 2012, 26: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto T, Saitoh Y, Hashimoto N, et al. : Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain, 2008, 140: 509–518. [DOI] [PubMed] [Google Scholar]
- 33.Hirayama A, Saitoh Y, Kishima H, et al. : Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain, 2006, 122: 22–27. [DOI] [PubMed] [Google Scholar]
- 34.Khedr EM, Kotb H, Kamel NF, et al. : Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry, 2005, 76: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, et al. : Neuropathic pain controlled for more than a year by monthly sessions of repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin, 2004, 34: 91–95. [DOI] [PubMed] [Google Scholar]
- 36.Lefaucheur JP, Drouot X, Keravel Y, et al. : Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport, 2001, 12: 2963–2965. [DOI] [PubMed] [Google Scholar]
- 37.Lefaucheur JP, Drouot X, Nguyen JP: Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin, 2001, 31: 247–252. [DOI] [PubMed] [Google Scholar]
- 38.Charvet LE, Kasschau M, Datta A, et al. : Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front Syst Neurosci, 2015, 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]