Abstract
Getting lost (GL) is a serious problem for people living with Alzheimer’s disease (PwAD), causing psychological distress in both PwAD and caregivers, and increasing the odds of being institutionalized. It is thus important to identify risk factors for the GL events in PwAD. Between April 2009 and March 2012, we invited 185 community-dwelling PwAD and their caregivers to participate in this study. At the baseline, 95 had experienced GL (Group B); the remaining 90 (Group A) had not. We focused on the incidence of GL events and the associated factors by way of demographic data, cognitive function assessed by the Cognitive Ability Screening Instrument (CASI), and spatial navigation abilities as assessed by the Questionnaire of Everyday Navigational Ability (QuENA). After a 2.5-year period, the incidence of GL in Group A was 33.3% and the recurrence of GL in Group B was 40%. Multiple logistic regression analysis revealed that the inattention item on the QuENA and orientation item on the CASI had independent effects on the GL incidence, while the absence of a safety range was associated with the risk of GL recurrence. During the 2.5 years, the PwAD with GL incidence deteriorated more in the mental manipulation item on the CASI than those without. We suggest that before the occurrence of GL, the caregivers of PwAD should refer to the results of cognitive assessment and navigation ability evaluation to enhance the orientation and attention of the PwAD. Once GL occurs, the caregivers must set a safety range to prevent GL recurrence, especially for younger people.
Introduction
Getting lost (GL) is a serious problem for people living with Alzheimer’s disease (PwAD), causing psychological distress in both PwAD and caregivers, increasing the odds of being institutionalized [1], and sometimes resulting even in fatal consequences [2, 3]. The prevalence of GL in PwAD ranges from 30% to 70% across countries [1, 4–7]. These PwAD may get lost in familiar environments, often when performing daily routine activities [2, 6]. Although the phenomenon is well known and has been widely studied, the predictors for GL are still unclear, making it difficult to arrange appropriate coping strategies.
Previous studies found that PwAD or people with amnesic mild cognitive impairment are more compromised in spatial navigational abilities, such as landmark and scene recognition [8], egocentric/ allocentric orientation [9, 10], and directed attention [11]. Some studies have addressed the risk factors of GL events in such persons, but most are cross-sectional. Kwok et al [4], for example, reported that PwAD with GL events ranked lower on the General Degenerative Scale (GDS). One of our previous studies [12] which examined the relationship between behavioral symptoms of topographical disorientation (TD) and GL events in PwAD revealed that those with a GL history had more severe TD symptoms. Bowen et al [6] conducted a 12-month follow-up study to evaluate the incident GL events among PwAD and described the antecedents and consequences of GL. However, they did not focus on the risk factors or long-term predictors of GL. Only a few longitudinal studies of GL predictors in PwADs [1, 13] have been done. The sample size, however, was small and no attempt was made to differentiate associated factors for incidence and recurrence of GL events. Another study focused on wandering behavior, and did not differentiate GL from wandering. Wandering behavior was defined as aimless or non-goal directed locomotion, including excessive ambulation, eloping behavior, and night-time walking [14–16]. Attention and consciousness may be impaired when the PwAD are wandering, contrast with GL events where PwAD are able to keep a specific goal in mind [17].
Incident and recurrent GL events are quite different. For the former, the PwAD encounter their first ever GL, and before the event their caregivers may be totally unaware that their PwAD are prone to GL, thus no prevention strategies are adopted. Because neither caregiver protection nor change in egression behavior act as confounders, we hypothesized that cognitive impairments and TD symptoms can predict GL incidence. On the contrary, the predictors for GL recurrence in PwAD might be different because of intervention from caregivers as well as restrictions. To this end, we carried out a longitudinal study to ascertain whether any demographic information, cognitive functions or spatial navigation impairments observed by caregivers can predict GL incidence or recurrence in a group of PwAD.
Methods
Participants
The baseline assessment was carried out from 1 April 2009 to 31 October in 2009, and a follow-up interview was done in the period 1 November 2011 to 31 March 2012 (Fig 1). At baseline, 218 community-dwelling PwADs and their caregivers joined the study [12] for the investigation of new GL events. All the PwAD regularly visited the Alzheimer’s Disease Center of a national university and had no ambulatory problems, aphasia, focal cerebral damage, or visual or auditory impairments. In addition, it was required that they had been living in their current residence before the development of AD. The caregiver had been living with the PwAD and had made adequate observation of their daily life. The PwADs were diagnosed by a senior behavioral neurologist according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS- ADRDA) and of the Diagnostic and Statistical Manual of Mental Disorder (Fourth Edition) and underwent neuropsychological assessment.
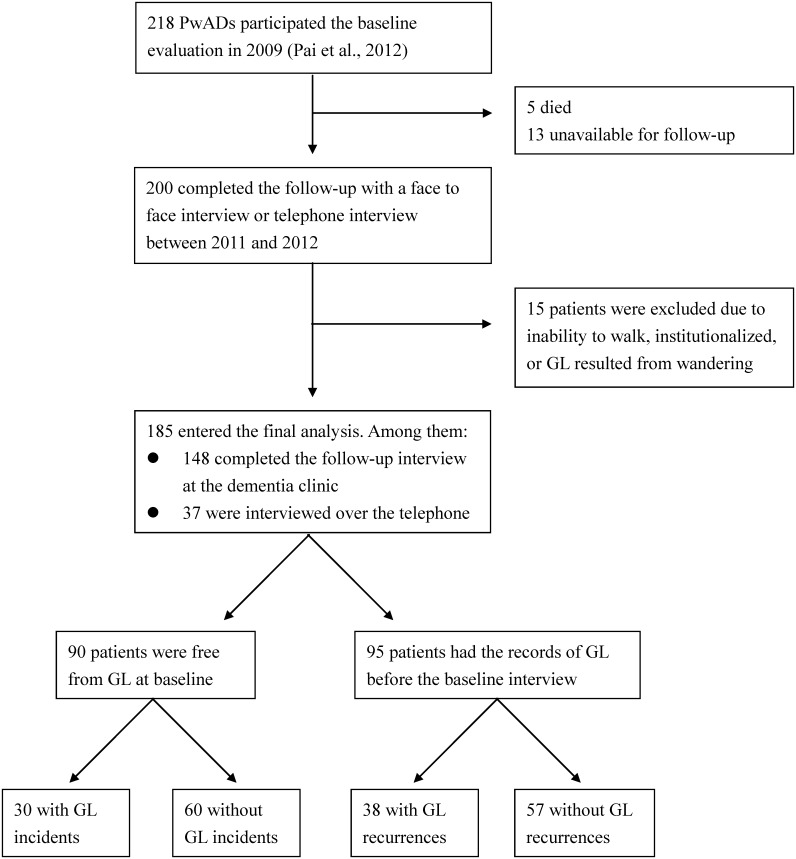
Fig 1. Flow chart of the study participants and Groups.
Abbreviation: AD = Alzheimer’s disease; QuENA = Questionnaire of Everyday Navigational Ability; GL = getting lost.
During the follow-up period, the PwAD with any of the following conditions were excluded: bed ridden or being restricted by caregivers due to physical handicaps, such as weakness, severe degenerative arthritis, or having been admitted to an institution or nursing home.
Standard protocol approvals and informed consents
The study was approved by the Institutional Review Board for the Protection of Human Subjects at the National Cheng Kung University Hospital. All subjects gave their written informed consent to participate.
Definition and assessment of GL
According to the observations of Rowe et al [2], the nature of GL is quite different from that of wandering as noted earlier. In the present study, GL events were assessed by a structural interview and the operational definition of GL was composed as follows: 1) the event occurred in a familiar environment; 2) the PwAD had the sense of “being lost”, or perceived difficulty reaching a goal or returning home; 3) the PwAD were unable to get to their destination without any aid from others; 4) the PwAD had a specific goal or purpose for the excursion and could keep the specific goal in mind along the way; 5) the information was provided by the caregivers because the PwAD may underreport GL events due to memory problems.
Demographic data and egression behavior
We collected information on age, sex, years of education, disease duration, and factors related to quality and quantity of the excursion, including egression frequency, presence or absence of safety range, and transportation restrictions. The egression frequency was recorded as number of days out per week. People who kept themselves in very familiar surroundings and rarely or never visited less familiar places alone were coded as “with safety range”. PwAD were classified according to transportation restrictions into four levels [18]. The people who could still operate a motorbike, scooter or motor vehicle were gauged minimum restriction (R1) and those who could go out by bicycle were defined as mild restriction (R2). People who went out only on foot were R3. People with the highest restriction (R4) were escorted by caregivers whenever they went out and the escort was usually a spouse, son/ daughter (-in-law), or a full time professional caregiver.
Global cognitive function
The cognitive function of the PwAD was evaluated by the Cognitive Ability Screening Instrument (CASI) [19] and Mini-Mental State Examination (MMSE) [20] administrated by an experienced clinical psychologist who was blind to the spatial navigation ability and GL records of the PwAD. The CASI includes 9 subscales: remote memory, recent memory, attention, mental manipulation, orientation, abstract thinking, language, visuospatial construction, and verbal fluency. In order to monitor the deteriorative patterns, the changes of the scores between the baseline and follow-up assessment were calculated. CASI is an appropriate tool to distinguish dementia from a cognitively healthy state. Its sensitivity and specificity were 0.80 to 0.90 from previous studies [19, 21, 22].
Spatial navigational difficulties
The behavioral perspectives of spatial navigation difficulties were evaluated using the Questionnaire of Everyday Navigational Abilities (QuENA) [12], a 10-item scale consisting of 4 factors: landmark and scene agnosia (LSA), egocentric disorientation (ED), heading disorientation (HD), and inattention (INA). Score on the QuENA ranges from 0 to 30, and a higher score reflects more severe TD symptoms. The QuENA has a caregiver’s version (QuENA-C) and patient’s version (QuENA-P). The QuENA-C was used as the main information source reflecting an individual’s true navigation abilities, while the QuENA-P was used to reveal the insight of the PwAD regarding their navigational impairment. The internal consistency, as evaluated by Cronbach’s alpha, is 0.91 and 0.87 for caregiver version and PwAD version respectively, indicating a good internal consistency [12].
Confounding factors
Many factors not mentioned above may influence the occurrence of new GL events. In order to minimize the possibilities, we collected two types of confounding factors. The first type includes the background variables gathered at the baseline interview, including medication, residential years, self-report maze dull, and caregiver’s educational level. The PwADs were mostly being treated with one of the cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and some with NMDA antagonist (memantine) or nicergoline, and some both. The self-report maze dull was provided by the PwAD and the caregivers confirmed whether the PwAD had difficulty traveling in new environments before the onset of AD. The educational levels of the caregivers were recorded because they may have an effect on the awareness and the care strategy for their PwAD.
The second type includes changes which might have developed during the follow-up period, including medication changes, moving, failure to follow-up and change in egression frequency. Medication change was defined as any change in generic drugs, including addition, removal, and alteration. We subtracted number of days out per week at follow-up evaluation from baseline data as the change of excursion frequency. Among the PwAD interviewed at baseline, 22.4% were lost to OPD follow-up. We interviewed these PwAD and their caregivers by telephone and evaluated the effect of their medical adherence on GL.
Statistical analysis
We compared the groups using a t-test with respect to GL incidence and GL recurrence. In order to compare the effect of predictors, we also provided the effect size (Cohen’s d). χ2 was used to compare the proportion among groups. Multiple logistic regression analysis was used to estimate the odds ratio (OR) of the GL predictors, and only significant predictors were chosen for further analysis. We conducted three logistic regression analyses separately using a cluster of independent variables: 1) cognitive functions (each subscales of the CASI), 2) spatial navigation difficulty (the severity of TD that derived from the QuENA), 3) self-awareness of spatial navigation difficulty (discrepancy in the QuENA-P and QuENA-C scores and the presence of safety range). Only the significant predictors revealed in the previous analysis qualified for the "composite analysis" to evaluate the magnitude of the predictive power across each cluster. A supplemental analysis was performed to ensure that the homogeneity of the interviews conducted face-to-face and by telephone. The analyses were performed using SPSS 15.0.
Results
As shown in Fig 1, during the follow-up period, among the 218 baseline participants, 5 died and 13 were unreachable. A total of 200 PwAD completed the follow-up evaluation. Among them, 15 were excluded because of weakness and severe degenerative arthritis resulting in inability to ambulate unaided in 7, admission to nursing home in 11, and GL while wandering in 6. Some PwAD met more than one exclusion criteria (inability to ambulate and admission to nursing home in 6, wandering and admission to nursing home in 3). As a result, 185 PwAD entered the final analysis; 148 of these were interviewed face-to-face, and the other 37 by telephone. The average follow-up duration was 2.5 (SD = 0.19) years. The mean age of the 185 participants was 74.9 (SD = 8.6) years, education was 5.9 (SD = 4.9) years, and 121 (65.4%) were female. Because the education level was relatively low in our subjects, the CASI (mean = 60.3, SD = 19.2) and the MMSE (mean = 17.3, SD = 5.6) total score was slightly lower. No difference was detected between the participants interviewed face-to-face and those by telephone across any variables (S1 Table).
Getting lost events
There were four sub-groups in this study. Group A (without GL events at baseline) and Group B (with GL events at baseline) differed in whether there was a GL event at the baseline. We examined the new GL events during the 2.5 years follow-up duration. In Group A, at follow-up interview, 30 (33.3%) had new GL (INC) and 60 remained free from GL (FFG). In the same way, in Group B, 38 (40%) had new or recurrent GL (REC) and 57 did not (free from recurrent, FFR).
Predictors of GL incidence in Group A
As shown in Table 1, the demographic data and egression behaviors failed to predict GL incidence, and no difference was detected between Group INC and Group FFG. Regarding global cognitive function, compared with Group FFG, Group INC was worse on baseline MMSE (d = .57, p = .009) and on baseline CASI total score (d = .66, p = .003). Among the CASI subscales, Group INC showed more impairment in remote memory (d = .55, p = .027), orientation (d = .82, p < .001), abstract thinking (d = .61, p = .004), and verbal fluency (d = .56, p = .012) compared with Group FFG. Group INC were worse on the QuENA-C total score (d = .91, p < .001) and on subscales landmark/scene agnosia (d = .78, p < .001), egocentric disorientation (d = .69, p = .002), and inattention (d = .98, p < .001). The discrepancy between the QuENA scores from PwAD and from caregivers were larger in Group INC (d = .63, p = .012). As shown in Table 2, after being adjusted for age, sex, and years of education, logistic regression revealed that GL incidence was predicted by the items of remote memory, orientation, abstract thinking, and verbal fluency on the CASI, and landmark agnosia, egocentric disorientation, inattention, and heading disorientation on the QuENA, and self-awareness of navigation impairments.
Table 1. Predictors of getting lost.
| Group A | Group B | |||||
|---|---|---|---|---|---|---|
| INC (30) | FFG(60) | p1 | REC (38) | FFR (57) | p2 | |
| Age, y, mean ± SD | 73.6 ± 9.3 | 75.9 ± 7.8 | .222 | 71.0 ± 9.3 | 77.1 ± 7.7 | .001 |
| Female, n (%) a | 17 (56.7) | 40 (66.7) | .353 | 25 (65.8) | 39 (68.4) | .788 |
| Years of education, mean ± SD | 5.2 ± 5.1 | 5.9 ± 4.9 | .548 | 6.1 ± 5.2 | 6.0 ± 4.6 | .959 |
| Disease duration, mean ± SD | 2.8 ± 2.8 | 2.7 ± 2.9 | .794 | 2.9 ± 2.6 | 3.0 ± 2.7 | .852 |
| Days out per week, mean ± SD | 4.1 ± 2.9 | 4.4 ± 3.0 | .477 | 3.4 ± 3.0 | 2.2 ± 3.0 | .098 |
| With safety range, n (%) a | 14 (46.7) | 34 (56.7) | .370 | 22 (57.9) | 46 (80.7) | .016 |
| Transport restriction, n(%) a | .609 | .039 | ||||
| R4, n (%) | 6 (20.0) | 12 (20.0) | 11 (28.9) | 32 (56.1) | ||
| R3, n (%) | 10 (33.3) | 27 (45.0) | 10 (26.3) | 13 (22.8) | ||
| R2, n (%) | 4 (13.3) | 4 (6.7) | 6 (15.8) | 3 (5.3) | ||
| R1, n (%) | 10 (33.3) | 17 (28.3) | 11 (28.9) | 9 (15.8) | ||
| MMSE, mean ± SD | 15.0 ± 5.8 | 18.0 ± 4.7 | .009 | 18.5 ± 5.7 | 17.0 ± 6.0 | .242 |
| (range) | (6–28) | (4–28) | (6–25) | (6–27) | ||
| CASI, mean ± SD | 51.9 ± 20.5 | 63.9 ± 15.6 | .003 | 63.3 ± 20.3 | 59.0 ± 20.1 | .310 |
| (range) | (12–89) | (15–91) | (10–92) | (12–92) | ||
| CASI sub-scales | ||||||
| Remote memory, mean ± SD | 7.7 ± 2.5 | 8.9 ± 1.8 | .027 | 8.5 ± 2.1 | 8.1 ± 2.3 | .396 |
| Recent memory, mean ± SD | 3.1 ± 3.3 | 4.3 ± 2.8 | .071 | 5.0 ± 3.7 | 4.0 ± 3.4 | .177 |
| Attention, mean ± SD | 6.4 ± 1.8 | 6.6 ± 1.1 | .474 | 6.5 ± 1.6 | 6.6 ± 1.5 | .686 |
| Mental manipulation, mean ± SD | 5.1 ± 3.5 | 6.0 ± 3.2 | .215 | 5.6 ± 3.4 | 5.3 ± 3.1 | .718 |
| Orientation, mean ± SD | 7.1 ± 5.2 | 11.2 ± 4.8 | .000 | 10.9 ± 4.8 | 9.8 ± 6.0 | .350 |
| Abstract thinking, mean ± SD | 4.8 ± 2.1 | 6.0 ± 1.8 | .004 | 5.9 ± 1.9 | 5.5 ± 2.1 | .394 |
| Language, mean ± SD | 7.2 ± 2.9 | 8.0 ± 1.3 | .131 | 8.2 ± 1.8 | 7.6 ± 2.1 | .157 |
| Drawing, mean ± SD | 6.7 ± 3.3 | 7.6 ± 2.9 | .199 | 7.5 ± 3.0 | 7.6 ± 2.8 | .794 |
| Verbal fluency, mean ± SD | 3.8 ± 2.5 | 5.1 ± 2.1 | .012 | 5.3 ± 2.8 | 4.4 ± 2.3 | .098 |
| QuENA-C, mean ± SD | 8.0 ± 5.6 | 3.4 ± 4.4 | .000 | 11.4 ± 7.1 | 11.5 ± 7.8 | .969 |
| LSA, mean ± SD | 2.7 ± 2.2 | 1.2 ± 1.6 | .000 | 3.2 ± 2.4 | 3.6 ± 2.9 | .473 |
| ED, mean ± SD | 1.7 ± 1.6 | 0.7 ± 1.3 | .002 | 2.8 ± 1.8 | 2.6 ± 2.0 | .659 |
| INA, mean ± SD | 1.8 ± 1.9 | 0.4 ± 0.7 | .000 | 2.3 ± 2.0 | 2.3 ± 2.0 | .967 |
| HD, mean ± SD | 1.7 ± 1.6 | 1.1 ± 1.7 | .085 | 3.1 ± 2.6 | 2.9 ± 2.7 | .745 |
| Discrepancy score ± SD | 5.3 ± 6.5 | 1.9 ± 4.1 | .012 | 5.0 ± 7.1 | 6.1 ± 7.9 | .486 |
Abbreviations: Group A = without any GL records at baseline; Group B = with one or more GL events before baseline; INC = with GL incidence; FFG = remaining free from GL; REC = with GL recurrence; FFR = free from GL recurrence; R4 = escorted by caregivers when going out; R3 = can only go out alone on foot; R2 = can still cycle a bike around the area; R1 = can still operate a motorbike or car; QuENA-C = Questionnaire of Everyday Navigational Ability, caregiver version; LSA = landmark and scene agnosia; ED = egocentric disorientation; INA = inattention; HD = heading disorientation; p1 = p value within Group A; p2 = p value within Group B.
a Analyzed by Pearson’s Chi-square and the percentage was within GL events.
Table 2. The predictive power of variables of interest for new GL ocurrence*.
| Risk factors for GL | GL incidence | GL recurrence | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p1 | OR | 95% CI | p2 | |
| Demographic data | ||||||
| Age (per year decrement) | 1.04 | .983~1.09 | .184 | 1.09 | 1.03~1.13 | .002 |
| Male vs. female | 1.95 | .715~5.31 | .192 | 1.42 | .501~4.01 | .511 |
| Years of education (per year increment) | .940 | .850~1.04 | .235 | .979 | .883~1.09 | .694 |
| MMSE (per point increment) a | .862 | .770~.965 | .010 | 1.01 | .926~1.10 | .812 |
| CASI subscales (per point increment) a, | ||||||
| Remote memory | .732 | .576~.930 | .011 | .996 | .795~1.25 | .971 |
| Recent memory | .838 | .707~1.02 | .062 | 1.03 | .900~1.18 | .676 |
| Attention | .883 | .635~1.23 | .462 | .845 | .623~1.15 | .280 |
| Mental manipulation | .881 | .743~1.05 | .147 | .960 | .819~1.13 | .612 |
| Orientation | .824 | .733~.926 | .001 | 1.00 | .920~1.10 | .934 |
| Abstract thinking | .651 | .486~.873 | .004 | .997 | .781~1.27 | .983 |
| Language | .795 | .628~1.06 | .066 | 1.04 | .810~1.33 | .754 |
| Visual construction | .907 | .771~1.07 | .237 | .954 | .804~1.13 | .590 |
| Verbal fluency | .735 | .588~.919 | .007 | 1.05 | .866~1.28 | .609 |
| QuENA-C (per point increment) a | ||||||
| Landmark and scene agnosia | 1.62 | 1.21~2.17 | .001 | .983 | .826~1.17 | .852 |
| Egocentric disorientation | 1.68 | 1.19~2.37 | .003 | 1.13 | .892~1.43 | .312 |
| Inattention | 2.46 | 1.55~3.90 | .000 | 1.02 | .812~1.27 | .891 |
| Heading disorientation | 1.36 | 1.01~1.81 | .040 | 1.04 | .880~1.23 | .636 |
| Self-awareness a | ||||||
| Discrepancy score (per point increment) | 1.15 | 1.05~1.27 | .004 | .981 | .921~1.04 | .543 |
| No safety range vs. with safety range | 1.23 | .447~3.41 | .685 | 3.36 | 1.07~10.53 | .037 |
| Egression behavior a | ||||||
| Days out per week (per day increment) | .920 | .784~1.08 | .307 | 1.18 | .960~1.30 | .152 |
| Restriction of transport vs. R4 | ||||||
| R3 | .405 | .117~1.40 | .154 | 2.29 | .743~7.05 | .194 |
| R2 | .640 | .121~3.40 | .600 | 3.85 | .843~20.14 | .062 |
| R1 | .390 | .101~1.50 | .172 | 1.93 | .470~5.34 | .306 |
Abbreviations: GL = getting lost; OR = odds ratio; QuENA = Questionnaire of Everyday Navigational Ability; R4 = escorted by caregivers when going out; R3 = can only go out alone on foot; R2 = can still cycle a bike around the area; R1 = can still operate a motorbike or car; p1 = p value within GL incidence; p2 = p value within GL recurrence.
a Adjusted for age, sex, and years of education.
*The predictors were entered into each model seperately.
Predictors of GL recurrence (Group B)
Regarding GL recurrence, the predictive variables in Group B were quite different from those in Group A. As shown in Tables 1 and 2, Group REC was younger (d = .71, p = .001) which carried a higher risk to develop recurrent GL. The absence of a safety range increased the risk of GL recurrence markedly as well. Although the transportation restriction had an effect on recurrence, the predictive power was diminished when age, sex, and education were adjusted. Neither general cognitive function nor the navigation impairments can predict GL recurrence.
The independent effect of predictors
To evaluate the magnitude of the power of predictors, we put the significant variables in the previous analysis into a model simultaneously and adjusted for age, sex, and education. As shown in Table 3, the GL incidence can be predicted by orientation subscale in the cognitive cluster (CASI), inattention in the behavioral cluster (QuENA), and the discrepancy score in self-awareness, while only the development of a safety range can predict GL recurrence. Again, we put those predictors into the composite analysis and found that the effect of the discrepancy score in self-awareness was diminished, and the orientation and inattention still had an effect on GL incidence. Meanwhile, the predictive power of the safety range remained robust in the final model regarding GL recurrence.
Table 3. Independent effects of predictors for new GL occurrence*.
| Risk factors for GL | GL incidence | GL recurrence | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p1 | OR | 95% CI | p2 | |
| Cognitive function (per point increment) a | ||||||
| MMSE | 1.17 | .920~1.50 | .196 | 1.01 | .833~1.23 | .908 |
| Remote memory | 1.03 | .700~1.51 | .895 | .968 | .708~1.33 | .836 |
| Orientation | .790 | .650~.961 | .018 | .990 | .851~1.15 | .895 |
| Abstract thinking | .746 | .482~1.15 | .187 | .952 | .668~1.36 | .788 |
| Verbal fluency | .817 | .614~1.09 | .163 | 1.09 | .809~1.46 | .580 |
| Behavioral manifestation (per point increment) a | ||||||
| Landmark and scene agnosia | 1.41 | .788~1.02 | .159 | .873 | .680~1.12 | .288 |
| Egocentric disorientation | 1.50 | .860~2.61 | .153 | 1.29 | .894~1.86 | .147 |
| Inattention | 2.13 | 1.28~3.56 | .004 | .964 | .731~1.27 | .793 |
| Heading disorientation | .700 | .416~1.18 | .181 | 1.01 | .793~1.29 | .937 |
| Self-awareness a | ||||||
| No safety range vs. with safety range | .867 | .297~2.53 | .749 | 3.80 | 1.14~12.66 | .029 |
| Discrepancy score (per point increment) | 1.13 | 1.02~1.24 | .015 | .972 | .909~1.04 | .401 |
| Composite analysis a, b | ||||||
| Orientation | .830 | .714~.964 | .015 | .974 | .875~1.08 | .633 |
| Inattention | 1.85 | 1.08~3.16 | .026 | .983 | .740~1.31 | .907 |
| No safety range | 1.03 | .313~3.39 | .960 | 4.26 | 1.27~14.29 | .019 |
| Discrepancy score | 1.06 | .928~1.22 | .379 | .969 | .899~1.04 | .402 |
Abbreviations: GL = getting lost; OR = odds ratio; p1 = p value within Group A; p2 = p value within Group B.
a Adjusted for age, sex, and years of education.
b Including only previous significant measures.
*Values were determined by putting the predictors into each model (or cluster) simultaneously.
Deteriorative patterns
The mental deterioration was derived by subtracting follow-up scores from baseline scores. 11 were excluded from the analysis because of no second neuropsychological assessment. As shown in Table 4, Group FFR and Group REC deteriorated on the CASI and MMSE scores equally. Similar findings were observed between INC and FFG, while INC deteriorated more in mental manipulation than FFG did (d = .57, p = .031).
Table 4. Deterioration in neuropsychological test*.
| Group A | Group B | |||||
|---|---|---|---|---|---|---|
| INC (27) | FFG (59) | p1 | REC (36) | FFR (52) | p2 | |
| MMSE, mean ± SD | 1.67 ± 4.6 | 1.1 ± 4.0 | .575 | .50 ± 4.6 | .84 ± 3.2 | .681 |
| CASI, mean ± SD | 8.3 ± 16.9 | 5.3 ± 11.2 | .403 | 3.8 ± 14.9 | 4.6 ± 9.8 | .745 |
| CASI sub-scales | ||||||
| Remote memory, mean ± SD | .56 ± 2.1 | .81 ± 2.2 | .590 | .42 ± 2.7 | .31 ± 1.6 | .810 |
| Recent memory, mean ± SD | .89 ± 1.8 | .18 ± 2.2 | .147 | .16± 2.8 | -.34 ± 2.0 | .320 |
| Attention, mean ± SD | .56 ± 1.7 | .34 ± 1.3 | .521 | .53 ± 1.4 | .13 ± .97 | .124 |
| Mental manipulation, mean ± SD | 1.6 ± 2.9 | .15 ± 2.1 | .031 | .58 ± 3.3 | .87 ± 2.5 | .648 |
| Orientation, mean ± SD | 1.4 ± 4.5 | 1.5 ± 4.8 | .941 | .33 ± 3.9 | 1.1 ± 3.0 | .283 |
| Abstract thinking, mean ± SD | .70 ± 2.1 | .46 ± 1.6 | .555 | .06 ± 1.6 | .27 ± 1.3 | .493 |
| Language, mean ± SD | .62 ± 2.2 | .22 ± 1.6 | .339 | .61 ± 2.1 | .25 ± 1.9 | .401 |
| Drawing, mean ± SD | 1.1 ± 3.6 | .86 ± 2.7 | .651 | .69 ± 2.1 | 1.3 ± 2.6 | .226 |
| Verbal fluency, mean ± SD | .77 ± 2.6 | -.18 ± 8.1 | .549 | .67 ± 1.8 | .67 ± 2.1 | .988 |
Abbreviations: Group A = without any GL records at baseline; Group B = with one or more GL events before baseline; INC = with GL incidence; FFG = remaining free from GL; REC = with GL recurrence; FFR = free from GL recurrence; p1 = p value within Group A; p2 = p value within Group B.
*The change in scores was derived by subtracting follow-up scores from baseline scores.
Confounding factors
Of the PwAD recruited in 2009, 11 had been admitted to nursing homes and were excluded from our final analysis. One who shifted residency among his sons was excluded according to the criteria (GL out of wandering). All of the PwAD entering the final analysis had not moved during the follow-up period. No effect of the confounding factors on either GL incidence or recurrence was detected and none of the factors can predict GL incidence and recurrence (see S2–S4 Tables).
Discussion
Incidence and recurrence
Previous studies have demonstrated that spatial navigation impairments in PwADs occur not simply in learning new environments [23–25] but also in tasks using familiar materials. [8, 26] Consequently, PwAD may get lost unexpectedly in familiar surroundings on a routine journey [2, 6]. In fact, GL is among the incipient symptoms in some PwAD, and most of the them had their first GL experience within two years of the clinical onset of AD [5, 17]. Our findings are consistent with these reports. Strikingly, at the 2.5-year follow-up interview, about two-thirds of the participants had had GL events in familiar surroundings. These PwAD usually remained competent in daily excursions and their GL events were not due to wandering.
The predictive values of factors
Incidence
The performance of subscale orientation on the baseline CASI and the presence of inattention on the QuENA are contributory to the risk of GL incidence in Group A. To complete the CASI orientation subscale successfully, participants need multiple cognitive abilities to update the information of their current situation including a consistent and reliable integration of attention, perception, and memory [27]. In PwAD, the impairments of orientation correlate well with hypometabolism in the posterior cingulate cortex (PCC) [28–30] which is known to play an important role in memory-related processes, including recognition of familiar places [31, 32] and retrieval of autobiographical memory [33]. In our previous cross-sectional study of a similar population, no difference in cognitive function was detected between PwADs who had experienced GL and those who had not [12]. Only a longitudinal study like the present one can reveal the effect of specific cognitive functions on the GL incidence. For example, compared with Group FFG, Group INC was worse in cognitive functions including remote memory, orientation, abstract thinking, verbal fluency, but not in recent memory. In addition, the score of mental manipulation of Group INC dropped markedly over the follow-up period compared with that of Group FFG, see Table 4. In spite of the complicated mechanisms contributing to spatial navigation impairment in PwAD, the role of specific cognitive functions was demonstrated in this study. Meanwhile, the inattention addressed in the QuENA reflects the careless mistakes PwAD may make during daily navigation, in particular when they are in less familiar or novel environments. When inattention and disorientation happens to a PwAD, he or she may fail to update and integrate the ongoing navigational events. Consequently, the individual may encounter difficulty getting back on the correct path and a GL outcome may ensue. Located in southern Taiwan, Tainan was the capital of Taiwan 350 years ago. Although Tainan has been modernized over the past decades, the streets and buildings in downtown Tainan are still highly complex and dense. Therefore, for successful navigation in downtown Tainan extensive decision making is required to negotiate the numerous intersections, which is a challenge to those PwAD with impaired executive functions [34] and the risk of GL rises.
Recurrence
As mentioned earlier, Group A and Group B differed in whether there was a GL event at the baseline. Before the first ever GL event, the PwAD and caregivers probably had no awareness of, or underestimated the risk of GL. Once GL occurred, however, not all PwAD and their caregivers would take action to prevent GL recurrence [17].
The factors relevant to GL recurrence are quite different from those relevant to GL incidence. The neuropsychological and behavioral variables failed to predict any GL recurrence in PwAD. On the contrary, a younger age was the most reliable predictor of GL recurrence while the development of a safety range was a protector. A safety range indicates that the PwAD restricts himself/ herself to a very familiar territory and refuses to go to less familiar places. However, the reasons leading to the establishment of a safety range remain unknown in the present study. In the process of interviewing, some PwAD claimed that the safety range resulted from "external" reasons, such as older age, poor physical condition, fear of traffic accidents, or restrictions imposed by the caregiver. Future studies might focus on the reasons leading to the establishment of a safety range. On the other hand, the insight of TD symptoms (the discrepancy score between QuENA-P and QuENA-C) which played an important role in the prevention of GL incidence was an "internal" cue serving to warn PwAD against GL. In other words, for those PwAD without GL experience, raising awareness of TD may decrease the risk of GL, whereas for those with a history of GL, the establishment of a safety range may be more effective in preventing GL recurrence.
As for the restrictions set by the caregivers, most caregivers acknowledged the GL risk and would do their best to accompany their PwAD in outdoor daily activities. In the present study, however, even when the PwAD were escorted by caregivers outside (R4), the risk of new GL occurrence was not entirely eliminated. Some PwAD had a strong desire to go out alone, especially the younger ones, while the caregivers could not provide 24-hour monitoring. Moreover, the caregivers might consider the PwAD’s familiar environments safe, but in fact GL events can still occur even in familiar environments as AD progresses [8, 16]. As a result, the caregivers should examine the wayfinding behavior of the PwAD even in their familiar territories. When a person with AD experiences one or more GL events and his/ her age is relatively young, the caregivers should provide some GL coping strategies for their PwAD, such as the use of GPS devices, to minimize the resulting inconvenience.
Interestingly, about one-third of the participants remained free of GL 5 years after their clinical onset. These participants, or Group FFG, performed better in global cognitive functions compared with those already having experienced GL within the 2.5-year follow-up. They also showed a better spatial navigation abilities (p < 0.001, df = 3) and better self-appraisal of TD symptoms (p = 0.005, df = 3) than the other groups. Likewise, Group B had their first GL event earlier than Group A in the clinical course of AD which indicates that GL was among the incipient symptoms for Group B but not for Group A. Whether these differences resulted from the progression of AD, PwAD's premorbid ability, or the effect of the subtype variation of clinical AD [35, 36] is unknown. Previous studies proposed the existence of subtypes under the diagnosis of AD according to the heterogeneity of neuropathology or clinical manifestation [35, 37, 38]. Although the number of subtypes may differ from one study to another because of methodological issues, the primary classifications are typical and atypical AD; the former is characterized by anterograde episodic memory impairment and the latter may include other cognitive deficits such as impairment in abstract reasoning, and verbal fluency [39]. The neuropathology of typical AD is manifested in greater damage to the hippocampi and related structures, while the atypical type shows is more damaged to the extra-hippocampal areas such as the inferior parietal cortex, middle frontal cortex, posterior cingulate cortex, and retrosplenial cortex [37, 40, 41]. The retrosplenial cortex, in particular, is related to heading orientation or the translation between different spatial representations which is very important in spatial navigation [42].
This study is of value due to its longitudinal observation which can help identify predictors of GL incidence and recurrence in PwAD. Even so, this study has some limitations. The cognitive tests used in the study, such as the Route Map Recall Test published elsewhere [43], were not specific to navigation ability. However, since the global cognitive test can be routinely administrated, it might be of help for early detection of GL risk. Secondly, some of the informants were not the same at baseline and follow-up interviews. Nevertheless, the new informants at the follow-up interview usually confirmed the history of previous GL occurrence of their PwAD given by the prior informants at the baseline. Finally, some GL events may be ignored by caregivers resulting in an underestimate of the incidence or recurrence of GL events. Moreover, we took only one assessment at the follow-up period which may raise issues of reliability. Thus the reporting of GL events should be interpreted coutiously. Future studies may use wearable devices such as GPS rings to record the actual GL events and give a more precise description of GL behavior, such as the distance away from familiar routes or details of ineffective wayfinding attempts.
In summary, GL incidence in PwADs can be predicted by neuropsychological and behavioral factors while the development of a safety range can help prevent GL recurrence. Caregivers are strongly urged to provide structured and friendly environments to enhance the orientation and attention of their PwAD, to remind them of the dangers of going out alone, and to evaluate their navigation ability carefully.
This paper was presented at Alzheimer's Association International Conference on July 13–18, 2013 in Boston, United States. No company provided support of any kind for this study. Dr. Pai reports having served as a consultant and/or having received lecture fees from Janssen, Lilly, Novartis, and Eisai. This does not alter our adherence to PLOS ONE policies on sharing data and materials. Mr. Lee has no financial conflicts of interest.
Supporting Information
Abbreviations: GL = getting lost; QuENA = Questionnaire of Everyday Navigational Ability; LSA = landmark and scene agnosia; ED = egocentric disorientation; INA = inattention; HD = heading disorientation. a Analyzed by Pearson’s Chi-square and the percentages were within GL events.
(DOC)
Abbreviation: GL = getting lost; p1 = p value within Group A; p2 = p value within Group B. a Analyzed by Pearson’s Chi-square, and the percentages were within GL events.
(DOC)
Abbreviations: GL = getting lost; PwAD = people with Alzheimer's disease; Group A = without any GL records at baseline; Group B = with one or more GL events before baseline; INC = with GL incidence; FFG = remaining free from GL; REC = with GL recurrence; FFR = free from GL recurrence; p1 = p value within Group A; p2 = p value within Group B. a Analyzed by Pearson’s Chi-square, and the percentages were within GL events. *The changes were derived by subtracting follow-up scores from baseline scores.
(DOC)
Abbreviations: GL = getting lost; OR = odds ratio; p1 = p value within Group A; p2 = p value within Group B. a Adjusted for age, sex, and years of education.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.McShane R, Gedling K, Keene J, Fairburn C, Jacoby R, Hope T. Getting lost in dementia: a longitudinal study of a behavioral symptom. Int Psychogeriatr. 1998;10(03):253–60. [DOI] [PubMed] [Google Scholar]
- 2.Rowe MA, Vandeveer SS, Greenblum CA, List CN, Fernandez RM, Mixson NE, et al. Persons with dementia missing in the community: Is it wandering or something unique? BMC Geriatrics. 2011;11(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt LA, Brown AE, Gilman IP. Drivers with dementia and outcomes of becoming lost while driving. Am J Occup Ther. 2010;64(2):225–32. [DOI] [PubMed] [Google Scholar]
- 4.Kwok TCY, Yuen KSL, Ho FKY, Chan WM. Getting lost in the community: a phone survey on the community‐dwelling demented people in Hong Kong. Int J Geriatr Psychiatry. 2010;25(4):427–32. 10.1002/gps.2361 [DOI] [PubMed] [Google Scholar]
- 5.Pai MC, Jacobs WJ. Topographical disorientation in community-residing patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19(3):250–5. [DOI] [PubMed] [Google Scholar]
- 6.Bowen ME, McKenzie B, Steis M, Rowe M. Prevalence of and antecedents to dementia-related missing incidents in the community. Dement Geriatr Cogn Disord. 2011;31(6):406–12. 10.1159/000329792 [DOI] [PubMed] [Google Scholar]
- 7.Walker AE, Livingston G, Cooper CA, Katona CLE, Kitchen GL. Caregivers’ experience of risk in dementia: The LASER-AD Study. Aging Ment Health. 2006;10(5):532–8. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum RS, Gao F, Richards B, Black SE, Moscovitch M. “Where to?” Remote memory for spatial relations and landmark identity in former taxi drivers with Alzheimer's disease and encephalitis. J Cogn Neurosci. 2005;17(3):446–62. [DOI] [PubMed] [Google Scholar]
- 9.Weniger G, Ruhleder M, Lange C, Wolf S, Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia. 2010;49(3):518–27. 10.1016/j.neuropsychologia.2010.12.031 [DOI] [PubMed] [Google Scholar]
- 10.Burgess N, Trinkler I, King J, Kennedy A, Cipolotti L. Impaired allocentric spatial memory underlying topographical disorientation. Rev Neurosci. 2006;17(1–2):239–51. [DOI] [PubMed] [Google Scholar]
- 11.Chiu YC, Algase D, Whall A, Liang J, Liu HC, Lin KN, et al. Getting lost: directed attention and executive functions in early Alzheimer's disease patients. Dement Geriatr Cogn Disord. 2004;17(3):174–80. [DOI] [PubMed] [Google Scholar]
- 12.Pai MC, Lee CC, Yang YC, Lee YT, Chen KC, Lin SH, et al. Development of a questionnaire on everyday navigational ability to assess topographical disorientation in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012;27(1):65–72. 10.1177/1533317512436805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope T, Keene J, McShane RH, Fairburn CG, Gedling K, Jacoby R. Wandering in dementia: a longitudinal study. Int Psychogeriatr. 2001;13(2):137–47. [DOI] [PubMed] [Google Scholar]
- 14.Lai KY, Arthur DG. Wandering behaviour in people with dementia. J Adv Nurs. 2003;44(2):173–82. [DOI] [PubMed] [Google Scholar]
- 15.Algase DL. What's new about wandering behaviour? An assessment of recent studies. Int J Older People Nurs. 2006;1(4):226–34. 10.1111/j.1748-3743.2006.00043.x [DOI] [PubMed] [Google Scholar]
- 16.Algase DL, Moore DH, Vandeweerd C, Gavin-Dreschnack DJ. Mapping the maze of terms and definitions in dementia-related wandering. Aging Ment Health. 2007;11(6):686–98. [DOI] [PubMed] [Google Scholar]
- 17.Tu MC, Pai MC. Getting lost for the first time in patients with Alzheimer's disease. Int Psychogeriatr. 2006;18(03):67–70. [DOI] [PubMed] [Google Scholar]
- 18.Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver route-following and safety errors in early Alzheimer disease. Neurology. 2004;63(5):832–7. [DOI] [PubMed] [Google Scholar]
- 19.Teng EL, Lin KN, Chou P, Fuh JL, Wang SJ, Liu HC. The Cognitive Abilities Screening Instrument and preliminary findings of its Chinese version, CASI-2.0. Chin J of Clin Psy. 1994;2:69–73. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 21.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 2005;6(01):45–58. [DOI] [PubMed] [Google Scholar]
- 22.Lin KN, Wang PN, Liu CY, Chen WT, Lee YC, Liu HC. Cutoff scores of the cognitive abilities screening instrument, Chinese version in screening of dementia. Dement Geriatr Cogn Disord. 2002;14(4):176–82. [DOI] [PubMed] [Google Scholar]
- 23.Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71(12):888–95. 10.1212/01.wnl.0000326262.67613.fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hort J, Laczó J, Vyhnálek M, Bojar M, Bureš J, Vlček K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci USA. 2007;104(10):4042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69(10):986–97. [DOI] [PubMed] [Google Scholar]
- 26.Lee YT, Pai MC. Recognition of personally familiar scenes in patients with very mild Alzheimer's disease: Effects of spatial frequency and luminance. J Alzheimers Dis. 2012;29(2):441–8. 10.3233/JAD-2011-111601 [DOI] [PubMed] [Google Scholar]
- 27.Lezak MD. Neuropsychological assessment: Oxford University Press, USA; 2004. [Google Scholar]
- 28.Giannakopoulos P, Gold G, Duc M, Michel JP, Hof PR, Bouras C. Neural substrates of spatial and temporal disorientation in Alzheimer's disease. Acta neuropathologica. 2000;100(2):189–95. [DOI] [PubMed] [Google Scholar]
- 29.Hirono N, Mori E, Ishii K, Ikejiri Y, Imamura T, Shimomura T, et al. Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64(4):552–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Wahlund LO, Svensson L, Winblad B, Julin P. Cingulate cortex hypoperfusion predicts Alzheimer's disease in mild cognitive impairment. BMC Neurol. 2002;2(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. J Cogn Neurosci. 2005;17(2):183–98. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum RS, Priselac S, Kohler S, Black SE, Gao F, Nadel L, et al. Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat Neurosci. 2000;3:1044–8. [DOI] [PubMed] [Google Scholar]
- 33.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–76. [DOI] [PubMed] [Google Scholar]
- 34.Rainville C, Passini R, Marchand N. A multiple case study of wayfinding in dementia of the Alzheimer type: decision making. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2001;8(1):54–71. [Google Scholar]
- 35.Jellinger KA. Neuropathological subtypes of Alzheimer’s disease. Acta neuropathologica. 2012;123(1):1–2. [DOI] [PubMed] [Google Scholar]
- 36.Helkala EL, Laulumaa V, Soininen H, Partanen J, Riekkinen PJ. Different patterns of cognitive decline related to normal or deteriorating EEG in a 3‐year follow‐up study of patients with Alzheimer's disease. Neurology. 1991;41(4):528–32. [DOI] [PubMed] [Google Scholar]
- 37.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. The Lancet Neurology. 2011;10(9):785–96. 10.1016/S1474-4422(11)70156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayeux R, Stern Y, Spanton S. Heterogeneity in dementia of the Alzheimer type. Neurology. 1985;35(4):453–61. [DOI] [PubMed] [Google Scholar]
- 39.Binetti G, Magni E, Padovani A, Cappa SF. Neuropsychological heterogeneity in mild Alzheimer's disease. Dementia. 1993;4(6):321–6. [DOI] [PubMed] [Google Scholar]
- 40.Shiino A, Watanabe T, Maeda K, Kotani E, Akiguchi I, Matsuda M. Four subgroups of Alzheimer's disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. NeuroImage. 2006;33(1):17–26. [DOI] [PubMed] [Google Scholar]
- 41.Frisoni GB, Testa C, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Structural correlates of early and late onset Alzheimer’s disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2005;76(1):112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pai MC, Yang YC. Impaired translation of spatial representation in young onset Alzheimer's disease patients. Curr Alzheimer Res. 2013;10(1):95–103. [PubMed] [Google Scholar]
- 43.Wang TY, Kuo YC, Ma HI, Lee CC, Pai MC. Validation of the Route Map Recall Test for getting lost behavior in Alzheimer's disease. Arch Clin Neuropsychol. 2012;27(7):781–789. 10.1093/arclin/acs073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations: GL = getting lost; QuENA = Questionnaire of Everyday Navigational Ability; LSA = landmark and scene agnosia; ED = egocentric disorientation; INA = inattention; HD = heading disorientation. a Analyzed by Pearson’s Chi-square and the percentages were within GL events.
(DOC)
Abbreviation: GL = getting lost; p1 = p value within Group A; p2 = p value within Group B. a Analyzed by Pearson’s Chi-square, and the percentages were within GL events.
(DOC)
Abbreviations: GL = getting lost; PwAD = people with Alzheimer's disease; Group A = without any GL records at baseline; Group B = with one or more GL events before baseline; INC = with GL incidence; FFG = remaining free from GL; REC = with GL recurrence; FFR = free from GL recurrence; p1 = p value within Group A; p2 = p value within Group B. a Analyzed by Pearson’s Chi-square, and the percentages were within GL events. *The changes were derived by subtracting follow-up scores from baseline scores.
(DOC)
Abbreviations: GL = getting lost; OR = odds ratio; p1 = p value within Group A; p2 = p value within Group B. a Adjusted for age, sex, and years of education.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.